Introduction
The neuronal system is complex, and synaptic
contacts between polarized neurons are fundamental structures
(1,2). During the polarization process,
neurons extend several neurites, the majority of which develop into
dendrites and only one of which becomes an axon (3). Neurites are tipped with motile growth
cones that receive extracellular guidance cues to reach their
targets, thus forming neural circuits (4). Growth cones maintain the mobility
predominantly due to the rich and flexible cytoskeleton, which
contains microtubules and actin (5). Bundled microtubules are distributed
predominantly in the central (C-) domain of the growth cone, while
splayed or looped microtubules are predominantly in the peripheral
(P-) domain and the transitional (T-) zone (6). Actin expresses predominantly in the
P-domain and T-zone (7). When the
growth cone is in a quiescent state, the polymerization and
depolymerization of microtubules and actin exhibit a dynamic
balance (8). If the balance is
disrupted, the development of growth cone is affected.
Collapsin response mediator proteins (CRMPs),
consisting of five cytosolic proteins (CRMP 1–5), are highly
expressed in developing and adult nervous systems (9–11).
CRMPs were originally identified as downstream of the Semaphorin-3A
(Sema3A) signaling pathway (12).
CRMP-5, as the first identified CRMP-associated protein, has the
lowest homology with other CRMP proteins (13). CRMP-5 is highly expressed in
post-mitotic neural precursors and the fasciculi of fibers in
developing brains (14). Hotta
et al (15) observed that
CRMP-5 is able to regulate filopodia dynamics and growth cone
development, negatively responding to Sema3A signaling. Yamashita
et al (16) demonstrated
that CRMP-5 regulated dendritic development and synaptic plasticity
in the cerebellar Purkinje cells. However, conversely, CRMP-5 has
been reported to inhibit neurite outgrowth (17). In addition, CRMP-5 antagonized the
promoting effect of CRMP-2 on axonal and dendritic growth through a
tubulin-based mechanism (17).
Thus, the role of CRMP-5 in neurite outgrowth remains
controversial. In addition, CRMP-5 is able to interact with other
proteins during brain development, including tyrosine kinase
Fes/Fps (18) and the
mitochondrial protein septin (19), however the functional significance
of these interactions remains unclear. The distribution of CRMP-5
in the growth cones suggests its potential role in regulating
growth cone development and neurite outgrowth (15). However, the detailed mechanisms of
CRMP-5 interaction with actin remain to be fully elucidated.
The current study aimed to determine whether CRMP-5
would interact with the actin cytoskeleton network to dynamically
regulate the distribution and remodeling of cytoskeleton, thus to
mediate growth cone development and neurite outgrowth.
Materials and methods
Animals
The experiments were conducted on 1-day-old pups of
Sprague-Dawley rats (n=8–10). Rats were purchased from the
Institute of Laboratory Animal Science of Jinan University (Jinan,
China) and were sacrificed immediately. The rats were anesthetized
with intraperitoneal injection of ketamine (30 mg/kg; Maijin
Biotechnology, Hubei, China). Normally, the rats were housed in a
temperature-controlled (20–22°C) room with a 12 h light/dark cycle,
and were provided with free access to food and water. All animal
procedures were performed in strict accordance with the
recommendations of the Guide for the Care and Use of Laboratory
Animals from the National Institutes of Health (20). The protocol was approved by the
Jinan University Institutional Animal Care and Use Committee
(approval no. SCXK20110029; Guangzhou, China). All efforts were
made to minimize the suffering and number of animals used.
Cell culture and transfection
Hippocampi were dissected from the postnatal rat
pups (days 0–1), and dissociated hippocampal neurons were obtained
using 0.125% trypsin (Gibco; Thermo Fisher Scientific Inc.,
Waltham, MA, USA), which were plated at a density of
1×104 cells/cm2 onto poly-D-lysine
(PDL)-coated glass coverslips. Cultures were maintained in
Neurobasal-A (Gibco; Thermo Fisher Scientific Inc.) medium
containing 2% B27 (Gibco; Thermo Fisher Scientific Inc.) and 0.5 mM
glutamine (Gibco; Thermo Fisher Scientific Inc.) supplement at 37°C
in a 5% CO2 humidified incubator. Half of the culture
media was replaced every 3 days. Calcium phosphate (Promega
Corporation, Madison, WI, USA) transfections with different
constructs were conducted on 6–7 days in vitro (DIV), and
all experiments were performed on 7–8 DIV. Human embryonic kidney
(HEK)293 cell (American Type Culture Collection, Manassas, VA, USA)
culture was performed as described previously (21). To determine the expression of
Flag-CRMP-5, the constructed pCMV-CRMP-5-Tag2 (pCMV-Tag2 vector was
obtained from Addgene, Cambridge, MA, USA) was transfected into
HEK293 cells using calcium phosphate. To verify the efficacy and
specificity of siRNAs, co-transfection of 100 pmol siRNAs or NC
together with 2 µg of the pCMV-CRMP-5-Tag2 plasmids into
HEK293 cells was performed using calcium phosphate. Following
transfection, cells were grown for 36 h prior to harvesting.
Plasmids and siRNA fragments were transfected using the ProFection
Mammalian Transfection system (Promega Corporation), performed
according to the manufacturer's instructions.
Growth cone particle isolation
The methods were performed according to previous
studies (22–24). Briefly, brains were dissected from
the rats at 18 days of gestation and homogenized by a Teflon-glass
homogenizer (Thomas Scientific, Swedesboro, NJ, USA) in ~8 volumes
(w/v) of 0.32 M sucrose (Sigma-Aldrich, St. Louis, MO, USA)
containing 1 mM MgC12 (Sigma-Aldrich) and 1 mM Tes-NaOH
(Sigma-Aldrich; pH 7.3), and the following protease inhibitors: 3
µM aprotinin (Calbiochem; EMD Millipore, Billerica, MA,
USA), 20 mM benzamidine, 1 mM leupeptin, 1 mM pepstatin A and 0.6
mM phenylmethylsulfonyl fluoride (all from Sigma-Aldrich, St.
Louis, MO, USA). The homogenate was centrifuged at 200 × g for 15
min. The low speed supernatant was loaded onto a discontinuous
sucrose density gradient consisting of three layers: 0.75, 1.0 and
2.66 M; the gradients were spun to equilibrium at 150,000 × g for
200 min in a Beckman SW 40 Ti Rotor (Beckman Coulter, Inc., Brea,
CA, USA). The isolated growth cone gradients were prepared as
previously described (23).
Neuronal samples subjected to immunocytochemistry were scanned by
confocal microscopy. The fraction at the load/0.75 M interface
(designated A as in the referred reference) contained the isolated
growth cones or growth cone particles (GCPs). Then 'A' fraction
samples were subjected to electron microscopy (H-7650, Hitachi,
Tokyo, Japan).
Electron microscopic procedures were performed as
preciously described (23).
Briefly, aliquots were mixed slowly with increasing amounts of
phosphate-buffered glutaraldehyde (1.5%). After 10–15 min, the
fixed material was pelleted by centrifugation at 2,000 × g for 5
min at room temperature into a conical embedding capsule (Electron
Microscopy Sciences, Hatfield, PA, USA). After further
glutaraldehyde fixation and washing with arsenate buffer, samples
were treated with osmium tetroxide, block-stained with magnesium
uranyl acetate and embedded in embedding mixture from the Low
Viscosity Embedding Media Spurr's kit (all from Electron Microscopy
Sciences). Thin sections were cut, stained and examined with the
electron microscope.
Western blotting
Western blot analysis was performed as previously
described (24). Briefly, lysates
were separated using 8, 10 and 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (Beyotime Institute of
Biotechnology, Jiangsu, China) and were then electrophoretically
transferred to a polyvinylidene difluoride membrane (Beyotime
Institute of Biotechnology). Membranes were blocked in
Tris-buffered saline (TBS; Beyotime Institute of Biotechnology)
with 5% milk and 0.05% Tween-20 and were then probed with the
following primary antibodies at 4°C overnight: Rabbit anti-CRMP-5
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA, cat. no.
sc-292382; 1:1,000) and mouse anti-β-actin (cat. no. A5441;
Sigma-Aldrich; 1:1,000). Subsequent to washing with TBS + 0.05%
Tween; the membranes were incubated with horseradish
peroxidase-conjugated goat anti-mouse (cat no. 115-001-008) or
anti-rabbit secondary antibodies (cat no. 711-001-003; Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA; 1:5,000)
and were then visualized using enhanced chemiluminescence reagents
(Beyotime Institute of Biotechnology).
Immunoprecipitation
Immunoprecipitation assays were performed as
described previously (21,25). For immunoprecipitation of
hippocampal neurons, extracts were prepared by solubilization in
400 µl of cell lysis buffer (Beyotime Institute of
Biotechnology) for 10 min at 4°C. Subsequent to brief sonication,
the lysates were cleared by centrifugation at 15,000 × g for 10 min
at 4°C, the cell extract was then immunoprecipitated with 4
µg of the antibodies against CRMP-5 (1:500) or β-actin
(1:1,000), then the samples were incubated with 60 µl of
protein G plus protein A-agarose (Calbiochem; EMD Millipore) for 16
h at 4°C by continuous inversion. Immunocomplexes were pelleted at
500 × g for 5 min at 4°C and washed three times. The precipitated
immunocomplexes were boiled in Laemmli buffer and assayed using
western blot analysis with anti-CRMP-5 or mouse monoclonal
anti-β-tubulin antibodies (Sigma-Aldrich; cat. no. T5201; dilution,
1:2,000).
Immunofluorescence
Hippocampal neurons were grown on coverslips (Thermo
Fisher Scientific, Inc.) and were processed for immunofluorescence
according to the standard protocol described previously (24). Cells were fixed with 4% (w/v)
paraformaldehyde (Sigma-Aldrich) for 5 min at room temperature and
permeabilized with 0.1% Triton X-100 in phosphate-buffered saline
(PBS) for 20 min. The cells were blocked in 3% normal donkey serum
(Jackson Immunoresearch Laboratories, Inc.) in TBS + 0.1% Triton
X-100 for 1 h at room temperature and incubated with the rabbit
anti-CRMP-5 and mouse anti-actin antibodies at 4°C overnight. The
cells were washed 3 times for 10 min with PBS + 0.1% Tween-20, and
were incubated with the monoclonal donkey anti-rabbit IgG Dylight
549 (Jackson ImmunoResearch Laboratories, Inc.; cat. no.
711-516-152; 1:1,000) or monoclonal donkey anti-mouse IgG Dylight
488 (Jackson ImmunoResearch Laboratories, Inc.; cat. no.
715-485-150; 1:1,000) for 2 h at room temperature. Subsequent to
three washes in lysis buffer, cells were mounted on glass slides
with Fluoro Gel II containing 4′,6-diamidino-2-phenylindole
(Electron Microscopy Sciences, Hatfield, PA, USA). For
co-localization analysis, confocal images were analyzed using
ImageJ software (version 1.48, National Institutes of Health,
Bethesda, MA, USA) with Just Another Colocalization Plugin (JACoP,
National Institutes of Health) (26). The random or codependent nature of
colocalizations were tested using Cytofluorogram and intensity
correlation analysis. All the co-localization calculations were
performed on >10 cells from at least three independent
experiments. Microscopy and image analysis were conducted using the
same optical slice thickness for every channel using a confocal
microscope (LSM 710; Carl Zeiss AG, Oberkochen, Germany).
RNA interference
A validated CRMP-5 short interfering RNA (siRNA)
(siCRMP-5) fragment and NC (scrambled sequence, negative control)
were synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China)
and validated previously (15,17,24).
Hippocampal neurons were transfected with siRNA using a calcium
phosphate protocol (27). To
transfect neurons in 24-well tissue culture plates, 100 pmol siRNA
was combined with 37 µl 2 M CaCl2 solution in
sterile, deionized water to a final volume of 300 µl and
then mixed well with 300 µl 2X
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid-buffered saline.
The mixtures were vortexed and incubated at 25°C for approximately
4 min. In each well, 30 µl mixture was added drop-wise to
the cells and allowed to incubate for an additional 25 min. The
green fluorescent protein expression plasmid (Addgene) was
co-transfected with the siRNAs to mark the transfected cells.
Statistical analysis
Data are presented as the mean ± standard error.
Significant differences were assessed with one-way analysis of
variance followed by the Bonferroni or Tamhane post hoc tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
CRMP-5 interacts with cytoskeleton
In order to separate GCPs from the soma, sucrose
gradient centrifugation was used. GCPs were then subjected to
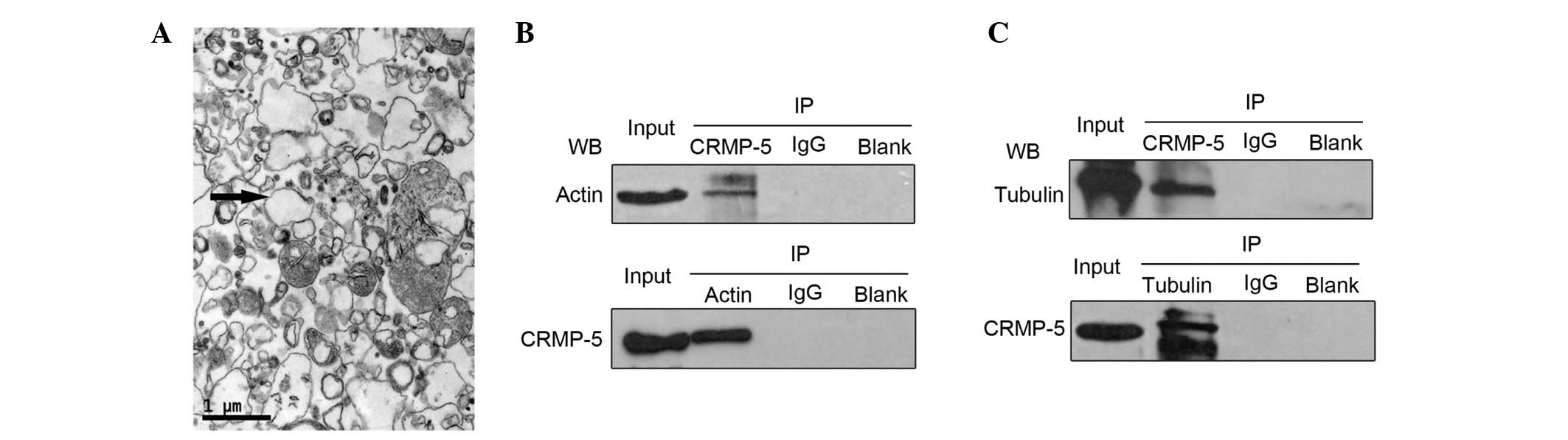
transmission electron microscopy. As presented in Fig. 1A, GCPs existed as vesicular
structures (indicated by arrows), with a uniform size (~1
µm) and loose distribution, which was consistent with
previously reported results (22).
Subsequently, co-immunoprecipitation was conducted in order to
determine whether CRMP-5 would interact with the actin and tubulin
cytoskeletons, rather than with tubulin alone as previously
reported (24). Using the CRMP-5
antibody, the actin immune-signal in the CRMP-5-precipitated
sediment from GCP lysates was detected, with no actin immune-signal
in the IgG and blank control groups (Fig. 1B, upper panel). Conversely, using
the actin antibody, a CRMP-5 signal was detected in the
actin-precipitated sediment (Fig.
1B, lower panel). Furthermore, CRMP-5 was indicated to interact
with tubulin using the CRMP-5 and tubulin antibodies (Fig. 1C). These data suggest that CRMP-5
interacts with the actin and tubulin cytoskeleton systems.
CRMP-5 colocalizes with actin in growth
cones of hippocampal neurons
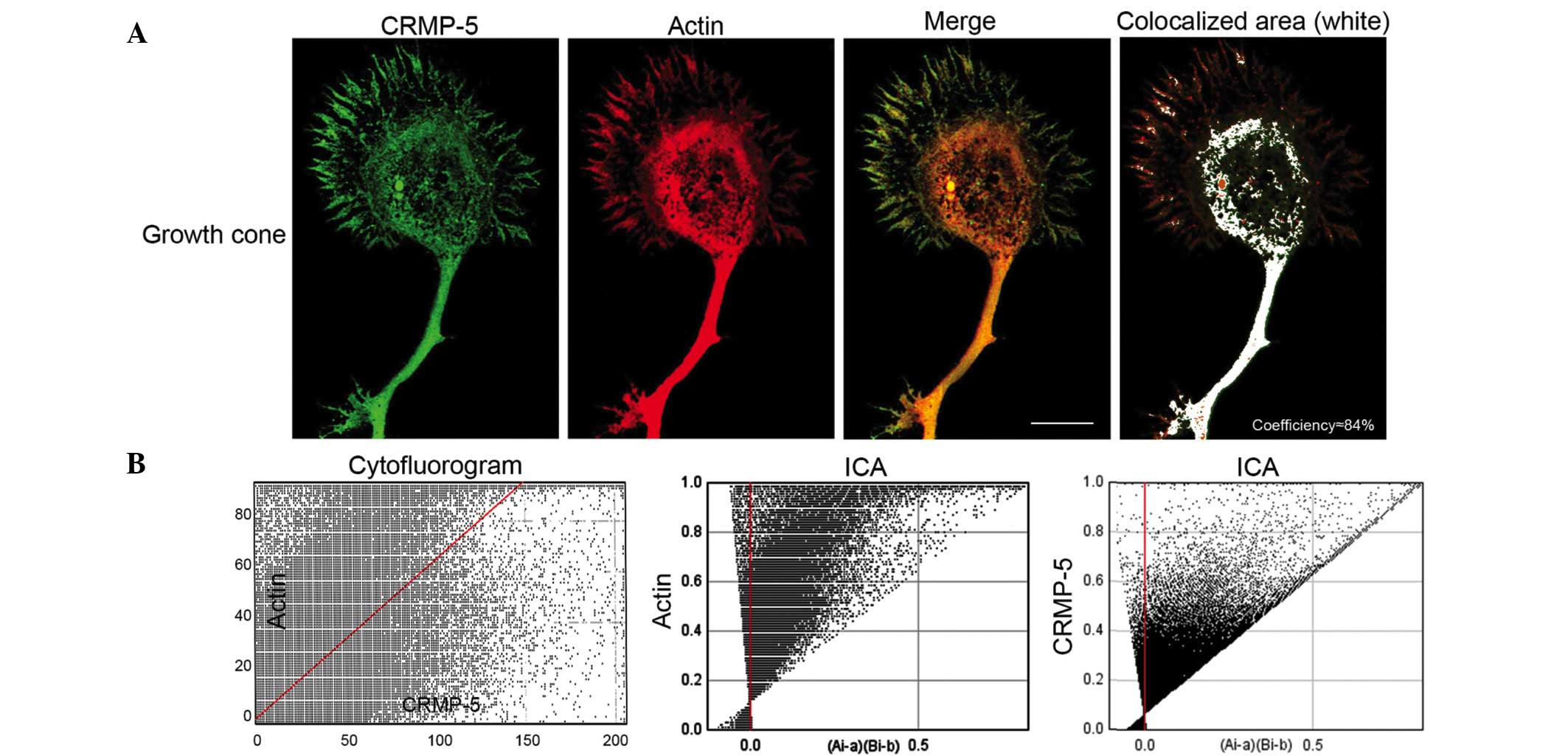
Furthermore, the distribution of actin and CRMP-5 in
growth cones of developing hippocampal neurons was investigated.
Neurons were cultured at a density of 1×104
cells/cm2 on a PDL-coated coverslip. At 3 DIV, neurons
were subjected to immunofluorescence with CRMP-5 and actin
antibodies. As presented in Fig.
2, actin was predominantly distributed in the P-domain and
T-zone, with fewer signals in the C-domain. Lamellipodia in the
P-domain exhibited a clear mesh and bundled filaments. CRMP-5 was
distributed predominantly in the C-domain and T-zone, however also
exhibited clear signals in the lamellipodia and filopodia of the
P-domain. CRMP-5 and actin were observed to exhibit colocalization
in the actin filaments in the P-domain and in the T-zone, with a
colocalization co-efficiency of 84% (Fig. 2, right panel). Using analysis with
ImageJ plus JACoP (26), a clear
cytofluorogram and ICA score between actin and CRMP-5 was obtained,
which additionally indicted colocalization of actin and CRMP-5.
These data suggest that CRMP-5 colocalizes with actin in the growth
cones of developing neurons.
CRMP-5 regulates actin dynamics and
growth cone development
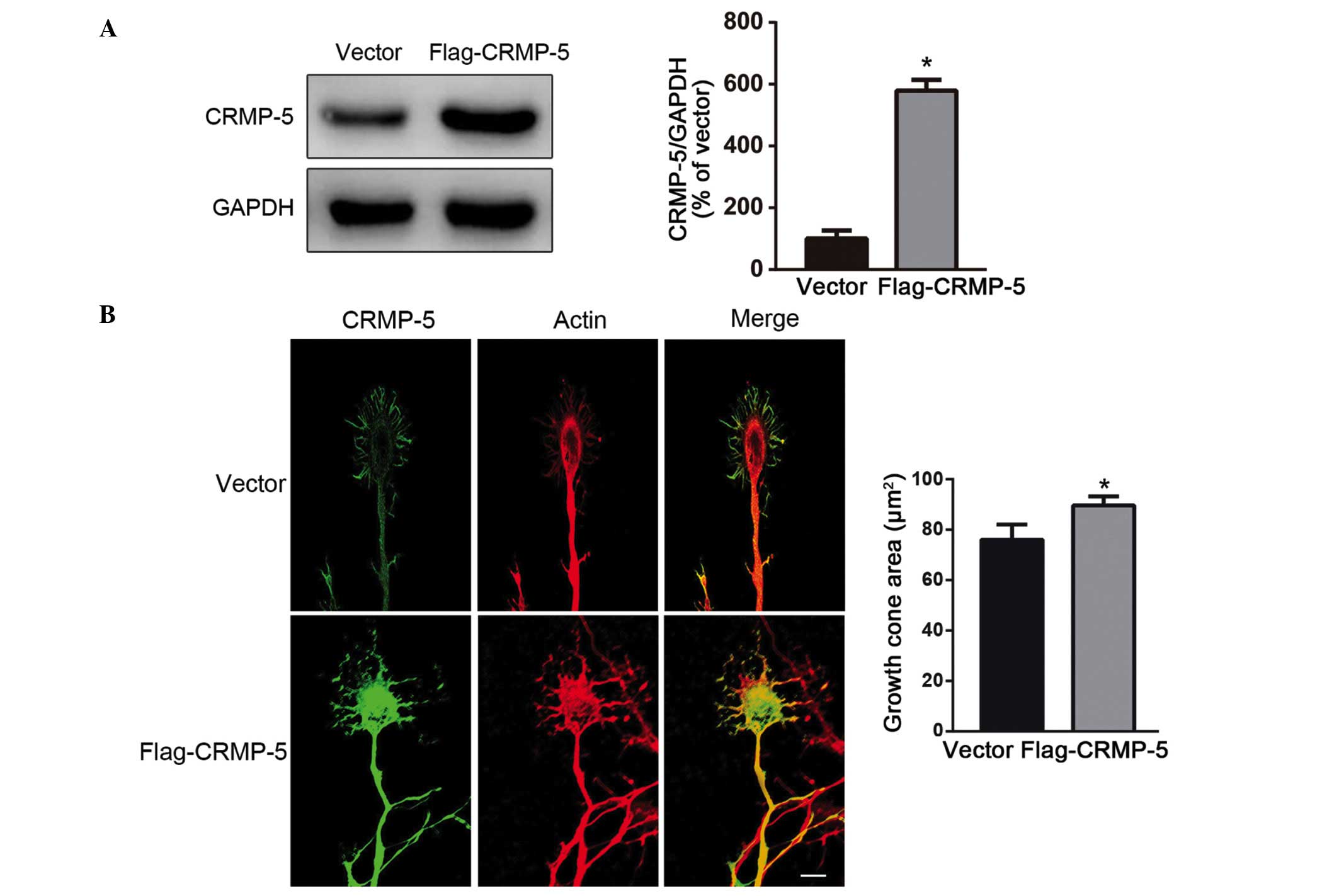
In order to determine the effect of CRMP-5 on actin
dynamics and growth cone development, a CRMP-5 expression plasmid
(pCMV-CRMP-5-Tag2) was constructed, which had been previously
reported (26). As presented in
Fig. 3A, the CRMP-5 plasmid was
successfully expressed in HEK293 cells by the detection of western
blot with the Flag antibody. The plasmid was then transfected into
hippocampal neurons. As presented in Fig. 3B, growth cones transfected with
CRMP-5 were fan-shaped, with actin predominantly distributed in the
P-domain with long actin filaments and increased branches.
Overexpression of CRMP-5 promoted growth cone development, with an
enlarged growth cone area (Fig.
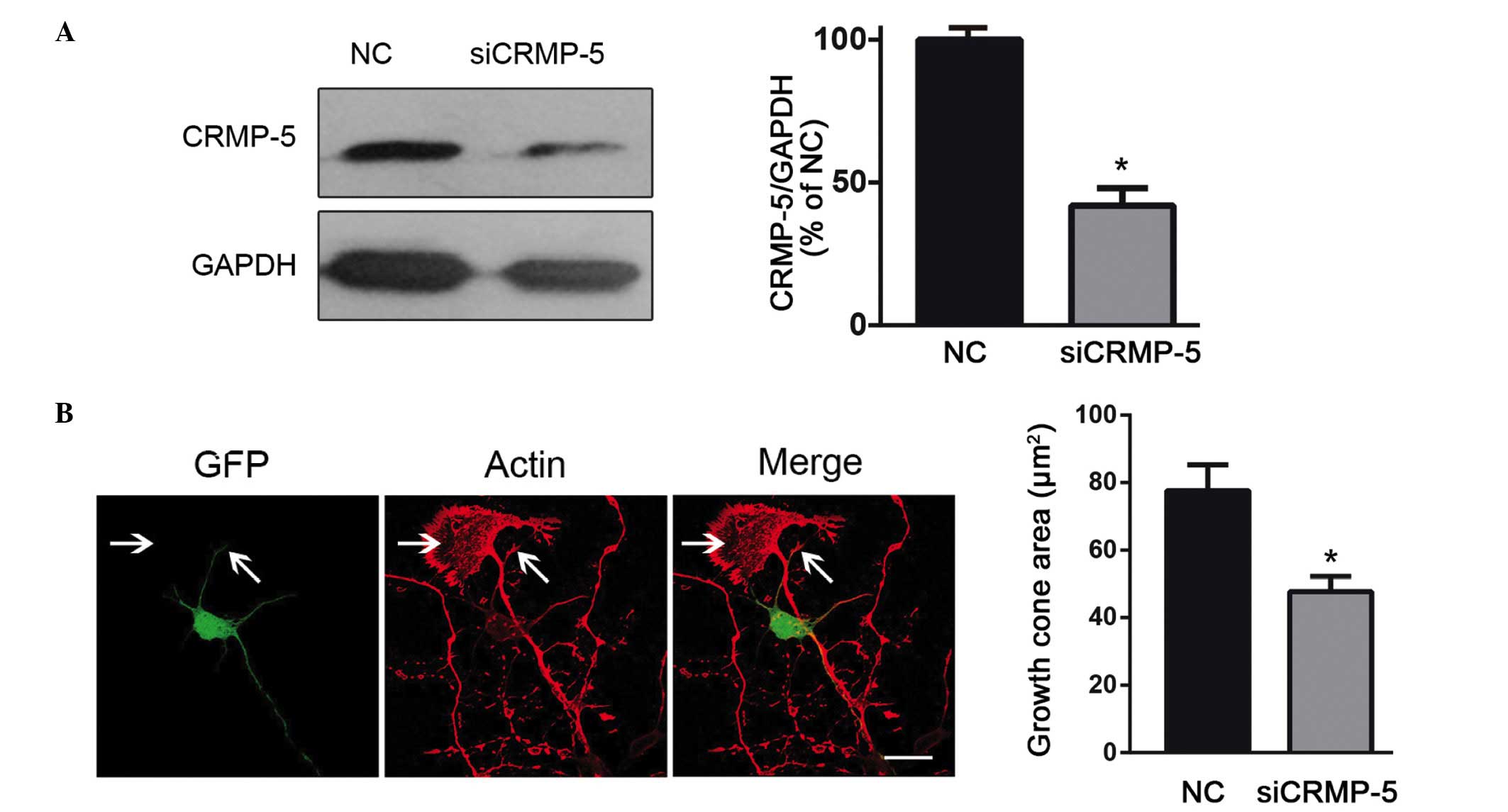
3B, right panel). The CRMP-5 siRNA experiment was then
conducted, where the siRNA efficiency was determined previously
(24) and the results are
presented in Fig. 4A. On CRMP-5
silencing, the actin filaments were observed to become fragile, and
exhibited collapsed growth cones, compared with those in
non-transfected neurons (Fig. 4B).
These data suggest that CRMP-5 regulates actin dynamics and growth
cone development.
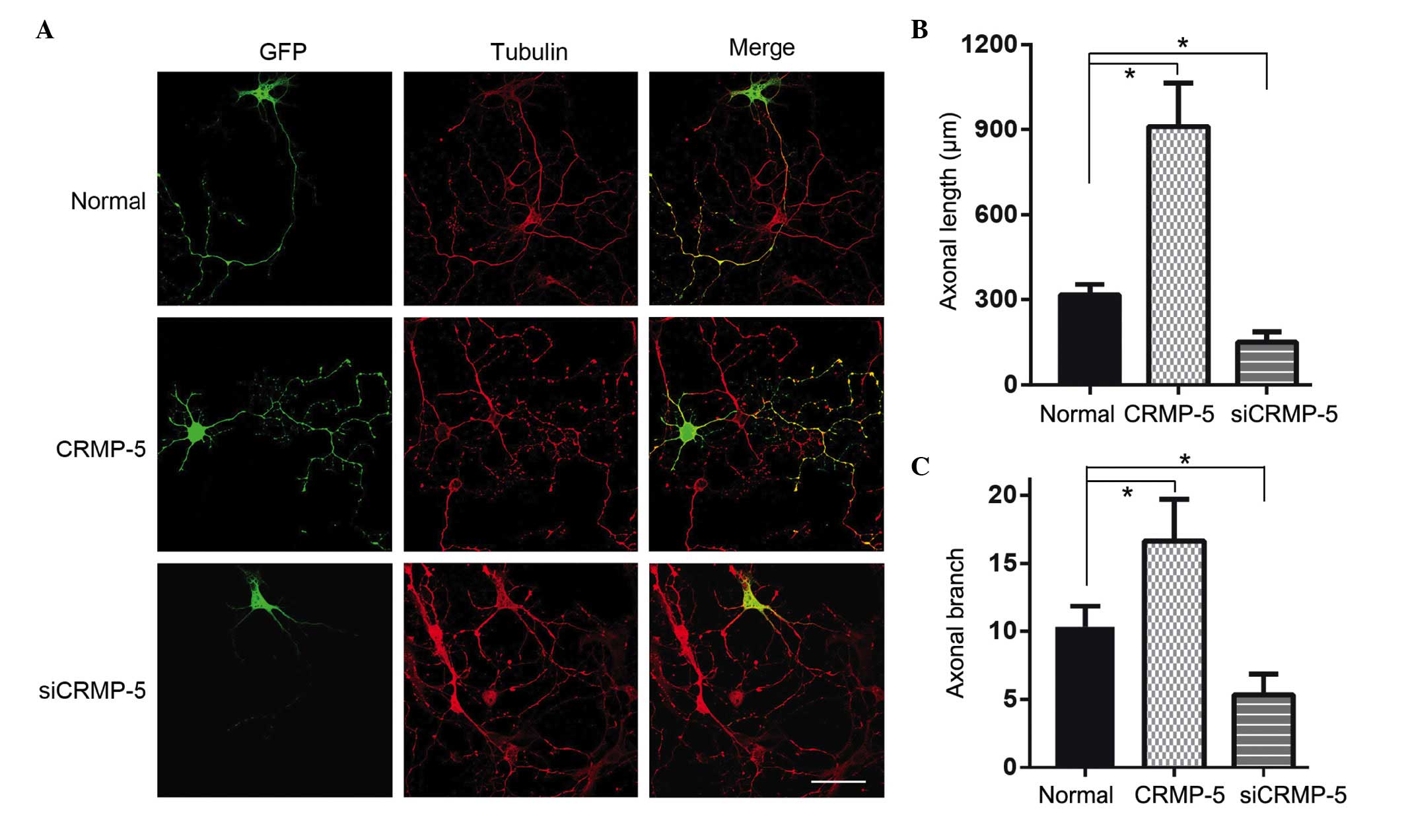
CRMP-5 regulates neurite outgrowth
It was then investigated whether CRMP-5 would
regulate neurite outgrowth. Hippocampal neurons were transfected
with the CRMP-5 overexpression plasmid or with CRMP-5 siRNA
fragments. Subsequent to transfection for 5 days, the neurons were
subjected to immunocytochemistry. As presented in Fig. 5, the CRMP-5-overexpressing neurons
were observed to have a longer neurite length and an increased
number of neurite branches than that of CRMP-5-silenced neurons,
which possessed short neurite length and few branches (Fig. 5B and C). These data suggest that
CRMP-5 regulates neurite outgrowth via modulating actin dynamics
and growth cone development.
Discussion
CRMPs are critical for growth cone development,
axonal guidance and neuronal polarity. CRMP proteins 1–5 have been
implicated to associated with tubulin (17,28).
CRMP-5, an isoform distinct from the other four CRMPs, was recently
reported to localize in the filopodia of growth cones (15), leaving the role of
CRMP-5-associated proteins in regulating growth cones to be further
elucidated. Thus, in the current study, it was identified that
CRMP-5 interacted with the actin cytoskeleton, colocalized with
actin in growth cones, regulated actin dynamics and modulated
growth cone development and neurite outgrowth.
Neurite outgrowth relies on the interaction between
the actin and microtubule cytoskeleton (29). Actin and microtubules within the
growth cone are not independent, however are associated, sensing
each other's movement (29).
Heidemann et al (30)
identified that coordinated cytoskeletal movement was important in
axon guidance. Numerous signaling transduction pathways and
proteins have been reported to be involved in this process. These
proteins include Rho family proteins (31–33),
plus-end tracking proteins (34,35),
spectraplakins (36,37) and microtubule-associated proteins
(38), particularly tau protein
(39–41). In addition, CRMPs may be the
structural mediator of actin and microtubules, CRMP-2 is able to
interact with tubulin promoting its assembly (28,42).
CRMP-4 interacts with actin and promotes neurite growth (43,44).
Previous studies have identified that CRMP-5 interacts with tubulin
in the growth cones (24). It has
additionally been identified that CRMP-2 and CRMP-4 interact to
coordinate cytoskeletal dynamics, regulating growth cone
development and axon elongation (45). Whether additional novel proteins or
members of the CRMP family, (e.g. CRMP-1 and CRMP-3) are involved
in this process remains unclear, thus requires further
investigation.
CRMP-5 is highly distributed in fetal and neonatal
rat brains and is markedly reduced in adult brains (13). In hippocampal neurons, the
spatiotemporal distribution of CRMP-5 varies at different
developmental stages; CRMP-5 is present in growth cones at stage 3
when neuronal polarity is developed, and reduces to a low level
when neurons are polarized. Thus, CRMP-5 contributes to the dynamic
regulation of neuronal polarity (17). The data of the current study are
consistent with previously reported data (15), in that CRMP-5 in the growth cone is
necessary and sufficient for growth cone development. The results
of the current study additionally suggest that CRMP-5 functions
through its interaction with the cytoskeleton. CRMP-5 has been
reported to inhibit dendritic growth in hippocampal neurons
(17). However, in cerebellar
Purkinje cells, CRMP-5 has been previously reported to promote
dendritic development and synaptic plasticity in studies using
CRMP-5−/− mice, where aberrant dendrite morphology was
observed (16,17). The different functions of CRMP-5
from these studies suggest that CRMP-5 acts to be cell-type
specific and this may be due to its efferent distribution or its
different interacting proteins. Taken together, the current study
has provided novel evidence for CRMP-5-regulated growth cone
development and neurite outgrowth.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 31170941 and
31300885), the Natural Science Foundation of Guangdong Province,
China (grant nos. S2013010014191, 2014A030310024 and
2014A030313357), Medical Scientific Research Foundation of
Guangdong Province, China (grant nos. A2014382 and A2015479) and
the Fundamental Research Funds for the Central Universities (grant
no. 21615477).
Abbreviations:
|
CRMP
|
collapsin response mediator
protein
|
|
MTs
|
microtubules
|
|
DIV
|
days in vitro
|
|
NC
|
negative control
|
|
T-zone
|
the transition zone
|
|
C-domain
|
the central domain
|
References
|
1
|
Huber AB, Kolodkin AL, Ginty DD and
Cloutier JF: Signaling at the growth cone: Ligand-receptor
complexes and the control of axon growth and guidance. Annu Rev
Neurosci. 26:509–563. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tessier-Lavigne M and Goodman CS: The
molecular biology of axon guidance. Science. 274:1123–1133. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rolls MM and Jegla TJ: Neuronal polarity:
an evolutionary perspective. J Exp Biol. 218:572–580. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nieto MA: Molecular biology of axon
guidance. Neuron. 17:1039–1048. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zou Y: Does planar cell polarity signaling
steer growth cones? Curr Top Dev Biol. 101:141–160. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pak CW, Flynn KC and Bamburg JR:
Actin-binding proteins take the reins in growth cones. Nat Rev
Neurosci. 9:136–147. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schaefer AW, Kabir N and Forscher P:
Filopodia and actin arcs guide the assembly and transport of two
populations of micro-tubules with unique dynamic parameters in
neuronal growth cones. J Cell Biol. 158:139–152. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buck KB and Zheng JQ: Growth cone turning
induced by direct local modification of microtubule dynamics. J
Neurosci. 22:9358–9367. 2002.PubMed/NCBI
|
|
9
|
Minturn JE, Fryer HJ, Geschwind DH and
Hockfield S: TOAD-64, a gene expressed early in neuronal
differentiation in the rat, is related to unc-33, a C. elegans gene
involved in axon outgrowth. J Neurosci. 15:6757–6766.
1995.PubMed/NCBI
|
|
10
|
Fukada M, Watakabe I, Yuasa-Kawada J,
Kawachi H, Kuroiwa A, Matsuda Y and Noda M: Molecular
characterization of CRMP5, a novel member of the collapsin response
mediator protein family. J Biol Chem. 275:37957–37965. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuasa-Kawada J, Suzuki R, Kano F, Ohkawara
T, Murata M and Noda M: Axonal morphogenesis controlled by
antagonistic roles of two CRMP subtypes in microtubule
organization. Eur J Neurosci. 17:2329–2343. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goshima Y, Nakamura F, Strittmatter P and
Strittmatter SM: Collapsin-induced growth cone collapse mediated by
an intracellular protein related to UNC-33. Nature. 376:509–514.
1995. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Inatome R, Tsujimura T, Hitomi T, Mitsui
N, Hermann P, Kuroda S, Yamamura H and Yanagi S: Identification of
CRAM, a novel unc-33 gene family protein that associates with CRMP3
and protein-tyrosine kinase(s) in the developing rat brain. J Biol
Chem. 275:27291–27302. 2000.PubMed/NCBI
|
|
14
|
Ricard D, Rogemond V, Charrier E, Aguera
M, Bagnard D, Belin MF, Thomasset N and Honnorat J: Isolation and
expression pattern of human Unc-33-like phosphoprotein 6/collapsin
response mediator protein 5 (Ulip6/CRMP5): Coexistence with
Ulip2/CRMP2 in Sema3a- sensitive oligodendrocytes. J Neurosci.
21:7203–7214. 2001.PubMed/NCBI
|
|
15
|
Hotta A, Inatome R, Yuasa-Kawada J, Qin Q,
Yamamura H and Yanagi S: Critical role of collapsin response
mediator protein-associated molecule CRAM for filopodia and growth
cone development in neurons. Molecular Biology of the Cell.
16:32–39. 2005. View Article : Google Scholar :
|
|
16
|
Yamashita N, Mosinger B, Roy A, Miyazaki
M, Ugajin K, Nakamura F, Sasaki Y, Yamaguchi K, Kolattukudy P and
Goshima Y: CRMP5 (collapsin response mediator protein 5) regulates
dendritic development and synaptic plasticity in the cerebellar
Purkinje cells. J Neurosci. 31:1773–1779. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brot S, Rogemond V, Perrot V,
Chounlamountri N, Auger C, Honnorat J and Moradi-Améli M: CRMP5
interacts with tubulin to inhibit neurite outgrowth, thereby
modulating the function of CRMP2. J Neurosci. 30:10639–10654. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mitsui N, Inatome R, Takahashi S, Goshima
Y, Yamamura H and Yanagi S: Involvement of Fes/Fps tyrosine kinase
in sema-phorin3A signaling. EMBO J. 21:3274–3285. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
National Research Council: Guide for the
Care and Use of Laboratory Animals. National Academy Press;
Washington DC: 1996, pp. 125
|
|
20
|
Takahashi S, Inatome R, Yamamura H and
Yanagi S: Isolation and expression of a novel mitochondrial septin
that interacts with CRMP/CRAM in the developing neurones. Genes
Cells. 8:81–93. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Fan J, Tian Q, Song Z, Zhang JF
and Chen Y: Characterization of two distinct modes of endophilin in
clathrin-mediated endocytosis. Cell Signal. 24:2043–2050. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pfenninger KH, Ellis L, Johnson MP,
Friedman LB and Somlo S: Nerve growth cones isolated from fetal rat
brain: Subcellular fractionation and characterization. Cell.
35:573–584. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lohse K, Helmke SM, Wood MR, Quiroga S, de
la Houssaye BA, Miller VE, Negre-Aminou P and Pfenninger KH: Axonal
origin and purity of growth cones isolated from fetal rat brain.
Brain Res Dev Brain Res. 96:83–96. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiao L, Liu W, Li J, Xie Y, He M, Fu J,
Jin W and Shao C: Irradiated U937 cells trigger inflammatory
bystander responses in human umbilical vein endothelial cells
through the p38 pathway. Radiat Res. 182:111–121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tian Q, Zhang JF, Fan J, Song Z and Chen
Y: Endophilin isoforms have distinct characteristics in
interactions with N-type Ca2+ channels and dynamin I. Neurosci
Bull. 28:483–492. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bolte S and Cordelières FP: A guided tour
into subcellular colocalization analysis in light microscopy. J
Microsc. 224:213–232. 2006. View Article : Google Scholar
|
|
27
|
Tan M, Ma S, Huang Q, Hu K, Song B and Li
M: GSK-3α/β-mediated phosphorylation of CRMP-2 regulates
activity-dependent dendritic growth. J Neurochem. 125:685–697.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fukata Y, Itoh TJ, Kimura T, Ménager C,
Nishimura T, Shiromizu T, Watanabe H, Inagaki N, Iwamatsu A, Hotani
H and Kaibuchi K: CRMP-2 binds to tubulin heterodimers to promote
microtubule assembly. Nat Cell Biol. 4:583–591. 2002.PubMed/NCBI
|
|
29
|
Dent EW, Gupton SL and Gertler FB: The
growth cone cytoskeleton in axon outgrowth and guidance. Cold
Spring Harb Perspect Biol. 3:a0018002011. View Article : Google Scholar
|
|
30
|
Heidemann SR, Landers JM and Hamborg MA:
Polarity orientation of axonal microtubules. J Cell Biol.
91:661–665. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ren XD, Kiosses WB and Schwartz MA:
Regulation of the small GTP-binding protein Rho by cell adhesion
and the cytoskeleton. EMBO J. 18:578–585. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Waterman-Storer CM, Worthylake RA, Liu BP,
Burridge K and Salmon ED: Microtubule growth activates Rac1 to
promote lamellipodial protrusion in fibroblasts. Nat Cell Biol.
1:45–50. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Watabe-Uchida M, Govek EE and Van Aelst L:
Regulators of Rho GTPases in neuronal development. J Neurosci.
26:10633–10635. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Watanabe T, Noritake J and Kaibuchi K:
Roles of IQGAP1 in cell polarization and migration. Novartis Found
Symp. 269:92–101; discussion 101–105, 223–230. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Komarova Y, Lansbergen G, Galjart N,
Grosveld F, Borisy GG and Akhmanova A: EB1 and EB3 control CLIP
dissociation from the ends of growing microtubules. Mol Biol Cell.
16:5334–5345. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Suozzi KC, Wu X and Fuchs E:
Spectraplakins: Master orchestrators of cytoskeletal dynamics. J
Cell Biol. 197:465–475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kodama A, Lechler T and Fuchs E:
Coordinating cytoskeletal tracks to polarize cellular movements. J
Cell Biol. 167:203–207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cueille N, Blanc CT, Popa-Nita S, Kasas S,
Catsicas S, Dietler G and Riederer BM: Characterization of MAP1B
heavy chain interaction with actin. Brain Res Bull. 71:610–618.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
He HJ, Wang XS, Pan R, Wang DL, Liu MN and
He RQ: The proline-rich domain of tau plays a role in interactions
with actin. BMC Cell Biol. 10:812009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Correas I, Padilla R and Avila J: The
tubulin-binding sequence of brain microtubule-associated proteins,
tau and MAP-2, is also involved in actin binding. Biochem J.
269:61–64. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Henríquez JP, Cross D, Vial C and Maccioni
RB: Subpopulations of tau interact with microtubules and actin
filaments in various cell types. Cell Biochem Funct. 13:239–250.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Arimura N, Menager C, Fukata Y and
Kaibuchi K: Role of CRMP-2 in neuronal polarity. J Neurobiol.
58:34–47. 2004. View Article : Google Scholar
|
|
43
|
Rosslenbroich V, Dai L, Baader SL, Noegel
AA, Gieselmann V and Kappler J: Collapsin response mediator
protein-4 regulates F-actin bundling. Exp Cell Res. 310:434–444.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Quinn CC, Chen E, Kinjo TG, Kelly G, Bell
AW, Elliott RC, McPherson PS and Hockfield S: TUC-4b, a novel TUC
family variant, regulates neurite outgrowth and associates with
vesicles in the growth cone. J Neurosci. 23:2815–2823.
2003.PubMed/NCBI
|
|
45
|
Tan M, Cha C, Ye Y, Zhang J, Li S, Wu F,
Gong S and Guo G: CRMP4 and CRMP2 interact to coordinate
cytoskeleton dynamics, regulating growth cone development and axon
elongation. Neural Plast. 2015:9474232015.PubMed/NCBI
|