Introduction
Contact dermatitis (CD), the predominant
inflammatory skin disease worldwide, is a type of eczematous
eruption that occurs following contact with foreign substances
(1,2). The histopathological features of CD
include epithelial hyperkeratosis, acanthosis and spongiosis that
present with perivascular inflammatory cell infiltration, primarily
consisting of T cells in the epidermis and upper dermis (3).
Following exposure to a skin irritant, various
cells, including activated keratinocytes, dendritic cells, dermal
fibroblasts, and endothelial cells, release proinflammatory and
inflammatory cytokines/chemokines (4). The cytokines/chemokines induce the
dilatation of blood vessels and inflammatory cell infiltration,
resulting in epidermal acanthosis and spongiosis, however, the
precise mechanism remains to be elucidated (4). Tumor necrosis factor (TNF)-α,
interferon (IFN)-γ and monocyte chemotactic protein (MCP)-1 are key
primary cytokines in the activation cascade and pathogenesis of CD
(4,5).
The fruit of Kochia scoparia (L.) Schrad.,
which is also designated Bassia scoparia, Bassia
sieversiana or Kochia alata, is administered to treat
skin diseases, diabetes mellitus and rheumatoid arthritis in
Chinese and Korean traditional medicine (6,7).
Furthermore, it is frequently administered to treat urticaria in
Taiwan (8). Previous studies have
indicated that K. scoparia and its components exert
anti-inflammatory (9) and
anti-allergic (10,11) activities. Choi et al
(12) recently reported that the
water extract of K. scoparia inhibits the development of CD
in mice.
The aim of the current study was to evaluate the
anti-inflammatory effects of methanol extracts of K.
scoparia dried fruit (MEKS) on 1-fluoro-2,4-dinitrobenzene
(DNFB)-induced CD. For this reason, the effects of MEKS on ear
thickness and weight, the histopathological changes in ear tissue
samples, and the cytokine and chemokine levels of inflamed tissues
were assessed in vivo.
Materials and methods
Preparation of MEKS
The mature fruit of K. scoparia was purchased
from Hwalim Medicinal Herbs (Pusan, Korea). A total of 100 g K.
scoparia dried fruit was immersed in 1,000 ml methanol and
sonicated for 30 min, following which the samples were extracted
for 48 h. The extract was subsequently filtered through Whatman
filter paper No. 20 (Advantech, Milpitas, CA, USA) and evaporated
under reduced pressure using a vacuum evaporator (N-1000V-W; Eyela
Co., Ltd., Tokyo, Japan), following which the condensed extract was
lyophilized using a freeze dryer (Labconco Corporation, Kansas
City, MO, USA) and 4.46 g lyophilized powder was obtained (yield,
4.46%). An aliquot of the extract (MEKS) was deposited at the
Department of Pharmacology, School of Korean Medicine, Pusan
National University (Yangsan, South Korea; voucher no.
MH2013-006).
Animals
A total of 44 male 6-week-old BALB/c mice were
purchased from Samtaco (Incheon, Korea). The mice were housed under
specific pathogen-free conditions with a 12-h light/dark cycle and
free access to standard rodent food and water. All animal
experiments were approved by the Animal Care and Use Committee of
Pusan National University and performed according to institutional
guidelines (PNU-2011-000406).
Induction of CD and experimental
design
Mice were sensitized by applying 50 µl DNFB
(0.1%, v/v; Sigma-Aldrich, St. Louis, MO, USA), in a vehicle of
acetone:olive oil (AOO; 4:1), onto the shaved back of each mouse
for three consecutive days. Four days following sensitization, each
mouse was challenged by applying 30 µl DNFB (0.2%, v/v) in
AOO onto the dorsal surface of each ear every two days (four
applications in total). MEKS solution (30, 100 or 300
µg/ear) was applied onto the dorsal surface of each ear for
seven consecutive days. Healthy mice (n=6) were treated with the
vehicle, AOO and only AOO was topically applied (non-treated normal
mice; NOR). Control mice (n=8) were sensitized and challenged with
DNFB, following which AOO was topically applied (non-treated CD
mice; CTL). MEKS-treated mice (n=8) were sensitized and challenged
with DNFB, and 30, 100 or 300 µg/ear (1%, w/v) of MEKS was
topically applied. Dexamethasone (DEX; Sigma-Aldrich)-treated mice
(n=6) were sensitized and challenged with DNFB, exposed to 75
µg/ear DEX and served as a positive control. The
experimental design is summarized in Fig. 1.
Measurement of ear thicknesses and
weights
Mice were anesthetized with 30 mg/kg
Zoletil® (Virbac, Carros, France) and sacrificed by
cervical dislocation, following which the thickness of each ear was
measured using vernier calipers (Mitutoyo, Kanagawa, Japan). The
pieces of ear (5 mm in diameter) obtained via dermal punch were
weighed using a microbalance (US/EL-2000S; Sartorius AG, Göttingen,
Germany).
Histopathological examination
Following assessment of ear thicknesses and weights,
ear tissues (4 µm) were resected, formalin-fixed
(Sigma-Aldrich) and embedded in paraffin (Leica Microsystems GmbH,
Wetzlar, Germany). Sections were cut and stained with hematoxylin
and eosin (Sigma-Aldrich) to observe histopathological changes,
such as epidermal acanthosis, spongiosis and immune cell
infiltration. Stained tissue sections were observed using a light
microscope (magnification, x200; DE/Axio Imager A1; Carl Zeiss AG,
Oberkochen, Germany).
Evaluation of epidermal acanthosis and
immune cell infiltration
To evaluate the epidermal acanthosis and immune cell
infiltration, five non-overlapping fields per slide were randomly
selected and images were captured with the light microscope. To
measure the thickness of the epithelium, the vertical length
between the basal lamina and top of the outermost stratum
granulosum was quantified. For each slide, five lengths were
measured at random using Motic Images Plus 2.0 (Motic Instruments,
Richmond, BC, Canada), following which the mean epithelial
thicknesses of all experimental groups were used for analysis. To
evaluate immune cell infiltration, the immune cells were counted
using a cell counting grid.
Measurement of cytokine production
Cytokine levels in the ear tissue samples were
measured according to the cytometric bead array (CBA) method with
the Mouse Inflammation CBA kit (BD Biosciences, San Jose, CA, USA).
Resected ear tissue samples were lysed and homogenized with protein
extraction solution (Pro-Prep; Intron Biotechnology, Seoul, Korea)
using a bullet blender (BB2516; Next Advance, Averill Park, NY,
USA) to obtain tissue lysates. The levels of TNF-α, IFN-γ,
interleukin (IL)-10 and MCP-1 were measured in 50 µg each
lysate using the CBA kit. All experimental procedures were
conducted according to the manufacturer's protocols.
Measurement of body and spleen
weights
Body and spleen weights were measured on day 15
using the microbalance. The effects of MEKS on changes in spleen
weights were analyzed as the spleen/body weight ratio.
Statistical analysis
A Mann-Whitney U test was used for all statistical
comparisons, and Prism 5 for Windows version 5.01 (GraphPad
Software Inc., La Jolla, CA, USA) was used for all analyses. All
data are presented as the mean ± standard deviation and P<0.05
was considered to indicate a statistically significant
difference.
Results
MEKS decreased the DNFB-induced changes
in ear thickness and weight
Topical application of DNFB induced ear swelling,
which is a major feature of CD. These increases in the thickness
and weight of ear tissues were inhibited in a dose-dependent manner
by topical application of MEKS (ear thickness: P<0.001, MEKS
treatment on left ear; P<0.01, MEKS treatment on right ear;
P<0.001, DEX treatment on both ears. Ear weight: P<0.05, 100
or 300 µg/ear MEKS treatment; P<0.001, 75 µg/ear
DEX treatment) as presented in Fig.
2.
MEKS inhibited epidermal spongiosis in
the inflamed tissue
Histological examination in the tissue sections of
the NOR group demonstrated normal epidermal thickness and a smaller
degree of immune cell infiltration into the dermis (Fig. 3A). Repeated application of DNFB
induced small and large subcorneal and intraepidermal vesicles,
diffused spongiotic changes and intercellular edema, which are
characteristics of CD (Fig. 3B).
Topical application of MEKS inhibited epidermal spongiosis in the
inflamed tissue (Fig. 3C–E).
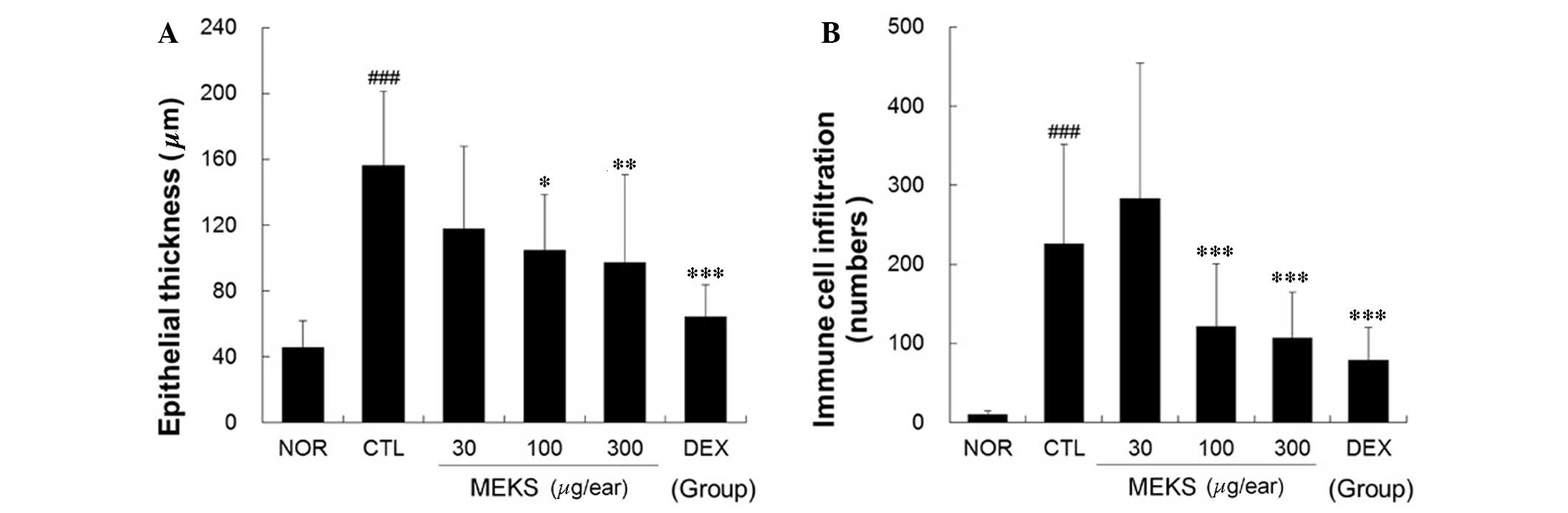
MEKS inhibited epidermal acanthosis and
immune cell infiltration
Repeated application of DNFB induced acanthosis,
hyperkeratosis and focal crust formation in the epidermis. In
addition, DNFB induced diffuse acute and chronic immune cell
infiltration, blood vessel dilation and perivascular eosinophil
infiltration into the dermis. Topical application of MEKS
effectively relieved epidermal acanthosis and immune cell
infiltration in a dose-dependent manner (epithelial thickness:
P<0.05, 100 µg/ear MEKS treatment; P<0.01, 300
µg/ear MEKS treatment; P<0.001, 75 µg/ear DEX
treatment. Immune cell infiltration: P<0.001, 100 or 300
µg/ear MEKS treatment or 75 µg/ear DEX treatment) as
presented in Fig. 4.
MEKS reduced expression levels of TNF-α,
IFN-γ and MCP-1 in ear tissue samples of CD mice
Marked increases in TNF-α, IFN-γ and MCP-1
production were observed in the CTL group. These increases were
effectively reduced in a dose-dependent manner by topical
application of MEKS (TNF-α and IFN-γ: P<0.05, 100 µg/ear
MEKS treatment; P<0.01, 300 µg/ear MEKS treatment;
P<0.001, 75 µg/ear DEX treatment. MCP-1: P<0.01, 300
µg/ear MEKS treatment or 75 µg/ear DEX treatment).
Treatment with MEKS did not affect the production of IL-10,
although DEX treatment reduced the IL-10 level significantly when
compared with that of the NOR and CTL groups (P<0.05, 75
µg/ear DEX treatment) as presented in Fig. 5.
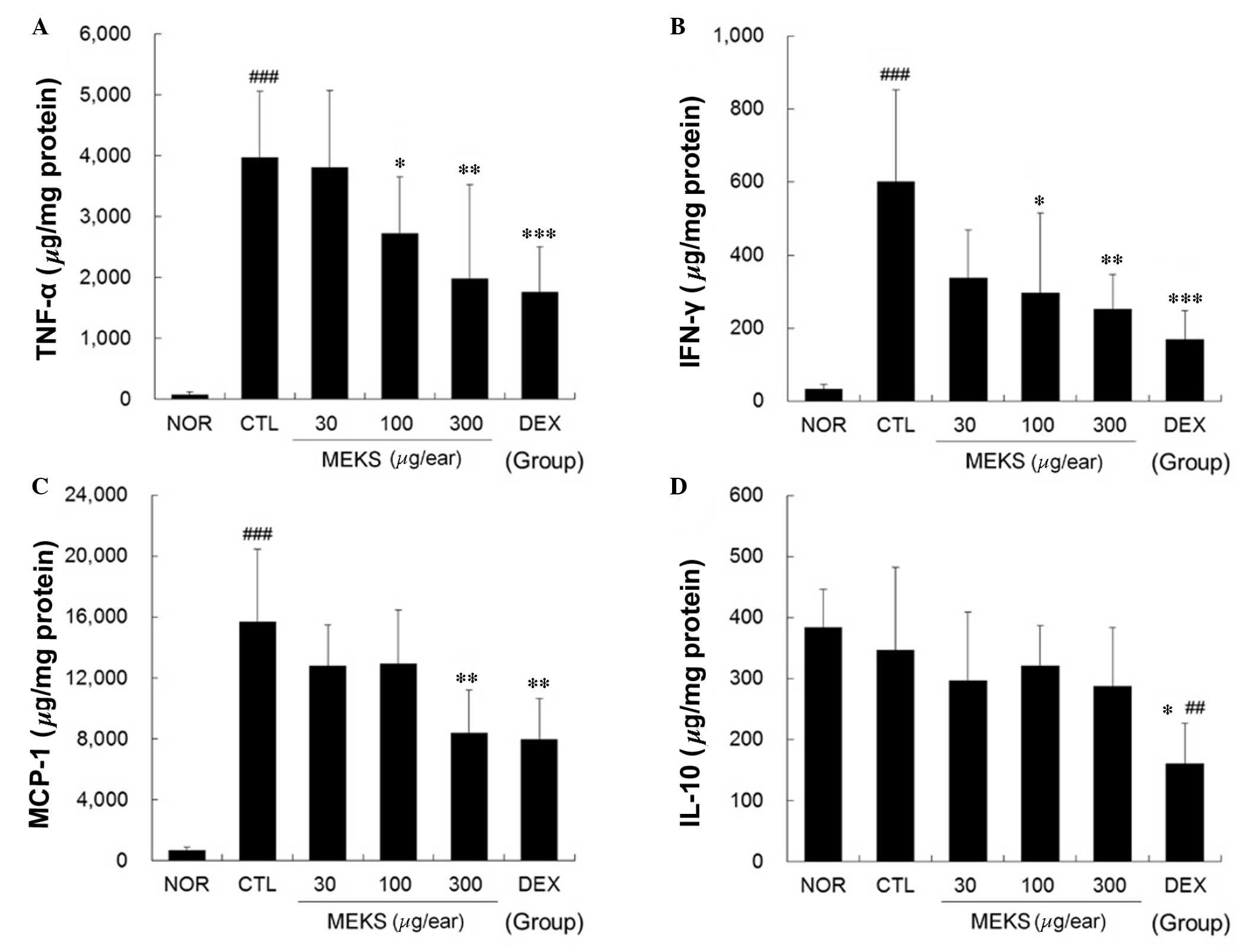 | Figure 5Effect of MEKS on expression levels of
TNF-α, IFN-γ, MCP-1 and IL-10 in CD mice. The expression levels of
(A) TNF-α, (B) IFN-γ, (C) MCP-1 and (D) IL-10 in the ear tissues
were analyzed using the cytometric bead array method. A total of 50
µg tissue lysates was used to measure the cytokine levels.
All values are presented as means ± standard deviation.
##P<0.01, ###P<0.001 vs. NOR;
*P<0.05, **P<0.01 and
***P<0.001 vs. CTL. TNF-α, tumor necrosis factor-α;
IFN-γ; interferon-γ; MCP-1, monocyte chemotactic protein-1; IL-10,
interleukin-10; NOR, non-treated normal mice; CD, contact
dermatitis; CTL, non-treated CD mice; MEKS, methanol extracts from
Kochia scoparia dried fruit; DEX, dexamethasone. |
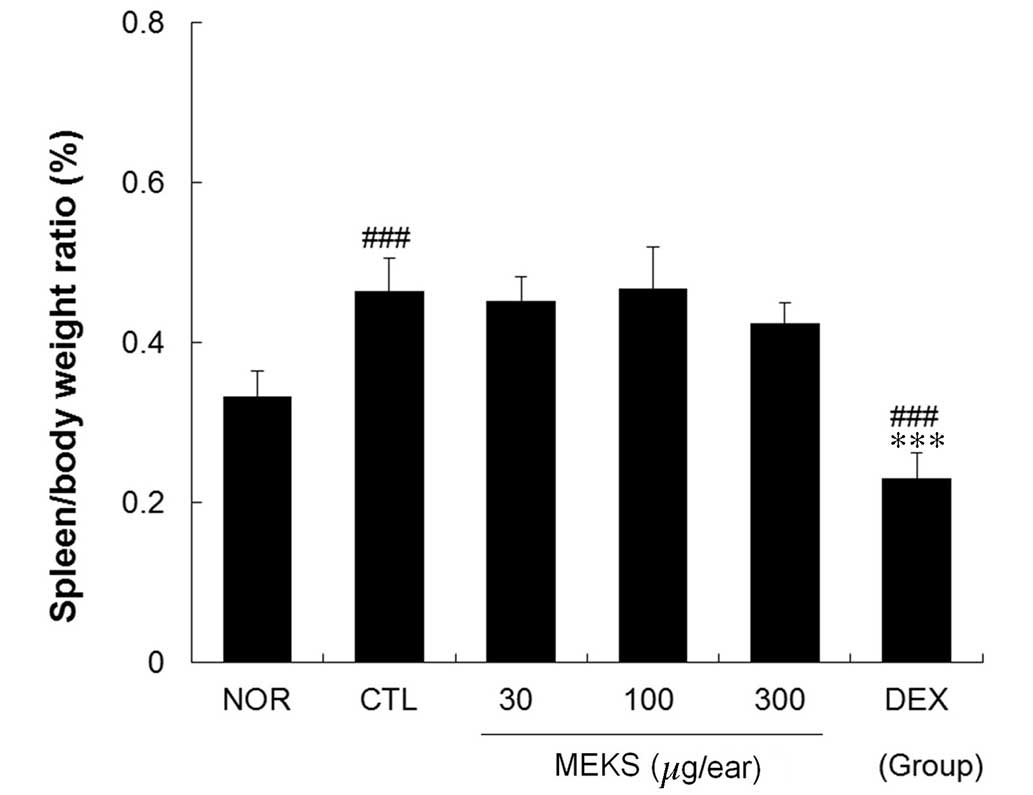
MEKS did not affect spleen/body weight
ratio in CD mice
The effects of MEKS on enlargement of the spleen
were estimated in terms of the spleen/body weight ratio. The
spleen/body weight ratio in the CTL group was significantly
elevated compared with the NOR group (P<0.001). Treatment with
MEKS did not affect the spleen/body weight ratio in CD mice.
However, this ratio was significantly reduced in the DEX group when
compared with that of the CTL and NOR groups (Fig. 6; P<0.001).
Discussion
The present study demonstrated the anti-inflammatory
effects of MEKS on CD. MEKS effectively prevented ear thickness and
weight increases, as well as epidermal acanthosis, spongiosis and
immune cell infiltration in inflamed tissues. In addition, the
expression levels of TNF-α, IFN-γ and MCP-1 were dose-dependently
reduced in response to MEKS. These findings indicate that MEKS
treatment prevents the inflammatory reactions, which leads to the
inhibition of ear thickness and weight increases in inflamed
tissues.
Inflammatory reactions, such as immune cell
infiltration and pro-inflammatory cytokine production, are
important in the pathophysiology of CD and may serve as therapeutic
targets. During progression of CD, IL-1 and TNF-α markedly
upregulate various chemokines, resulting in the recruitment of
leukocytes (5). In addition, IFN-γ
is indicative of type 1 T helper skewing reaction of T cells and
rapidly promotes the secretion of mediators, including chemokine
(C-X-C motif) receptor 3 agonist and MCP-1, when it is present
alone or together with TNF-α (13,14).
The results of the present study are consistent with those of a
previous study by Choi et al (12), who demonstrated that topical
application of K. scoparia extract inhibited the expression
of IL-1β and TNF-α messenger RNA. In the present study, the
production of TNF-α, IFN-γ, MCP-1 and IL-10 was evaluated using
proteins obtained from samples of lysed tissues. Treatment with
MEKS effectively decreased expression levels of TNF-α and IFN-γ in
inflamed tissues. MCP-1, also termed chemokine (C-C motif) ligand
2, is a small chemokine that belongs to the CC chemokine family,
which recruits monocytes, T cells and dendritic cells to
inflammatory sites (15,16). In the present study, treatment with
300 µg/ear of MEKS significantly inhibited MCP-1 production
in samples of ear tissue. These results indicate that the
underlying mechanism of MEKS inhibition of immune cell infiltration
involves decreasing MCP-1 production.
The effects of MEKS on CD were further demonstrated
by histopathological analysis, which indicated that epidermal
hyperplasia, spongiosis and immune cell infiltration were decreased
by topical application of MEKS. Keratinocytes at the site of CD
overexpress numerous cytokines and chemokines, such as TNF-α,
IFN-γ, MCP-1 and IL-10 (17–19).
It has been previously reported that IFN-γ is important in the
development of skin hypertrophy, and TNF-α and MCP-1 function as
stimulants of immune cell recruitment around blood vessels
(17–19). In the present study, the topical
application of MEKS inhibited the histopathological features of CD
via suppression of TNF-α, IFN-γ and MCP-1 expression. These
observations and the changes in cytokine production in the
MEKS-treated tissue samples, suggest that MEKS is an
anti-inflammatory agent against the Th1 skewing reaction, thus,
reducing inflammatory reactions, such as epidermal acanthosis,
spongiosis and immune cell infiltration.
DEX and MEKS prevented ear swelling, diminished
epidermal acanthosis, spongiosis and immune cell infiltration and
decreased the levels of TNF-α, IFN-γ and MCP-1 in ear tissue
samples. MEKS did not affect the expression of IL-10, however, DEX
significantly reduced the expression levels of all cytokines
examined in the present study, including IL-10, which has a
suppressive role in CD and atopic dermatitis (20). Treatment with DEX also led to a
significant reduction in the spleen/body weight ratio when compared
with the CTL and the NOR groups, which is indicative of an immune
reaction. These findings indicate that the underlying therapeutic
mechanism of MEKS differs to that of DEX, particularly with regards
to general immune suppression, which is one of the major side
effects of corticosteroids, such as DEX.
The findings of the present study indicate that MEKS
may be administered for the treatment of inflammatory skin
diseases. In addition, the current study suggests that an
anti-inflammatory mechanism of MEKS is involved in the inhibition
of Th1 skewing reactions.
In conclusion, the present study demonstrated that
MEKS reduces Th1 skewing reactions, such as production of TNF-α,
IFN-γ and MCP-1, resulting in decreased epidermal acanthosis,
spongiosis and immune cell infiltration. Repeated administration of
MEKS resulted in anti-inflammatory reactions leading to the
inhibition of ear swelling. The effects of MEKS were similar to
those of DEX, however, no general immune suppression was observed
in response to MEKS treatment. The findings of the present study
indicate that MEKS may be used to reduce or replace corticosteroid
use with relative safety.
Acknowledgments
This research was supported by the National Research
Foundation of Korea grant funded by the Korean government (MSIP;
grant no. 2015R1A2A2A04005619).
References
|
1
|
English JS: Current concepts of irritant
contact dermatitis. Occup Environ Med. 61:722–726. 6742004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Usatine RP and Riojas M: Diagnosis and
management of contact dermatitis. Am Family Physician. 82:249–255.
2010.
|
|
3
|
Streit M and Braathen LR: Contact
dermatitis: Clinics and pathology. Acta Odontol Scand. 59:309–314.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee HY, Stieger M, Yawalkar N and Kakeda
M: Cytokines and chemokines in irritant contact dermatitis.
Mediators of Inflamm. 2013:9164972013. View Article : Google Scholar
|
|
5
|
Grabbe S and Schwarz T: Immunoregulatory
mechanisms involved in elicitation of allergic contact
hypersensitivity. Immunol Today. 19:37–44. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim NY, Lee MK, Park MJ, Kim SJ, Park HJ,
Choi JW, Kim SH, Cho SY and Lee JS: Momordin Ic and oleanolic acid
from Kochiae fructus reduce carbon tetrachloride-induced
hepatotoxicity in rats. J Med Food. 8:177–183. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi J, Lee KT, Jung H, Park HS and Park
HJ: Anti-rheumatoid arthritis effect of the Kochia scoparia fruits
and activity comparison of momordin lc, its prosapogenin and
sapogenin. Arch Pharm Res. 25:336–342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin YH, Chen YC, Hu S, Chen HY, Chen JL
and Yang SH: Identifying core herbal treatments for urticaria using
Taiwan's nationwide prescription database. J Ethnopharmacol.
148:556–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shin KM, Kim YH, Park WS, Kang I, Ha J,
Choi JW, Park HJ and Lee KT: Inhibition of methanol extract from
the fruits of Kochia scoparia on lipopolysaccharide-induced nitric
oxide, prostaglandin [correction of prostagladin] E2, and tumor
necrosis factor-alpha production from murine macrophage RAW 264.7
cells. Biol Pharm Bull. 27:538–543. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee MY, Shin IS, Lim HS, Seo CS, Ha H and
Shin HK: Kochia scoparia fruit attenuates allergic airway
inflammation in ovalbumin (OVA)-induced murine asthma model. Inhal
Toxicol. 23:938–946. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsuda H, Dai Y, Ido Y, Yoshikawa M and
Kubo M: Studies on kochiae fructus. IV Anti-allergic effects of 70%
ethanol extract and its component, momordin Ic from dried fruits of
Kochia scoparia L. Biol Pharm Bull. 20:1165–1170. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi YY, Kim MH, Lee JY, Hong J, Kim SH
and Yang WM: Topical application of Kochia scoparia inhibits the
development of contact dermatitis in mice. J Ethnopharmacol.
154:380–385. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Albanesi C, Cavani A and Girolomoni G:
IL-17 is produced by nickel-specific T lymphocytes and regulates
ICAM-1 expression and chemokine production in human keratinocytes:
Synergistic or antagonist effects with IFN-gamma and TNF-alpha. J
Immunol. 162:494–502. 1999.PubMed/NCBI
|
|
14
|
Sebastiani S, Albanesi C, De PO, Puddu P,
Cavani A and Girolomoni G: The role of chemokines in allergic
contact dermatitis. Arch Dermatol Res. 293:552–559. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carr MW, Roth SJ, Luther E, Rose SS and
Springer TA: Monocyte chemoattractant protein 1 acts as a
T-lymphocyte chemoattractant. Proc Natl Acad Sci USA. 91:3652–3656.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu LL, Warren MK, Rose WL, Gong W and Wang
JM: Human recombinant monocyte chemotactic protein and other C-C
chemokines bind and induce directional migration of dendritic cells
in vitro. J Leukoc Biol. 60:365–371. 1996.PubMed/NCBI
|
|
17
|
Tung D, Cheung PH, Kaur P, Foreman O,
Kavirayani A, Hain HS and Saha S: Anti-inflammatory and
immunomodulatory effects of bortezomib in various in vivo models.
Pharmacology. 88:100–113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fukuda S, Midoro K, Kamei T, Gyoten M,
Kawano Y, Ashida Y and Nagaya H: Inhibition of allergic dermal
inflammation by the novel imidazopyridazine derivative TAK-427 in a
guinea pig experimental model of eczema. J Pharmacol Exp Ther.
303:1283–1290. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gaffal E, Cron M, Glodde N, Bald T, Kuner
R, Zimmer A, Lutz B and Tüting T: Cannabinoid 1 receptors in
keratinocytes modulate proinflammatory chemokine secretion and
attenuate contact allergic inflammation. J Immunol. 190:4929–4936.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boyman O, Werfel T and Akdis CA: The
suppressive role of IL-10 in contact and atopic dermatitis. J
Allergy Clin Immunol. 129:160–161. 2012. View Article : Google Scholar
|