Introduction
First-degree burns and superficial wounds undergo a
similar wound repair process involving reconstruction of the
epithelial barrier, the underlying dermis and extracellular matrix
(ECM), organized by granulation tissue (1). However, second- and third-degree
burns present a broad range of additional challenges for
restoration of normal dermal function (2,3). The
extensive skin defects induced by severe burns are dangerous and
can be fatal to patients. A common therapy used to treat extensive
burns is skin grafting following tangential excision to remove the
necrotic or denatured skin. However, sources of skin transplants
are often limited. Xenogeneic dermal substitutes, such as porcine
acellular dermal matrix (ADM), can be used to cover the burn
wounds, prevent infection, facilitate skin renewal and
vascularization, and accelerate wound healing (4–7).
However, foreign or xenogeneic tissues have partial
histoincompatibility, which can induce rejection and allergic
reaction (8,9). Engineered human skin matrices,
including Dermagraft, DermACELL and Integra, have exhibited
satisfactory effects in the clinic (10–12).
However, these skin products are expensive and rare, therefore,
their clinical application is restricted. It is important for the
clinical therapy of patients with deep second- and third-degree
burns to investigate novel dermal substitutes that avoid the
limitations associated with currently used dermal matrices.
The necrotic or denatured skin of burn wounds is
typically discarded during treatment due to the formation of
cutaneous burn toxins (13). Burn
toxins, which include free radicals, lipoproteins,
lipopolysaccharides and lipid peroxides, are formed as a result of
thermal injury to skin and are released into the interstitial fluid
and serum during the early stages of burns (14–17).
These hazardous substances have been shown to exert various
effects, including immunosuppression, mitochondrial destruction,
cellular energy metabolic disorders and increasing the permeability
of the cell membrane, which in turn have been implicated in the
various complications associated with burns, including sepsis and
multiple organ dysfunction syndrome (16,18–25).
In addition, bacteria can rapidly proliferate in necrotic tissue
resulting in wound infection (13). It has been previously observed that
sections of the skin removed from deep burn wounds partially
maintained the integrity of their collagen structure, and may have
the potential to be repaired under the appropriate conditions.
Therefore, we hypothesized that burned skin with partial biological
activity may be recycled to produce an autologous acellular dermal
matrix, termed 'deep-degree burned dermal matrix (DDBDM)̓, and
applied for the treatment of deep burn wounds (26). In theory, DDBDM, which originates
from human skin, may avoid the histoincompatibility associated with
foreign and xenogeneic dermal matrices. Furthermore, DDBDM may
reduce therapeutic costs by making full use of the discarded skin.
Thus, successful clinical application of DDBDM may be an improved
method of treatment for patients with deep burn wounds.
In the current study, DDBDM was successfully
prepared using a mouse model of a deep-degree burn wound. To
determine if DDBDM and ADM produced burn toxin with different
compositions, the protein expression levels of 308 cytokines were
analyzed. The DDBDM was subcutaneously implanted in mice to observe
whether an inflammatory reaction was induced. The aim of the
present study was to investigate the use of DDBDM as a dermal
matrix and evaluate its potential clinical significance for the
treatment of patients with deep-degree burns.
Materials and methods
Animals and ethics statement
A total of 150 healthy male Balb/c mice (age, 12
weeks; weight, 27–33 g) were obtained from the Animal Center of
Shandong University (Jinan, China). The mice were maintained under
a 12 h light/dark cycle with ad libitum access to animal
chow and water in the animal quarter at the Animal Laboratory of
the Second Hospital of Shandong University (Jinan, China) at
20–24°C and 50–60% humidity. All experimental procedures were
conducted according to the criteria outlined in the Guide for the
Care and Use of Laboratory Animals published by the National
Institutes of Health (NIH Publication no. 85–23, revised 1996). All
experimental protocols were approved by Animal Care and Use
Committee of the Second Hospital of Shandong University.
Establishment of burn animal model
A total of 60 healthy male Balb/c mice were used to
establish the burn animal model. Following anesthesia by
intraperitoneal injection of 10% chloral hydrate (0.3 ml/kg; Qilu
Hospital, Jinan, China), the fur on the dorsum of each mouse was
shaved. The shaved edges were protected by plastic wrap and a thin
foam board. A deep-degree burn wound of 5×4 cm was created by hot
water-bath burn, as follows: Using a water bath (Shanghai Jing Hong
Experimental Equipment Co. Ltd, Shanghai, China) maintained at a
constant temperature of 80°C, the dorsum of each mouse was bathed
in the hot water for 8 sec. All the burned mice received anti-shock
therapy with Lactated Ringer's solution (Baxter International Inc.,
Deerfield, IL, USA) by intraperitoneal injection (40 ml/kg) and
were treated with 1% povidone iodine solution (Lircon Disinfection
Science Technology Inc., Dezhou, China) to protect the wound. The
mice were resuscitated in a warm environment by subcutaneous
injection of physiological saline solution (10 ml/kg; Baxter
International, Inc.) until they were fully conscious. After 72 h,
the mice were anesthetized and sacrificed by decapitation, after
which burned skin was immediately harvested for further
experimentation.
ADM preparation method
Following anesthesia and shaving of the fur on the
dorsum of 40 healthy Balb/c mice, normal skin specimens were
removed and washed with sterile saline solution (Baxter
International Inc.). The subcutaneous tissue was removed leaving
skin sections of 0.05–1.00 mm thickness. The skin sections were
placed into a mixed solution of 0.25% trypsin (Sigma-Aldrich, St.
Louis, MO, USA) and Triton X-100 (Sigma-Aldrich), and shaken 100
times/min for 2 h at 37°C. Samples were repeatedly washed and
shaken with phosphate-buffered saline (PBS) until the cells and
trypsin/Triton X-100 solution were removed. The ADM was maintained
in saline solution with 800 U/ml gentamicin (Shandong Lukang
Chenxin Pharmaceuticals Co., Ltd., Jining, China) at 4°C. The whole
preparation process was conducted under aseptic conditions.
DDBDM preparation method
Burned mouse skin specimens were obtained from 60
burned mice and washed with sterile saline solution. The
subcutaneous tissues were removed leaving skin sections of
0.05–1.00 mm thickness. The skin sections were placed into a mixed
solution of 0.25% trypsin (Sigma-Aldrich) and Triton X-100
(Sigma-Aldrich) and shaken 100 times/min for 1 h at 37°C. Samples
were repeatedly washed and shaken with PBS until the cells and
trypsin/Triton X-100 solution were removed. The DDBDM was
maintained in saline solution with 800 U/ml gentamicin at 4°C. The
whole preparation process was conducted under aseptic
conditions.
Physical evaluation of ADM and DDBDM
At room temperature and in a humid environment, the
prepared ADM and DDBDM were trimmed to 1×1-cm sections and measured
with a Benchtop Tester (H10K-T; Tinius Olsen Testing Machine
Company, Horsham, PA, USA). The samples were stretched at a rate of
1 mm/min until the actual load reached 3 MPa, then released at the
same rate until the actual load was 0 MPa. After repeating the
process three times, the stress-strain curve became stable. The
strain value at 3 MPa in the fourth stretch was calculated using
the following formula: Strain (%) = [(L −
L0)/L0] × 100 = (L − 1) × 100, where L was
the length of the dermal matrix during stress in cm and
L0 was the initial length of the dermal matrix, which
was 1 cm in the present study. Subsequently, new samples were
stretched at a rate of 1 mm/min until broken, recording the values
of ultimate tensile strength, maximum tension and elongation at
break. All the data were recorded and calculated using a JBK
Measure-Control System (Beijing Jincun Electromechanical Technology
Research Institute, Beijing, China).
Biotinylated antibody-based cytokine
microarray assay
Total protein was extracted from skin samples using
RIPA buffer (Beyotime Institute of Biotechnology, Haimen, China),
after which the supernatants were obtained by centrifugation at
12,000 × g for 5 min at 4°C. The protein concentrations of the
supernatants were detected using a BCA kit (Thermo Fisher
Scientific Inc., Waltham, MA, USA), according to the manufacturer's
protocol. A total of 308 different mouse proteins, including
cytokines, chemokines, adipokines, growth factors, angiogenic
factors, proteases, soluble receptors and soluble adhesion
molecules, were detected using a mouse cytokine array kit
(AAM-BLM-1-4; RayBiotech, Inc., Norcross, GA, USA), according to
the manufacturer's protocol. Following subtraction of local
background signals, the microarray signals were recorded using a
GenePix 4000B microarray scanner (Molecular Devices, LLC,
Sunnyvale, CA, USA) using the Cy3 channel and with a scanning
wavelength of 532 nm. The positive control value was the mean
fluorescent signal intensity minus the background of all the
positive control spots. Following background subtraction, negative
signal intensities were assigned a value of 1. If the value of all
the samples tested was 1, those cytokines were removed from further
analysis. The mean and the standard error of each cytokine were
calculated separately.
Histological staining
The skin specimens were embedded in paraffin blocks
after they had been fixed in 4% paraformaldehyde solution (Beyotime
Institute of Biotechnology). Sections of 5 µm were
deparaffinized and stained using the hematoxylin and eosin
(H&E; Beyotime Institute of Biotechnology) staining method. The
skin specimens were examined and evaluated in a blinded manner
under a standard light microscope (CX31; Olympus Corporation,
Tokyo, Japan).
DAPI fluorescence staining
Sections (5-µm thickness) were obtained,
deparaffinized and stained with DAPI solution (Beyotime Institute
of Biotechnology) at room temperature for 3–5 min, washed three
times using PBS and observed with a fluorescence microscope.
Subcutaneous implantation of skin
matrix
ADM and DDBDM were trimmed to 1×1-cm sections and
implanted subcutaneously into 48 mice. Briefly, following
anesthetization by intraperitoneal injection with 10% chloral
hydrate (0.3 ml/kg), the fur on the dorsum of the mice was shaved,
a vertical incision was made in the middle of the dorsum and the
skin was separated from the subcutaneous tissue by a blunt
dissection in order to create two 15 mm-deep subcutaneous bursae.
Subsequently, the ADM was implanted in the left bursa and the DDBDM
in the right. The incision was closed and the gap in the
subcutaneous tissue was sutured to prevent the ADM and DDBDM from
touching. The wound was disinfected using 1% povidone iodine
solution every day. At 1, 2, 3 and 4 weeks of subcutaneous
implantation, three mice were randomly selected for sacrifice by
decapitation, prior to immersion in 75% alcohol for 3 min.
Subsequently, the dorsal skins of the mice were cut open and
separated from the subcutaneous tissue in order to expose the
implanted skin matrices, which were then stained using H&E and
DAPI. Images of the skin matrices were captured under a microscope
(BX53; Olympus Corporation).
Statistical analysis
All data are presented as the mean ± standard
deviation. Dual comparisons between groups were evaluated with the
Student's t-test, using the SPSS software, version 19.0 (IBM SPSS,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
DDBDM preparation and its biological
properties
Images of ADM and DDBDM prior to implantation are
presented in Fig. 1. The ADM and
DDBDM were white in color, and had good elasticity and tenacity.
However, the DDBDM was softer and thinner than ADM. The physical
properties of ADM and DDBDM are presented in Table I. There was a statistically
significant difference between ADM and DDBDM in each of the
properties measured (P<0.05).
 | Table IPhysical properties of acellular and
deep-degree burned dermal matrix. |
Table I
Physical properties of acellular and
deep-degree burned dermal matrix.
| Dermal matrix | Ultimate tensile
strength, MPa | Maximum tension,
N | Elongation at
break, % | Strain at stress of
3 MPa, % |
|---|
| Acellular dermal
matrix | 15.0±2.1 | 84±4 | 200±7 | 60.1±2.7 |
| Deep-degree burned
dermal matrix | 10.2±1.8 | 57±3 | 182±5 | 41.6±1.4 |
| P-value | <0.05 | <0.01 | <0.05 | <0.01 |
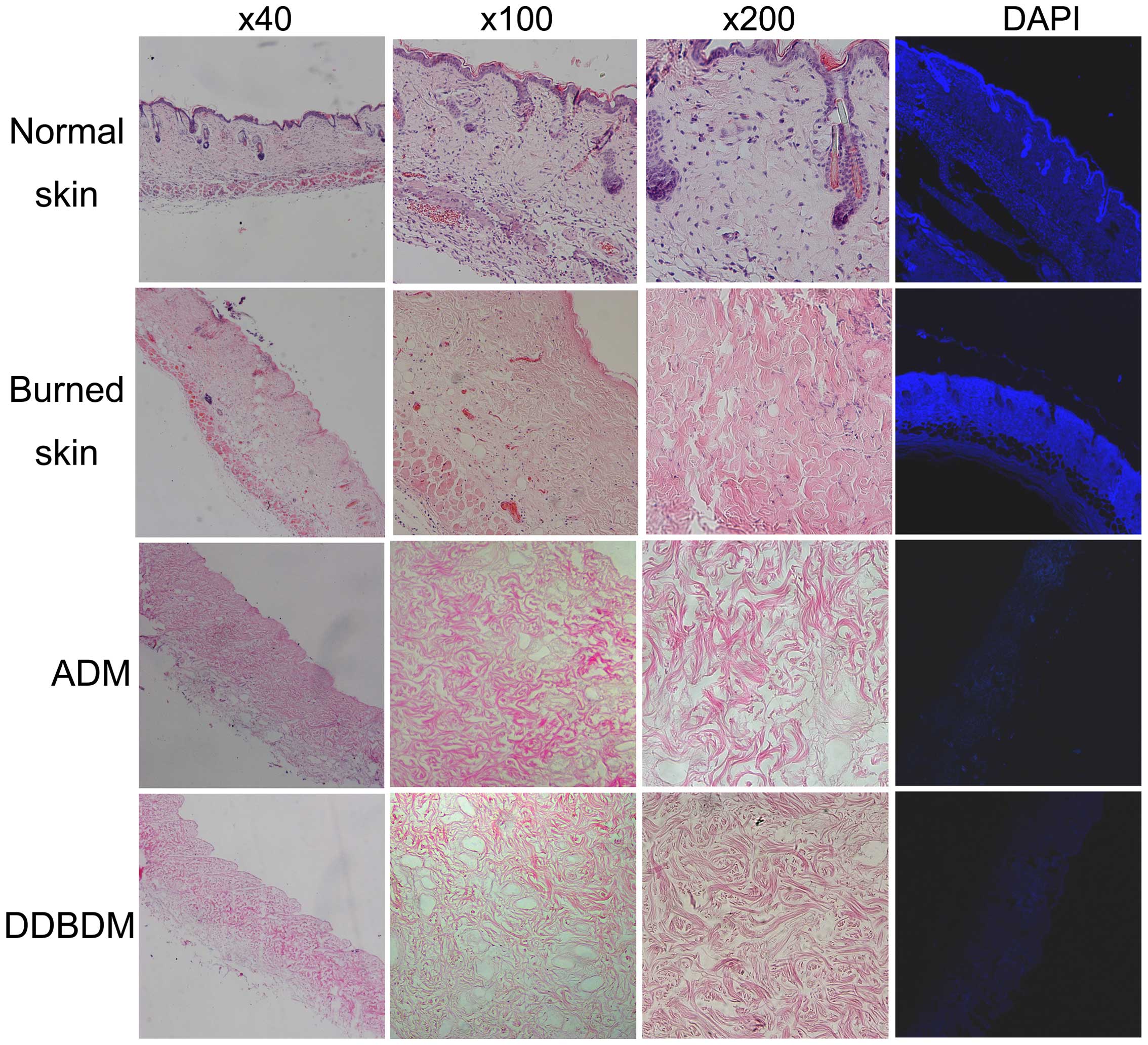
Under microscopic observation, normal skin samples
exhibited an integrated structure, including epidermis, dermis and
appendages. DAPI staining demonstrated the presence of high DNA
levels in the nuclei of the normal skin. By contrast, collagen
fibers in the burned skin samples were thickened and swollen,
coagulated necrosis was observed, and the number of integrated
nuclei decreased. DAPI staining demonstrated that the DNA was
diffusely distributed due to karyorrhexis and karyolysis in the
burned skin. No DNA was observed in ADM and DDBDM samples as they
underwent acellular disposal. H&E staining demonstrated that
the collagens in the ADM were loose but well-organized, and no
residual cellular nuclei were observed. In the DDBDM, the collagens
were thickened, disorganized and fractured; however, the reticulate
structure was maintained (Fig.
2).
Cytokine analysis using biotinylated
antibody microarrays
Images of the biotinylated antibody arrays are
presented in Fig. 3A, and the
names of all cytokines analyzed are presented in Fig. 3B–E. If expression of a cytokine was
negative in all samples, it was eliminated from further analysis.
Subsequently, 227 of the 308 cytokines remained for analysis. The
values from the two groups were compared. The following conditions
were considered to be significant: i) A fold change in cytokine
level of <0.66 or >1.5; ii) at least one value >200 and
iii) P<0.05.
Effects of burn on the expression level
of cytokines
To explore whether the expression of cytokines is
affected by burn, the values of cytokine expression were compared
between normal and burned skin, and between ADM and DDBDM. If the
results demonstrated a significant increase or decrease in both
comparisons of the same cytokine, the cytokine was considered to be
affected by burn. Results showed that the expression of 14
cytokines were altered by burn (Table
II).
 | Table IIExpression values of cytokines with
significant differences between burned and normal skin samples. |
Table II
Expression values of cytokines with
significant differences between burned and normal skin samples.
| Cytokine | Normal skina | Burned skina | P-value | Fold change | ADMa | DDBDMa | P-value | Fold change |
|---|
| Activin βC | 393.5±61.5 | 1.0±0.0 | <0.01 | 393.500 | 76.5±0.5 | 34.0±3.0 | <0.01 | 2.250 |
| 6Ckine | 193.5±18.5 | 1.0±0.0 | <0.01 | 193.500 | 49.0±12.0 | 5.5±0.5 | <0.01 | 8.909 |
| Decorin | 49.0±11.0 | 1.0±0.0 | <0.01 | 49.000 | 913.5±76.5 | 180.0±6.0 | <0.01 | 5.075 |
| IL-10 | 1,460.0±94.0 | 336.0±8.0 | <0.01 | 4.345 | 221.0±7.0 | 98.0±1.0 | <0.01 | 2.255 |
| IL-17 | 1,057.5±46.5 | 268.5±60.5 | <0.01 | 3.939 | 180.0±8.0 | 107.5±3.5 | <0.01 | 1.674 |
| MMP-14 | 929.0±37.0 | 372.0±15.0 | <0.01 | 2.497 | 162.0±2.0 | 81.0±7.0 | <0.01 | 2.000 |
| HVEM | 1,193.5±118.5 | 499.0±212.0 | <0.01 | 2.392 | 219.5±1.5 | 68.5±1.5 | <0.01 | 3.204 |
| TNF-α | 1,327.0±32.0 | 637.5±18.5 | <0.01 | 2.082 | 184.0±17.0 | 107.0±4.0 | <0.01 | 1.720 |
| IL-3 | 2,519.0±33.0 | 1,356.5±56.5 | <0.01 | 1.857 | 303.0±15.0 | 145.5±3.5 | <0.01 | 2.082 |
| TGF-β1 | 1,205.5±111.5 | 688.5±39.5 | <0.01 | 1.751 | 212.0±22.0 | 115.5±4.5 | <0.01 | 1.835 |
| RELM β | 139.0±3.0 | 562.0±28.0 | <0.01 | 0.247 | 41.5±0.5 | 75.0±1.0 | <0.01 | 0.553 |
| IL-9 | 148.5±60.5 | 921.5±267.5 | <0.01 | 0.161 | 65.5±9.5 | 258.0±32.0 | <0.01 | 0.254 |
| IL-23 R | 12.8±13.2 | 157.5±46.5 | <0.01 | 0.081 | 2.5±2.2 | 14.5±4.5 | <0.05 | 0.172 |
| Thrombospondin | 20.5±26.0 | 907.0±46.0 | <0.01 | 0.023 | 25.5±14.5 | 65.5±9.5 | <0.05 | 0.389 |
Effects of cell extraction on the
expression level of cytokines
The expression levels of cytokines were compared
between the normal skin and ADM, and between burned skin and DDBDM.
If the results demonstrated a significant increase or decrease in
both comparisons of the same cytokine, the cytokine was then
considered to be affected by acellular treatment. Results showed
that the levels of 23 cytokines were affected by cell extraction
(Table III).
 | Table IIIExpression values of the cytokines
with significant differences following cell extraction. |
Table III
Expression values of the cytokines
with significant differences following cell extraction.
| Cytokine | Normal skina | ADMa | P-value | Fold change | Burned skina | DDBDMa | P-value | Fold change |
|---|
| RANTES | 115.0±7.0 | 9.0±7.0 | <0.01 | 12.778 | 208.5±102.5 | 19.0±10.0 | <0.05 | 10.974 |
| IL-6 | 2,195.0±32.0 | 226.0±20.0 | <0.01 | 9.712 | 3,129.0±4.0 | 131.5±9.5 | <0.01 | 23.795 |
| IL-3 | 2,519.0±33.0 | 303.0±15.0 | <0.01 | 8.314 | 1,356.5±56.5 | 145.5±3.5 | <0.01 | 9.323 |
| TNF-α | 1,327.0±32.0 | 184.0±17.0 | <0.01 | 7.212 | 637.5±18.5 | 107.0±4.0 | <0.01 | 5.958 |
| IL-10 | 1,460.0±94.0 | 221.0±7.0 | <0.01 | 6.606 | 336.0±8.0 | 98.0±1.0 | <0.01 | 3.429 |
| GDF-1 | 316.5±6.5 | 50.5±14.5 | <0.01 | 6.267 | 155.5±23.5 | 53.0±1.0 | <0.01 | 2.934 |
| IL-12 p70 | 2,000.5±120.5 | 322.0±11.0 | <0.01 | 6.213 | 1,984.5±21.5 | 150.0±16.0 | <0.01 | 13.230 |
| IFN-β | 1,117.5±6.5 | 182.0±9.0 | <0.01 | 6.140 | 1,252.0±85.0 | 136.0±5.0 | <0.01 | 9.206 |
| MCP-1 | 1,125.0±20.0 | 187.5±12.5 | <0.01 | 6.000 | 829.5±89.5 | 89.5±0.5 | <0.01 | 9.268 |
| IFN-γ | 1,990.5±81.5 | 332.5±6.5 | <0.01 | 5.986 | 1,357.5±75.5 | 163.0±1.0 | <0.01 | 8.328 |
| CD11b | 2,094.0±231.0 | 355.5±4.5 | <0.01 | 5.890 | 1,527.0±80.0 | 152.0±6.0 | <0.01 | 10.046 |
| IL-17 | 1,057.5±46.5 | 180.0±8.0 | <0.01 | 5.875 | 268.5±60.5 | 107.5±3.5 | <0.05 | 2.498 |
| MMP-14 | 929.0±37.0 | 162.0±2.0 | <0.01 | 5.735 | 372.0±15.0 | 81.0±7.0 | <0.01 | 4.593 |
| TGF-β1 | 1,205.5±111.5 | 212.0±22.0 | <0.01 | 5.686 | 688.5±39.5 | 115.5±4.5 | <0.01 | 5.961 |
| IL-5 | 1,112.0±44.0 | 202.0±4.0 | <0.01 | 5.505 | 1,073.5±90.5 | 118.0±4.0 | <0.01 | 9.097 |
| IL-12 p40/p70 | 1,288.0±44.0 | 235.5±3.5 | <0.01 | 5.469 | 1,111.5±80.5 | 118.5±12.5 | <0.01 | 9.380 |
| HVEM | 1,193.5±118.5 | 219.5±1.5 | <0.01 | 5.437 | 499.0±212.0 | 68.5±1.5 | <0.05 | 7.285 |
| IL-5 R α | 339.0±65.0 | 81.5±0.5 | <0.01 | 4.160 | 370.0±37.0 | 50.0±3.0 | <0.01 | 7.400 |
| RELM β | 139.0±3.0 | 41.5±0.5 | <0.01 | 3.349 | 562.0±28.0 | 75.0±1.0 | <0.01 | 7.493 |
| IL-1 R9 | 48.5±49.6 | 465.0±5.0 | <0.01 | 0.104 | 1.0 ±0.0 | 29.0±3.0 | <0.01 | 0.034 |
| MMP-9 | 1.0±0.0 | 15.0±3.0 | <0.01 | 0.067 | 1.0±0.0 | 219.5±21.5 | <0.01 | 0.005 |
| Decorin | 49.0±11.0 | 913.5±76.5 | <0.01 | 0.054 | 1.0±0.0 | 180.0±6.0 | <0.01 | 0.006 |
| IL-2 R γ | 1.0±0.0 | 164.5±3.5 | <0.01 | 0.006 | 1.0±0.0 | 109.0±3.0 | <0.01 | 0.009 |
Differences in cytokine expression
between ADM and DDBDM
The expression levels of cytokines in ADM and DDBDM
were compared. The results are indicated in Table IV. The levels of 17 cytokines were
decreased in DDBDM compared with ADM, however IL-9 and MMP-9 levels
were increased.
 | Table IVExpression values of the cytokines
with significant differences between ADM and DDBDM. |
Table IV
Expression values of the cytokines
with significant differences between ADM and DDBDM.
| Cytokine | ADMa | DDBDMa | Fold change | P-value |
|---|
| IL-1 R9 | 465.0±5.0 | 29.0±3.0 | 16.034 | <0.01 |
| Decorin | 913.5±76.5 | 180.0±6.0 | 5.075 | <0.01 |
| HVEM | 219.5±1.5 | 68.5±1.5 | 3.204 | <0.01 |
| CD11b | 355.5±4.5 | 152.0±6.0 | 2.339 | <0.01 |
| IL-10 | 221.0±7.0 | 98.0±1.0 | 2.255 | <0.01 |
| IL-12 p70 | 322.0±11.0 | 150.0±16.0 | 2.147 | <0.01 |
| MCP-1 | 187.5±12.5 | 89.5±0.5 | 2.095 | <0.01 |
| IL-3 | 303.0±15.0 | 145.5±3.5 | 2.082 | <0.01 |
| IFN-γ | 332.5±6.5 | 163.0±1.0 | 2.040 | <0.01 |
| MMP-14 | 162.0±2.0 | 81.0±7.0 | 2.000 | <0.01 |
| IL-12 p40/p70 | 235.5±3.5 | 118.5±12.5 | 1.987 | <0.01 |
| TGF-β1 | 212.0±22.0 | 115.5±4.5 | 1.835 | <0.01 |
| TNF-α | 184.0±17.0 | 107.0±4.0 | 1.720 | <0.01 |
| IL-6 | 226.0±20.0 | 131.5±9.5 | 1.719 | <0.01 |
| IL-5 | 202.0±4.0 | 118.0±4.0 | 1.712 | <0.01 |
| IL-17 | 180.0±8.0 | 107.5±3.5 | 1.674 | <0.01 |
| IL-2 R γ | 164.5±3.5 | 109.0±3.0 | 1.509 | <0.01 |
| IL-9 | 65.5±9.5 | 258.0±32.0 | 0.254 | <0.01 |
| MMP-9 | 15.0±3.0 | 219.5±21.5 | 0.068 | <0.01 |
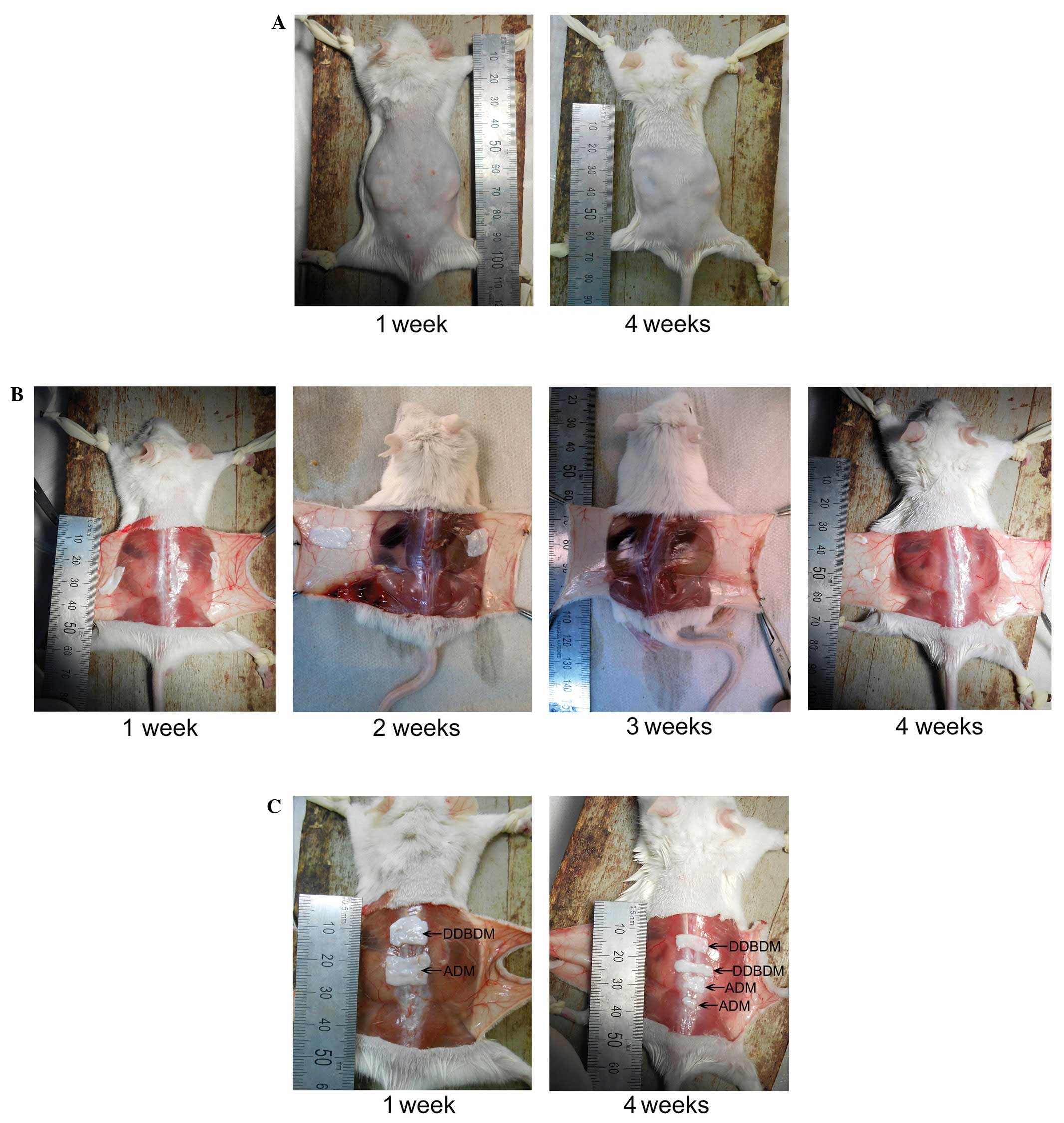
General observation of the ADM and DDBDM
following subcutaneous implantation
Following subcutaneous implantation, the skin wounds
of the mice healed well (Fig. 4A).
Four weeks after implantation, ADM and DDBDM degraded incompletely
and maintained the some integrity (Fig. 4B and C). No marked inflammatory
reaction or rejection was observed, demonstrating
histocompatibility of ADM and DDBDM. After one week, the ADM and
DDBDM could be easily separated from the subcutaneous tissue
(Fig. 4B and C). Two weeks after
implantation, the ADM adhered more tightly to the subcutaneous
tissue than the DDBDM, due to the formation of a fibrous coat. By
the third week, the ADM and DDBDM were both covered by the dense
fibrous coats and were difficult to separate from the surrounding
tissues (Fig. 4B). At week four,
the dense fibrous coats became thinner and the ADM and DDBDM began
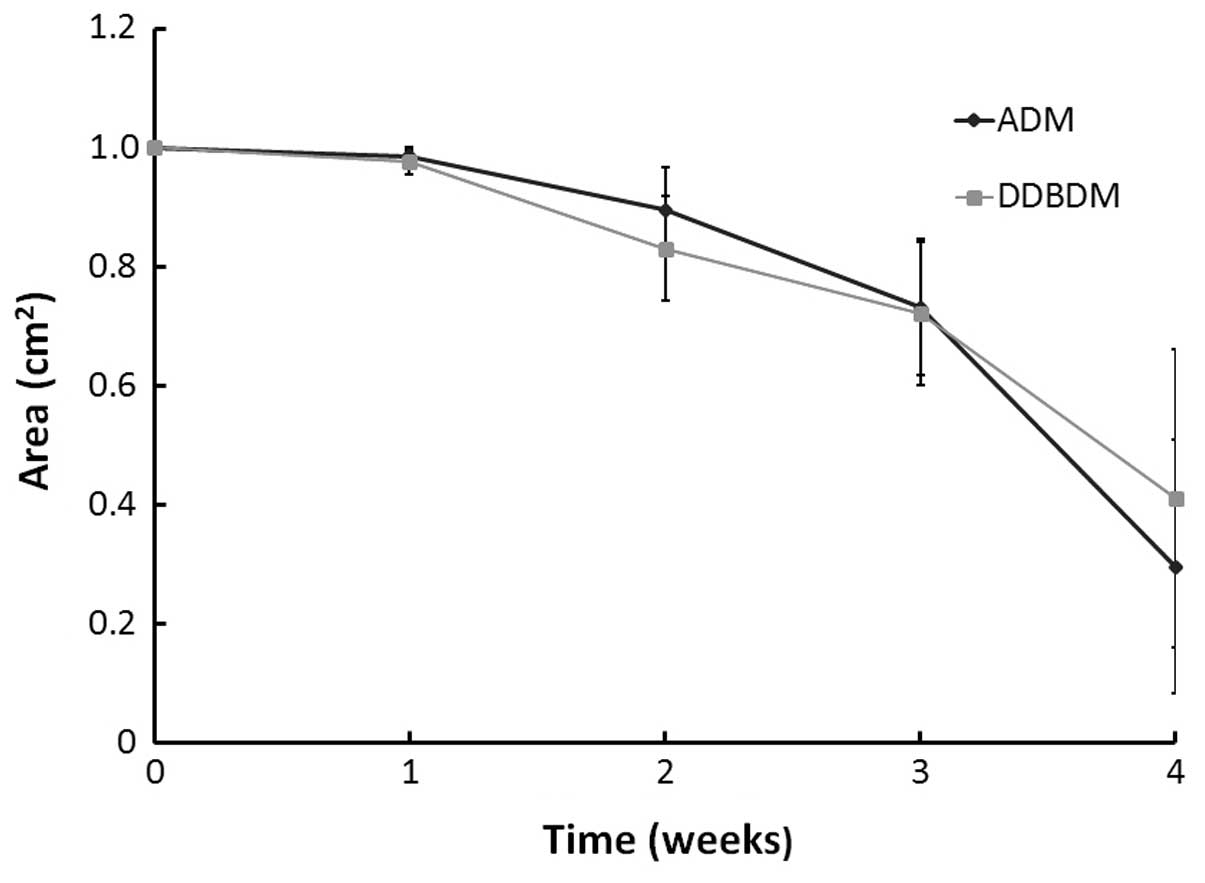
to be absorbed (Fig. 4B and C);
the ratio of absorption area between them was not significantly
different (P=0.615; Fig. 5).
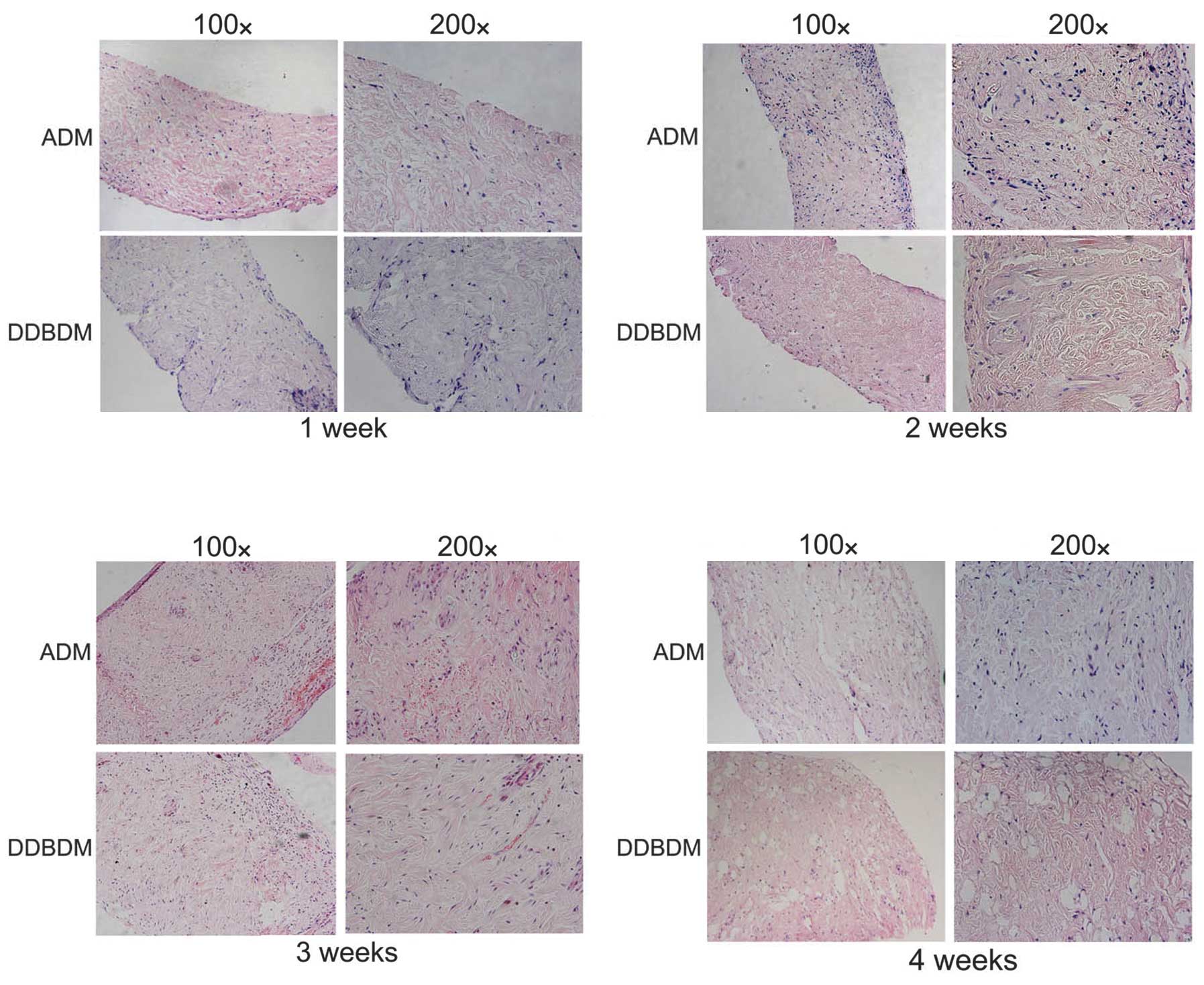
Histological observation of the ADM and
DDBDM following subcutaneous implantation
After one week of subcutaneous implantation,
infiltration of inflammatory cells from the surface to the space
among the collagen was observed in both tissues; however, the DDBDM
exhibited more inflammatory cell infiltration than ADM near the
surface. There was no obvious change in collagen distribution
between the ADM and the DDBDM. The growth of fibroblasts and
neovascularization were not observed in ADM or DDBDM (Fig 6A). After two weeks, the number of
infiltrating inflammatory cells was markedly increased in the ADM
but decreased in the DDBDM. A small number of new blood vessels
were observed, which suggested neovascularization of the dermal
matrix. Meanwhile, the fibroblasts began to infiltrate and produce
new collagen (Fig. 6B). In the
third week, granulation tissue had formed on the ADM surface and
more new blood vessels were observed. The number of fibroblasts and
newly formed collagen increased while the old collagen began to
degrade. In comparison, the DDBDM had thinner granulation tissue on
the surface, and the process of neovascularization appeared to be
slower, with a smaller number and smaller diameter of new blood
vessels than in the ADM samples. Notably, numerous fibroblasts and
the emergence of ordered new collagen fibers were observed in the
DDBDM (Fig. 6C). Four weeks after
subcutaneous implantation, more new collagen fibers emerged in
place of the old ones, the majority of which had been degraded,
with some forming cavities. It was observed that the DDBDM had more
new collagen and less undegraded old collagen than the ADM
(Fig. 6D).
Discussion
Deep-degree burn injuries can result in serious
disability due to hypertrophic scar formation and skin contracture
(27,28). The eschar and denatured skin are
typically removed following burn injury due to adverse reactions
that may result in further loss of dermis. Tissue-engineered skin
substitutes are generally applied to cover the burn wound following
removal of the eschar, improving wound healing (12,29,30).
The clinical application of tissue-engineered skin substitutes or
an ADM may avoid damage caused by multiple wound dressing changes
and thus, accelerate wound healing and reduce scar formation
(31,32).
The biological mechanical properties of the dermal
matrix are very important. The common treatment method of the
dermal matrix is to cover the burn wound following tangential
excision. As a barrier between the wound and the external
environment, the dermal matrix should have adequate intensity to
bear pressure and tensile. In addition, it must possess suitable
elasticity such that it can return to its normal length following
some degree of elongation. At present, there is no standard method
for evaluating the mechanical properties of tissue-engineered skin
substitutes (33–36). In the present study, there was a
significant difference in the mechanical intensities of ADM and
DDBDM. As compared with DDBDM, ADM exhibited enhanced tensile
strength and maximum tension. Furthermore, ADM had better
elasticity, which was reflected by its elongation at break and
strain under stress of 3 MPa. This may have occurred due to the
thermal denaturation of collagen, which may have decreased its
tensile strength and extensibility. In addition, the thermal injury
may have damaged the network structure of the collagen. Despite
this, the gaps of these parameters between ADM and DDBDM were
acceptable and it was demonstrated that DDBDM prepared in the right
conditions may still possess good mechanical properties.
Tissue-engineered skin substitutes are predominantly
composed of seed cells and cellular supporting structures, termed
the cytoskeleton. The seed cells of the skin are fibro-blasts,
which produce collagen to form connective tissue, and
keratinocytes, which form the epithelium. The cytoskeleton is
essential to the growth of seed cells and the regeneration of
tissues. It is known that the collagen is one of the most important
components of the ECM. ADM is a collagen skeleton without any
cells. ADM can protect burn wounds from bacterial infection and
maintain the appropriate environments to accelerate the dermis
reconstruction (7,37).
When the skin is deeply burned, the entire
epithelium and parts of the dermis are damaged. Pathological
changes to the dermis include hyaline degeneration, swelling,
enlargement and disordered arrangement of collagen. When burned,
the hydrogen bonds between collagen fibers break, however, the
intermolecular cross-linking is not disrupted due to its low
thermal sensitivity. Thus, the collagen is shortened, while the
integrity of its structure is maintained (38,39).
When the skin is burned unevenly, there may be certain areas in
which the cells are necrotic, however, the collagens are merely
denatured and maintain a complete structure. Under certain
conditions, these collagens may be repaired to become the ideal
dermal matrix (40).
In the present study, it was observed that the
collagen of DDBDM exhibited swelling and degradation, however, its
structure was partially maintained. Therefore, it was hypothesized
that the DDBDM may act as a satisfactory cytoskeleton with good
tension and ductility when the digestion time of trypsin is
sufficiently shortened. When observed microscopically, certain
areas of collagen were enlarged, partially fractured and loosely
arranged, but predominantly remained continuous in the DDBDM. No
cells remained in the ADM and DDBDM following their removal with
trypsin and Triton-X, which may explain why neither ADM nor DDBDM
showed marked histoincompatibility. Almost all antigens able to
induce allograft rejection were removed in the process of removing
cells, and the main cells involved in allograft rejection,
including T-cells and NK-cells (41,42),
were destroyed completely. Good histocompatibility is a critical
feature of tissue-engineered tissues or organs, since they are
transplanted into the human body (43–46).
It has previously been demonstrated that the ECM possesses almost
all the features of an ideal tissue-engineered biological material,
including histocompatibility, degradability, non-toxicity and
mechanical properties that match those of the original tissue
(47,48). In the present study, the key
components of ADM or DDBDM were collagens, which are a key
component of the ECM produced by fibroblasts.
A key characteristic of good histocompatibility is
an inability to induce significant immune rejection responses
(49–51). In the subcutaneous implantation
experiment, no significant rejection response was observed for
either ADM nor DDBDM, although the ADM induced a greater
inflammatory reaction than DDBDM, which suggested that the
so-called burn toxin did not induce adverse reactions to the DDBDM,
as previously presumed. Although neovascularization was not clearly
observed in the DDBDM, it exhibited a stronger ability to promote
fibroblast chemotaxis and the production of new collagen. Four
weeks after subcutaneous implantation with DDBDM, almost all old
degenerated collagens were degraded and replaced by new collagen
fibers with normal structure. Therefore, the DDBDM may be
considered as a substitute to ADM to promote wound healing of
patients with burns.
Burn wound healing is a complicated process
involving inflammatory responses, neovascularization, granulation
tissue formation, and epithelium and connective tissue remodeling
(52,53). When severe burns occur, a series of
harmful substances, termed burn toxins are released. Burn toxins
are typically products of ECM degradation that are considered to
induce adverse effects in patients with burns, including methyl
guanidine, histamine, putrescine, indoles and inflammatory factors.
However, beneficial burn healing factors may also be produced by
degradation of the ECM, possibly resulting in chemotaxis,
angiogenesis, growth factor signaling and anti-microbial activities
(14,54). To investigate whether DDBDM,
constructed from burned skin, contains reduced burn toxins and
beneficial factors compared with ADM, the present study analyzed
the expression of 308 cytokines in the mouse skin models.
The current study demonstrated that the expression
level of certain cytokines in the skin tissue decreased
significantly following burn injury, including interleukin (IL)-3,
IL-10, IL-17 and decorin. By contrast, resistin-like molecule β
(RELM β), IL-9, IL-23 R and thrombospondin levels were increased.
These cytokines are closely associated with inflammatory responses.
For example, IL-10 is a known anti-inflammatory cytokine (55) and tumor necrosis factor-α (TNF-α)
is a primary mediator of the host response to inflammation
(56). Decorin is expressed by
fibroblasts (57) and has
important biological functions, including regulating collagen
formation, maintaining collagen arrangement and inhibiting the
activity of transforming growth factor-β (TGF-β). Reducing the
level of decroin in the deep dermis may lead to hypertrophic
scarring (58). A previous study
demonstrated that suppressors of cytokine signaling-3 (SOCS-3) may
be important in regulating the balance between immunosuppression
and inflammation following thermal injury (59). IL-9 induced the expression of
SOCS-3 and SOCS-3 over-expression suppressed IL-9 signaling
(60). In addition, IL-9 inhibits
TNF-α release in lipopolysaccharide-stimulated human monocytes
through TGF-β (61). TGF-β is
involved in a number of wound healing processes, including
inflammation, stimulation of angiogenesis, fibroblast
proliferation, collagen synthesis, and deposition and remodeling of
new ECM (62,63). Thrombospondin-1 and 2 are best
known for their anti-angiogenic properties and their ability to
modulate cell-matrix interactions (64). Thrombospondin-1 suppresses wound
healing and granulation tissue formation (65), and blocks thrombospondin-1 binding
to CD47, markedly increasing skin graft survival (66).
A previous study demonstrated that the expression of
certain cytokines, including TNF-α, IL-1β and IL-6, were
significantly increased in the serum of animal models (67) and human patients with burns
(68). Thus, the serum levels of
TNF-α, IL-1, IL-6 and IL-10 may indicate the severity of this fatal
condition following burn injury (69). However, in local skin tissue, the
levels of these cytokines were decreased. This may be because the
deep burn destroyed a large number of cells resulting in necrosis
and degeneration, and the subsequent loss of cytokine expression.
However, it is still unclear why RELM β, IL-9, IL-23 R and
thrombospondin levels were increased in the burned skin in the
current study.
To avoid the immune response induced by the genetic
material of the skin donator, acellularization was performed on the
normal and burned skin to decrease histoincompatibility. In the
present study, the trypsin digestion and shock-elution method was
successfully used to remove cells from the dermal matrix.
Undergoing this process significantly decreased the levels of the
majority of cytokines compared with normal and burned skin.
Notably, the level of decorin was increased significantly in ADM
and DDBDM compared with normal and burned skin, respectively.
Decorin is a component of connective tissue that binds to type 1
collagen fibrils and is important in matrix assembly (70,71).
It may regulate the activity of TGF-β as recombinant human decorin
inhibits TGF-β1-induced contraction of collagen lattices by
hypertrophic scar fibroblasts (70).
In comparison, the levels of cytokines in DDBDM were
lower than those in ADM, with the exception of IL-9 and MMP-9,
which may explain the observation that the DDBDM induced a smaller
inflammatory response than ADM in the subcutaneous implantation
experiments. MMP-9 is secreted by keratinocytes and inflammatory
cells, and is associated with epithelialization. A previous study
also observed an increased level of MMP-9 during the early
inflammatory phase of wound healing (72).
During the formation of new granulation tissue,
TGF-β1 stimulation induces the deposition of fibronectin extra
domain A and α-smooth muscle actin expression by fibroblasts,
resulting in enhanced synthesis of new ECM (73). TGF-β1 is also a crucial factor in
the regulation of myofibroblast differentiation, facilitating the
contraction of granulation tissue (74). TGF-β has different temporal effects
on wound healing and scarring, and any disruption in this
expression pattern may result in hypertrophic scar formation
(75). In the present study, the
level of TGF-β1 in DDBDM was lower than in ADM, which may explain
the thinner granulation tissue on the surface of DDBDM compared
with ADM in the second week. Excess TGF-β1 may be responsible for
the overproduction of ECM, leading to tissue fibrosis and scar
formation. Therefore, the DDBDM may decrease the formation of scar
tissue compared with ADM in the treatment of burns.
In conclusion, the current study describes the
preparation method of DDBDM. The differences in cytokine expression
between the ADM and DDBDM indicated that DDBDM did not produce
increased levels of harmful burn toxin. Therefore, the DDBDM may be
useful as a dermal matrix. If this method is confirmed to decrease
harmful inflammatory factors and utilize beneficial factors, it may
be used to alleviate adverse responses and promote wound healing
processes, including seed cell infiltration, angiogenesis and
matrix remodeling. The DDBDM is derived from autologous dermal
matrix, therefore, the histoincompatibility associated with
xenogeneic or allogeneic dermal matrices is avoided. However, the
present study has a number of limitations. For example, further
analysis regarding the regulation key cytokines in DDBDM were not
conducted, therefore, the effect of these cytokines on the body is
unknown. Additionally, it is unclear whether burn healing would be
promoted using the DDBDM as a dermal matrix instead of the ADM.
Future investigation should aim to resolve these questions.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 30772258, 81071560
and 81372074), the Scientific and Technological Project of Shandong
Province (grant no. 2009GG10002078), and Jinan Young Star Project
of Science and Technology (grant no. 2013031).
References
|
1
|
Reinke JM and Sorg H: Wound repair and
regeneration. Eur Surg Res. 49:35–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Orgill DP and Ogawa R: Current methods of
burn reconstruction. Plast Reconstr Surg. 131:827e–836e. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kagan RJ, Peck MD, Ahrenholz DH, Hickerson
WL, Holmes J IV, Korentager R, Kraatz J, Pollock K and Kotoski G:
Surgical management of the burn wound and use of skin substitutes:
an expert panel white paper. J Burn Care Res. 34:e60–e79. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chiu T and Burd A: “Xenograft” dressing in
the treatment of burns”. Clin Dermatol. 23:419–423. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chiu T, Pang P, Ying SY and Burd A:
Porcine skin: Friend or foe? Burns. 30:739–741. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng X, Shen R, Tan J, Chen X, Pan Y, Ruan
S, Zhang F, Lin Z, Zeng Y, Wang X, et al: The study of inhibiting
systematic inflammatory response syndrome by applying xenogenic
(porcine) acellular dermal matrix on second-degree burns. Burns.
33:477–479. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feng X, Tan J, Pan Y, Wu Q, Ruan S, Shen
R, Chen X and Du Y: Control of hypertrophic scar from inception by
using xenogenic (porcine) acellular dermal matrix (ADM) to cover
deep second degree burn. Burns. 32:293–298. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Allman AJ, McPherson TB, Badylak SF,
Merrill LC, Kallakury B, Sheehan C, Raeder RH and Metzger DW:
Xenogeneic extra-cellular matrix grafts elicit a TH2-restricted
immune response. Transplantation. 71:1631–1640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang D, Chen B, Xu M, Hu D, Tang C and
Zhu X: The manufacturing and clinical application of heterogenous
acellular dermal matrix. Zhonghua Shao Shang Za Zhi. 18:15–18.
2002.In Chinese.
|
|
10
|
Philandrianos C, Andrac-Meyer L, Mordon S,
Feuerstein JM, Sabatier F, Veran J, Magalon G and Casanova D:
Comparison of five dermal substitutes in full-thickness skin wound
healing in a porcine model. Burns. 38:820–829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Truong AT, Kowal-Vern A, Latenser BA,
Wiley DE and Walter RJ: Comparison of dermal substitutes in wound
healing utilizing a nude mouse model. J Burns Wounds. 4:e42005.
|
|
12
|
Chen SG, Tzeng YS and Wang CH: Treatment
of severe burn with DermACELL(®), an acellular dermal matrix. Int J
Burns Trauma. 2:105–109. 2012.
|
|
13
|
Solomon JR: Early surgical excision and
grafting of burns including tangential excision. Prog Pediatr Surg.
14:133–149. 1981.PubMed/NCBI
|
|
14
|
Sparkes BG, Monge G, Marshall SL, Peters
WJ, Allgöwer M and Schoenenberger GA: Plasma levels of cutaneous
burn toxin and lipid peroxides in thermal injury. Burns.
16:118–122. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hiramatsu M, Izawa Y, Hagihara M,
Nishigaki I and Yagi K: Serum lipid peroxide levels of patients
suffering from thermal injury. Burns Incl Therm Inj. 11:111–116.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schoenenberger GA: Burn toxins isolated
from mouse and human skin. Their characterization and immunotherapy
effects. Monogr Allergy. 9:72–139. 1975.PubMed/NCBI
|
|
17
|
Allgöwer M, Cueni LB, Städtler K and
Schoenenberger GA: Burn toxin in mouse skin. J Trauma. 13:95–111.
1973. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Constantian MB: Association of sepsis with
an immunosuppressive polypeptide in the serum of burn patients. Ann
Surg. 188:209–215. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ozkan AN and Ninnemann JL: Circulating
mediators in thermal injuries: Isolation and characterization of a
burn injury-induced immunosuppressive serum component. J Burn Care
Rehabil. 6:147–151. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schoenenberger GA, Burkhardt F, Kalberer
F, Müller W, Städtler K, Vogt P and Allgöwer M: Experimental
evidence for a significant impairment of host defense for
gram-negative organisms by a specific cutaneous toxin produced by
severe burn injuries. Surg Gynecol Obstet. 141:555–561.
1975.PubMed/NCBI
|
|
21
|
Kremer B, Allgöwer M, Scheidegger AM,
Schmidt KH, Schölmerich J, Wüst B and Schoenenberger GA:
Toxin-specific ultra-structural alterations of the mouse liver
after burn injuries and the possibility of a specific antitoxic
therapy. Scand J Plast Reconstr Surg. 13:217–222. 1979. View Article : Google Scholar
|
|
22
|
Schölmerich J, Kremer B, Schmidt K,
Setyadharma H, Richter IE and Schoenenberger GA: Effects of peptide
hormones on urea- and glycogen-synthesis of isolated hepatocytes
and the influence of a toxic factor from burnt mouse and human
skin. Horm Metab Res. 14:80–84. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ninnemann JL and Stein MD: Suppressor cell
induction by povidone-iodine: In vitro demonstration of a
consequence of clinical burn treatment with betadine. J Immunol.
126:1905–1908. 1981.PubMed/NCBI
|
|
24
|
Sparkes BG, Gyorkos JW, Gorczynski RM and
Brock AJ: Comparison of endotoxins and cutaneous burn toxin as
immunosuppressants. Burns. 16:123–127. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sparkes BG: Mechanisms of immune failure
in burn injury. Vaccine. 11:504–510. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang XC, Li C, Shan F, Wang WT, Zhu XG and
Jiang DY: Experimental study on the recycling of denatured
acellular dermal matrix after burn. Zhonghua Shao Shang Za Zhi.
28:201–206. 2012.In Chinese. PubMed/NCBI
|
|
27
|
Lu KH and Li HY: Study on the management
of postburn pathological scars. Zhonghua Shao Shang Za Zhi.
20:65–66. 2004.In Chinese. PubMed/NCBI
|
|
28
|
Eldad A, Din A, Weinberg A, Neuman A,
Lipton H, Ben-Bassat H, Chaouat M and Wexler MR: Cryopreserved
cadaveric allografts for treatment of unexcised partial thickness
flame burns: Clinical experience with 12 patients. Burns.
23:608–614. 1997. View Article : Google Scholar
|
|
29
|
Purdue GF, Hunt JL, Still JM Jr, Law EJ,
Herndon DN, Goldfarb IW, Schiller WR, Hansbrough JF, Hickerson WL,
Himel HN, et al: A multicenter clinical trial of a biosynthetic
skin replacement, Dermagraft-TC, compared with cryopreserved human
cadaver skin for temporary coverage of excised burn wounds. J Burn
Care Rehabil. 18:52–57. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anderson JR, Fear MW, Phillips JK, Dawson
LF, Wallace H, Wood FM and Rea SM: A preliminary investigation of
the reinnervation and return of sensory function in burn patients
treated with INTEGRA®. Burns. 37:1101–1108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu SL, Liao ZJ, Xiang J, Wang ZY, Yang LY
and Shi JX: Clinical observation of the effect of tangential
excision within 24 postburn hours on the patients with deep partial
thickness burn. Zhonghua Shao Shang Za Zhi. 19:326–328. 2003.In
Chinese.
|
|
32
|
Wang ZQ, Cai BR, Xiao J, Hao GH, Wu JB and
Zhao XH: The clinical staging and tissue bacterial quantification
in the diagnosis of burn wound sepsis. Zhonghua Shao Shang Za Zhi.
19:282–284. 2003.In Chinese. PubMed/NCBI
|
|
33
|
Zhang Z, Lv L, Mamat M, Chen Z, Liu L and
Wang Z: Xenogenic (porcine) acellular dermal matrix is useful for
the wound healing of severely damaged extremities. Exp Ther Med.
7:621–624. 2014.PubMed/NCBI
|
|
34
|
Lavine M, Frisk M and Pennisi E:
Biomaterials. Introduction. Science. 338:8992012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mehrali M, Shirazi FS, Mehrali M,
Metselaar HS, Kadri NA and Osman NA: Dental implants from
functionally graded materials. J Biomed Mater Res A. 101:3046–3057.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Leong KF, Chua CK, Sudarmadji N and Yeong
WY: Engineering functionally graded tissue engineering scaffolds. J
Mech Behav Biomed Mater. 1:140–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen X, Shi Y, Shu B, Xie X, Yang R, Zhang
L, Ruan S, Lin Y, Lin Z, Shen R, et al: The effect of porcine ADM
to improve the burn wound healing. Int J Clin Exp Pathol.
6:2280–2291. 2013.PubMed/NCBI
|
|
38
|
Pierce MC, Sheridan RL, Hyle Park B, Cense
B and de Boer JF: Collagen denaturation can be quantified in burned
human skin using polarization-sensitive optical coherence
tomography. Burns. 30:511–517. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cuttle L, Kempf M, Phillips GE, Mill J,
Hayes MT, Fraser JF, Wang XQ and Kimble RM: A porcine deep dermal
partial thickness burn model with hypertrophic scarring. Burns.
32:806–820. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hayashi K and Markel MD: Thermal
capsulorrhaphy treatment of shoulder instability: Basic science.
Clin Orthop Relat Res. 390:59–72. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hall BM: Cells mediating allograft
rejection. Transplantation. 51:1141–1151. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Alegre ML, Florquin S and Goldman M:
Cellular mechanisms underlying acute graft rejection: Time for
reassessment. Curr Opin Immunol. 19:563–568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
van Minnen B, van Leeuwen MB, Stegenga B,
Zuidema J, Hissink CE, van Kooten TG and Bos RR: Short-term in
vitro and in vivo biocompatibility of a biodegradable polyurethane
foam based on 1,4-butanediisocyanate. J Mater Sci Mater Med.
16:221–227. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pomahac B, Svensjö T, Yao F, Brown H and
Eriksson E: Tissue engineering of skin. Crit Rev Oral Biol Med.
9:333–344. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Prasad T, Shabeena EA, Vinod D, Kumary TV
and Anil Kumar PR: Characterization and in vitro evaluation of
electrospun chitosan/polycaprolactone blend fibrous mat for skin
tissue engineering. J Mater Sci Mater Med. 26:53522015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mañez R, White LT, Linden P, Kusne S,
Martin M, Kramer D, Demetris AJ, Van Thiel DH, Starzl TE and
Duquesnoy RJ: The influence of HLA matching on cytomegalovirus
hepatitis and chronic rejection after liver transplantation.
Transplantation. 55:1067–1071. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Borschel GH, Dennis RG and Kuzon WM Jr:
Contractile skeletal muscle tissue-engineered on an acellular
scaffold. Plast Reconstr Surg. 113:595–602; discussion 603–604.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Badylak SF: The extracellular matrix as a
biologic scaffold material. Biomaterials. 28:3587–3593. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Low PS, Tjin MS and Fong E: Design and
Construction of Artificial Extracellular Matrix (aECM) Proteins
from Escherichia coli for Skin Tissue Engineering. J Vis Exp.
e528452015.PubMed/NCBI
|
|
50
|
Sundaramurthi D, Krishnan UM and
Sethuraman S: Biocompatibility of
poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) nanofibers for
skin tissue engineering. J Biomed Nanotechnol. 9:1383–1392. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Müller WE and Müller IM: Origin of the
metazoan immune system: Identification of the molecules and their
functions in sponges. Integr Comp Biol. 43:281–292. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Singer AJ and Clark RA: Cutaneous wound
healing. N Engl J Med. 341:738–746. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Velnar T, Bailey T and Smrkolj V: The
wound healing process: An overview of the cellular and molecular
mechanisms. J Int Med Res. 37:1528–1542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chang KC, Ma H, Liao WC, Lee CK, Lin CY
and Chen CC: The optimal time for early burn wound excision to
reduce pro-inflammatory cytokine production in a murine burn injury
model. Burns. 36:1059–1066. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Drost AC, Burleson DG, Cioffi WG Jr, Mason
AD Jr and Pruitt BA Jr: Plasma cytokines after thermal injury and
their relationship to infection. Ann Surg. 218:74–78. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Beutler B and Cerami A: The biology of
cachectin/TNF–a primary mediator of the host response. Annu Rev
Immunol. 7:625–655. 1989. View Article : Google Scholar
|
|
57
|
Tan EM, Hoffren J, Rouda S, Greenbaum S,
Fox JW IV, Moore JH Jr and Dodge GR: Decorin, versican, and
biglycan gene expression by keloid and normal dermal fibroblasts:
differential regulation by basic fibroblast growth factor. Exp Cell
Res. 209:200–207. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Honardoust D, Varkey M, Marcoux Y,
Shankowsky HA and Tredget EE: Reduced decorin, fibromodulin, and
transforming growth factor-β3 in deep dermis leads to hypertrophic
scarring. J Burn Care Res. 33:218–227. 2012. View Article : Google Scholar
|
|
59
|
Ogle CK, Kong F, Guo X, Wells DA, Aosasa
S, Noel G and Horseman N: The effect of burn injury on suppressors
of cytokine signalling. Shock. 14:392–399. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lejeune D, Demoulin JB and Renauld JC:
Interleukin 9 induces expression of three cytokine signal
inhibitors: Cytokine-inducible SH2-containing protein, suppressor
of cytokine signalling (SOCS)-2 and SOCS-3, but only SOCS-3
overexpression suppresses interleukin 9 signalling. Biochem J.
353:109–116. 2001. View Article : Google Scholar
|
|
61
|
Pilette C, Ouadrhiri Y, Van Snick J,
Renauld JC, Staquet P, Vaerman JP and Sibille Y: IL-9 inhibits
oxidative burst and TNF-alpha release in
lipopolysaccharide-stimulated human monocytes through TGF-beta. J
Immunol. 168:4103–4111. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Nall AV, Brownlee RE, Colvin CP, Schultz
G, Fein D, Cassisi NJ, Nguyen T and Kalra A: Transforming growth
factor beta 1 improves wound healing and random flap survival in
normal and irradiated rats. Arch Otolaryngol Head Neck Surg.
122:171–177. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Roberts AB, Sporn MB, Assoian RK, Smith
JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V and
Kehrl JH: Transforming growth factor type beta: rapid induction of
fibrosis and angiogenesis in vivo and stimulation of collagen
formation in vitro. Proc Natl Acad Sci USA. 83:4167–4171. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bornstein P, Agah A and Kyriakides TR: The
role of thrombospondins 1 and 2 in the regulation of cell-matrix
interactions, collagen fibril formation, and the response to
injury. Int J Biochem Cell Biol. 36:1115–1125. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Streit M, Velasco P, Riccardi L, Spencer
L, Brown LF, Janes L, Lange-Asschenfeldt B, Yano K, Hawighorst T,
Iruela-Arispe L and Detmar M: Thrombospondin-1 suppresses wound
healing and granulation tissue formation in the skin of transgenic
mice. EMBO J. 19:3272–3282. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Isenberg JS, Pappan LK, Romeo MJ, Abu-Asab
M, Tsokos M, Wink DA, Frazier WA and Roberts DD: Blockade of
thrombos-pondin-1-CD47 interactions prevents necrosis of full
thickness skin grafts. Ann Surg. 247:180–190. 2008. View Article : Google Scholar :
|
|
67
|
Emanuele NV, LaPaglia N, Kovacs EJ and
Emanuele MA: The impact of burn injury and ethanol on the cytokine
network of the mouse hypothalamus: Reproductive implications.
Cytokine. 30:109–115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
de Bandt JP, Chollet-Martin S, Hernvann A,
Lioret N, du Roure LD, Lim SK, Vaubourdolle M, Guechot J, Saizy R,
Giboudeau J and Cynober L: Cytokine response to burn injury:
Relationship with protein metabolism. J Trauma. 36:624–628. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Drost AC, Burleson DG, Cioffi WG Jr,
Jordan BS, Mason AD Jr and Pruitt BA Jr: Plasma cytokines following
thermal injury and their relationship with patient mortality, burn
size, and time postburn. J Trauma. 35:335–339. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhang Z, Garron TM, Li XJ, Liu Y, Zhang X,
Li YY and Xu WS: Recombinant human decorin inhibits
TGF-beta1-induced contraction of collagen lattice by hypertrophic
scar fibroblasts. Burns. 35:527–537. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chen S, Young MF, Chakravarti S and Birk
DE: Interclass small leucine-rich repeat proteoglycan interactions
regulate collagen fibrillogenesis and corneal stromal assembly.
Matrix Biol. 35:103–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Gillard JA, Reed MW, Buttle D, Cross SS
and Brown NJ: Matrix metalloproteinase activity and
immunohistochemical profile of matrix metalloproteinase-2 and -9
and tissue inhibitor of metalloproteinase-1 during human dermal
wound healing. Wound Repair Regen. 12:295–304. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Serini G, Bochaton-Piallat ML, Ropraz P,
Geinoz A, Borsi L, Zardi L and Gabbiani G: The fibronectin domain
ED-A is crucial for myofibroblastic phenotype induction by
transforming growth factor-beta1. J Cell Biol. 142:873–881. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Smith PC, Cáceres M and Martinez J:
Induction of the myofibroblastic phenotype in human gingival
fibroblasts by transforming growth factor-beta1: Role of RhoA-ROCK
and c-Jun N-terminal kinase signaling pathways. J Periodontal Res.
41:418–425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lu L, Saulis AS, Liu WR, Roy NK, Chao JD,
Ledbetter S and Mustoe TA: The temporal effects of anti-TGF-beta1,
2, and 3 monoclonal antibody on wound healing and hypertrophic scar
formation. J Am Coll Surg. 201:391–397. 2005. View Article : Google Scholar : PubMed/NCBI
|