Introduction
Colorectal cancer (CRC) is the second leading cause
of cancer-associated mortality worldwide, according to the
International Agency for Research on Cancer (1). Cancer reports reveal that ~1 million
new cases of CRC were detected worldwide in 2008, establishing it
as a key focus for the study of cancer and therapeutic approaches
(1,2). In CRC and numerous other types of
cancer, it has been established that the aberration of the growth
factor signaling pathways can function in tumor initiation,
progression and metastasis (3–6).
Transforming growth factor β (TGF-β) has been identified as a key
growth factor triggering varied biological processes, including
proliferation, differentiation and programmed cell death
(apoptosis). Studies indicate that this factor functions in
signaling during the later stages of certain types of cancer and
promotes tumor development, progression and metastasis (7,8).
TGF-β binds heterodimeric receptor complexes of
transmembrane serine/threonine kinases known as type I and type II
receptors (TGβRI and TGβRII). Cell signaling triggers interaction
of TGF-β with TGβRII, followed by TGβRI activation. Activated TGβRI
initiates the recruitment and phosphorylation of a family of signal
transducers, termed Smad factors. A complex of phosphorylated Smad2
and Smad3 components associates with Smad4, and these translocate
to the nucleus to activate the transcription of downstream target
genes (7–9).
It is well-established that growth factors with a
broad range of cellular effects, such as TGF-β, must be subject to
extensive regulation to control their expression and function. This
regulation includes mechanisms that occur during a diversity of
growth events that are dependent on cell and tissue contexts; it
may be apt, therefore, to utilize cell growth inhibition as a form
of cancer therapy (8,9). Several reports have indicated that
the targeting of TGF-β signaling during the late stages of
carcinogenesis may be a useful tool for the treatment of human
cancer, including CRC, glioblastoma and breast cancer; repressing
the function of the TGF-β signal transduction pathway components
may thus provide an effective therapeutic strategy for the
treatment of CRC (9,10).
For hundreds of years, it has been reported that
certain infectious diseases exert a beneficial, therapeutic effect
upon malignancy (11–13). Bacteria and associated molecules
have been utilized in diverse fields of cancer therapy, including
their use as vectors for gene therapy, as carriers of tumoricidal
agents and bacterial toxins have been used in tumor repression
within their role of binding tumor surface antigens. Toxins are
unique bacterial factors with a suggested protective role in
carcinogenesis and in cancer remission (14–17).
Previous studies have demonstrated that bacterial toxins may lead
to the cytolysis and death of a range of malignant cells; in this
regard, the bacterial toxin-based vaccine was widely used to
successfully treat sarcomas, carcinomas, lymphomas, melanomas and
myelomas (13,14). There is evidence that certain types
of bacterial toxins may aid the prevention or treatment of cancer;
it is this that inspired the development of the earliest
toxin-based cancer therapies (11–13).
Certain bacteria secrete enterotoxins able to
modulate cellular signaling processes, controlling proliferation,
apoptosis and differentiation during carcinogenesis (18–21).
Although it has demonstrated successful results in vivo,
further investigation into the targeting mechanisms of bacteria is
required in order to develop a complete therapeutic approach for
cancer treatment. Regarding the basic function of bacterial
enterotoxins in tumor repression, it is rational to hypothesize
that anticancer properties may partially be associated with their
regulation of the cell signaling genes involved in cancer
development and progression. However, modulation of a distinct
signaling pathway to explain the possible inhibitory action of
staphylococcal enterotoxins in cancer, including CRC, has yet to be
elucidated.
Staphylococcal enterotoxins are a family of
structurally-related proteins produced by Staphylococcus
aureus. Staphylococcal enterotoxin B (SEB) belongs to the
superantigen protein family. These are proteins or peptides
produced by various microorganisms, including bacteria, mycoplasma
or viruses, and induce T lymphocytes clonally (22–24).
It has been demonstrated that SEB exerts anticancer and
anti-metastatic effects due to its ability to modify cell immunity
processes and cancer cell signaling pathways (22,23).
These promising characteristics prompted study into whether SEB
reduces CRC cell proliferation. As bacterial enterotoxins have, to
a certain extent, previously been harnessed for cancer treatment
(22–24), we hypothesized that the anticancer
properties of the enterotoxin may be partially recapitulated by
manipulating growth signaling pathways.
The aim of the present study was to investigate the
growth inhibitory effect of SEB on CRC growth through the in
vitro manipulation of the TGF-β signaling pathway transduction
components Smad2/3, in vitro. The study was designed to
provide an insight into the molecular mechanism of SEB in colon
cancer cell signaling pathways, emphasizing the potential for novel
toxin -based cancer therapies.
Materials and methods
Cell culture
HCT116, a human colorectal adenocarcinoma cell line
from original tumors of pathological differentiation grade II, was
selected from a panel of CRC cell lines to examine the downstream
effects of SEB on TGF-β.
The cell line was obtained from the National Cell
Bank of Iran, affiliated to the Pasteur Institute (Tehran, Iran).
The cells were grown in RPMI-1640 medium containing 25 mM
D-glucose, 4 mM L-glutamine and 1 mM sodium pyruvate, supplemented
with 5% (v/v) heat-inactivated fetal bovine serum (FBS), 2 mM
GlutaMAX, 100 U/ml penicillin, 100 µg/ml streptomycin and
250 ng/ml amphotericin (all Gibco; Thermo Fisher Scientific,
Darmstadt, Germany) in 25-cm2 culture flasks (SPL Life
Sciences, Pocheon, Korea). The cells were maintained at 37°C in a
humidified, 95% air/5% CO2 atmosphere incubator in
steady-state conditions. Cell viability was assessed using a trypan
blue (Sigma-Aldrich, St. Louis, MO, USA) exclusion test and
routinely demonstrated >95% viable cells in all flasks.
SEB preparation
SEB (Sigma-Aldrich) was dissolved in distilled water
according to the manufacturer's protocols. The enterotoxin was
prepared as stock solutions of 20 µg/ml and stored at −20°C
until use.
MTT assay
To quantify cell proliferation, the in vitro
growth inhibitory effect of SEB following incubation for 24, 48 or
72 h was measured using an MTT assay (Roche Applied Science,
Mannheim, Germany). Monolayer cultures were trypsinized in the
exponential growth phase and viable cell counts were assessed using
trypan blue exclusion assay. The cells were then seeded in 96-well
flat-bottom microtitration plates (SPL Life Sciences) at a density
of 105 cells/well (200 µl media/well). After 24
h, upon reaching ~85% confluence, the cells were treated with
different concentrations of SEB (0.5, 1 and 2 µg/ml). In all
in vitro experiments, untreated and distilled water-treated
cells were used as controls (with a final volume of 5
µl).
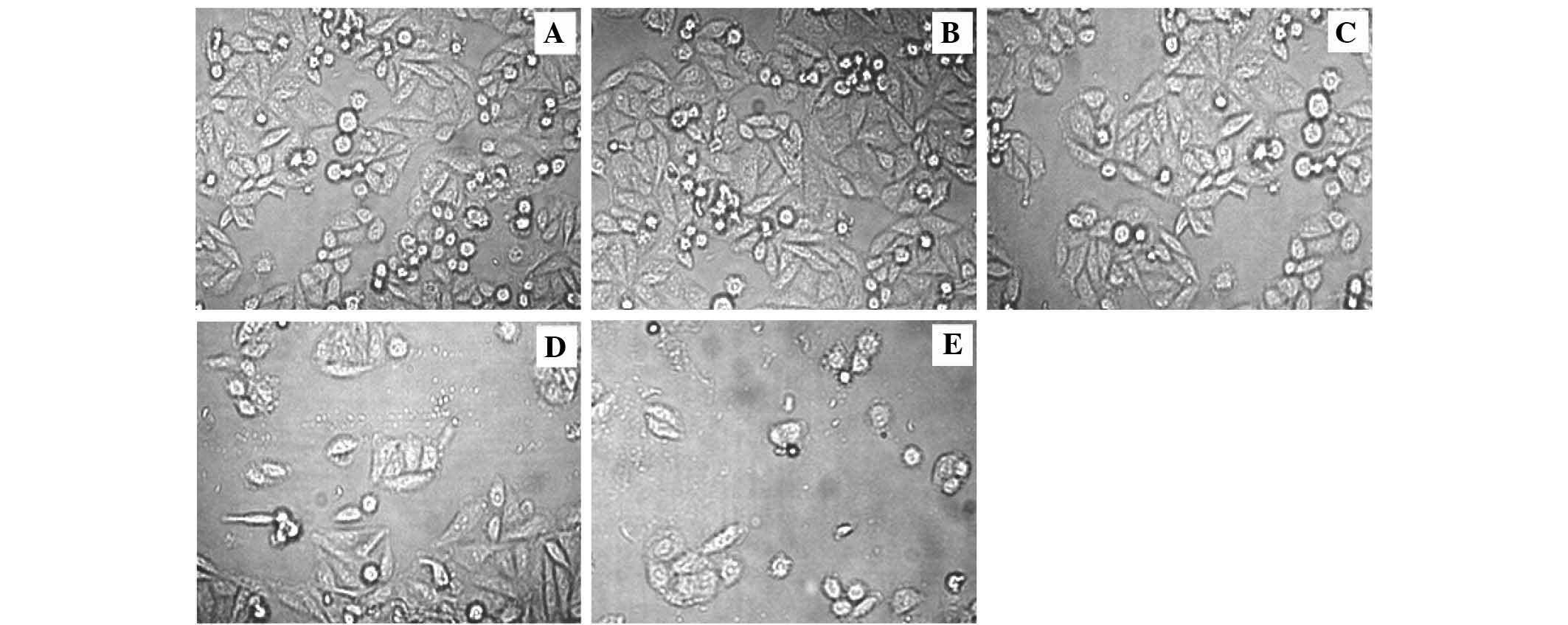
Following 24 h of drug application, for the recovery
period, the cells were washed twice with fresh, FBS-free medium and
the culture was continued (Fig.
1). This medium was then replaced with FBS-containing medium to
remove unbound SEB.
Complete medium was replaced with 100 µl MTT
after 24, 48 and 72 h of treatment. The cells were incubated for 3
h at 37°C, and then 100 µl dimethyl sulfoxide was added to
each well. The optical density (OD) was measured at a wavelength of
570 nm with background subtraction at 630 nm using an ELX808
spectrophotometric microplate reader (BioTek Instruments, Inc.,
Winooski, VT, USA). Cell viability was calculated using the
following formula: Cell viability (%) = (OD drug exposure / OD
control) × 100.
Total RNA extraction from cells
Total RNA was extracted from the cultured HCT116
cells prior to or subsequent to 24, 48 and 72 h of treatment with
SEB. Total cellular RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Karlsruhe, Germany)
according to the manufacturer's protocols. Extracted total RNA was
stored at −70°C until use.
Gene expression analysis by
reverse-transcription quantitative polymerase chain reaction
(RT-qPCR)
Smad2/3 gene expression level was analyzed by qPCR
using the SYBR-Green method, with specific forward and reverse
primers used to amplify the relevant genes (Table I) supplied by Genfanavaran (Tehran,
Iran). GAPDH primers were used as an endogenous, positive control,
and the data were normalized to the expression level of this
housekeeping gene. This method included two steps as follows: i)
RT-qPCR. RNA was transcribed to complementary DNA (cDNA) using the
oligo(dT) procedure. Briefly, the cDNA was synthesized using total
RNA and specific primers in a reverse transcription reaction. This
reaction was performed in a volume of 10 µl containing 1
µl total RNA, 1 µl 0.5 mM oligo(dT) RT primer, 1
µl 10 mM dNTP, 1 µl reverse transcriptase and 6
µl reaction buffer. The reaction was incubated at 42°C for
60 min, then terminated by heating at 85°C for 5 min.
 | Table IPrimer sequences used for quantitative
polymerase chain reaction in the present study. |
Table I
Primer sequences used for quantitative
polymerase chain reaction in the present study.
| Target gene | Primer
sequence | Product size,
bp |
|---|
| Smad2 | | 480 |
| Forward |
5′-TCAAGCTTGAGTGTAAACCCTTACCACTATC-3′ | |
| Reverse |
5′-TAGCGGCCGCGAAAGCTATGATTAACAG48GGG-3′ | |
| Smad3 | | 340 |
| Forward |
5′-TCAAGCTTGAACACCAGTTCTACCTCCTG-3′ | |
| Reverse |
5′-TAGCGGCCGCGAAATGTCTCCCCGACGCGCTG-3 | |
| GAPDH | | 190 |
| Forward |
5′-CGTTCCCAAAGTCCTCCTGTTTC-3′ | |
| Reverse |
5′-TTTTTTTCCGCAGCCGCCTG-3′ | |
Subsequently, diluted cDNAs were amplified in a
20-µl reaction containing SYBR-Green Master mix (Takara,
Kyoto, Japan), forward and reverse Smad2/3-specific primers (each 1
µl) and diethylpyrocarbonate-treated distilled water, using
35 cycles of PCR amplification under the following conditions:
Denaturing at 95°C for 1 min, annealing at 56°C for 1 min and
extension at 72°C for 1 min. The PCR was performed on a CFX96 Touch
Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
All reactions were performed in triplicate.
Specificity of primers was verified by observing a single peak
dissociation curve for each run. The quantification cycle (Cq) was
defined as the fractional cycle number at which the fluorescence
passes the fixed threshold. Cq values were converted into total
copy numbers using a standard curve. The absence of contamination
was verified using distilled water as non-template controls. PCR
products were visualized by electrophoresis on a 2% agarose gel in
TAE buffer using GelRed (Biotium, Inc., Hayward, CA, USA) (Fig. 2).
Statistical analysis
PCR data analysis was performed using the
2−ΔΔcq method via GraphPad Prism 5.0 software (GraphPad
Software, Inc., La Jolla, CA, USA). The mean, standard deviation,
standard error of the mean and ranges of each parameter were
calculated. A comparison was made of the mean and variance of gene
expression using an analysis of variance (ANOVA). Significant
differences were also determined (Prism) using ANOVA and Tukey's
post-hoc test, or the unpaired Student's t-test, when applicable.
P<0.05 was considered to indicate a statistically significant
difference between data sets.
Results
SEB reduces the growth of the colon
adenocarcinoma HCT116 cell line
In order to test SEB antiproliferative activities,
an MTT assay was used to evaluate the cell growth inhibitory effect
upon altering duration and concentration of treatment. The results
indicated that 1 and 2 µg/ml SEB significantly decreased
HCT116 cell viability after 48 h of treatment (P=0.0021 and
P=0.0017, respectively). It was concluded that SEB exerts its
growth inhibitory effects in a concentration- and time-dependent
manner. Overall, the data demonstrated that SEB was an effective
inhibitor of human colon cancer cell proliferation (Fig. 3).
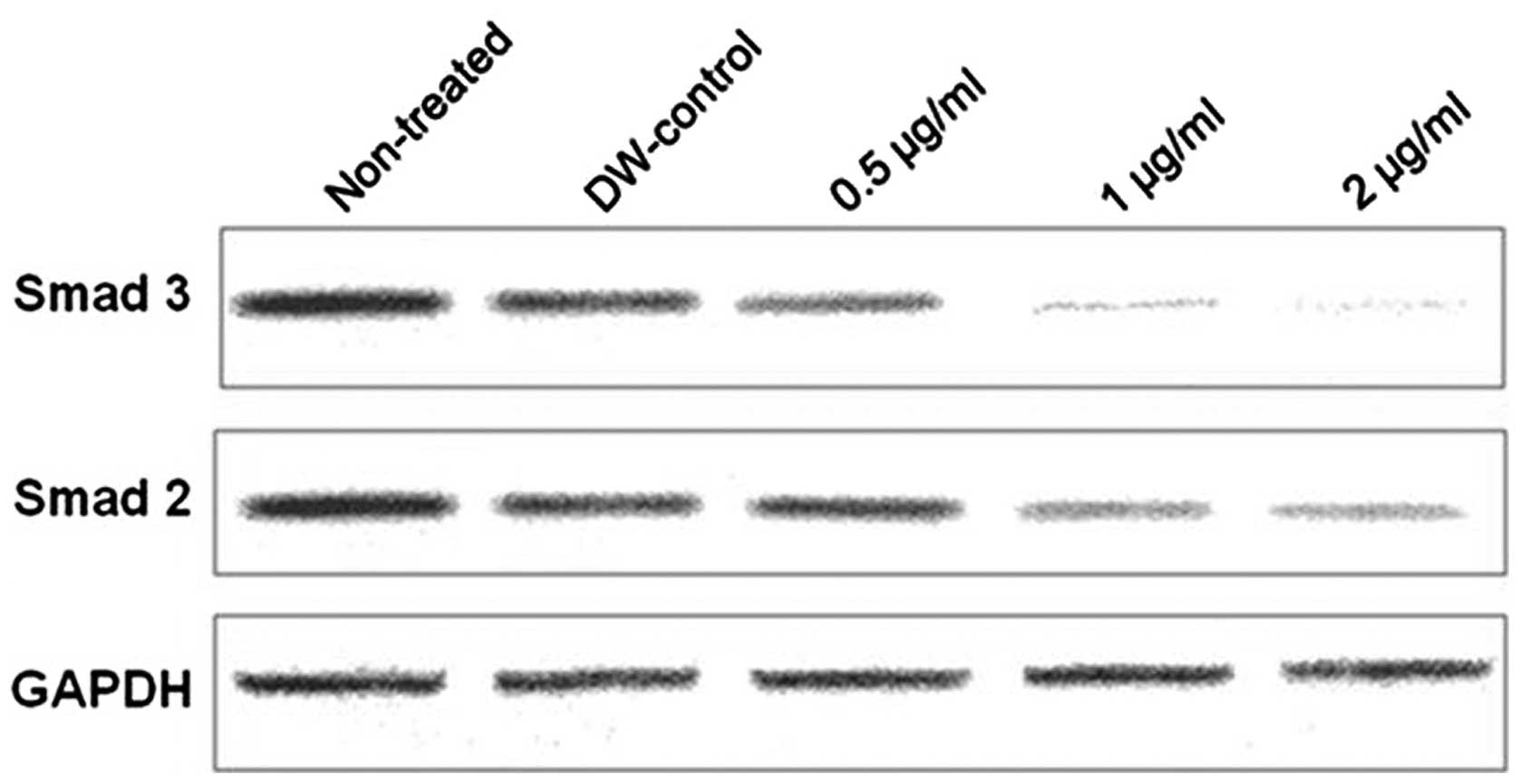
SEB downregulates Smad2/3 expression
The expression levels of TGF-β signaling targets
Smad2 and -3 were evaluated by qPCR. The results revealed that 1
and 2 µg/ml of SEB reduced Smad2/3 expression in the human
colon adenocarci-noma HCT116 cell line. SEB treatment at 2
µg/ml for 72 h significantly reduced Smad2 expression
(P=0.006), while Smad3 expression was significantly reduced by SEB
treatment at concentrations of 2 µg/ml for 48 h (P=0.0075),
and at 1 and 2 µg/ml for 72 h (P=0.011 and P=0.004,
respectively). SEB reduced Smad2/3 expression in a dose- and
time-dependent manner (Fig. 4).
SEB inhibited the growth of HCT116 cells at high concentrations,
which may be a partial consequence of the downregulation of TGF-β
signaling pathway components.
It was predicted that SEB, as a potent inhibitor of
colon cancer cell proliferation, would regulate the expression of
key transducer genes controlling TGF-β cancer cell signaling. The
SEB concentrations effective at inhibiting Smad2/3 expression were
correlated with those used to inhibit the proliferation of the
HCT116 cells.
According to the data presented in the current
study, Smad2/3 downregulation in the presence of SEB may precede
the inhibitory effects of SEB on proliferation; however, this
proposal requires further empirical evaluation prior to trial as a
CRC therapy.
Discussion
Malignancies such as CRC are currently considered an
important area of study, due to the burden of the disease and the
mortality rate (2–5). In numerous types of cancer, it has
been established that aberration in genes encoding TGF-β signaling
components can contribute to colon carcinogenesis in humans
(7,8). This signaling pathway controls
numerous cellular functions, including epithelial cell
proliferation, apoptosis and migration, in addition to tumor
initiation, progression and metastasis (8,9),
making it a suitable target for cancer therapy (9,10).
Bacterial toxins are widely studied for their
anticancer activities and certain examples are currently in
clinical development, inciting anticipation for their
pharmacological use in cancer treatment (15–17).
These toxins can function to kill cells or alter cellular processes
controlling proliferation, apoptosis and differentiation in
carcinogenesis, and these salient roles have stimulated study into
whether these may be useful anticancer agents (14,15,17).
Despite successful results in vivo, further investigation
into the targeting mechanisms used by bacteria are required to
generate a complete therapeutic approach in cancer treatment. In
several studies, however, the cancer-promoting signaling pathways
instigated by bacterial toxins have been evaluated (19–21).
SEB belongs to the family of superantigens. These
proteins bind the β-chain of the T cell receptor and the major
histocompatibility complex class II dimer (22,23).
It has been suggested that SEB exerts anticancer and antimetastatic
advantages via the modification of cancer signaling pathways and
cell immunity (24). We therefore
hypothesized that the anticancer functions of the enterotoxin may
be partially due to changes to cancer signaling pathways.
SEB, the potent inhibitor of colon cancer cell
proliferation analyzed in the current study, was predicted to
modulate the expression of key transducer genes controlling TGF-β
cancer cell signaling. The present study aimed to provide an
insight into this molecular mechanism, with an overall objective of
promoting toxin use in CRC therapy and, potentially, other
malignancies involving TGF-β signaling.
As previous studies implicated functionally active
SEB-binding structures in mediating target cell killing in a range
of human colon carcinoma cells (25), HCT116 cells were thus selected as a
promising CRC model in the present study. It has also previously
been demonstrated that these receptors are distinct from the
conventional MHC class II molecules and bind to SEB in a class
II-independent manner (25).
Treatment of HCT116 cells at different
concentrations for varying durations indicated that SEB treatment
resulted in the time- and concentration-dependent inhibition of
Smad2/3 expression. The SEB inhibitory action upon Smad2/3
expression occurred at concentrations as low as 1 µg/ml. It
was presumed that this observable phenomenon resulted from the
downregulated expression of TGF-β signaling components. SEB was
more effective at reducing Smad3 expression than Smad2 expression
(Fig. 2) and SEB was also
demonstrated to exert an inhibitory effect on HCT116 cell
proliferation subsequent to 48 h treatment at concentrations of 1
and 2 µg/ml (P=0.0021 and P=0.0017, respectively). The SEB
concentrations effective at inhibiting Smad2/3 expression
correlated with those able to inhibit HCT116 cell proliferation.
According to the data presented in the current study, it was
hypothesized that Smad2/3 downregulation may precede the SEB
inhibition of cell proliferation, but further evaluation is
required to confirm this.
Results of the present study are consistent with
those from previous studies, indicating that SEB exerts
anti-angiogenic effects (22–24).
In these studies, SEB was demonstrated to be effective at inducing
apoptosis and attenuating cancer cell proliferation.
In accordance with the present study, a previous
study revealed that SEB induced the Fas/Fas ligand-mediated
cytolysis of target cells (26),
postulating that Fas/Fas ligand may be a key mediator for
SEB-mediated cell death (26). In
this regard, it should be noted that TGF-β also activates other
downstream signaling pathways, including Rho GTPases, the
extracellular signaling-regulated kinases, c-Jun NH2-terminals
kinase and phosphatidylinositol-3 kinase (8–10),
and it is probable that these pathways are also affected by
enterotoxin activity. The use of SEB in the complete reduction of
CRC proliferation, acting solely through Smad2/3 downregulation,
therefore requires comprehensive examination.
Additionally, it has previously been reported that
anthrax toxin (a dangerous bacterial toxin secreted by Bacillus
anthracis) inhibits the growth of Ras-transformed cancerous
cells by disturbing mitogen-activated protein kinase (MAPK)
signaling pathways. It has therefore been suggested that this toxin
may also be used against cancer cells in which MAPKs are activated
by oncogenic proteins; this specificity ensures the selective
damage of tumors at a low dosage (27). Furthermore, bacterial toxins may be
used in targeted cancer therapy or synergistically potentiate the
activity of anticancer drugs (28–30).
It is therefore recommended that additional studies further analyze
the synergistic activity of enterotoxin with anticancer drugs.
Although SEB is proposed to be an attractive
biomolecule in cancer treatment, a significant drawback of using
the enterotoxin as an anticancer agent is its toxicity at the dose
required for therapeutic efficacy. Furthermore, sufficient
experimental evidence to justify the conclusion that SEB has
therapeutic value in TGβRI/II-positive cancer cells is yet to be
demonstrated.
In conclusion, identification of molecular
mechanisms involved in beneficial functions of biotoxins to treat
cancer may provide a novel insight into immunotoxin-based cancer
therapy; further investigation and development in these studies may
add a further dimensions to cancer treatment. Nonetheless, the
successful translation of these approaches into scientific practice
is likely to depend on the outcome of clinical trials.
In the present study, SEB significantly reduced the
expression of Smad2/3, which are components of the TGF-β signaling
pathway. It was further demonstrated that SEB may successfully
suppress CRC proliferation, and that this suppression may be
partially attributable to TGF-β signaling pathway inhibition. The
continued examination of these salient molecular features may yet
facilitate the development of the effective immunotoxin-based
therapy of malignancies.
Acknowledgments
The current study was supported by AJA University of
Medical Sciences (Tehran, Iran).
Abbreviations:
|
TGF-β
|
transforming growth factor-β
|
|
CRC
|
colorectal cancer
|
|
SEB
|
staphylococcal enterotoxin B
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carmona FJ and Esteller M: Epigenomics of
human colon cancer. Mutat Res. 693:53–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim E, Coelho D and Blachier F: Review of
the association between meat consumption and risk of colorectal
cancer. Nutr Res. 33:983–994. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mishra J, Drummond J, Quazi SH, Karanki
SS, Shaw JJ, Chen B and Kumar N: Prospective of colon cancer
treatments and scope for combinatorial approach to enhanced cancer
cell apoptosis. Crit Rev Oncol Hematol. 86:232–250. 2013.
View Article : Google Scholar :
|
|
5
|
Mohan HM, O'Connor DB, O'Riordan JM and
Winter DC: Prognostic significance of detection of microscopic
peritoneal disease in colorectal cancer: A systematic review. Surg
Oncol. 22:e1–6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Loh KW, Majid HA, Dahlui M, Roslani AC and
Su TT: Sociodemographic predictors of recall and recognition of
colorectal cancer symptoms and anticipated delay in help-seeking in
a multiethnic asian population. Asian Pac J Cancer Prev.
14:3799–3804. 2013. View Article : Google Scholar
|
|
7
|
Ma J, Gao HM, Hua X, Lu ZY and Gao HC:
Role of TGF-β1 in human colorectal cancer and effects after
cantharidinate intervention. Asian Pac J Cancer Prev. 15:4045–4048.
2014. View Article : Google Scholar
|
|
8
|
Bierie B and Moses HL: TGF-β and cancer.
Cytokine Growth Factor Rev. 17:29–40. 2006. View Article : Google Scholar
|
|
9
|
Jean-Jacques L: The dual role of TGF in
human cancer: From tumor suppression to cancer metastasis. ISRN Mol
Biol. 7:1–28. 2012.
|
|
10
|
Bierie B and Moses HL: TGFbeta: The
molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 6:506–520.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramasamy S, Nattarayan V, Jayaraj GG,
Arulanandh MD and Jaiswal A: Bacterial infection-mediated
anticancer activity (BIMAc) - revisiting the molecular mechanisms.
J Med Hypoth Ideas. 6:19–22. 2012. View Article : Google Scholar
|
|
12
|
Nauts HC, Fowler GA and Bogatko FH: A
review of the influence of bacterial infection and of bacterial
products (Coley's toxins) on malignant tumors in man; a critical
analysis of 30 inoperable cases treated by Coley's mixed toxins, in
which diagnosis was confirmed by microscopic examination selected
for special study. Acta Medica Scandinavica. 276:1–103. 1953.
|
|
13
|
Nauts HC: The beneficial effects of
bacterial infections on host resistance to cancer: End result in
449 cases. (Monograph no. 8). 2nd edition. Cancer Research
Institute; New York, NY, USA: 1980
|
|
14
|
Zacharski LR and Sukhatme VP: Coley's
toxin revisited: Immunotherapy or plasminogen activator therapy of
cancer? J Thromb Haemost. 3:424–427. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hoption Cann SA, van Netten JP and van
Netten C: Dr William Coley and tumour regression: A place in
history or in the future. Postgrad Med J. 79:672–680. 2003.
|
|
16
|
Zhong L, Zhang X and Covasa M: Emerging
roles of lactic acid bacteria in protection against colorectal
cancer. World J Gastroenterol. 20:7878–7886. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Patyar S, Joshi R, Byrav DS, Prakash A,
Medhi B and Das BK: Bacteria in cancer therapy: A novel
experimental strategy. J Biomed Sci. 17:21–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Michl P, Buchholz M, Rolke M, Kunsch S,
Löhr M, McClane B, Tsukita S, Leder G, Adler G and Gress T:
Claudin-4: A new target for pancreatic cancer treatment using
Clostridium perfringens enterotoxin. Gastroenterology. 121:678–684.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kominsky SL, Vali M, Korz D, Gabig TG,
Weitzman SA, Argani P and Sukumar S: Clostridium
perfringensenterotoxin elicits rapid and specific cytolysis of
breast carcinoma cells mediated through tight junction proteins
claudin 3 and 4. Am J Pathol. 164:1627–1633. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao Z and McClane BA: Use of Clostridium
perfringens enterotoxin and the enterotoxin receptor-binding domain
(C-CPE) for cancer treatment: Opportunities and challenges. J
Toxicol. 2012:9816262012. View Article : Google Scholar
|
|
21
|
Ansiaux R and Gallez B: Use of botulinum
toxins in cancer therapy. Expert Opin Investig Drugs. 16:209–218.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mahmoodzadeh Hosseini H, Ali Imani Fooladi
A, Soleimanirad J, Reza Nourani M and Mahdavi M:
Exosome/staphylococcal enterotoxin B, an anti tumor compound
against pancreatic cancer. J BUON. 19:440–448. 2014.PubMed/NCBI
|
|
23
|
Hui J, Xiao F, Li H, Cui X, Liu H and Hu
F: Inhibiting tumor-cell growth by novel truncated staphylococcal
enterotoxin C2 mutant. Sheng Wu Gong Cheng Xue Bao. 27:891–899.
2011.PubMed/NCBI
|
|
24
|
Reis LO, Ferreira U, Billis A, Cagnon VH
and Fávaro WJ: Anti-angiogenic effects of the superantigen
staphylococcal enterotoxin B and bacillus Calmette-Guérin
immunotherapy for nonmuscle invasive bladder cancer. J Urol.
187:438–445. 2012. View Article : Google Scholar
|
|
25
|
Dohlsten M, Hedlund G, Segren S, Lando PA,
Herrmann T, Kelly AP and Kalland T: Human major histocompatibility
complex class II-negative colon carcinoma cells present
staphylococcal superantigens to cytotoxic T lymphocytes: Evidence
for a novel enterotoxin receptor. Eur J Immunol. 21:1229–1233.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fuller CL and Braciale VL: Selective
induction of CD8+ cytotoxic T lymphocyte effector
function by Staphylococcus enterotoxin B. J Immunol. 161:5179–5186.
1998.PubMed/NCBI
|
|
27
|
Ascenzi P, Visca P, Ippolito G,
Spallarossa A, Bolognesi M and Montecucco C: Anthrax toxin: A
tripartite lethal combination. FEBS Lett. 531:384–388. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Engedal N, Skotland T, Torgersen ML and
Sandvig K: Shiga toxin and its use in targeted cancer therapy and
imaging. Microb Biotechnol. 4:32–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bandala C, Perez-Santos JL, Lara-Padilla
E, Delgado Lopez G and Anaya-Ruiz M: Effect of botulinum toxin A on
proliferation and apoptosis in the T47D breast cancer cell line.
Asian Pac J Cancer Prev. 14:891–894. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brigotti M, Arfilli V, Carnicelli D,
Rocchi L, Calcabrini C, Ricci F, Pagliaro P, Tazzari PL, Alfieri
RR, Petronini PG and Sestili P: Shiga toxin 1, as DNA repair
inhibitor, synergistically potentiates the activity of the
anticancer drug, mafosfamide, on raji cells. Toxins (Basel).
5:431–444. 2013. View Article : Google Scholar
|