Introduction
The liver in vertebrates performs a number of vital
functions, including metabolic and detoxification activities
(1). Reactive oxygen species
(ROS), generated under conditions of oxidative stress, are
considered to be involved in the liver damage, which is induced
under a variety of conditions, including alcohol abuse,
fibrosis/cirrhosis, hepatocellular carcinoma, ischemia/reperfusion
liver injury, paracetamol overdose and viral hepatitis (2). ROS are produced and degraded by all
aerobic organisms, and exert beneficial effects, including
cytotoxicity against bacteria and other pathogens during the
maintenance of normal cell function. However, when ROS are present
in excess, the state called 'oxidative stress' arises, which is
associated with DNA damage, protein oxidation, carbonylation, lipid
peroxidation, mitochondrial dysfunction, calcium homeostasis, actin
reorganization, NAD depletion, impairment of energy metabolism and
glutathione depletion in various cell types (3–5). To
protect the human body against highly toxic ROS, various
antioxidative stress mechanisms have been acquired, including an
antioxidant defense system, which comprises intracellular
antioxidant enzymes, including superoxide dismutase (SOD) and
catalase (CAT), and glutathione (4,5).
Hydrogen peroxide (H2O2), one
of the ROS molecules, is a by-product of oxidative stress, which is
considered to act as a trigger of apoptosis in various cell types
(3,6). Previous studies reported that
H2O2-induced apoptotic cell death was
associated with caspase-3 (7).
Various processes activate apoptosis and, in particular, caspase-3
activation may ensure the efficient completion of apoptotic cell
death (7). Therefore, the
prevention of oxidative stress may reduce apoptotic cell death.
Previous studies indicated that antioxidant herbal
supplements are promising agents in therapeutic intervention
strategies for the prevention and treatment of hepatic disorders
(8,9). Rhus javanica Linn (R.
javanica), from the family Anacardiaceae, is a deciduous,
arborescent plant widely grown at the foot of mountains and in
ravines in China, Korea and other Asian countries (10,11).
Traditionally, the fruit of the R. javanica plant has been
used variously as an antidiarrheal, antitussive, anticoagulant and
an antiperspirant. The leaves of R. javanica have been used
as a detoxicant and as an antivenom for snake bites, and are
renowned for their liver-protecting and antioxidative effects. The
parasitic cocoon, termed 'Gallunt', on R. javanica, which
contains gallotannin as its major component, also functions as an
antidiarrheal, antibiotic and as a detoxicant, and exerts effects
in hemostasis and in liver protection (12). Furthermore, it was reported that
R. javanica has been widely used for centuries to treat
cancer, dysentery, diarrhea, parasitic and bacterial infections in
Korea, China, Japan and other Asian countries (11,13,14).
However, at present, the detailed mechanism underlying the action
of the active agent(s) of R. javanica, and its beneficial
effects on H2O2-induced oxidative stress,
remain to be fully elucidated. In the present study, the
anti-oxidative properties of R. javanica were investigated,
as well as the mechanism underlying these antioxidative effects in
human Chang liver cells.
Materials and methods
Plant material
Dried plant material of R. javanica was
purchased from a local herb market in Daejeon, South Korea. The
plant material was authenticated by Professor Jung-Bo Kim, a
taxonomist at Konkuk University, South Korea, and a voucher
specimen (MDS-KKU/RJ2012) was deposited in our department herbarium
at Konkuk University for future reference. The extraction was
performed with the assistance of the Plant Extract Bank (Daejeon,
South Korea). Briefly, the plant material (100 g) was extracted
with 75% ethanol three times under reflux. The extract was filtered
and concentrated by rotary evaporation at 50°C under a vacuum. The
resulting residue was moved to a vacuum oven at 40°C and dried for
48 h to yield a solid extract [R. javanica ethanolic extract
(RJE); yield, 10.344 g]. RJE was dissolved in dimethylsulfoxide
(DMSO; Sigma-Aldrich, St. Louis, MO, USA) and was subsequently
diluted to the required concentrations (0.1, 0.3 and 0.5 mg/ml)
with complete medium (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) for experimental use.
Cell culture
Human Chang liver cells were obtained from American
Type Culture Collection (Manassas, VA, USA). Gibco™ Dulbecco's
modified Eagle's medium (DMEM), penicillin/streptomycin and the
other materials required for the culture of cells were purchased
from Thermo Fisher Scientific, Inc. All other chemicals were of
analytical grade, or of the highest grade available commercially.
Cell lines were maintained at 37°C in Gibco™ RPMI-1640 medium
(Thermo Fisher Scientific, Inc.), supplemented with Invitrogen™ 10%
fetal bovine serum, penicillin and streptomycin (100 units/ml;
Thermo Fisher Scientific, Inc.) in a humidified 5%
CO2/95% air atmosphere.
Determination of the ferric reducing
antioxidant power (FRAP) assay
A FRAP assay was performed, according to the
procedure described previously, with slight modifications (15). Briefly, FRAP reagent was prepared
from 300 mM acetate buffer (pH 3.6), 10 mM
2,4,6-tripyridyl-s-triazine solution (Sigma-Aldrich) in 40 mM HCl
and 20 mM iron (III) chloride solution (Sigma-Aldrich) in the
proportions of 10:1:1 (v/v), respectively. A total of 950 µl
FRAP reagent was added 50 µl RJE. Following a period of 4
min, the absorbance of the colored product (ferrous
tripyridyltriazine complex) was measured at 593 nm using a
Hewlett-Packard UV-Vis spectrophotometer (Palo Alto, CA, USA). The
results were expressed as the µM Fe(II)/mg dry mass, and
were compared against those of butylated hydroxytoluene (BHT),
which was used as a reference compound. All measurements were
recorded in triplicate and the mean values were calculated.
2,2′-azinobis
[3-ethylbenzthiazoline]-6-sulfonic acid (ABTS) free radical
scavenging assay
The total antioxidant status of the RJE was measured
using an ABTS assay (16). ABTS
was dissolved in deionized water to a final concentration of 7 mM,
and potassium persulfate (Sigma-Aldrich) was subsequently added to
a concentration of 2.45 mM. The working solution was subsequently
prepared by mixing the two stock solutions in equal quantities, and
allowing them to react for 12 h at room temperature in the dark.
The solution was subsequently diluted by mixing 1 ml ABTS solution
with 60 ml methanol to obtain an absorbance of 0.706±0.001 units at
734 nm, as measured spectrophotometrically (Ultrospec 2100 Pro; GE
Healthcare Bio-Sciences, Pittsburgh, PA, USA). Fresh ABTS solution
was prepared for each assay. RJE (1 ml) was allowed to react with 1
ml ABTS solution, and the absorbance was measured at 734 nm
following an incubation of 7 min using the spectrophotometer. The
ABTS scavenging capacity of the extract was compared with that of
BHT. The total antioxidant activity was expressed as mM Trolox
(Sigma-Aldrich) equivalent antioxidant capacity.
Determination of the total polyphenol
content
The total phenolic acid content in RJE was estimated
using a modified Folin-Ciocalteu method (17). An aliquot of the extract was mixed
with 5 ml Folin-Ciocalteu reagent (previously diluted with water at
1:10, v/v; Sigma-Aldrich) and 4 ml (75 g/l) sodium carbonate
solution (Sigma-Aldrich). The tubes were vortexed for 15 sec and
allowed to stand for 30 min at 40°C to enable color development.
The absorbance was subsequently measured at 765 nm using the
Hewlett-Packard UV-Vis spectrophotometer. The total polyphenol
content in the RJE was compared with gallic acid equivalents (GAEs)
using a gallic acid (0–0.6 mg/ml) standard calibration curve.
Additional dilutions were performed when the absorbance value
measured was determined to be over the linear range of the standard
curve. The results were expressed as mg GAEs. All measurements were
performed in triplicate and the mean values were calculated.
Determination of the total flavonoid
content
The total flavonoid content was determined using the
aluminum chloride colorimetric assay, as previously described
(18). Briefly, distilled water (4
ml) was added to 1 ml RJE, and subsequently 5% sodium nitrite
solution (0.3 ml; Sigma-Aldrich) was added, followed by 10%
aluminium chloride solution (0.3 ml; Sigma-Aldrich). The mixtures
were incubated at room temperature for 5 min, and 2 ml 1 mM/l
sodium hydroxide solution was subsequently added. The volume of the
reaction mixture was made up to 10 ml straight away with distilled
water. The mixture was thoroughly vortexed and the absorbance of
the pink color, which had developed, was determined at 510 nm. A
calibration curve was prepared using catechin as the reference
compound and the results were expressed as mg catechin equivalents
(CEs).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The cytotoxicity of RJE in Chang liver cells was
measured using an MTT assay. The assay is based on the cleavage of
yellow tetrazolium salt dye into purple formazan by metabolically
active cells, which can be photometrically quantified. An increase
in the number of living cells results in an increase in total
metabolic activity, which consequently leads to a higher formation
of purple coloration. At 24 h following cell treatment, 20
µl MTT dye (5 mg/ml) was added into each well and incubated
for a duration of 4 h. The medium was subsequently discarded and
the intracellular formazan product was dissolved in 150 µl
DMSO. The absorbance of each well was measured at 540 nm using an
enzyme-linked immunosorbent assay reader (Bio-Rad Model 680
Microplate reader; Bio-Rad, Hercules, CA, USA), and the cell
viability was expressed as the percentage of the control cells.
Determination of SOD enzyme activity
The total proteins were extracted from cultured
cells using Pro-Prep™ reagent (Intron Biotechnology, Inc., Seoul,
South Korea) and the protein content was measured using a Bradford
assay, with bovine serum albumin (BSA) as the standard. The level
of SOD enzyme activity in the Chang liver cells was measured using
a SOD determination kit (Sigma-Aldrich). Assays were performed in
the manufacturer-provided 96-well plates. Briefly, samples (10
µl) were added to 200 µl diluted radical detector.
The reaction was initiated by adding 20 µl diluted xanthine
oxidase (Sigma-Aldrich) to each well using a multichannel pipette.
The plate was carefully agitated and incubated at room temperature
for 20 min. The absorbance was measured at 450 nm using a plate
reader, and the SOD activity was expressed as U/mg cellular
protein.
Determination of the CAT enzyme
activity
The total proteins were extracted from cultured
cells using Pro-Prep™ reagent (Intron Biotechnology, Inc.), and the
protein content was measured using the Bradford assay, with BSA as
the standard. The level of CAT enzyme activity in the Chang liver
cells was measured using a CAT assay kit (Sigma-Aldrich). One unit
of CAT was defined as the quantity of enzyme required to decompose
1 µM H2O2 in 1 min. The rate of
decomposition of H2O2 was measured
spectrophotometrically at a wavelength of 570 nm, and the enzyme
activity was expressed as U/mg protein.
Cell cycle analysis
Chang liver cells were collected following
treatments with RJE and H2O2, centrifuged
(180 × g, 10 min, 4°C), resuspended in phosphate-buffered saline
(PBS) and incubated with 80% ethyl alcohol overnight at −4°C, prior
to further analysis. The cells were subsequently washed twice with
PBS, suspended in 1 ml cold propidium iodide (PI) solution (50
µg/ml PI and 100 µg/ml ribonuclease). Subsequently,
the cells were incubated on ice for 30 min in the dark prior to
flow cytometric analysis using a Fluorescence-Activated Cell
Sorting Calibur flow cytometry (BD Biosciences, Franklin Lakes, NJ,
USA) and analyzed by Cell Quest software version 5.1 (BD
Biosciences).
Western blot analysis
The total proteins were extracted from cultured
cells using Pro-Prep™ reagent (Intron Biotechnology, Inc.) and
stored at −80°C until further use. Total protein concentration was
determined using an Invitrogen™ Quant-iT protein assay kit (Thermo
Fisher Scientific, Inc.). Equal amounts of protein (20 µg)
were separated using 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (Sigma-Aldrich), and transferred onto
nitrocellulose membranes (Bio-Rad). The membranes were subsequently
blocked for 90 min at room temperature in Tris-buffered saline and
Tween-20 buffer, comprising 20 mM Tris-HCl (pH 7.6), 135 mM sodium
chloride, 1% Tween-20 and 5% non-fat dried milk. The blots were
subsequently incubated at 4°C for 8 h separately with specific
mouse monoclonal anti-p53 (1:1,000; cat. no. 554167; BD
Biosciences), mouse monoclonal anti-Bax (1:1,000; cat. no. BS2538;
Bioworld Technology, Inc., St. Louis Park, MN, USA), mouse
monoclonal anti-caspase-3 (1:1,000; cat. no. 9661; Cell Signaling
Technology, Inc., Danvers, MA, USA), and mouse monoclonal
anti-Bcl-2 (1:1,000; cat. no. 610746; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) antibodies. Following incubation, the
membrane was washed three times for 5 min in Tris-Buffered saline
and Tween-20 buffer, prior to subsequent treatment with
peroxidase-conjugated anti-rabbit immunoglobulin G antibody (Vector
Laboratories, Inc., Burlingame, CA, USA) at a dilution of 1:1,000
for ~2 h at room temperature. The membrane was washed and incubated
with the substrate from an enhanced chemiluminescence reagent kit
(DuPont NEN, Boston, MA, USA). The proteins were detected using a
Fujifilm LAS-3000 Imager (Tokyo, Japan). As an internal control,
the expression of β-actin was analyzed using a β-actin antibody
(1:5,000; cat. no. sc-1615; Santa Cruz Biotechnology, Inc.). In
order to remove the antibodies and substrates, the blots were
stripped using Restore™ Western Blot Stripping buffer (Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Following stripping, the blot was reprobed with β-actin antibody to
monitor the loading control.
Statistical analysis
The data were evaluated for statistical significance
using SPSS 14.0 software for Windows (SPSS, Inc., Chicago, IL,
USA). The data are expressed as the mean ± standard error of the
mean. The mean values were compared using a one-way analysis of
variance, followed by Duncan's multiple-range test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of RJE on
H2O2-induced cytotoxicity in the Chang liver
cells
Initially, to investigate the RJE-induced
cytotoxicity of the cells, Chang liver cells were exposed to RJE at
concentrations of 0, 0.1, 0.3, 0.5 or 1.0 mg/ml for 24 h, and the
cytotoxicity was determined using an MTT assay. As shown in
Fig. 1, RJE exhibited no signs of
cytotoxicity up to a concentration of 0.5 mg/ml. Therefore, the
subtoxic concentrations of 0.1, 0.3 and 0.5 mg/ml were selected for
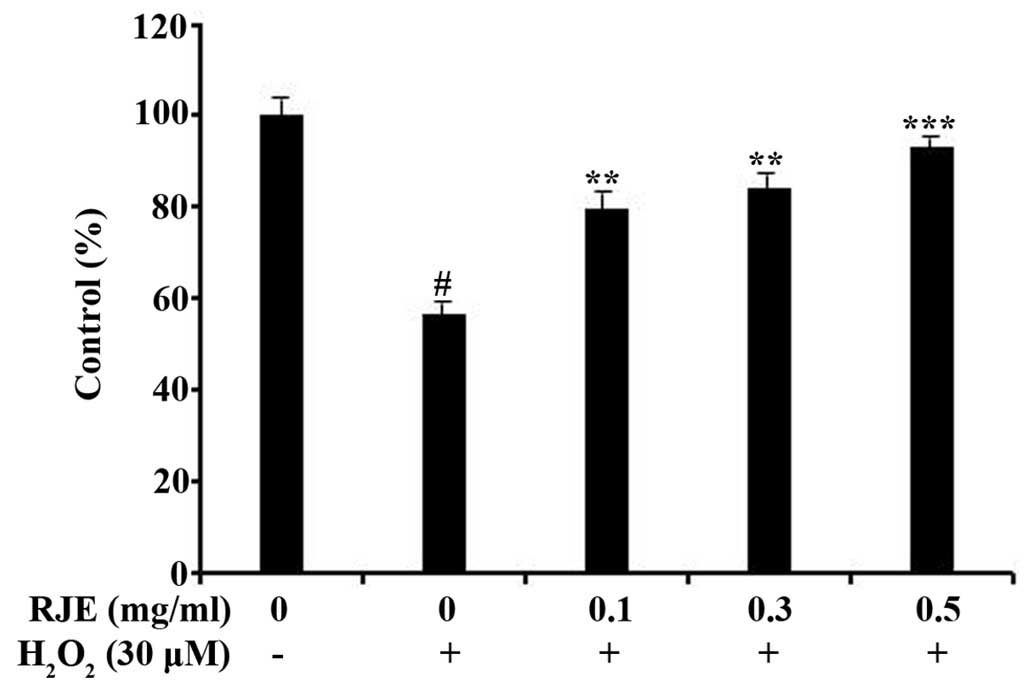
subsequent experiments. H2O2 (30 µM)
significantly (P<0.05) reduced the cell viability of Chang liver
cells (Fig. 2). However, RJE
pretreatment (at concentrations of 0.1, 0.3 or 0.5 mg/ml)
significantly (P<0.05, P<0.01 and P<0.001 for 0.1, 0.3 and
0.5 mg/ml, respectively) inhibited in a concentration-dependent
manner the cytotoxicity induced by H2O2 in
Chang liver cells (Fig. 2).
RJE enhances the
H2O2-induced CAT and SOD activity in Chang
liver cells
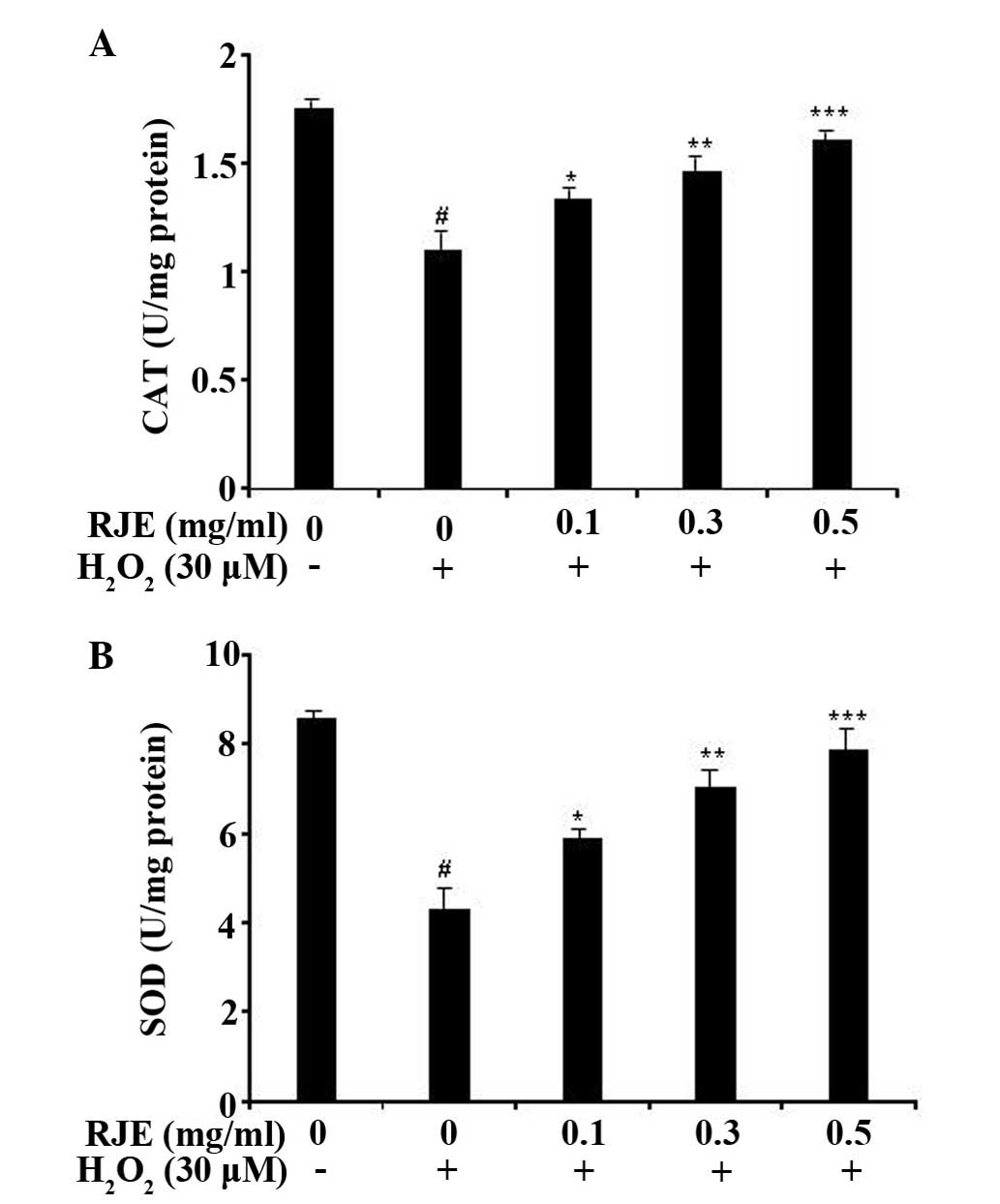
It is well known that the activity of endogenous
antioxidant enzymes, including CAT and SOD, protects cells from
ROS-induced oxidative damage (4,5). In
order to investigate whether RJE was mediated by the activities of
antioxidant enzymes, CAT and SOD enzyme activities were measured in
H2O2-damaged Chang liver cells.
H2O2 (30 µM) significantly (P<0.05)
decreased the activities of CAT and SOD compared with the control
cells. However, RJE-treated groups increased the enzyme activity of
CAT and SOD in a concentration-dependent manner (Figs. 3A and B). Treatment of the cells
with RJE at a concentration of 0.5 mg/ml attenuated the
H2O2-induced suppression of CAT and SOD
enzyme activities to almost normal levels (P<0.001). These
results supported the hypothesis that RJE protects Chang liver
cells from H2O2-induced cytotoxicity by
increasing the levels of the endogenous antioxidant enzymes.
Effect of RJE on the cell cycle in
H2O2-induced Chang liver cells
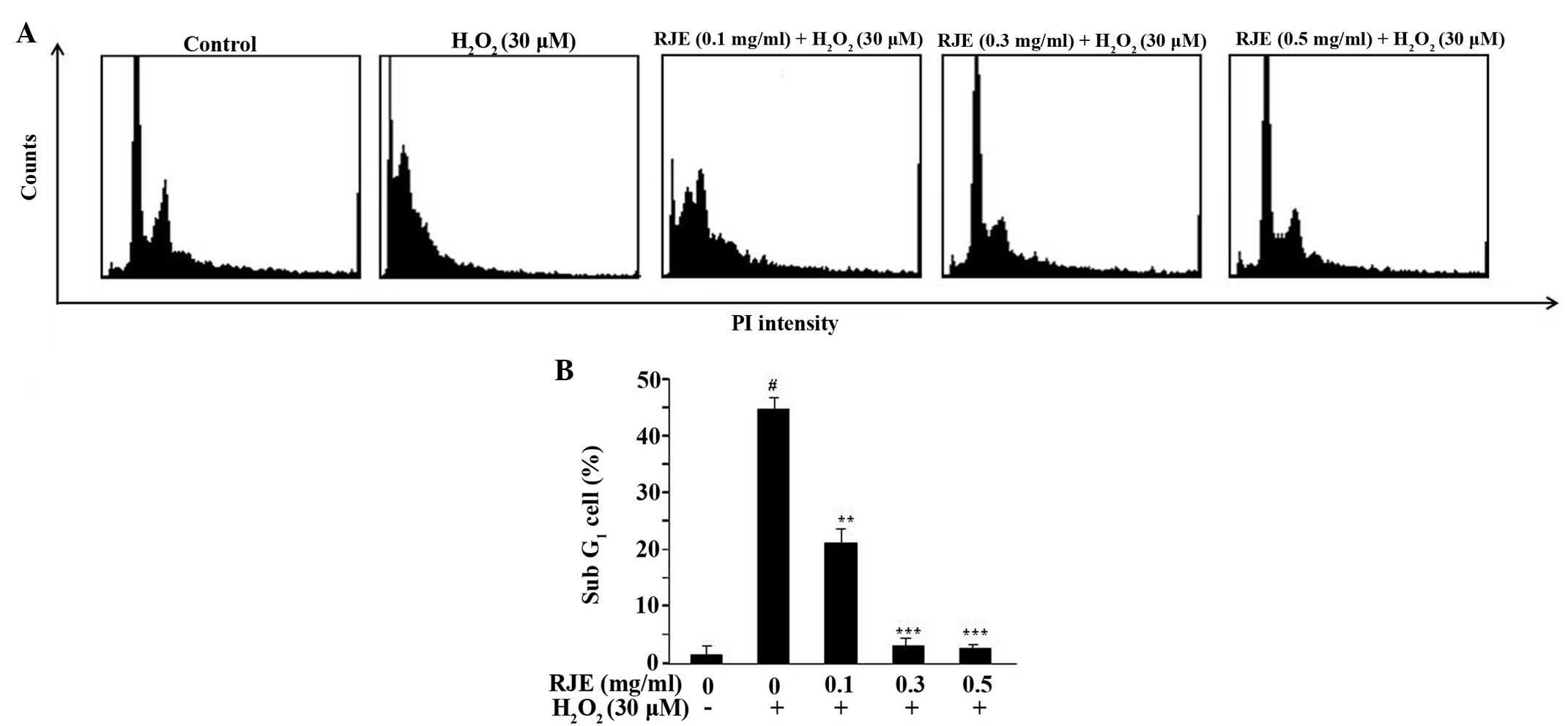
The flow cytometric analysis of the
H2O2-treated cells, treated with or without
RJE, is shown in Fig. 4A. A
significant effect (P<0.001) was observed on treatment of the
cells with 0.3 and 0.5 mg/ml RJE, with 3.09 and 2.02% of the cells
being counted in the sub-growth (G)1 phase,
respectively, compared with the H2O2-treated
cells without RJE treatment (44.86% of the cells counted in the
sub-G1 phase; Fig. 4B).
These results suggested that RJE treatment exerted a marked effect
by reducing the death of the Chang liver cells.
Antioxidant potential of RJE in FRAP and
ABTS free radical scavenging assay
The antioxidant capacity of RJE was determined using
the ABTS method and the FRAP assay. With the ABTS assay, an
inhibition of the generation of the ABTS·+ radical
cation provides the basis of the spectrophotometric method, which
may be applied in order to measure the total antioxidant activities
of solutions of pure substances, aqueous mixtures and beverages.
Trolox was used as a positive control. The total antioxidant
activity was expressed as the mM Trolox equivalent antioxidant
capacity, by reference to the Trolox standard calibration curve.
The ABTS radical scavenging activity was determined to be 0.93±0.06
mM Trolox equivalents/mg extract, and that of BHT was 0.94±0.02 mM
Trolox equivalents/mg dry weight.
Furthermore, the FRAP assay was used, as it is quick
and simple to perform, and the reaction is reproducible and
linearly correlated with the molar concentration of the
antioxidants (19). The ferric
reducing antioxidant power of RJE was expressed as the mM ferrous
iron equivalents per mg dry weight of RJE. The antioxidant activity
of RJE using FRAP assay was determined to be 0.88±0.09 mM
FeSO4 equivalents/mg extract, and 1.88±0.16 mM
FeSO4 equivalents/mg dry weight for BHT. These results
indicated that RJE exhibited antioxidant effects.
Determination of the total polyphenol and
flavonoid content in RJE
Polyphenols, including flavonoids, have received
considerable attention due to their marked antioxidant activities
(20). Therefore, we measured the
total polyphenolic and flavonoid content in RJE. The quantity of
the total polyphenol and flavonoid content in RJE was determined to
be 56.12±0.06 mg GAEs/g extract and 47.5±2.33 mg CEs/g extract,
respectively.
Effect of RJE on the expression levels of
apoptotic signaling molecules in H2O2-induced
Chang liver cells
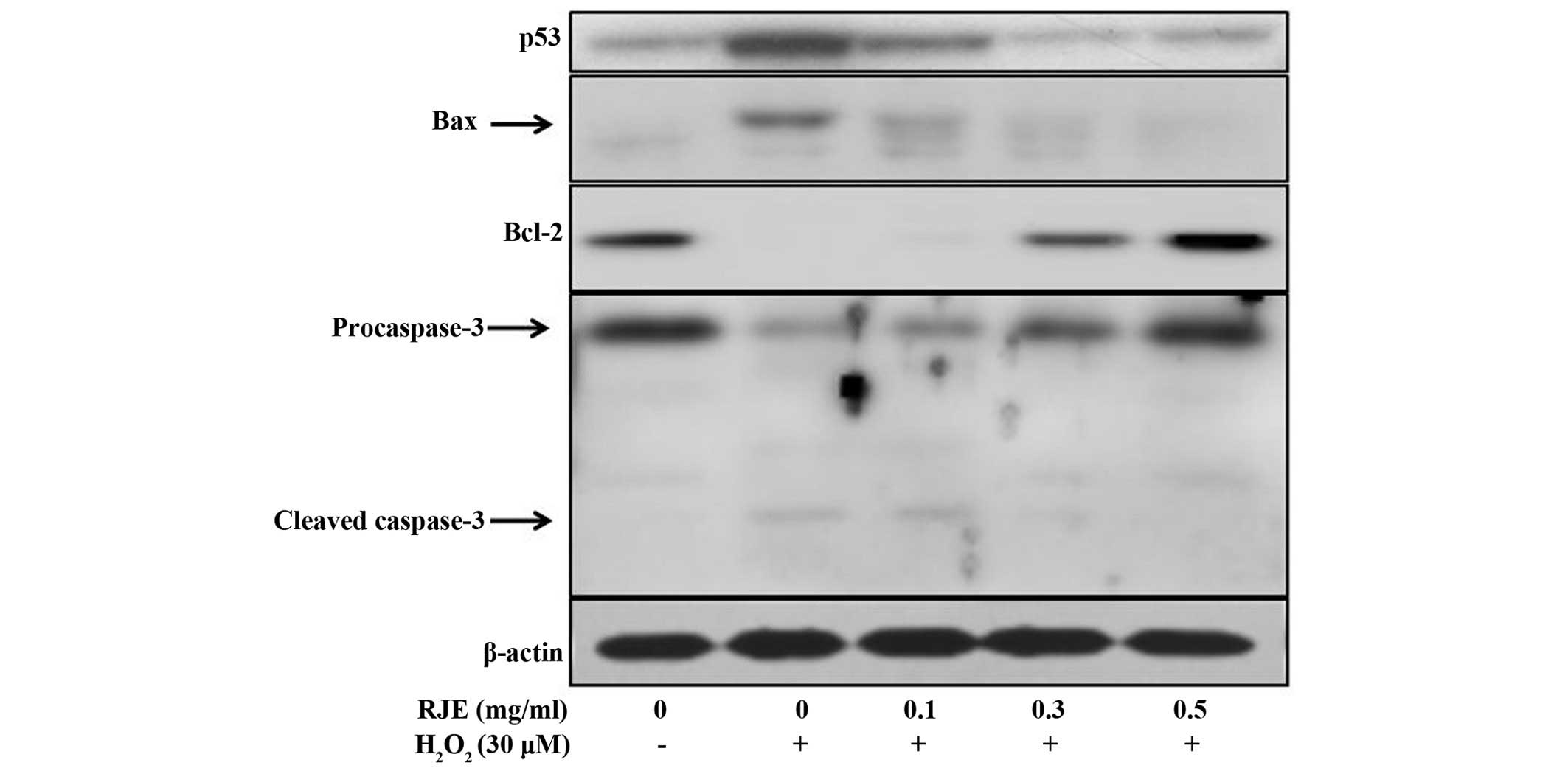
ROS induce a variety of physiological and cellular
events, including DNA damage and apoptosis. Several genes exert
important roles in apoptotic pathways. The p53 gene is able to
activate the cell cycle checkpoint, DNA repair and apoptosis. The
ratio of Bax protein to Bcl-2 protein functions as a cell death
'switch'. Therefore, the present study investigated the effect of
RJE on H2O2-induced apoptotic signaling
mediators in Chang liver cells. As shown in Fig. 5, RJE treatment regulated the
protein expression levels of the apoptotic genes in
H2O2-induced cytotoxicity in Chang liver
cells. The expression levels of proapoptotic proteins, including
p53, Bax and cleaved caspase-3, were downregulated, whereas the
expression level of the antiapoptotic protein, Bcl-2, was markedly
upregulated. These results, therefore, provided the evidence that
RJE reduced apoptosis in Chang liver cells via a decrease in the
protein expression levels of p53, Bax and cleaved caspase-3, and an
increase in the protein expression of Bcl-2.
Discussion
Oxidative stress, which is produced by ROS, provides
one of the major determining factors of cellular injuries in a
variety of aberrant clinical conditions, including hepatoprotection
(21). In the present study, RJE
elicited protective effects against
H2O2-induced cytotoxicity via an inhibition
of the generation of intracellular ROS in Chang liver cells. The
natural antioxidant system comprises various antioxidant compounds
and antioxidant enzymes, including SOD, CAT and glutathione
peroxidase. ROS is converted into H2O2 by
SOD. H2O2, in turn, is converted into
molecular oxygen and H2O by CAT (22). In addition, previous studies
indicated that CAT is considered to be the most important enzyme
involved in the detoxification of H2O2 and
the protection of hepatocytes from oxidative stress (23). In the present study, the decreased
enzyme activity of SOD and CAT upon H2O2
induction was markedly increased in RJE-treated Chang liver cells.
These results supported the hypothesis that RJE protected Chang
liver cells from H2O2-induced cytotoxicity by
regulating the activities of the intracellular antioxidative
enzymes.
The cell cycle comprises a series of events, which
occur in a cell, leading to its division and replication, thereby
producing two daughter cells. It is divided into four different
phases: Synthesis (S) phase (chromosomal replication), mitotic (M)
phase (chromosomal condensation and separation), G1
phase (existing between M phase and S phase) and G2
phase (existing between S phase and M phase). The predominant
control point of the cell cycle is situated in the late
G1 phase. As shown in the present study, the increased
sub-G1 phase associated with H2O2
cytotoxicity was reduced by treatment with RJE, indicating that RJE
may inhibit H2O2-induced apoptosis in Chang
liver cells.
H2O2 leads to a variety of
physiological and cellular events, including inflammation, DNA
damage and apoptosis. Apoptosis is induced by extracellular or
intracellular signals, which trigger the onset of a signaling
cascade characterized by specific biochemical and cytological
signatures, including nuclear condensation and DNA fragmentation
(24). Several genes are known to
be involved in apoptotic pathways. The p53 gene activates cell
cycle checkpoints, DNA repair and apoptosis to maintain genomic
stability (25). The ratio of Bax
to Bcl-2 functions as a cell death 'switch', which determines
whether cells live or die in response to an apoptotic stimulus. An
increased Bax/Bcl-2 ratio decreases the cellular resistance to
apoptotic stimuli, leading to apoptosis (26,27).
Furthermore, destabilization of the mitochondrial integrity by
apoptotic stimuli precedes activation of caspases, leading to
apoptosis (28,29). Several genes, including those for
p53, Bax, Bcl-2 and caspase-3, are known to be involved in the
apoptotic pathway (30). In the
present study, the increased protein expression levels of p53,
proapoptotic Bax and caspase-3 in the
H2O2-induced Chang liver cells were inhibited
on treatment with RJE. By contrast, the decreased expression of the
antiapoptotic protein, Bcl-2, was increased in Chang liver cells
compared with H2O2-treated cells. These
results provided evidence that RJE inhibited
H2O2-induced apoptosis.
The use of numerous herbal and other natural
products worldwide for the prevention and treatment for oxidation
is gaining in popularity. According to the Illustrated Book of
Korean Medicinal Herbs (12), each
component part of the R. javanica plant is associated with
various medicinal efficacies. In particular, R. javanica
exerts liver protection and detoxification effects (12). Previous phytochemical studies on
RJE revealed the presence of several polyphenolic constituents,
including gallic acid, triterpenes and semialactic acid (11,31).
These compounds have been previously described to possess
antioxidant properties (11,32,33).
The data in the present study concurred with the previously
published studies that RJE exhibits marked antioxidant activity, as
determined by the ABTS free radical scavenging assay and the FRAP
assay. The present study also demonstrated that RJE has phenolic
content.
In conclusion, the present study indicated that RJE
exhibited marked protective effects against
H2O2-induced cytotoxicity and oxidative
stress in human Chang liver cells. Treatment of the cells with RJE
enhanced the activities of the antioxidative enzymes, including SOD
and CAT. Furthermore, changes in the levels of the
H2O2-induced apoptotic signaling genes were
regulated by RJE in the Chang liver cells. RJE also exhibited
marked antioxidant capacity, as determined by the ABTS and the FRAP
assays. The putative antioxidant compounds present in the extract
may act individually or in combination in delivering such
beneficial effects, thereby providing insights into the mechanism
which underpins traditional claims made for RJE in the treatment of
oxidative stress-mediated hepatic diseases.
Acknowledgments
The present study was supported by Konkuk University
in 2015.
References
|
1
|
Saladin KS: Anatomy & Physiology. The
Unity of Form and Function. 6th ed. The McGraw-Hill Companies; USA:
pp. 887–925. 2011
|
|
2
|
Muriel P: Role of free radicals in liver
diseases. Hepatol Int. 3:526–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nordberg J and Arnér ES: Reactive oxygen
species, antioxidants, and the mammalian thioredoxin system. Free
Radic Biol Med. 31:1287–1312. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Medina J and Moreno-Otero R:
Pathophysiological basis for antioxidant therapy in chronic liver
disease. Drugs. 65:2445–2461. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cerella C, Coppola S, Maresca V, De Nicola
M, Radogna F and Ghibelli L: Multiple mechanisms for hydrogen
peroxide-induced apoptosis. Ann N Y Acad Sci. 1171:559–563. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clément MV, Ponton A and Pervaiz S:
Apoptosis induced by hydrogen peroxide is mediated by decreased
superoxide anion concentration and reduction of intracellular
milieu. FEBS Lett. 440:13–18. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim MH, Chung J, Yang JW, Chung SM, Kwag
NH and Yoo JS: Hydrogen peroxide-induced cell death in a human
retinal pigment epithelial cell line, ARPE-19. Korean J Ophthalmol.
17:19–28. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sharma A, Chakraborti KK and Handa SS:
Anti-hepatotoxic activity of some Indian herbal formulations as
compared to silymarin. Fitoterapia. 62:229–235. 1991.
|
|
9
|
Britton RS and Bacon BR: Role of free
radicals in liver diseases and hepatic fibrosis.
Hepatogastroenterology. 41:343–348. 1994.PubMed/NCBI
|
|
10
|
Cha BC, Lee SB, Rhim TJ and Lee KH:
Constituents of anti-oxidative activity and free radical scavenging
effect from Galla Rhois (Rhus javanica Linne). Korean J Pharmacogn.
31:185–189. 2000.
|
|
11
|
Lee IS, Oh SR, Ahn KS and Lee HK:
Semialactone, isofou-quierone peroxide and fouquierone, three new
dammarane triterpenes from Rhus javanica. Chem Pharm Bull (Tokyo).
49:1024–1026. 2001. View Article : Google Scholar
|
|
12
|
Ahn DK: Illustrated Book of Korean
Medicinal Herbs. Kyo-Hak Publisher; Seoul, South Korea: 1998
|
|
13
|
You YO, Choi NY, Kang SY and Kim KJ:
Antibacterial Activity of Rhus javanica against
Methicillin-Resistant Staphylococcus aureus. Evid Based Complement
Alternat Med. 2013:5492072013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Djakpo O and Yao W: Rhus chinensis and
Galla chinensis - folklore to modern evidence: Review. Phytother
Res. 24:1739–1747. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Szeto YT, Chu WK and Benzie IFF:
Antioxidants in fruits and vegetables: A study of cellular
availability and direct effects on human DNA. Biosci Biotechnol
Biochem. 70:2551–2555. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Re R, Pellegrini N, Proteggente A, Pannala
A, Yang M and Rice-Evans C: Antioxidant activity applying an
improved ABTS radical cation decolorization assay. Free Radic Biol
Med. 26:1231–1237. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wolfe K, Wu X and Liu RH: Antioxidant
activity of apple peels. J Agric Food Chem. 51:609–614. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim DO, Jeong SW and Lee CY: Antioxidant
capacity of phenolic phytochemicals from various cultivars of
plums. Food Chem. 81:321–326. 2003. View Article : Google Scholar
|
|
19
|
Muller L, Gnoyke S, Popken AM and Bohm V:
Antioxidant capacity and related parameters of different fruit
formulations. LWT-Food Sci Technol. 43:992–999. 2010. View Article : Google Scholar
|
|
20
|
Othman A, Ismail A, Ghani AN and Adenan I:
Antioxidant capacity and phenolic content of cocoa beans. Food
Chem. 100:1523–1530. 2007. View Article : Google Scholar
|
|
21
|
Zhang R, Kang KA, Piao MJ, Kim KC, Kim AD,
Chae S, Park JS, Youn UJ and Hyun JW: Cytoprotective effect of the
fruits of Lycium chinense Miller against oxidative stress-induced
hepatotoxicity. J Ethnopharmacol. 130:299–306. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sindhu RK, Koo JR, Roberts CK and Vaziri
ND: Dysregulation of hepatic superoxide dismutase, catalase and
glutathione peroxide in diabetes: Response to insulin and
antioxidant therapies. Clin Exp Hypertens. 26:43–53. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Bleser PJ, Xu G, Rombouts K, Rogiers V
and Geerts A: Glutathione levels discriminate between oxidative
stress and transforming growth factor-beta signaling in activated
rat hepatic stellate cells. J Biol Chem. 274:33881–33887. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gopinath P, Gogoi SK, Sanpui P, Paul A,
Chattopadhyay A and Ghosh SS: Signaling gene cascade in silver
nanoparticle induced apoptosis. Colloids Surf B Biointerfaces.
77:240–245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sherr CJ: Principles of tumor suppression.
Cell. 116:235–246. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chiu CL, Lee TH, Shao YY and Kuo YH: Three
new triterpenes from the roots of Rhus javanica L. var.
roxburghuna. J Asian Nat Prod Res. 10:684–688. 2008. View Article : Google Scholar
|
|
27
|
Gao C and Wang AY: Significance of
increased apoptosis and Bax expression in human small intestinal
adenocarcinoma. J Histochem Cytochem. 57:1139–1148. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Timmer JC and Salvesen GS: Caspase
substrates. Cell Death Differ. 14:66–72. 2007. View Article : Google Scholar
|
|
29
|
Youle RJ and Strasser A: The Bcl-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View Article : Google Scholar
|
|
30
|
Ahmad J, Ahamed M, Akhtar MJ, Alrokayan
SA, Siddiqui MA, Musarrat J and Al-Khedhairy AA: Apoptosis
induction by silica nanoparticles mediated through reactive oxygen
species in human liver cell line HepG2. Toxicol Appl Pharmacol.
259:160–168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chung SC, Hwang BY, Oh GJ, Kang SJ, Kim
MJ, Choi WH, Lee KS and Ro JS: Chemical components from the stem
bark of Rhus javanica. L Kor J Pharmacogn. 30:295–300. 1999.
|
|
32
|
Aruoma O, Murcia A, Butler J and Halliwell
B: Evaluation of the antioxidant and prooxidant actions of gallic
acid and its derivatives. J Agric Food Chem. 41:1880–1885. 1993.
View Article : Google Scholar
|
|
33
|
Devbhuti P, Roy S, Sarkar RG and Devbhut
D: An in vitro study on effect of lactic acid and ascorbic acid on
etoposide-induced lipid peroxidation. J Pharma Sci Technol.
2:91–95. 2013.
|