Introduction
Neurogenesis is coordinated by cell proliferation,
migration, differentiation, synaptogenesis and apoptosis (1). The subventricular zone (SVZ) of the
lateral ventricle is one of the restricted neurogenesis regions in
the central nervous system (2–5).
Under normal conditions, neurogenesis is modulated by
N-methyl-D-aspartate receptor (NMDAR) (6). NMDAR is a subtype of ionotropic
glutamate receptors. NMDAR is a heterodimer primarily comprising
NR1 and NR2 (A–D) subunits (7),
while the NR3 subunit is identified less frequently (8). Functional properties of NMDAR are
strongly influenced by the type of NR2 subunits (7). NMDAR comprises NR2A and NR2B, which
are involved in synaptic plasticity and in pathological conditions
(9–11). We recently demonstrated that NR2A
and NR2B are expressed starting postnatal day 1 to postnatal day 28
in the SVZ, with each subunit showing a distinct expression pattern
(12). Furthermore, NR2A and NR2B
are differentially expressed in hypoxic-ischemic (13) and ischemia-reperfusion (14) injuries of the hippocampus. In
addition, NMDAR plays an important role in glutamate neurotoxicity
following hypoxic-ischemic injury (15). However, whether hypoxic-ischemic
injury exerts an effect on the expression of NMDAR subunits in the
SVZ remains to be determined.
In the normal adult hippocampus, the NR2B-containing
NMDAR subtypes negatively regulate neurogenesis (16), while those with NR2A promote
neurogenesis (17). This indicates
that NMDAR subunits differentially regulate neurogenesis. The
effects of NMDAR subunits on neurogenesis under pathological
conditions, such as hypoxic-inschemic injury, remain to be
elucidated. To address this knowledge gap, in the present study, we
assessed the effects of NMDAR subunits on neurogenesis in the SVZ
during hypoxic-ischemic injury.
Materials and methods
Animals
A total of 160, 7-day-old newborn Sprague-Dawley
rats weighing between 12 and 18 g, of both genders, were provided
by the Laboratory Animal Center of Xuzhou Medical College (Jiangsu,
China). Animal use was regulated by the Animal Research Principles
and Procedures established by the University's Animal Care
Committee, as well as in accordance with the National Institute of
Health Guide for the Care and Use of Laboratory Animals. In the
first experiment, 60 animals were randomly divided into three
groups of 20 animals (control, sham and hypoxic-ischemic injury
groups). The animals in each group were fed normally until a
specific time point (2, 6, 24 or 48 h after hypoxic-ischemic
injury), and then sacrificed. In the second experiment, 100 rats
were randomly divided into five groups: sham group,
hypoxic-ischemic injury group, hypoxic-ischemic injury+MK-801
(selective non-competitive NMDAR antagonist) group,
hypoxic-ischemic injury+NVP-AAM077 (NR2A antagonist) group, and
hypoxic-ischemic injury+Ro25-6981 (NR2B antagonist) group. The
animals in each group were sacrificed at a specific time point (2,
6, 24 or 48 h after hypoxic-ischemic injury).
Equipment and reagents
Selective non-competitive NMDAR antagonist MK-801
(M107), NR2A antagonist NVP-AAM077 (P1999), and NR2B antagonist
Ro25-6981 (R7150) were purchased from Sigma-Aldrich (St. Louis, MO,
USA). The 3,3′-diaminobenzidine (DAB) solution was obtained from
Beijing Jinqiao Biotechnology Co., Ltd., (Beijing, China). The
Polink-2 plus polymer horseradish peroxidase detection systems for
rabbit and mouse primary antibodies were also purchased from
Beijing Jinqiao Biotechnology Co., Ltd.. Mouse monoclonal
anti-Nestin antibody (ab11306), rabbit polycloncal anti-DCX
antibody (ab18723), rabbit polycloncal anti-NR2A antibody
(ab84181), and rabbit polyclonal anti-NR2B antibody (ab65875) were
purchased from Abcam (Hong Kong, China). The paraffin wax slicing
machine RM2235 was purchased from Leica (Wetzlar, Germany), the
photomicrography system DP25 was purchased from Olympus Corp.
(Osaka, Japan), and the image analysis software Image-Pro Plus 6.0
was purchased from Media Cybernetics, Inc. (Chicago, IL, USA).
Hypoxic-ischemic injury
The hypoxic-ischemic injury was modeled with minor
modifications as previously described (18). The animals in the control group
were anesthetized with ether and not subjected to hypoxia-ischemia.
Animals in the sham and hypoxic-ischemic injury groups received
intraperitoneal injections of sterile saline, while animals in the
drug intervention groups (MK, NVP and Ro groups) received
intraperitoneal injections of selective non-competitive NMDAR
antagonist MK-801 (0.5 mg/kg), NR2A antagonist NVP-AAM077 (5
mg/kg), and NR2B antagonist Ro25-6981 (5 mg/kg) 30 min prior to the
induction of hypoxia-ischemia. The animals of the sham,
hypoxic-ischemic, and drug intervention groups were anesthetized
with ether and subjected to hypoxia-ischemia. Specifically, after
drug injection for 30 min, the right common carotid artery was
clamped. The animals were then placed in a container and perfused
for 2 h at 37°C with a gas mixture consisting of 8% oxygen and 92%
nitrogen, at 1.5–2.5 l/min. After the treatment, the rats were fed
again.
Immunohistochemical staining
The rats were deeply anesthetized with chloral
hydrate and perfused intracardially with physiological saline
solution, followed by 4% paraformaldehyde. At the end of perfusion,
the brains were removed and fixed overnight in 4% paraformaldehyde
at 4°C. The tissues were rinsed with water, dehydrated through an
ascending series of alcohol, and embedded in paraffin through
xylene. Sections (4-μm) were cut using a Leica paraffin wax
slicing machine. Antigen retrieval was performed in a citrate
antigen retrieval solution using a microwave oven (Galanz Corp.,
Guangzhou, China). The sections were cooled to room temperature and
transferred to 3% hydrogen peroxide for 10 min to block endogenous
peroxidase. The sections were washed three times with 0.01 M
phosphate buffered saline (PBS; 5 min each wash) and blocked with
appropriate serum for 1 h at 37°C. The slices were incubated with
primary antibodies overnight at 4°C. The primary antibodies were
diluted 1:100 (anti-NR2A), 1:200 (anti-NR2B), 1:500 (anti-Nestin),
and 1:200 (anti-DCX). Subsequently, the sections were equilibrated
to room temperature, washed with PBS (pH 7.4) for 5 min, and
incubated with appropriate secondary antibodies for 30 min at 37°C.
This step was followed by 3×5 min washes in PBS. The sections were
then treated with DAB solution according to the manufacturer's
instructions. The slices were then washed with distilled water to
remove the reagent. The slices were microscopically examined, and
the images captured using the Olympus DP25 photomicrography system
(Olympus. Corp. Japan). To control for the specificity of the
staining, parallel slices were stained identically but without
primary antibodies.
Positive cell count
Cells were counted in at least five 100×100
μm2 areas of each section of three serially
sectioned brains. The number of NR2A-, NR2B-, or DCX-positive cells
in the SVZ was counted at each time point. The Nestin
immunoexpression images were subsequently processed by densitometry
with the Image-Pro Plus image analysis software, and integrated
optical density (IOD) of at least five 100×100
μm2 areas of each section of three serially
sectioned brains were obtained.
Statistical analysis
Data are presented as mean ± standard deviation. The
two-tailed Student's t-test and one-way analysis of variance tests
were used for statistical comparisons. The Student's Newman-Keuls
and Dunnet tests were used for post hoc analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of hypoxia-ischemia on the
expression of NR2A and NR2B subunits in the SVZ
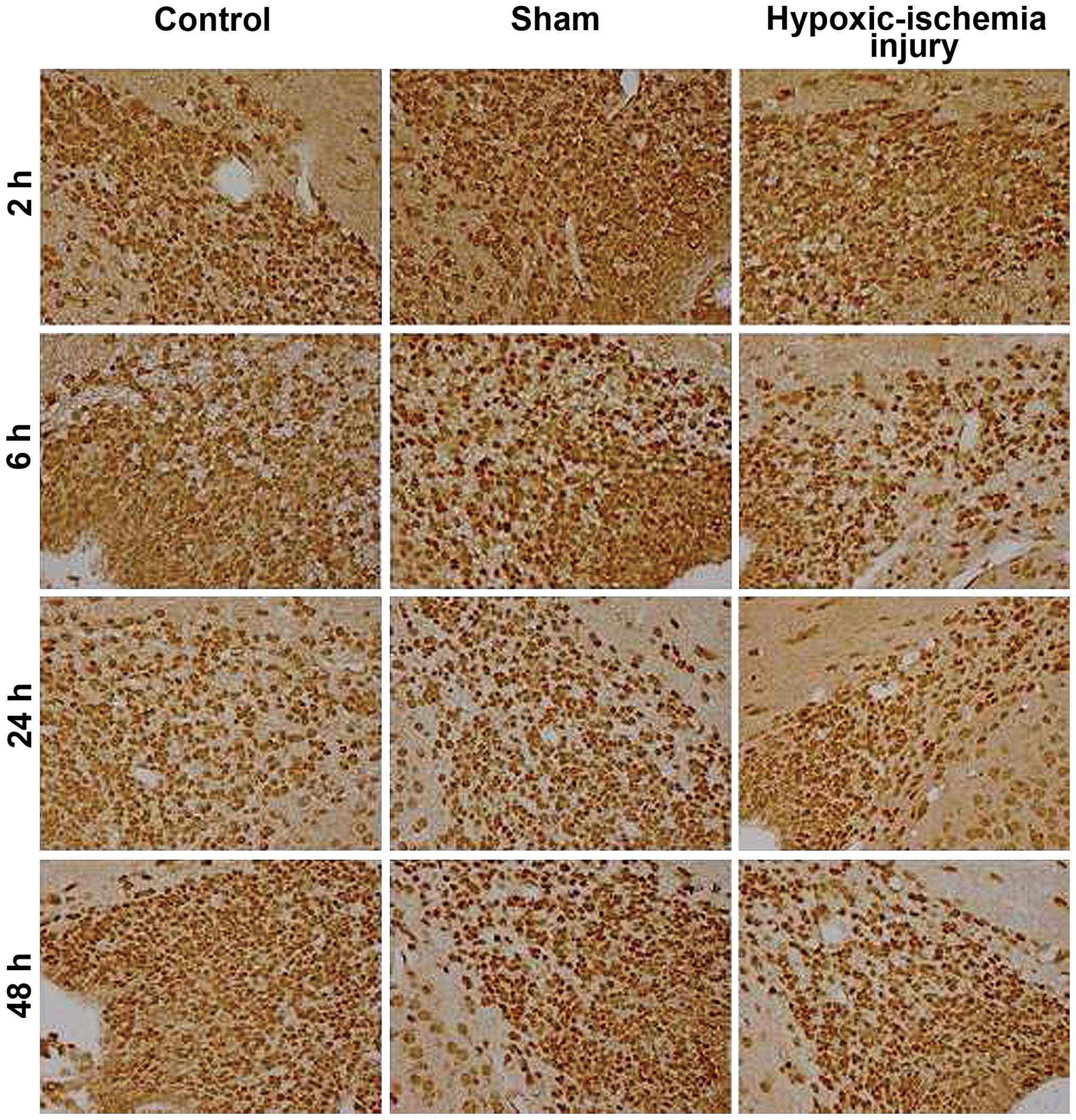
NMDAR subunits NR2A and NR2B were expressed in the
SVZ cells of rat brain at 2, 6, 24 and 48 h following
hypoxic-ischemic injury. NR2A-positive cells were mainly located in
the dorsal lateral horn of the lateral ventricle (Fig. 1). The majority of the positive
cells were distributed irregularly in the SVZ, with the exception
of the ependymal layer. NR2A-positive cells in the membrane and
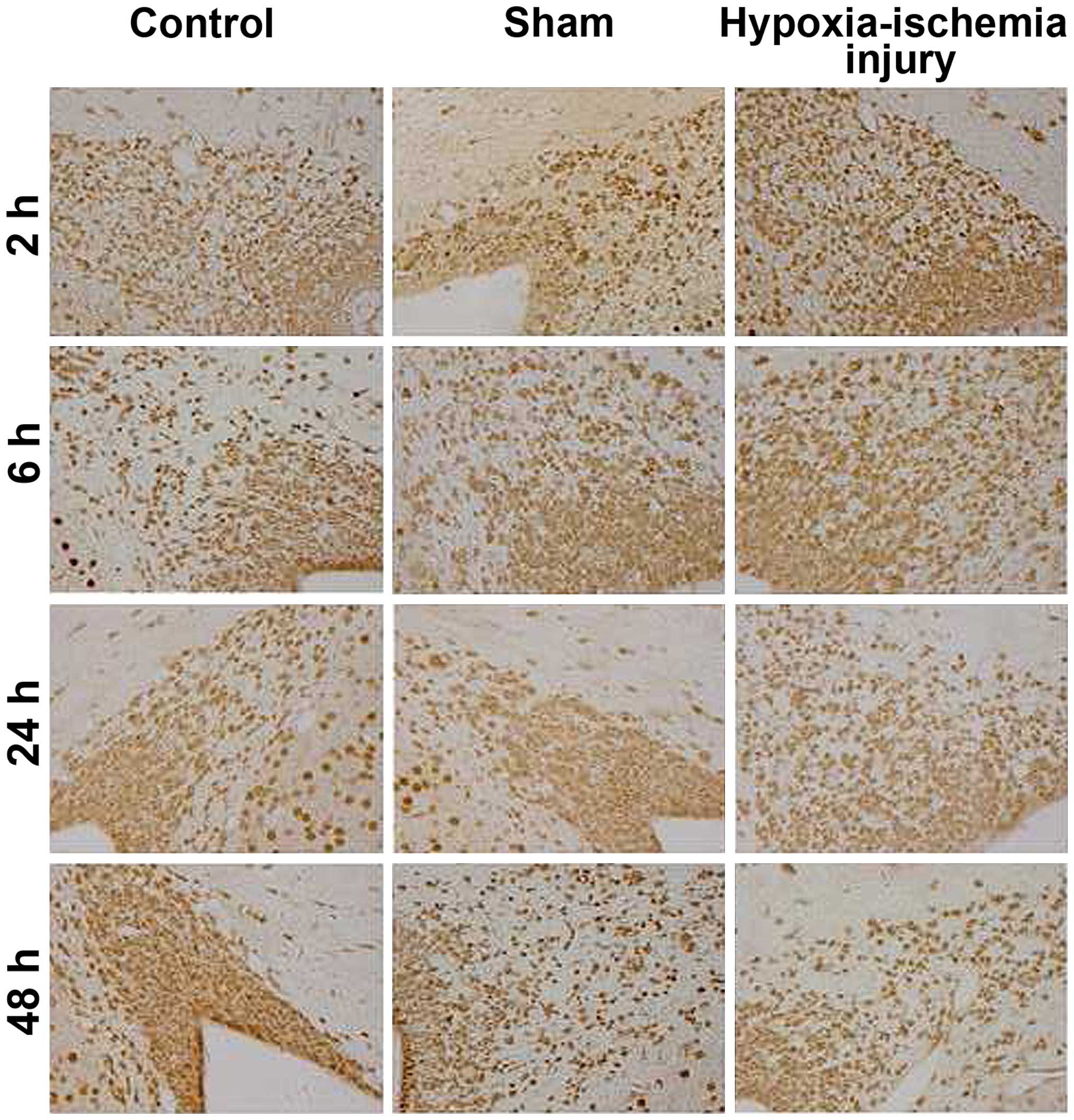
cytoplasm showed strong immunopositivity. NR2B immuno-expression
was also detected in a similar location (Fig. 2). The immunoreactivity of NR2B was
weaker than that of NR2A at each tested time point.
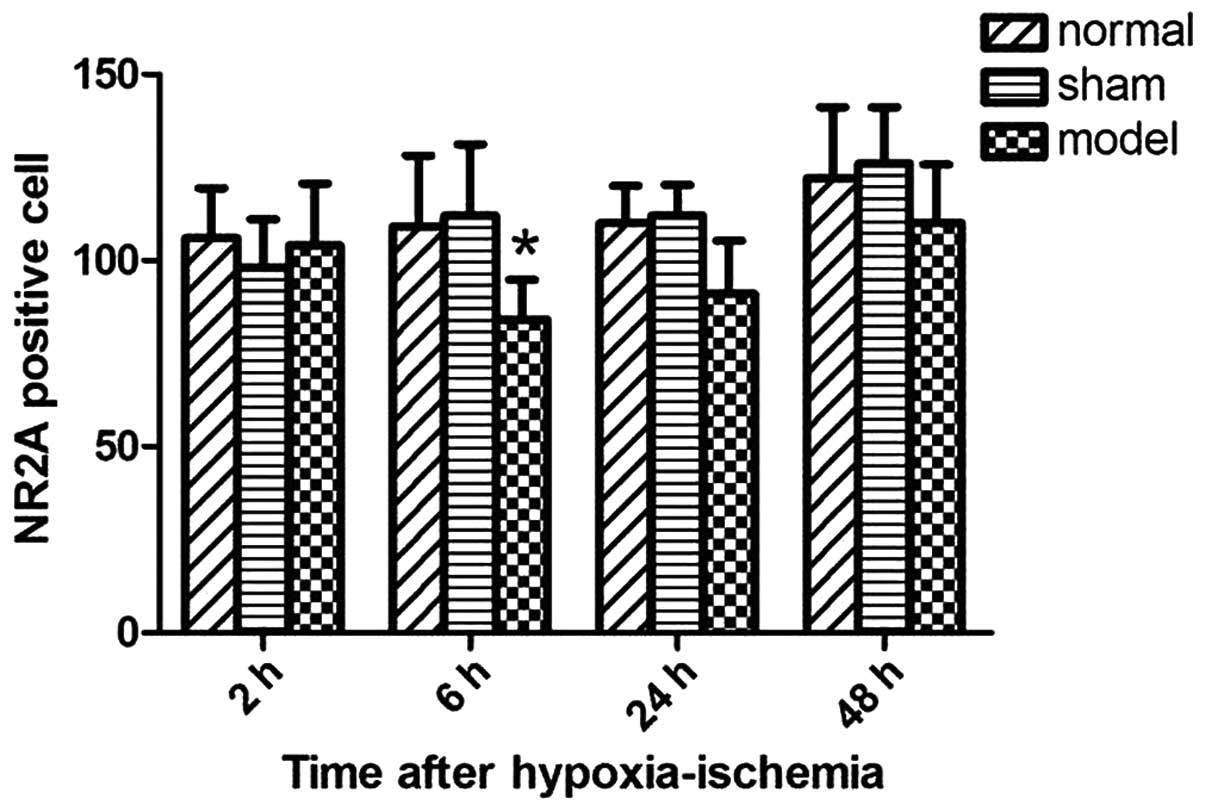
Expression of NR2A showed a 'decreased first and
then increased' pattern. At 6 h after hypoxic-ischemia injury, NR2A
expression significantly decreased (P<0.05 vs. control group;
Fig. 3). The difference between
the hypoxic-ischemic injury and control groups did not reach
statistical significance at 24 and 48 h after induction of
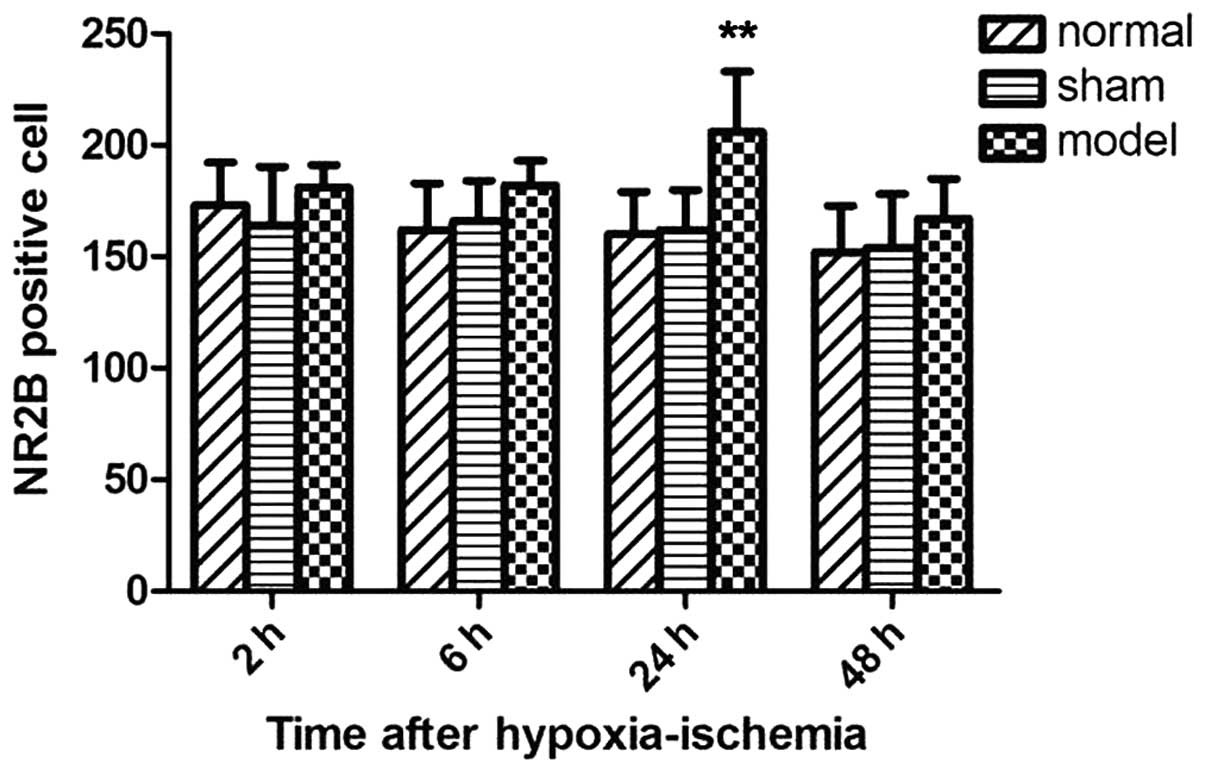
hypoxic-ischemia injury. By contrast, the expression of NR2B showed
the opposite trend and reached the maximum at 24 h after
hypoxia-ischemia (P<0.01 vs. control group; Fig. 4).
Effects of NMDAR antagonists on the
expression of Nestin in SVZ
The cytoplasm and neurites of Nestin-positive cells
showed strong immunoreactivity. Nestin-positive cells were mainly
located in the dorsal lateral horn of the lateral ventricle
(Fig. 5).
At 48 h after hypoxic-ischemia injury, the
Nestin-positive IOD value of the hypoxic-ischemic group was
increased significantly compared with the sham group (P<0.05;
Fig. 6). Hypoxic-ischemia injury
exerted no significant effect on Nestin expression at 2, 6 and 24 h
after hypoxia-ischemic injury compared with the sham group
(Fig. 6). Compared with the
hypoxic-ischemic injury group, the animals treated with MK-801 or
Ro25-6981 showed a reduced expression of Nestin in the SVZ at 6 and
24 h after hypoxia-ischemia (Fig.
6), and the animals treated with Ro25-6981 compound
demonstrated a more pronounced downregulation (P<0.05 vs. MK
group; Fig. 6). At 48 h after
hypoxia-ischemia, the IOD value in the Ro group was significantly
decreased compared with the hypoxic-ischemic injury group
(P<0.05; Fig. 6). This result
was in contrast to the MK group (Fig.
6). In addition, compared with the MK group, the NVP group
(animals pre-treated with NVP-AAM077) showed a marked increase at 6
and 24 h after hypoxia-ischemia (P<0.05; Fig. 6).
Effect of NMDAR antagonists on the
expression of DCX in SVZ
DCX-positive cells were mainly located in the dorsal
lateral horn of the lateral ventricle as well as in the basal
ganglia. The outline shape of the positive cells was regular and
intensive in arrangement. Membranes and cytoplasms of the
DCX-positive cells showed strong immunopositivity in DAB staining
(Fig. 7).
Compared with the hypoxic-ischemic group, the
animals pre-treated with MK-801 or Ro25-6981 (MK and Ro groups,
respectively) showed a markedly reduced number of DCX
protein-positive cells in the SVZ at 2, 6 and 24 h after
hypoxia-ischemia (Fig. 8).
Additionally, fewer positive cells were identified in the Ro group
compared with the MK group (Fig.
8). At 48 h after hypoxia-ischemia, the number of DCX
protein-positive cells in the Ro group was significant lower
compared with the sham group (Fig.
8). This was not the case for the MK group (Fig. 8). In addition, compared with
animals in the MK group, animals pre-treated with NVP-AAM077 (NVP
group) showed a markedly increased number of positive cells at 24
and 48 h after hypoxia-ischemia (Fig.
8). The difference between the sham and MK groups reached
statistical significance at 2, 24 and 48 h after hypoxia-ischemia
(Fig. 8).
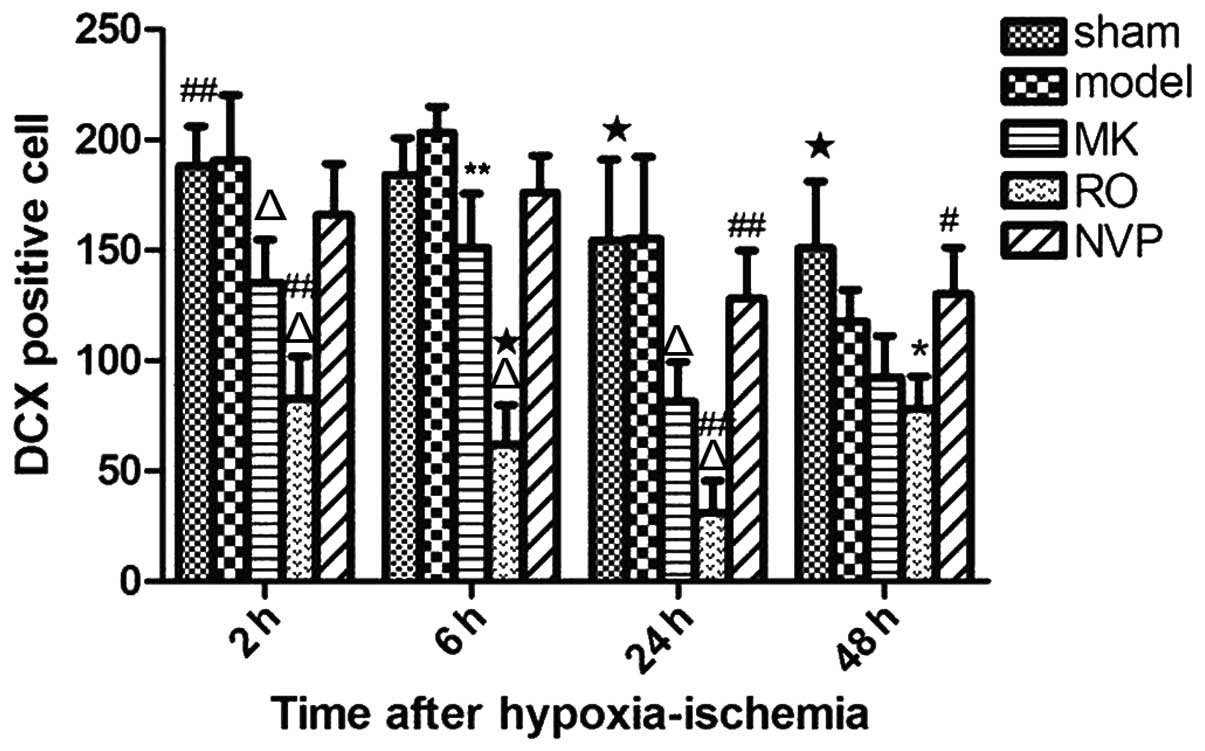 | Figure 8Comparison of the number of
DCX-positive cells in the subventricular zone (SVZ) at different
time points (2, 6, 24 and 48 h) after hypoxic-ischemic in the sham
group, hypoxic-ischemia (labeled 'model') group,
hypoxic-ischemia+treatment with MK-801 group,
hypoxic-ischemia+treatment with Ro25-6981 group, and
hypoxic-ischemia+treatment with NVP-AAM077 group. Data are
presented as mean ± standard deviation. Positive cell counts in
five 100×100 μm2 areas in the SVZ of three
serially sectioned brains at each tested time point.
*P<0.05, **P<0.01, and
△P<0.001 vs. hypoxic-ischemia group. #P<0.05,
##P<0.01, and ★P<0.001 vs.
hypoxic-ischemia+treatment with MK group. |
Discussion
NMDARs play an important role in normal brain
development and are involved in excitotoxicity in hypoxic-ischemic
injury (19). In the present
study, we report that hypoxic-ischemic injury leads to an increased
expression of NR2B and a decreased expression of NR2A subunits in
the SVZ of neonatal rats. Furthermore, in neonatal rats,
hypoxic-ischemic injury stimulates neurogenesis in the SVZ, which
is inhibited by NMDA receptor antagonists. Based on these
observations, NMDAR may promote neurogenesis in the SVZ of neonatal
rats.
In our study, the animals exposed to
hypoxia-ischemia demonstrated a significant upregulation of NR2B
subunits 24 h after induction of the injury. This finding is in
concordance with those of previous studies which described
developmentally related changes in NMDAR expression (13,14,20).
As reported previously (21), NR2A
levels were markedly reduced at 6 h after hypoxia-ischemia, a
finding that is consistent with our results. There are several
mechanisms accounting for the observed developmental changes of
NMDAR subunits in hypoxic-ischemic injury. The first mechanism
involves glutamate excitotoxicity inducing truncation of the NR2A
subunit and cleavage of the scaffolding protein PSD-95 (22). This NR2A subunit truncation may
lead to a rapid uncoupling of synaptic NMDAR from the survival
pathways and a decrease in synaptic NMDAR functionality. The second
mechanism involves calcium overload being responsible for
transcriptional blockage of the NMDAR obligatory subunit NR1
(23), which is involved in the
downregulation of NMDAR functionality. Furthermore, the currents
mediated by NR1/NR2A heteromers develop 3- to 4-fold faster than
the NR1/NR2B-mediated currents (24). Therefore, a preferential decrease
in NR2A may lead to an increase in the duration of NMDAR-mediated
excitatory post-synaptic currents that may cause elevated calcium
levels and greater sensitivity to excitotoxic cell damage. While
these mechanisms have not been directly addressed in the present
study, alterations in the NMDAR subunit expression may play a
significant role in modulating the response of brain development
follownig hypoxic-ischemic injury.
As for the NMDAR subunit of neonatal brain in the
SVZ, whether this is a 'functional' or a 'silent' receptor remains
to be determined. Additionally, whether a potential function of
this receptor is associated with neurogenesis has yet to be
investigated. In the present study, we observed that Nestin
expression was significantly increased at 48 h after
hypoxic-ischemic injury. This observation indicates that
hypoxic-ischemic injury stimulates neural stem cell proliferation
in the SVZ, as previously reported (25). In a previous study, we demonstrated
that MK-801, a selective non-competitive NMDAR antagonist inhibits
cell proliferation in the SVZ of neonatal rats (12). However, it was not clear whether
MK-801 exerted its effect on neurogenesis in the SVZ under
hypoxic-ischemic injury. We extended our previous observations in
the current study and demonstrated that MK-801 inhibits the protein
expression of Nestin and DCX in the SVZ. This finding is supported
by previous studies showing that NMDAR activation increases
proliferation in neural stem/progenitor cells in vitro and
in vivo (26–31). At the same time, several studies
suggest that NMDAR blockade in adult or aged hippocampus increases
precursor proliferation and subsequent neuron production (16,32,33).
It is also unclear whether NMDA receptor antagonism
inhibits neurogenesis mainly through inhibition of the NR2A or NR2B
subunits. Our results show that the NR2B antagonist Ro25-6981
decreases Nestin and DCX protein expression in the SVZ. Therefore,
NR2B-containing NMDAR may promote neurogenesis in the SVZ of
neonatal rats. This hypothesis is supported by previous studies
which showed that the NR2B-containing NMDARs promote neural
progenitor cell proliferation (34). Our study demonstrates that the NR2A
antagonist NVP-AAM077 exerted no significant effect on the protein
expression of Nestin and DCX. Thus, blocking through NR2A NMDAR has
no significant effect on neurogenesis in the SVZ. However, previous
findings have shown that NVP-AAM077 reduced spatial learning by
downregulating neurogenesis in the adult hippocampus (17). However, there is inconsistency in
the literature regarding the role of NMDAR subunits in regulating
neurogenesis. A number of mechanisms potentially account for the
different effect of NMDAR subunits on neurogenesis. First, NMDAR
subunit composition undergoes a change during postnatal
development, with a high NR2B and low NR2A expression at postnatal
early stage, and an increased expression of NR2A during postnatal
development (10,35). A similar observation was made in
our previous study (12). In the
present study, at the early stage after the hypoxic-ischemic
injury, the pattern of high NR2B and low NR2A expression was
evident in the SVZ. The protein expression of Nestin and DCX was
completely eliminated by Ro25-6981, an antagonist of
NR2B-containing receptors, but not affected by NVP-AAM077, an
NR2A-containing receptor antagonist. Second, the NR2A- and
NR2B-containing NMDAR subtypes have opposing roles in the
modulation of the direction of synaptic plasticity (36,37)
or mediation of the NMDA-elicited neuronal survival and apoptosis
(38), and are differently
involved in ischemic neuronal cell death and ischemic tolerance
(39). However, the mechanisms
regarding NMDAR promotion of neurogenesis are poorly understood,
and remain to be investigated.
In conclusion, hypoxic-ischemic injury upregulates
the expression of NR2B and downregulates the expression of NR2A in
the SVZ of neonatal rats. NMDA receptor antagonists (specifically
NR2B) significantly decreased the expression of Nestin and DCX in
this region in the neonatal brain. Therefore, the result show that
NR2B-containing NMDA receptors promote neurogenesis in the SVZ of
neonatal brain.
Acknowledgments
This study was partly supported by the Department of
Clinical Pharmacology, School of Pharmacy, Xuzhou Medical College
(Xuzhou, China).
References
|
1
|
Monk CS, Webb SJ and Nelson CA: Prenatal
neurobiological development: Molecular mechanisms and anatomical
change. Dev Neuropsychol. 19:211–236. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Charvet CJ, Owerkowicz T and Striedter GF:
Phylogeny of the telencephalic subventricular zone in sauropsids:
Evidence for the sequential evolution of pallial and subpallial
subventricular zones. Brain Behav Evol. 73:285–294. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Curtis MA, Connor B and Faull RL:
Neurogenesis in the diseased adult human brain - new therapeutic
strategies for neurodegenerative diseases. Cell Cycle. 2:428–430.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grote HE and Hannan AJ: Regulators of
adult neurogenesis in the healthy and diseased brain. Clin Exp
Pharmacol Physiol. 34:533–545. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khodosevich K, Seeburg PH and Monyer H:
Major signaling path ways in migrating neuroblasts. Front Mol
Neurosci. 2:72009. View Article : Google Scholar
|
|
6
|
Nacher J and McEwen BS: The role of
N-methyl-D-asparate receptors in neurogenesis. Hippocampus.
16:267–270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cull-Candy S, Brickley S and Farrant M:
NMDA receptor subunits: Diversity, development and disease. Curr
Opin Neurobiol. 11:327–335. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prybylowski K and Wenthold RJ:
N-Methyl-D-aspartate receptors: Subunit assembly and trafficking to
the synapse. J Biol Chem. 279:9673–9676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krapivinsky G, Krapivinsky L, Manasian Y,
Ivanov A, Tyzio R, Pellegrino C, Ben-Ari Y, Clapham DE and Medina
I: The NMDA receptor is coupled to the ERK pathway by a direct
interaction between NR2B and RasGRF1. Neuron. 40:775–784. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu XB, Murray KD and Jones EG: Switching
of NMDA receptor 2A and 2B subunits at thalamic and cortical
synapses during early postnatal development. J Neurosci.
24:8885–8895. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim MJ, Dunah AW, Wang YT and Sheng M:
Differential roles of NR2A- and NR2B-containing NMDA receptors in
Ras-ERK signaling and AMPA receptor trafficking. Neuron.
46:745–760. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan H, Gao J, Wang W, Li X, Xu T and Yin
X: Expression of NMDA receptor and its effect on cell proliferation
in the subventricular zone of neonatal rat brain. Cell Biochem
Biophys. 62:305–316. 2012. View Article : Google Scholar
|
|
13
|
Ma ZL, Gu XP, Zeng YM and Zhang Y: Effect
of sodium hydroxybutyrate on the expression of hippocampal
N-methyl-D-aspartate receptor 2B subunit mRNA in neonatal rats with
hypoxic-ischemic insult. Ann Clin Lab Sci. 36:307–311.
2006.PubMed/NCBI
|
|
14
|
Sutcu R, Altuntas I, Eroglu E and Delibas
N: Effects of ischemia-reperfusion on NMDA receptor subunits 2a and
2b level in rat hippocampus. Int J Neurosci. 115:305–314. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Savignon T, Costa E, Tenorio F, Manhães AC
and Barradas PC: Prenatal hypoxic-ischemic insult changes the
distribution and number of NADPH-diaphorase cells in the
cerebellum. PLoS One. 7:e357862012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu M, Sun YJ, Zhou QG, Chen L, Hu Y, Luo
CX, Wu JY, Xu JS, Li LX and Zhu DY: Negative regulation of
neurogenesis and spatial memory by NR2B-containing NMDA receptors.
J Neurochem. 106:1900–1913. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu M, Sun YJ, Zhou QG, Auberson YP, Chen
L, Hu Y, Luo CX, Wu JY, Zhu DY and Li LX: Reduced spatial learning
in mice treated with NVP-AAM077 through downregulating
neurogenesis. Eur J Pharmacol. 622:37–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vannucci RC and Vannucci SJ: Perinatal
hypoxic-ischemic brain damage: Evolution of an animal model. Dev
Neurosci. 27:81–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lynch DR and Guttmann RP: Excitotoxicity:
Perspectives based on N-methyl-D-aspartate receptor subtypes. J
Pharmacol Exp Ther. 300:717–723. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guerguerian AM, Brambrink AM, Traystman
RJ, Huganir RL and Martin LJ: Altered expression and
phosphorylation of N-methyl-D-aspartate receptors in piglet
striatum after hypoxia-ischemia. Brain Res Mol Brain Res.
104:66–80. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gurd JW, Bissoon N, Beesley PW, Nakazawa
T, Yamamoto T and Vannucci SJ: Differential effects of
hypoxia-ischemia on subunit expression and tyrosine phosphorylation
of the NMDA receptor in 7- and 21-day-old rats. J Neurochem.
82:848–856. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gascón S, Sobrado M, Roda JM,
Rodríguez-Peña A and Díaz-Guerra M: Excitotoxicity and focal
cerebral ischemia induce truncation of the NR2A and NR2B subunits
of the NMDA receptor and cleavage of the scaffolding protein
PSD-95. Mol Psychiatry. 13:99–114. 2008. View Article : Google Scholar
|
|
23
|
Gascón S, Deogracias R, Sobrado M, Roda
JM, Renart J, Rodríguez-Peña A and Díaz-Guerra M: Transcription of
the NR1 subunit of the N-methyl-D-aspartate receptor is
downregulated by excitotoxic stimulation and cerebral ischemia. J
Biol Chem. 280:35018–35027. 2005. View Article : Google Scholar
|
|
24
|
Monyer H, Burnashev N, Laurie DJ, Sakmann
B and Seeburg PH: Developmental and regional expression in the rat
brain and functional properties of four NMDA receptors. Neuron.
12:529–540. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang Z and Levison SW: Hypoxia/ischemia
expands the regenerative capacity of progenitors in the perinatal
subventricular zone. Neuroscience. 139:555–564. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arvidsson A, Kokaia Z and Lindvall O:
N-methyl-D-aspartate receptor-mediated increase of neurogenesis in
adult rat dentate gyrus following stroke. Eur J Neurosci. 14:10–18.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luk KC, Kennedy TE and Sadikot AF:
Glutamate promotes proliferation of striatal neuronal progenitors
by an NMDA receptor-mediated mechanism. J Neurosci. 23:2239–2250.
2003.PubMed/NCBI
|
|
28
|
Deisseroth K, Singla S, Toda H, Monje M,
Palmer TD and Malenka RC: Excitation-neurogenesis coupling in adult
neural stem/progenitor cells. Neuron. 42:535–552. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Joo JY, Kim BW, Lee JS, Park JY, Kim S,
Yun YJ, Lee SH, Lee SH, Rhim H and Son H: Activation of NMDA
receptors increases proliferation and differentiation of
hippocampal neural progenitor cells. J Cell Sci. 120:1358–1370.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mochizuki N, Takagi N, Kurokawa K, Kawai
T, Besshoh S, Tanonaka K and Takeo S: Effect of NMDA receptor
antagonist on proliferation of neurospheres from embryonic brain.
Neurosci Lett. 417:143–148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Toriumi K, Mouri A, Narusawa S, Aoyama Y,
Ikawa N, Lu L, Nagai T, Mamiya T, Kim HC and Nabeshima T: Prenatal
NMDA receptor antagonism impaired proliferation of neuronal
progenitor, leading to fewer glutamatergic neurons in the
prefrontal cortex. Neuropsychopharmacology. 37:1387–1396. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nacher J, Alonso-Llosa G, Rosell DR and
McEwen BS: NMDA receptor antagonist treatment increases the
production of new neurons in the aged rat hippocampus. Neurobiol
Aging. 24:273–284. 2003. View Article : Google Scholar
|
|
33
|
Maekawa M, Namba T, Suzuki E, Yuasa S,
Kohsaka S and Uchino S: NMDA receptor antagonist memantine promotes
cell proliferation and production of mature granule neurons in the
adult hippocampus. Neurosci Res. 63:259–266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li M, Zhang DQ, Wang XZ and Xu TJ:
NR2B-containing NMDA receptors promote neural progenitor cell
proliferation through CaMKIV/CREB pathway. Biochem Biophys Res
Commun. 411:667–672. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sans N, Petralia RS, Wang YX, Blahos J II,
Hell JW and Wenthold RJ: A developmental change in NMDA
receptor-associated proteins at hippocampal synapses. J Neurosci.
20:1260–1271. 2000.PubMed/NCBI
|
|
36
|
Liu L, Wong TP, Pozza MF, Lingenhoehl K,
Wang Y, Sheng M, Auberson YP and Wang YT: Role of NMDA receptor
subtypes in governing the direction of hippocampal synaptic
plasticity. Science. 304:1021–1024. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Massey PV, Johnson BE, Moult PR, Auberson
YP, Brown MW, Molnar E, Collingridge GL and Bashir ZI: Differential
roles of NR2A and NR2B-containing NMDA receptors in cortical
long-term potentiation and long-term depression. J Neurosci.
24:7821–7828. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu
L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, et al: NMDA receptor
subunits have differential roles in mediating excitotoxic neuronal
death both in vitro and in vivo. J Neurosci. 27:2846–2857. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen M, Lu TJ, Chen XJ, Zhou Y, Chen Q,
Feng XY, Xu L, Duan WH and Xiong ZQ: Differential roles of NMDA
receptor subtypes in ischemic neuronal cell death and ischemic
tolerance. Stroke. 39:3042–3048. 2008. View Article : Google Scholar : PubMed/NCBI
|