Introduction
Epidemiological studies have indicated that
hypertension is an independent risk factor for stroke in patients
with cardiovascular disorders. There is a significant positive
correlation between blood pressure (BP) and stroke, even in the
prehypertensive stage (1). A
previous study demonstrated that extensive management of BP using
antihypertensive medications significantly reduced the risk of
stroke during the prehypertensive stage by 15–22% (2). Results from studies conducted using
the spontaneously hypertensive stroke-prone (SHRSP) rat model have
suggested that angiotensin receptor blockers (ARBs) significantly
attenuated BP-induced brain damage by reducing the increased BP
level (3,4). Among the ARB antihypertensive
medications which are currently available, losartan has
demonstrated clear efficacy in lowering morbidity and mortality
from disorders such as myocardial infarction (MI) and stroke
(5). In addition to its
antihypertensive activity, losartan reverses left ventricle
hypertrophy and atrial fibrillation (6). Together, these results suggest that
losartan may prevent cardiovascular system deterioration through
additional mechanisms.
Amlodipine is a long-acting calcium channel blocker
(CCB) of the dihydropyridine class, and has been widely used as an
antihypertensive agent in a clinical setting (7). While certain preliminary studies have
indicated that CCBs may reduce stroke risk to a greater extent than
ARBs (8), overall the data is
inconclusive. A meta-analysis has indicated that compared with
alternative antihypertensive drugs, amlodipine has the additional
advantages of controlling central arterial pressure, and reducing
the risk of stroke and MI (9). In
addition to its antihypertensive effects, studies have suggested
that amlodipine may significantly impact blood circulation in the
brain by influencing calcium concentration in blood vessels, which
may attenuate vasospasms and facilitate the reperfusion of ischemic
areas in the brains of patients (10). However, the results of previous
studies conflict with this conclusion (11,12).
A previous study observed that losartan exhibited improved effects
for the long-term control of BP in SHRSP rats, in particular
following treatment discontinuation. However, it was not
investigated whether prehypertensive intervention with either of
these two agents was able to reduce the risk of stroke.
Therefore, in the present study the effects of
losartan and amlodipine on hypertension and stroke were studied and
compared in a spontaneously hypertensive rat model.
Materials and methods
Animal treatments
All animals (96 males; weight, 100–120 g) were
purchased from the Shanghai Laboratory Animal Center Laboratory
Animal Co., Ltd. (Shanghai, China). The animals were housed under
conditions of a 12/12 h light/dark cycle, a temperature of 21±1°C
and 60% humidity. Following acclimation for 1 week, 4-week old
SHRSP rats were randomly assigned to 1 of 3 groups: Vehicle group,
SHRSP-Veh (n=24); losartan group, SHRSP-Los (20 mg/kg/day; n=24);
and the amlodipine group, SHRSP-Aml (10 mg/kg/day; n=24). Age- and
gender-matched Wistar rats were assigned to a corresponding control
group (WKY; n=24). Following 10 weeks of gavage administration of
either losartan, amlodipine or the vehicle (0.9% normal saline,
Sichuan Kelun Pharmaceutical Co., Ltd., Sichuan, China), the
systolic blood pressure (SBP), clinical stroke score and levels of
angiotensin II (Ang II) and aldosterone (Aldo) in the brain cortex,
in addition to the expression of Ang II receptors type 1 and 2
(AT1R and AT2R, respectively) were measured. Amlodipine was
purchased from Pfizer, Inc., (New York, NY, USA) and losartan was
from Merck & Co., Inc. (Whitehouse Station, NJ, USA). The
current study was approved by the Animal Ethics Committee of Fujian
Medical University (Fuzhou, China) and conducted in accordance with
institutional guidelines.
SBP measurement
Systolic pressure in the tail artery of animals in
all groups was measured at 4, 10, 16, 20, 24, 28, 32, 36 and 40
weeks of dosing using a Rat Noninvasive Blood Pressure Measurement
Analysis System (Chengdu TME Technology Co., Ltd., Chengdu,
China).
Measurement of the mesenteric
arterioles
Abdominal aortic cannulation was conducted and the
blood vessels (8 µm; 1512 microtome; Leica Microsystems,
Wetzlar, Germany) were perfused with formalin (ZSGB-BIO, Beijing,
China) and fixed with paraffin (ZSGB-BIO) prior to staining with
hematoxylin and eosin (H&E; Sigma-Aldrich, St. Louis, MO, USA).
Images of the blood vessels were captured using a microscope
(Olympus IX70; Olympus Corporation, Tokyo, Japan) The ratio of the
thickness of the blood vessel wall to the lumen (W/L) in the third
branch of the mesenteric arterioles was analyzed using Image Pro
Plus Version 4.5 analysis software (B-Colored Multifunction Imaging
Analyzing system; Media Cybernetics, Inc.). A 3 mm section of the
third branch of the mesenteric arterioles was immersed in a bath
solution containing 118 mmol/l NaCl, 4.7 mmol/l KCl, 2.5 mmol/l
CaCl2, 1.2 mmol/l MgSO4, 1.2 mmol/l
NaH2PO4, 20 mmol/l NaHCO3, and
11.1 mmol/l glucose (Beyotime Institute of Biotechnology, Shanghai,
China; 95% O2 and 5% CO2 saturated; 37°C) and
vasodilation and vasoconstriction were measured following treatment
with gradually increasing concentrations of norepinephrine
(10−10, 10−9, 10−8,
10−7, 10−6, and 10−5 mol/l; 100
µl; Tianjin Jinyao Amino Acid Co., Ltd., Tianjin, China),
acetylcholine (10−10, 10−9, 10−8,
10−7, 10−6, 10−5 and
10−4 mol/l; 100 µl; Sigma-Aldrich) and sodium
nitroprusside (10−10, 10−9, 10−8,
10−7, 10−6, 10−5 and
10−4 mol/l; 100 µl; China Resources Double-Crane
Pharmaceuticals Co., Ltd., Beijing, China). A ML870 PowerLab 30
eight-channel recorder and LabChart software 6.0 (AD Instruments,
Bella Vista. Australia) were used for data analysis.
Clinical stroke score
The clinical scores for stroke in each group were
evaluated according to the symptomatological classification system
(13), with minor modifications as
follows: Level 0, normal activity; level 1, slightly reduced
activity and/or slightly agitated; level 2, significantly reduced
activity and/or highly agitated; level 3, lethargic and
depression-like symptoms; level 4, paralyzed (either one or two
sides).
Radioimmunoassay (RIA)
RIA kits for Ang II and Aldo were purchased from
Beijing North Institute of Biological Technology (Beijing, China)
and used according to the manufacturer's instructions. Briefly,
samples of lysed tissue from 100 mg cortex pellet were boiled and
then centrifuged at 1,000 × g at 4°C for 10 min. The supernatant
fractions were used for measurements of Ang II and Aldo content in
the brain cortex. For serum tests, 1 ml blood samples were
extracted from the rats and stored in tubes containing heparin
anticoagulant. The samples were then centrifuged at 1,000 × g at
4°C for 10 min, and the resultant serum samples were analyzed for
levels of Ang II and Aldo according the manufacturer's
instructions.
Western blot
Samples containing 100 mg cerebral cortex tissue
were homogenized in lysis buffer (Beyotime Institute of
Biotechnology), and equal amounts of total protein (1 ml) were
applied to 10% SDS-polyacrylamide gels (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and separated by electrophoresis. The proteins
were then transferred onto a nitrocellulose membrane (EMD
Millipore, Billerica, MA, USA), blocked with 5% nonfat milk in
Tris-buffered saline containing 0.05% Tween-20 (TBST; Beyotime
Institute of Biotechnology) prior to incubation with polyclonal
rabbit anti-AT1R antibodies (1:500; ab18801; Abcam, Cambridge, MA,
USA), and rabbit polyclonal anti-AT2R antibody (1:800; ab19134;
Abcam) overnight at 4°C. Goat anti-β-actin antibodies were used as
an internal control (1:1,000; sc-1616; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA). The membranes were washed 3 times with
TBST, and then incubated for 1 h at room temperature with
horseradish peroxidase-conjugated goat anti-mouse IgG (1,000; Santa
Cruz Biotechnology, Inc.) and goat anti-rabbit IgG (1,000; Santa
Cruz Biotechnology, Inc.) secondary antibodies. Following a further
three washes with TBST, labeled proteins were visualized using
enhanced chemiluminescence (sc-2048; Santa Cruz Biotechnology,
Inc.) on high-performance chemiluminescence film (Eastman Kodak
Company, Rochester, NY, USA). The band intensity was quantified by
densitometry using image analysis software (Tanon Science &
Technology Co., Ltd., Shanghai, China). Results for AT1R and AT2R
were expressed as a ratio of AT1R or AT2R density divided by
β-actin density.
Statistical analysis
Stroke scores were analyzed using the Kruskal-Wallis
H test, followed by the Mann-Whitney U test. Comparisons between
groups were made using the least significant difference test and
SPSS software, version 16.0 (SPSS, Inc., Chicago, IL, USA). All
data with the exception of stroke scores are presented as the mean
± standard error. P<0.05 was considered to indicate a
statistically significant difference.
Results
Prehypertensive treatment with losartan
resulted in long-term effects on BP and blood vessel
pathophysiology in SHRSP rats
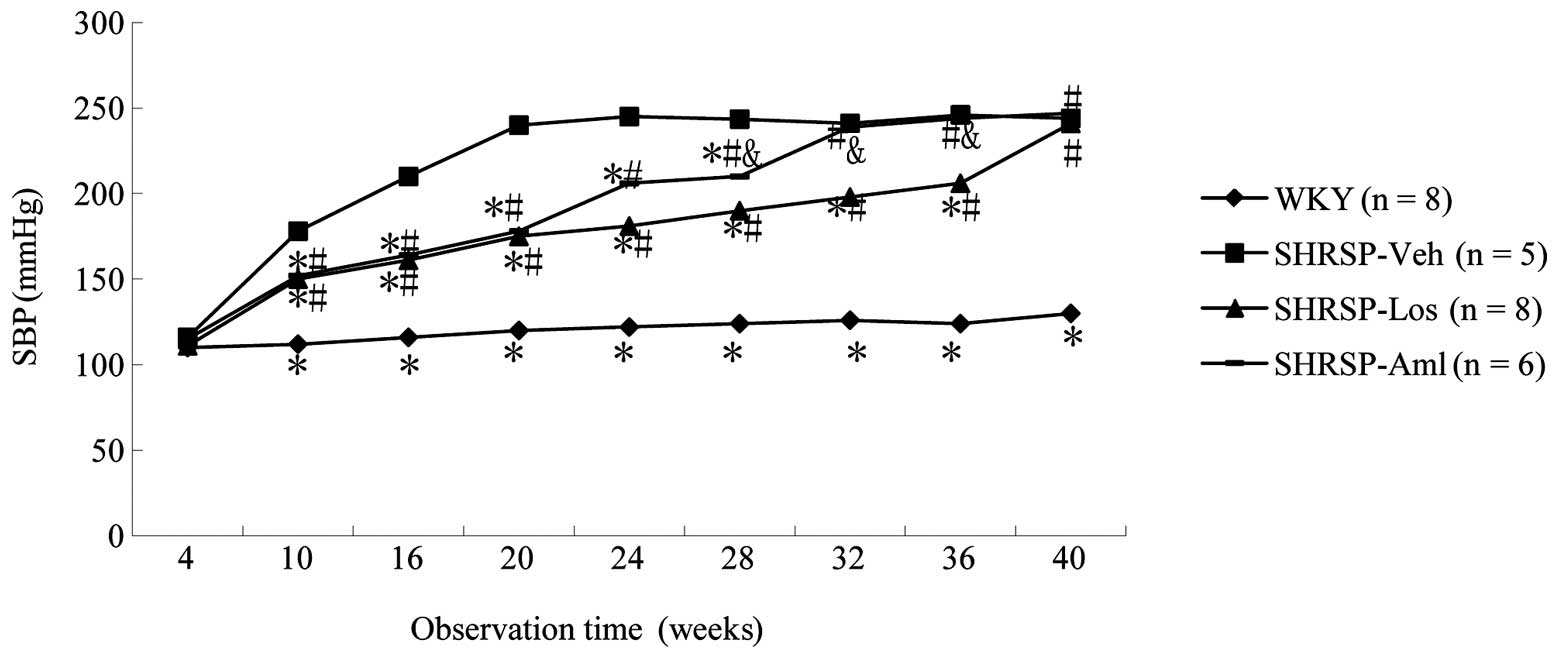
No significant difference in SBP was observed
between rats treated with losartan and amlodipine. However, rats
treated with losartan or amlodipine exhibited significantly reduced
SBP values compared with rats in the SHRSP-Veh group (Fig. 1). Additionally, rats in the
SHRSP-Los group maintained lower SBP readings for longer than their
counterparts in the SHRSP-Aml group, SBP readings in the SHRSP-Los
group were lower than those in the SHRSP-Aml group up to and
including 36 weeks, following the suspension of drug treatment at
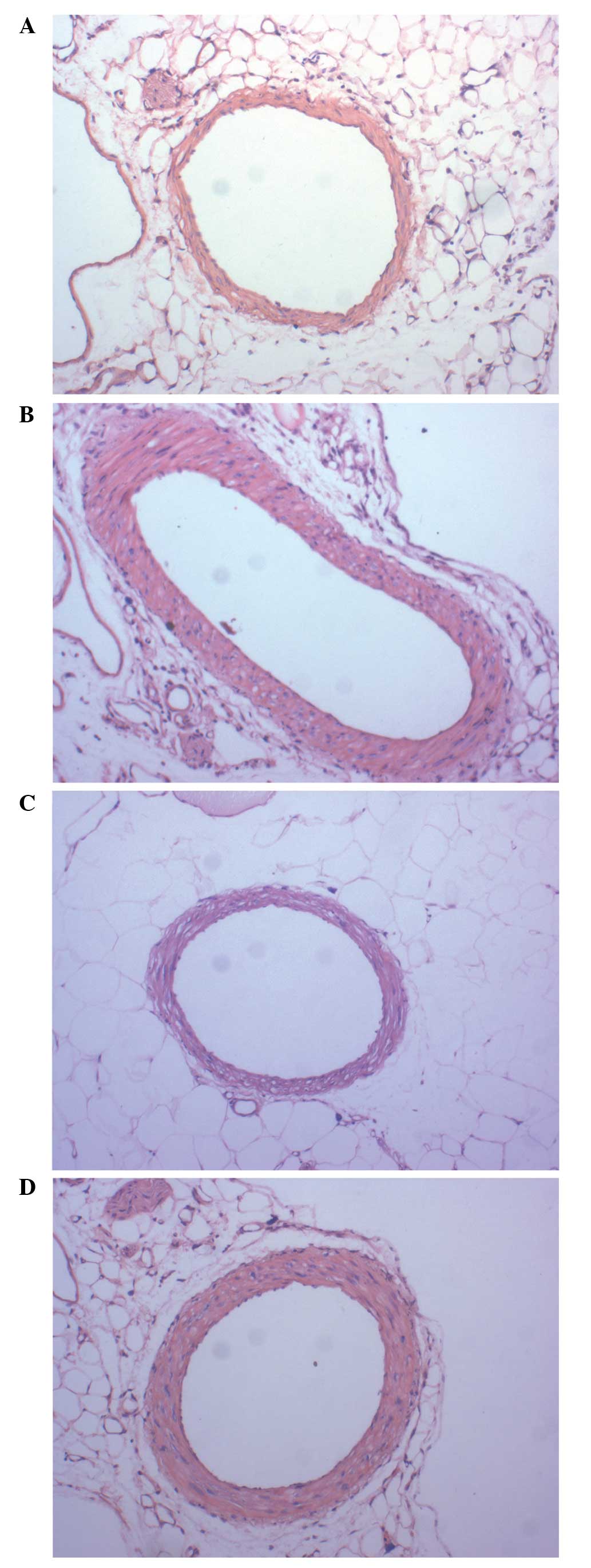
10 weeks (Fig. 1). To investigate
the direct effects of losartan on blood vessels, the third branch
of the mesenteric arterioles was studied using H&E staining.
Higher W/L values were observed in the vessels of mice in the
SHRSP-Veh and SHRSP-Aml groups (Table
I), however, the values in the SHRSP-Los groups were higher
compared with those in the WKY group. Losartan effectively reversed
the narrowed lumen of blood vessels, however amlodipine did not
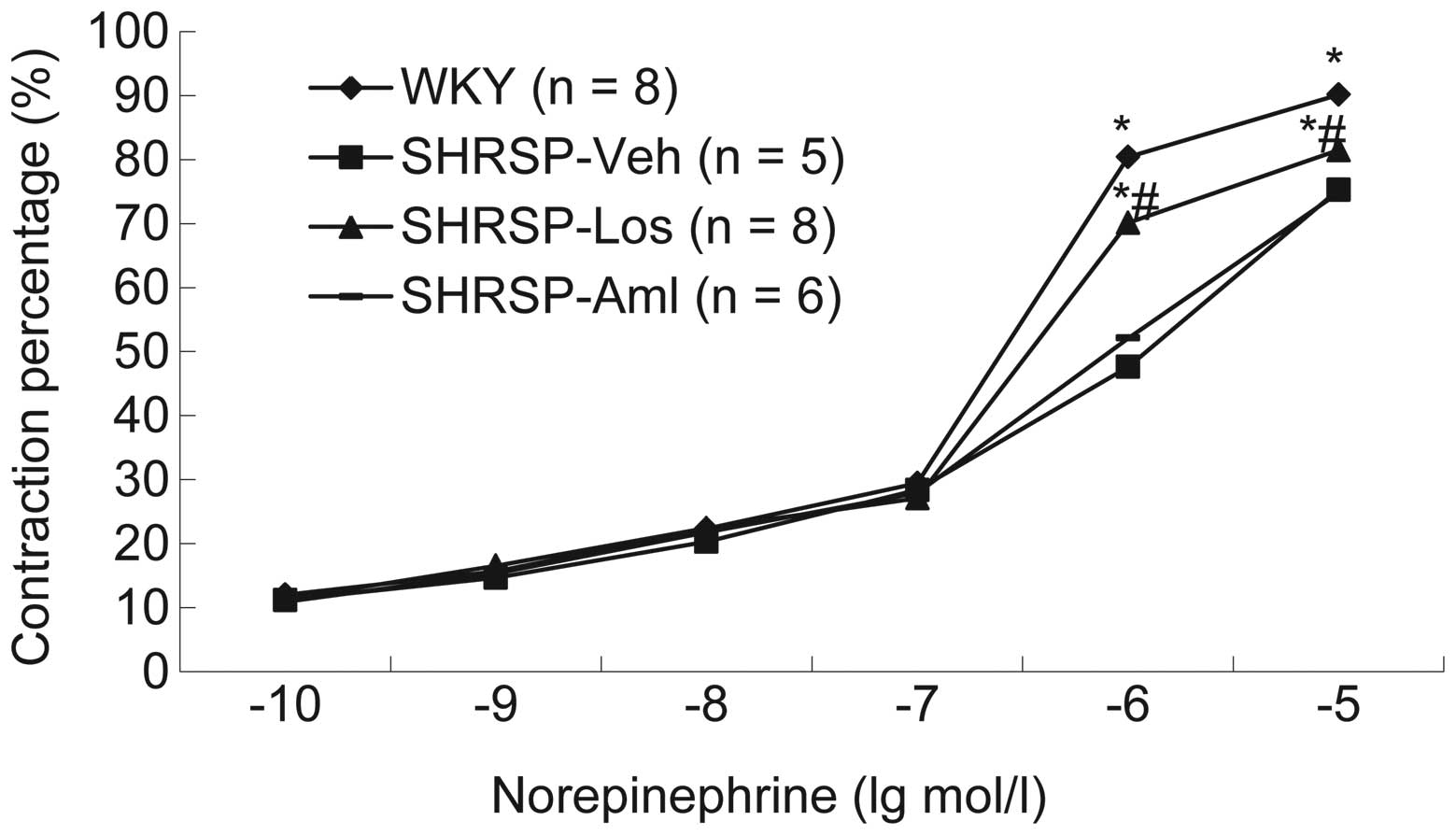
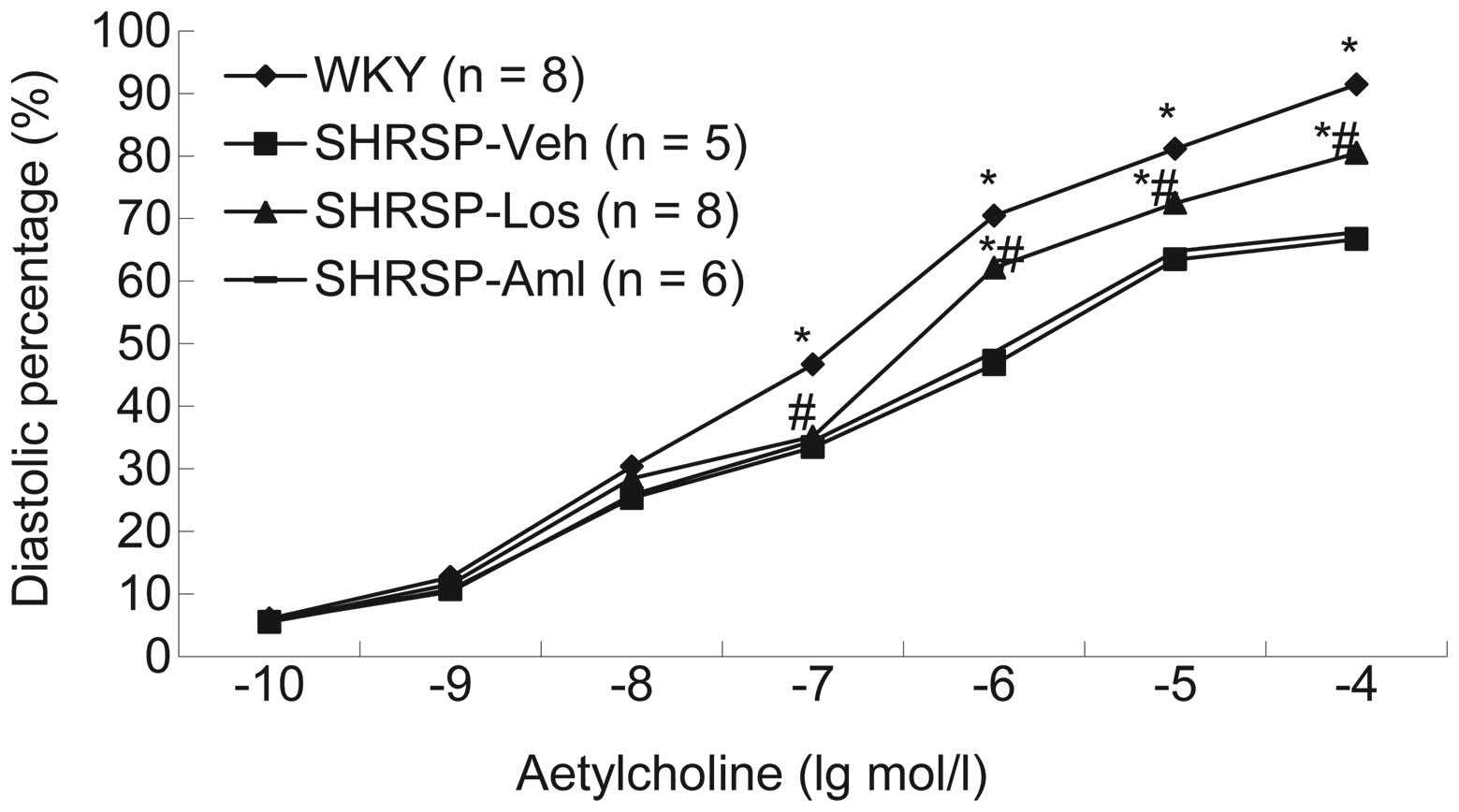
(Fig. 2). Additionally, the
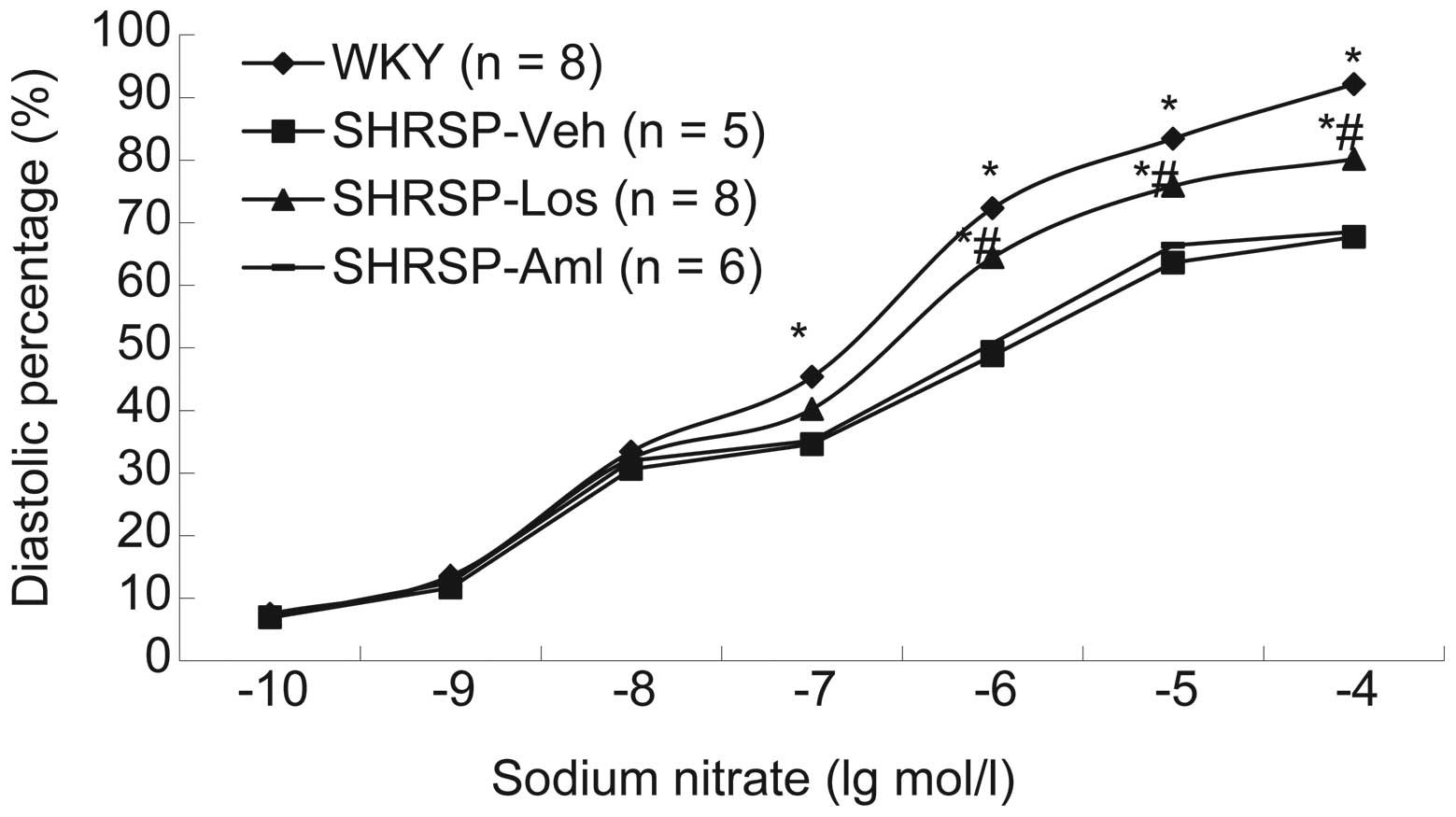
systolic and diastolic function in each group was measured. Results
demonstrated that compared with rats in the SHRSP-Aml and SHRSP-Veh
groups, rats from the SHRSP-Los group exhibited thinner vascular
walls and improved systolic and diastolic function following
treatment with norepinephrine (Fig.
3), acetylcholine (Fig. 4) or
sodium nitroprusside (Fig. 5).
 | Table IW/L in third branch of mesenteric
arterioles of 40-week old rats in all groups (mean ± standard
deviation). |
Table I
W/L in third branch of mesenteric
arterioles of 40-week old rats in all groups (mean ± standard
deviation).
| Group | W/L |
|---|
| WKY (n=8) | 0.33±0.02a |
| SHRSP-Veh (n=5) | 0.92±0.08 |
| SHRSP-Los (n=8) | 0.51±0.04a,b |
| SHRSP-Aml (n=6) | 0.89±0.06b,c |
Prehypertensive treatment with losartan
reduced the risk of stroke in SHRSP rats
Hypertension predisposes patients to the occurrence
of stroke. Therefore, the long-term effects of losartan and
amlodipine on the risk of stroke in rats were investigated in the
present study. As presented in Table
II, the mean clinical stroke score in the SHRSP-Los group was
significantly reduced compared with the SHRSP-Aml (P=0.001) and
SHRSP-Veh (P=0.002) groups, suggesting that prehypertensive
treatment with Los may reduce the risk of stroke in these rats.
 | Table IIStroke score evaluation of 40-week old
rats in all groups. |
Table II
Stroke score evaluation of 40-week old
rats in all groups.
| Group | Stroke scale
|
|---|
| WKY (n=8) | SHRSP-Veh (n=5) | SHRSP-Los (n=8) | SHRSP-Aml (n=6) |
|---|
| 0 | 8.00 | 0.00 | 3.00 | 0.00 |
| 1 | 0.00 | 0.00 | 5.00 | 0.00 |
| 2 | 0.00 | 0.00 | 0.00 | 1.00 |
| 3 | 0.00 | 2.00 | 0.00 | 3.00 |
| 4 | 0.00 | 3.00 | 0.00 | 2.00 |
| Mean rank | 6.00 | 23.00 | 11.00 | 21.17 |
Prehypertensive treatment with losartan
regulated the levels of Ang II and Aldo in SHRSP rats
The above data demonstrate that losartan is more
effective than amlodipine in reducing BP and the risk of stroke,
thus, the possible underlying mechanisms for this improvement were
investigated. One possible explanation for the improved performance
of losartan may involve BP-associated hormones, therefore the
potential roles of Ang II and Aldo in these processes were
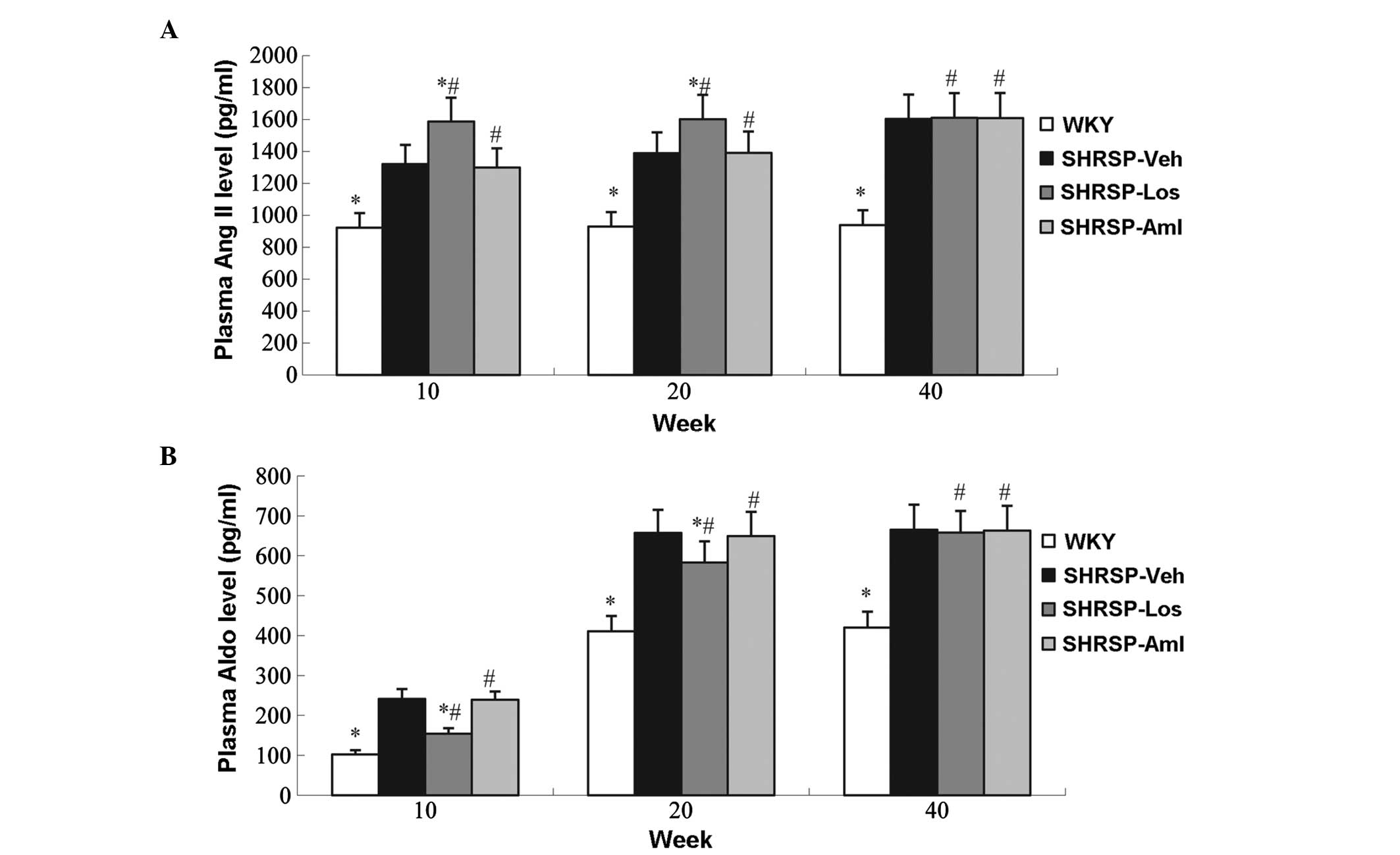
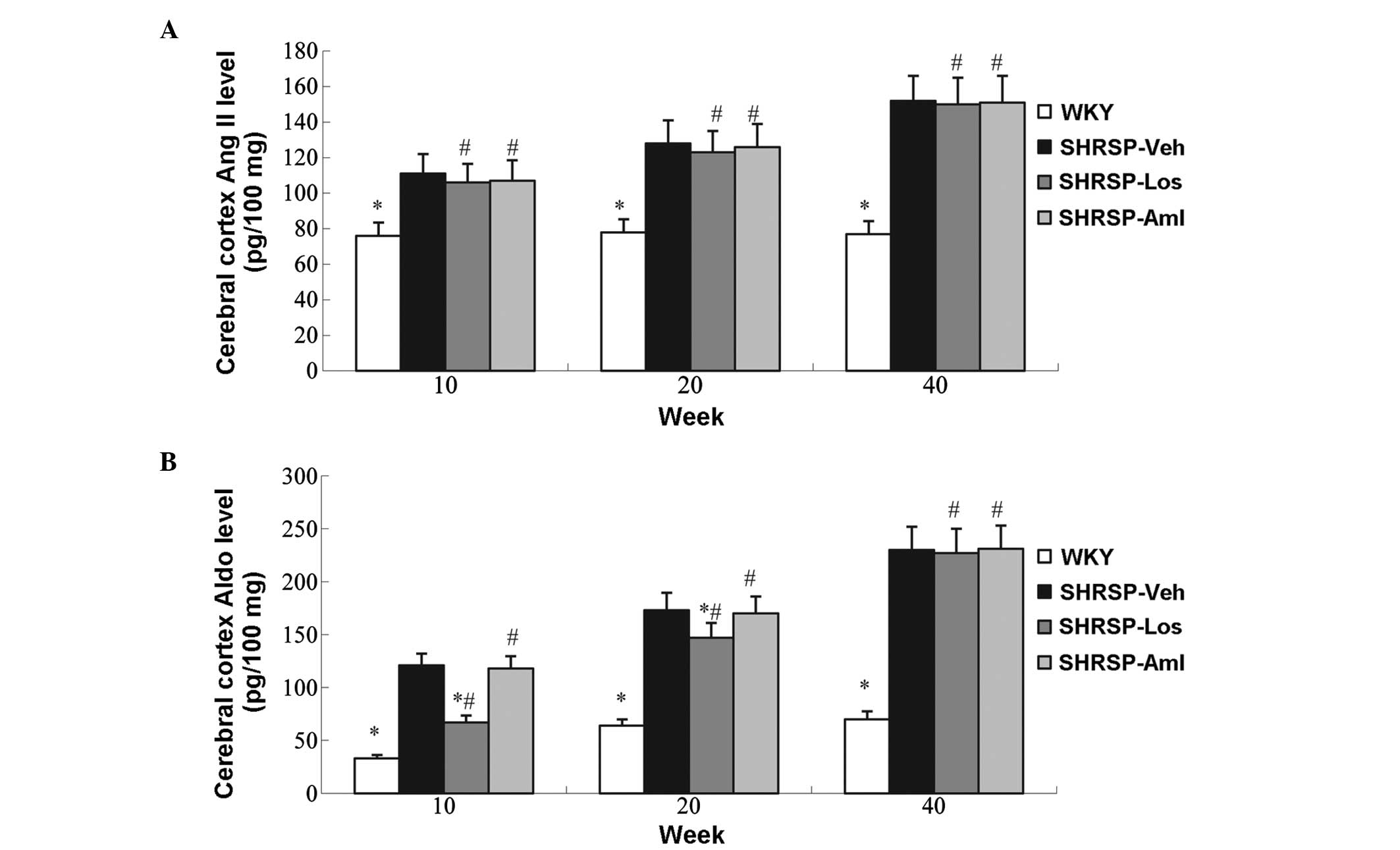
investigated. Increased levels of Ang II and Aldo were observed in
the SHRSP-Veh group compared with WKY animals (Figs. 6 and 7). Compared with the control group,
losartan increased the serum levels of Ang II, and reduced levels
of aldosterone; treatment with losartan in the cerebral cortex had
no effect on Ang II levels, but decreased the levels of
aldosterone. However amlodipine had no effect on Ang II and
aldosterone levels (Figs 6 and
7).
Prehypertensive treatment with losartan
regulated the expression of AT1R/AT2R proteins in SHRSP rats
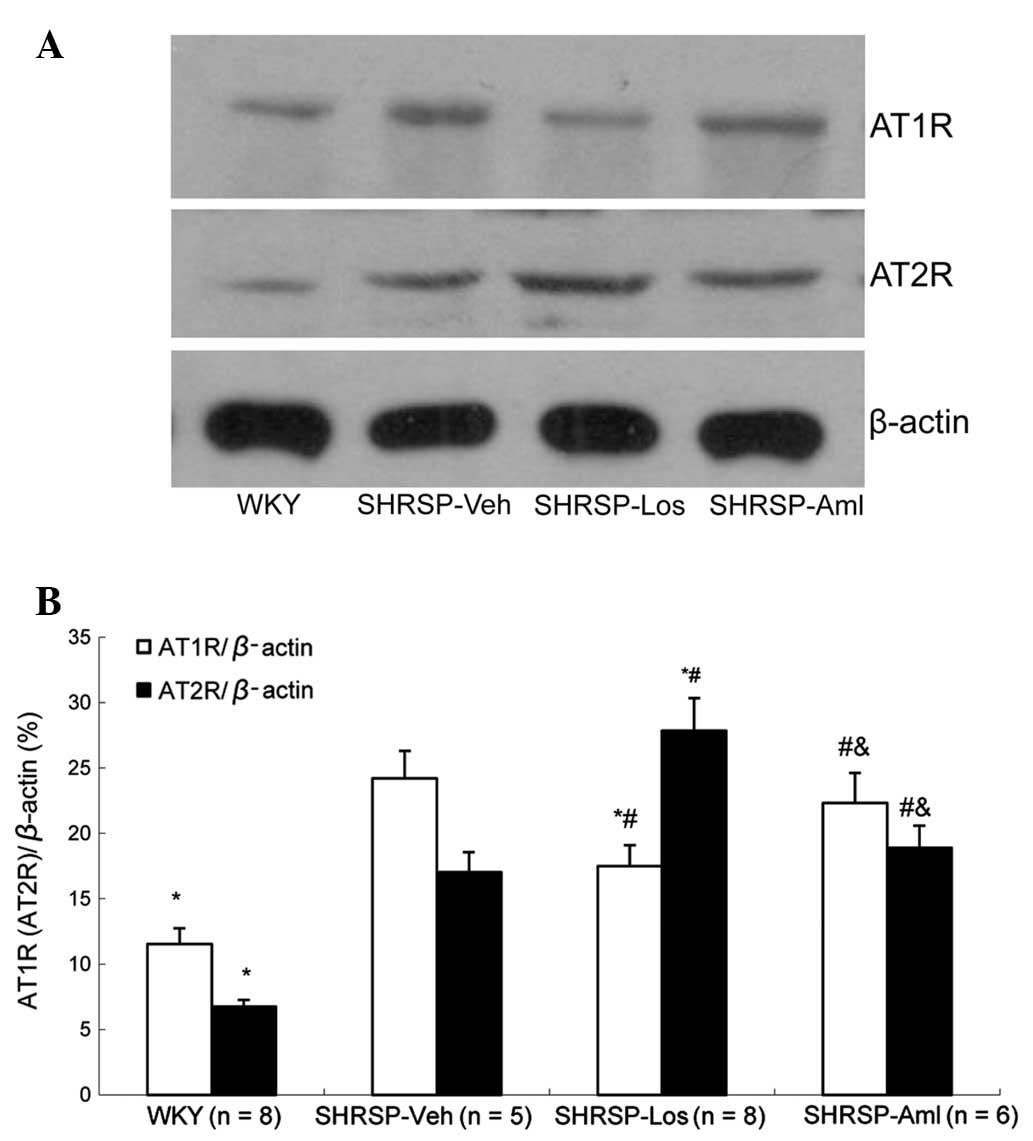
Ang II and Aldo are the ligands of AT1R and AT2R,
respectively. Ligand binding to AT1R and AT2R activates the
receptors, and subsequently activates intracellular responses such
as the stimulation of protein kinase C, which induces
vasoconstriction (14). Therefore,
it was suggested that these two receptors may be involved in these
processes. To investigate this hypothesis, western blot analysis
was conducted, which indicated a significant downregulation of AT1R
expression and upregulation of AT2R expression in the SHRSP-Los
group compared with the SHRSP-Veh group (Fig. 8). However, these alterations in the
protein levels of AT1R and AT2R were not observed in the SHRSP-Aml
group.
Discussion
As a common and chronic condition, hypertension
places a significant burden on society. However, despite the
development of numerous therapeutic strategies, hypertension
remains an unsolved medical problem, due to the numerous associated
complications. Studies have suggested that prehypertensive
intervention may be beneficial for patient prognosis, thus
represents novel targets to reduce the impact of this disease. In
the current study, the effects of losartan and amlodipine on BP and
stroke risk were compared in an SHRSP rat model. It was observed
that all SHRSP animals developed high blood pressure at 10 weeks of
age compared with zero in the WKY group. However, losartan and
amlodipine significantly reduced the increase in BP whilst not
resulting in a significant difference regarding anti-hypertensive
capacity. Notably, following the suspension of the drug treatments,
rats in the SHRSP-Los group demonstrated a slower rise in BP
compared with rats in the SHRSP-Aml group. These data indicate that
the two antihypertensive drugs exhibited comparable capacities in
preventing the development of hypertension, however, losartan may
have improved long-term efficacy for regulating BP. These results
are consistent with those reported in previous studies (15,16).
The potential underlying mechanisms for these results were
investigated, with thinner vessel walls of the mesenteric
arterioles in the SHRSP-Los group observed compared with the
SHRSP-Aml and SHRSP-Veh groups. Furthermore, the mesenteric
arterioles in rats in the SHRSP-Los group demonstrated improved
systolic and diastolic function and reduced clinical stroke scores
compared with the SHRSP-Aml group. These observations are
consistent with a previous study in which visual observations and
microscopic analysis observed tissue abnormalities in the brains of
SHRSP rats treated with amlodipine, including loss of neurons and
hemorrhage foci. However, these abnormalities were not observed in
brain sections from Wistar control rats and SHRSP rats treated with
losartan (17).
In the current study, investigations using
radioimmunoassays and western blotting indicated increased Ang II
levels and reduced Aldo levels in the SHRSP-Los groups, while there
was no difference in Ang II and Aldo levels between the SHRSP-Aml
and SHRSP-Veh groups. Furthermore, the expression of receptors for
Ang II, AT1R and AT2R, were significantly altered following
losartan treatment, however not following amlodipine treatment.
These results suggest that the renin angiotensin system (RAS) may
be activated by losartan, which may contribute to the therapeutic
mechanism of this antihypertensive drug. This would be consistent
with the theory of “RAS block memory” (17–20)
in which Ang II is suggested to act as the conduit for translating
blood vessel alterations into the pathogenesis of hypertension. As
a blocker of Ang II receptors, losartan may prevent the further
damage induced by constant stimulation of Ang II by reducing BP and
rates of blood vessel remodeling.
Previous studies have reported that AT1R/AT2R
expression levels were strongly correlated with an improved
prognosis in patients with cardiovascular diseases, including
hypertension and stroke (21–24).
The current study observed a downregulation of AT1R protein
expression and upregulation of AT2R protein expression in the
SHRSP-Los group compared with the SHRSP-Veh group. AT1R/AT2R may
mediate the neuroprotective effects of losartan in SHRSP animals.
Whilst losartan blocks AT1R and thereby attenuates brain
angiospasm, it may additionally improve blood supply to the brain
via activating AT2R. A meta-analysis of clinical studies suggested
that CCBs were more efficacious than ARBs in reducing the risk of
stroke, which conflicts with the results of the present study
(9). However, in the majority of
human studies, the patient population consisted predominantly of
elderly people, and such patients commonly have an underactive RAS
system (25–27). Thus, during the prehypertensive
stage, endothelial function in these patients may be compromised
due to high RAS activity, which may result in a reduced response of
the RAS system.
The were several limitations of the current study.
Firstly, the current conclusion was reached on the basis of animal
studies, and the results may not apply to human subjects. Secondly,
the current study investigated a potential mechanism by focusing on
the role of vasoconstricting hormones and provided evidence that
Ang II and Aldo were responsible for the superior effects of
losartan compared with amlodipine. However, additional biological
pathways and molecules may affect these processes and further
studies are required to fully elucidate the mechanisms
involved.
Taken together, for the first time to the best of
our knowledge, the current study demonstrated that the
prehypertensive administration of losartan was more efficacious
than amlodipine for the long-term maintenance of normal BP and
brain function in SHRSP rats. Anti-hypertensive compounds that
target the ABR system may exert improved efficacy by affecting Ang
II and its corresponding receptors. While presenting novel evidence
for the advantage of using losartan to reduce the risk of stroke in
the prehypertensive stage, the present study additionally suggested
that the brain-protective effect of losartan may be independent of
its antihypertensive role.
Acknowledgments
The current study was supported by a grant from the
National Natural Science Foundation of China (grant no. 81070207)
to Dr Jinxiu Lin. The authors would like to thank Medjaden
Bioscience Ltd. for assisting in the preparation of the
manuscript.
References
|
1
|
Lee M, Saver JL, Chang B, Chang KH, Hao Q
and Ovbiagele B: Presence of baseline prehypertension and risk of
incident stroke: A meta-analysis. Neurology. 77:1330–1337. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sipahi I, Swaminathan A, Natesan V,
Debanne SM, Simon DI and Fang JC: Effect of antihypertensive
therapy on incident stroke in cohorts with prehypertensive blood
pressure levels: A meta-analysis of randomized controlled trials.
Stroke. 43:432–440. 2012. View Article : Google Scholar
|
|
3
|
Takemori K, Ishida H and Ito H: Continuous
inhibition of the renin-angiotensin system and protection from
hypertensive end-organ damage by brief treatment with angiotensin
II type 1 receptor blocker in stroke-prone spontaneously
hypertensive rats. Life Sci. 77:2233–2245. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hamaguchi R, Takemori K, Inoue T, Masuno K
and Itox H: Short-term treatment of stroke-prone spontaneously
hypertensive rats with an AT1 receptor blocker protects against
hypertensive end-organ damage by prolonged inhibition of the
renin-angiotensin system. Clin Exp Pharmacol Physiol. 35:1151–1155.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dahlöf B, Devereux RB, Kjeldsen SE, Julius
S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K,
Lederballe-Pedersen O, et al LIFE Study Group: Cardiovascular
morbidity and mortality in the Losartan Intervention For Endpoint
reduction in hypertension study (LIFE): A randomised trial against
atenolol. Lancet. 359:995–1003. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McLachlan J, Beattie E, Murphy MP, Koh-Tan
CH, Olson E, Beattie W, Dominiczak AF, Nicklin SA and Graham D:
Combined therapeutic benefit of mitochondria-targeted antioxidant,
MitoQ10, and angiotensin receptor blocker, losartan, on
cardiovascular function. J Hypertens. 32:555–564. 2014. View Article : Google Scholar :
|
|
7
|
Judd E and Jaimes EA: Aliskiren,
amlodipine and hydrochlorothiazide triple combination for
hypertension. Expert Rev Cardiovasc Ther. 10:293–303. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Messerli FH and Staessen JA: Amlodipine
better than lisinopril? How one randomized clinical trial ended
fallacies from observational studies. Hypertension. 48:359–361.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang JG, Li Y, Franklin SS and Safar M:
Prevention of stroke and myocardial infarction by amlodipine and
Angiotensin receptor blockers: A quantitative overview.
Hypertension. 50:181–188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He DH, Zhang LM, Lin LM, Ning RB, Wang HJ,
Xu CS and Lin JX: Long-term prehypertension treatment with losartan
effectively prevents brain damage and stroke in stroke-prone
spontaneously hypertensive rats. Int J Mol Med. 33:301–309.
2014.
|
|
11
|
Julius S, Kjeldsen SE, Weber M, Brunner
HR, Ekman S, Hansson L, Hua T, Laragh J, McInnes GT, Mitchell L, et
al VALUE trial group: Outcomes in hypertensive patients at high
cardiovascular risk treated with regimens based on valsartan or
amlodipine: The VALUE randomised trial. Lancet. 363:2022–2031.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ogihara T, Nakao K, Fukui T, Fukiyama K,
Ueshima K, Oba K, Sato T and Saruta T; Candesartan Antihypertensive
Survival Evaluation in Japan Trial Group: Effects of candesartan
compared with amlodipine in hypertensive patients with high
cardiovascular risks: Candesartan antihypertensive survival
evaluation in Japan trial. Hypertension. 51:393–398. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamori Y, Horie R, Akiguchi I, Kihara M,
Nara Y and Lovenberg W: Symptomatological classification in the
development of stroke in stroke-prone spontaneously hypertensive
rats. Jpn Circ J. 46:274–283. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Higuchi S, Ohtsu H, Suzuki H, Shirai H,
Frank GD and Eguchi S: Angiotensin II signal transduction through
the AT1 receptor: Novel insights into mechanisms and
pathophysiology. Clin Sci (Lond). 112:417–428. 2007. View Article : Google Scholar
|
|
15
|
Lin JX and Lin LM: Prehypertensive
treatment in spontaneously hypertensive rats: A comparison of
losartan and amlodipine regarding blood pressure control and
cardiovascular protection after drug withdrawal. Int J Cardiol.
137:S1322009. View Article : Google Scholar
|
|
16
|
Morton JJ, Beattie EC and MacPherson F:
Angiotensin II receptor antagonist losartan has persistent effects
on blood pressure in the young spontaneously hypertensive rat: Lack
of relation to vascular structure. J Vasc Res. 29:264–269. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harrap SB, Van der Merwe WM, Griffin SA,
Macpherson F and Lever AF: Brief angiotensin converting enzyme
inhibitor treatment in young spontaneously hypertensive rats
reduces blood pressure long-term. Hypertension. 16:603–614. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ishiguro K, Sasamura H, Sakamaki Y, Itoh H
and Saruta T: Developmental activity of the renin-angiotensin
system during the “critical period” modulates later L-NAME-induced
hypertension and renal injury. Hypertens Res. 30:63–75. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sasamura H, Hayashi K, Ishiguro K, Nakaya
H, Saruta T and Itoh H: Prevention and regression of hypertension:
Role of renal microvascular protection. Hypertens Res. 32:658–664.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bergström G, Johansson I, Wickman A, Gan L
and Thorup C: Brief losartan treatment in young spontaneously
hypertensive rats abates long-term blood pressure elevation by
effects on renal vascular structure. J Hypertens. 20:1413–1421.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edvinsson L: Cerebrovascular angiotensin
AT1 receptor regulation in cerebral ischemia. Trends Cardiovasc
Med. 18:98–103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kasahara Y, Taguchi A, Uno H, Nakano A,
Nakagomi T, Hirose H, Stern DM and Matsuyama T: Telmisartan
suppresses cerebral injury in a murine model of transient focal
ischemia. Brain Res. 1340:70–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iwai M, Liu HW, Chen R, Ide A, Okamoto S,
Hata R, Sakanaka M, Shiuchi T and Horiuchi M: Possible inhibition
of focal cerebral ischemia by angiotensin II type 2 receptor
stimulation. Circulation. 110:843–848. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McCarthy CA, Vinh A, Callaway JK and
Widdop RE: Angiotensin AT2 receptor stimulation causes
neuroprotection in a conscious rat model of stroke. Stroke.
40:1482–1489. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Denker MG and Cohen DL: What is an
appropriate blood pressure goal for the elderly: Review of recent
studies and practical recommendations. Clin Interv Aging.
8:1505–1517. 2013.PubMed/NCBI
|
|
26
|
Umemoto S, Ogihara T, Rakugi H, Matsumoto
M, Kitagawa K, Shimada K, Higaki J, Ito S, Suzuki H, Ohashi Y, et
al Combination Therapy of Hypertension to Prevent Cardiovascular:
Effects of a benidipine-based combination therapy on the risk of
stroke according to stroke subtype: The COPE trial. Hypertens Res.
36:1088–1095. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aronow WS: Hypertension-related stroke
prevention in the elderly. Curr Hypertens Rep. 15:582–589. 2013.
View Article : Google Scholar : PubMed/NCBI
|