Introduction
A stroke or cerebrovascular accident occurs when an
area of the brain is suddenly deprived of blood flow. This may be
due to the occlusion of a blood vessel (ischemic stroke) accounting
for ~80% of all strokes, or to a local intracranial hemorrhage
(hemorrhagic stroke). The lack of nutrients and oxygen alters the
metabolism of the affected neurons and glial cells and results in
the appearance of neurological symptoms and signs that may become
irreversible, depending on the time prior to circulation being
re-established (1,2). Stroke constitutes a important health
and social problem; according to the World Health Organization, ~15
million people suffer a stroke every year and, of these, 5 million
are fatal and 5 million result in permanent incapacitation
(3). Recent data suggests that up
to 85% of all strokes may be preventable using medical intervention
and lifestyle modifications (4),
however, urgent care is required at emergency stroke units.
A search for biomarkers that may predict clinical
outcomes in stroke patients is ongoing (5,6). As
stroke is a vascular disease, vasoactive substances are
particularly notable. Thus, the present study investigated nitric
oxide (NO) and adrenomedullin (AM). NO is a gaseous free radical
that is synthesized from L-arginine by nitric oxide synthases
(NOS). NO may exert opposite functions in the central nervous
system depending on its concentration, site of production, and the
NOS isoform that produces it. Low concentrations produced by
endothelial NOS are often beneficial and contribute to local
vasodilatation. However, large concentrations of NO, such as those
produced by the inducible NOS isoform result in the excessive
generation of free radicals and the destruction of biological
molecules, including proteins, lipids, and nucleic acids (7–9). It
has been demonstrated that NO production increases under hypoxia
(10), a common situation in
tissues affected by stroke. In periods of ischemia, NO production
is reduced due to oxygen deficiency, however, immediately following
reperfusion, synthesis of this molecule is triggered by activation
of neuronal and endothelial NOS. A number of hours later, NO
concentration increases again due to the activation of the
inducible NOS isoform (11). Thus,
modulators of NO may provide neuroprotection following
neurovascular accidents. NO donors markedly reduce infarction
volume in experimental models (12,13).
Recently, circulating NO levels have been proposed as a biomarker
to predict mortality in ischemic stroke patients (14). However, as NO is a free radical
with a short half life, only its chemical products, nitrate/nitrite
and S-nitroso compounds (NOx) are measurable in clinical samples
(7).
AM is a vasodilatatory peptide produced by numerous
areas of the central nervous system (15) and peripheral tissues (16). As with NO, AM expression is also
upregulated under hypoxia via activation of the hypoxia inducible
factor-1 pathway (17) and it has
been demonstrated to increase following ischemic insults to the
brain (18–20). Previous studies in mice lacking AM
expression suggest that this peptide is neuroprotective in the
context of stroke, and may exert its effects via regulation of
inducible NOS, matrix metalloproteinases, and inflammatory
mediators (21).
The present study aimed to perform a longitudinal
follow-up of stroke patients measuring blood pressure, NO, and AM
levels and investigating whether these values may be predictive of
clinical parameters, including infarct volume growth, neurological
severity, or functional prognosis.
Materials and methods
Ethical issues
All procedures were approved by the local review
board (Comité Ético de Investigación Clínica de La Rioja, Logroño,
Spain) and all described procedures adhere to the tenets of the
Declaration of Helsinki.
Patients
The present study was designed as a prospective,
observational, and longitudinal clinical study with patients
diagnosed with acute ischemic stroke at the Neurology Service of
the Hospital San Pedro (Logroño, Spain) from October 2014 to April
2015. Consecutive patients (n=76) fulfilling inclusion criteria
signed the written informed consent documents and were recruited
into the current study. Inclusion criteria required patients to be
suffering from acute ischemic stroke demonstrated by magnetic
resonance imaging (MRI), and an evolution of <24 h. Exclusion
criteria were the same used for the hospital's stroke unit and were
as follows: Contraindications for performing MRI; age, <18
years; dementia; previous stroke within 3 months; cranial
traumatism; central nervous system infection; cardiac
insufficiency; renal failure; sepsis; active neoplasia; active
inflammatory or autoimmune disease; pregnant or lactating women;
and patients who would not be able to complete proper
follow-up.
In this time period, 140 patients were diagnosed and
treated at the stroke unit. Of these, 127 were suspected of
suffering ischemic stroke and 120 arrived within 24 h of the first
symptoms. A total of 29 patients were excluded from the present
study due to fulfilling at least one of the exclusion criteria and
15 additional patients were excluded following performing MRI as
the ischemic lesion could not be confirmed. Finally, 76 patients
were included in the study and all required data was collected for
70 of them.
Variables of the present study
Patients received standard care following the
approved protocols of the stroke unit. General characteristics of
the patient were collected as part of the clinical history
(including age, gender, risk factors, current medical treatment and
previous functional situation). During their stay at the stroke
unit, numerous parameters were continuously monitored, including
electrocardiogram, systolic arterial pressure (SAP) and diastolic
arterial pressure (DAP), temperature, and hypoxemia. Neurological
severity was measured with the National Institutes of Health Stroke
Scale (NIHSS) (22) at 0, 24 h, 7
days, and 3 months. Functional prognosis was also evaluated with
the Rankin scale at 3 months (23). In addition, blood plasma samples
were taken on day 1, day 2, and day 7 to quantify circulating
levels of NO and AM. Infarct volume evolution was established by
comparing the images captured by MRI on day 1 and at day 7 with a 3
Tesla instrument (Discovery MR 750w; GE Healthcare Life Sciences,
Chalfont, UK).
Determination of NOx levels
NO production was indirectly quantified by
determining NOx, using an ozone chemiluminescence-based assay
adapted to plasma samples (24).
Briefly, plasma samples were deproteinized with 0.8 N NaOH and 16%
ZnSO4 solutions (1/0.5/0.5, v/v/v; Sigma-Aldrich,
Madrid, Spain). Following centrifugation at 10,000 × g for 5 min at
room temperature, the resulting supernatants were removed for
chemiluminescence analysis in a NO analyzer (NOA™ 280i; GE
Analytical Instruments, Boulder, CO). NOx concentration was
calculated by comparison with standard solutions of sodium nitrate.
Final NOx values were expressed in µM.
Determination of AM levels
The concentration of AM present in blood plasma was
determined using a commercially available radioimmunoassay (RIA)
kit (Phoenix Europe GmbH, Karlsruhe, Germany). Samples (1 ml) were
initially diluted in an equal volume of 0.1% alkali-treated casein
in phosphate-buffered saline at pH 7.4, and applied to pre-washed
reverse-phase Sep-Pak C-18 cartridges (Waters Corporation, Milford,
MA, USA) to remove the AM-binding protein, complement factor H. The
peptide fraction was eluted from the C-18 matrix with 3 ml 80%
isopropanol containing 0.125 N HCl and freeze-dried overnight, as
previously described (25). AM
levels contained in lyophilized extracts were then determined by
RIA following the manufacturer's protocols.
Statistical analysis
All statistical analyses were performed using the
SPSS version 21 software package (IBM SPSS, Armonk, NY, USA). A
descriptive analysis of all variables was performed and categorical
variables were expressed as absolute and relative frequencies.
Continuous variables were defined by their mean and standard
deviation (SD) when their distribution was normal (as tested by the
Shapiro-Wilk test) or as the median and interquartile range when
the distribution was not normal. Univariate analyses were performed
with Pearson's χ2 test, modified by Fisher's exact test,
or in the case of continuous variables with Student's t-test or
analysis of variance. When samples did not follow a normal
distribution, non-parametric tests, including Kruskal Wallis
followed by Mann-Whitney's U test were performed. Parameters that
were marked as significant in univariate analysis entered
multivariate binary logistic regression to investigate independent
effects. A receiving operating characteristic (ROC) analysis was
performed to assess the potential predictive value of binary
classifier systems. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical characteristics of patients
The clinical sample included 76 stroke patients, 33
women (43.4%) and 43 men (56.6%), with a mean age of 73.3±12.3
years (Table I). Age-matched
healthy controls were 5 women (35.7%) and 9 men (64.3%) with a mean
age of 73.4±12.2 years. Certain patients had been exposed to
relevant risk factors, including arterial hypertension,
dyslipidemia, diabetes, atrial fibrillation, ischemic cardiopathy,
or a previous stroke (Table I). A
marked proportion of these patients had been treated with
antiaggregants, anticoagulants, antihypertensives, and statins
(Table I). Following completion of
the etiological profile, ~1/3 of the patients were diagnosed with
atherothrombotic stroke, another third with cardioembolic stroke,
and 14.5% with lacunar stroke (Table
I). The median NIHSS taken at admission was 3, with a median
infarct volume of 3.8 cm3 (Table I). Intracranial or extracranial
occlusion of a large vessel was demonstrated in 17 patients
(22.3%).
 | Table IClinical characteristics of the 76
patients included in the present study. |
Table I
Clinical characteristics of the 76
patients included in the present study.
| Clinical
characteristic | Total |
|---|
| Age, mean ± SD | 73.31±12.33 |
| Gender (M) | 43 (56.6%) |
| Risk factors | |
| Arterial
hypertension | 53 (69.7%) |
| Diabetes
mellitus | 24 (31.6%) |
| Dyslipidemia | 38 (50%) |
| Ischemic
cardiopathy | 9 (11.8%) |
| Atrial
fibrillation | 16 (21,1%) |
| Previous
stroke | 14 (18.5%) |
| Previous
treatment | |
|
Antiagreggants | 25 (32.9%) |
|
Anticoagulants | 10 (13.2%) |
|
Antihypertensives | 54 (71.1%) |
| Statins | 30 (39.5%) |
| Previous
Rankin | |
| 0–1 | 67 (88.2 %) |
| 2 | 6 (7.9 %) |
| 3 | 3 (3.9 %) |
| Basal NIHSS, median
(Q1-Q3) | 3 (2–11) |
| Temperature at ER
(°C), mean ± SD | 36.1±0.4 |
| Glycemia at ER
(mg/dl), mean ± SD | 124.9±37.4 |
| SAP at ER (mm Hg),
mean ± SD | 160.1±30.0 |
| DAP at ER (mm Hg),
mean ± SD | 84.8±18.7 |
| TOAST | |
|
Atherothrombotic | 25 (32.9%) |
|
Cardioembolism | 27 (35.5%) |
| Lacunar | 11 (14.5%) |
| Other | 13 (17.1%) |
| Basal infarct
volume (cm3), median (Q1-Q3) | 3.8 (0.9–17.2) |
| Time from symptoms
to MRI (min), median (Q1-Q3) | 960 (540–1260) |
| Basal AM (pg/ml),
median (Q1-Q3) | 488.7
(411.5–631.6) |
| Basal NOx
(µM), median (Q1-Q3) | 9.26
(6.88–14.34) |
Blood pressure variability was associated
with neurological severity and infarct volume growth
At admission in the emergency room (ER), mean values
for SAP and DAP were 160.1±30.0 mmHg and 84.8±18.7 mmHg,
respectively (Table I).
Hypertension was observed at the admission of 59 (77.6%) patients.
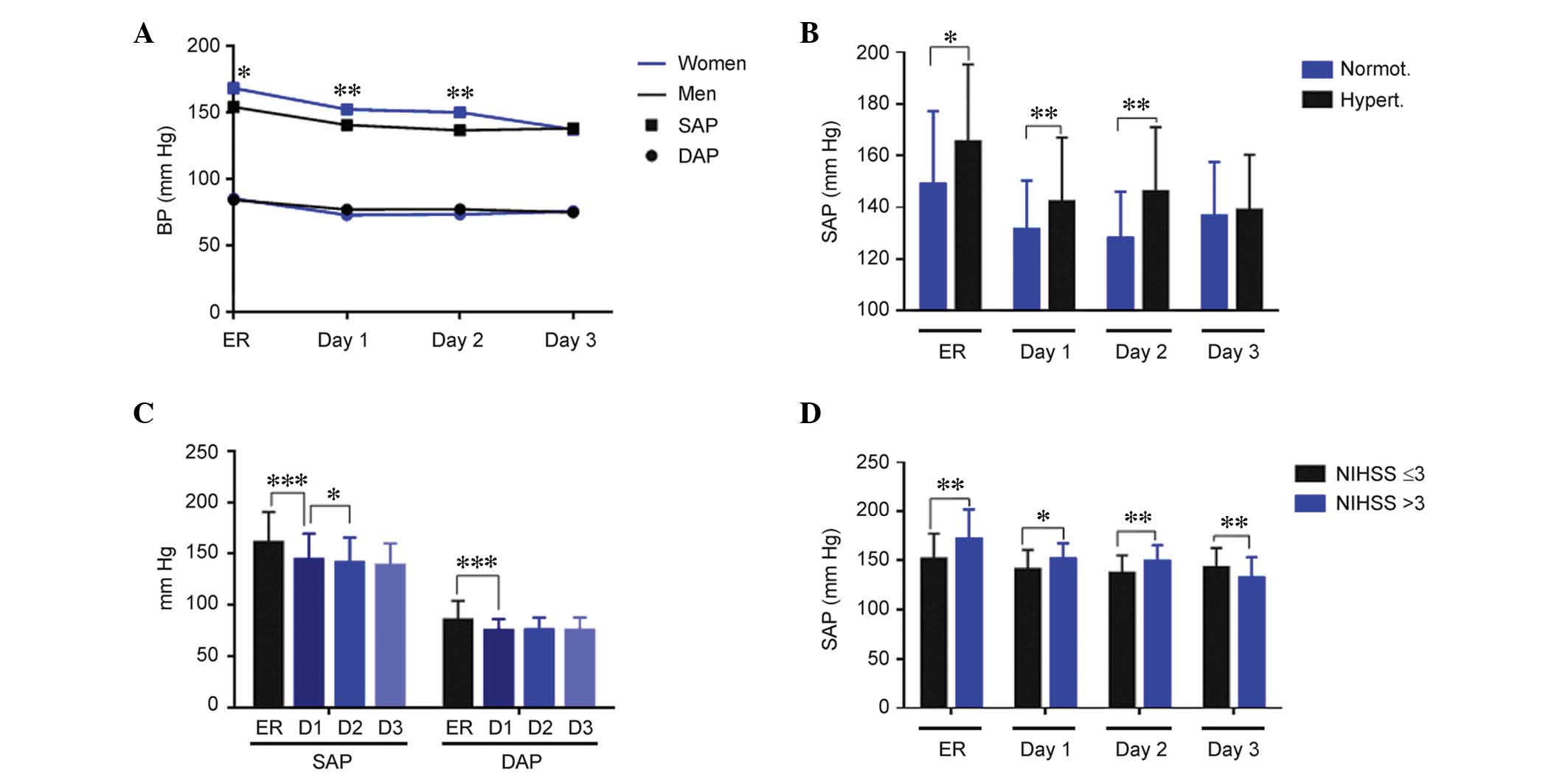
Women exhibited significantly higher SAP values than men at
admission (P=0.04), and on day 1 (P=0.008) and day 2 (P=0.003) of
treatment; however, this difference was corrected by day 3. No
significant differences by gender were observed for DAP (Fig. 1A). Patients with a history of
hypertension exhibited significantly higher SAP values, as compared
with normotensive patients at admission (P=0.03), and on days 1
(P=0.004) and 2 (P=0.002) of treatment (Fig. 1B). DAP values were similar
independently of hypertension history. There was a significant
reduction in SAP and DAP (P<0.001), during the first day of
treatment, and a further reduction in SAP (P<0.05) from day 1 to
day 2 (Fig. 1C). When comparing
blood pressure and neurological severity measured by the NIHSS at
24 h, patients with more severe symptoms (NIHSS >3) had
significantly higher SAP (Fig. 1D;
ER, P=0.002; day 1, P=0.014; and day 2, P=0.005) and DAP (data not
shown) until day 2, with this trend reversing at day 3 (Fig. 1D; P=0.009), as compared with
patients with less severe symptoms (NIHSS ≤3).
Variability in blood pressure (BP) values has been
proposed as a possible cause of brain deterioration in ischemic
stroke patients (26). This
variability is established by measuring the SD, the successive
variation, and the coefficient of variation of either the SAP or
the DAP. Notably, almost all variability parameters were
significantly higher in women when compared with men (Table II). This higher variability was
also observed in patients with increased neurological severity as
measured by the NIHSS at 24 h and at 7 days (Table II). In addition, patients whose
infarct volume grew during the first 7 days had significantly
higher BP variability than those whose infarct volume did not grow
(Table II). There was no
association between the variability in BP during the stay of the
patients in the stroke unit and their performance at 3 months,
measured by either the NIHSS or the Rankin scale (data not
shown).
 | Table IIVariability of BP compared with
gender, NIHSS at 24 h and 7 days, and with infarct volume
growth. |
Table II
Variability of BP compared with
gender, NIHSS at 24 h and 7 days, and with infarct volume
growth.
A, BP variability
vs. gender
|
|---|
| Variability | Men | Women | P |
|---|
| SAP-SD | 12.5
(10.1–14.56) | 14.6
(12.3–18.9) | 0.003 |
| SAP-SV | 13.1
(11.9–15.3) | 17.2 (12.9–20) | 0.020 |
| SAP-CV | 9.3 (7.4–11.1) | 9.8 (7.9–13.8) | 0.090 |
| DAP-SD | 8.4 (7.3–10) | 9.8 (8.1–12.3) | 0.030 |
| DAP-SV | 10.9 (8.5–13) | 12.5 (10–15.8) | 0.030 |
| DAP-CV | 9.2 (6.7–14.4) | 14.3
(10.7–17.8) | 0.003 |
B, BP variability
vs. NIHSS at 24 h
|
|---|
| Variability | NIHSS≤3 | NIHSS>3 | P |
|---|
| SAP-SD | 12.5
(10.4–15.4) | 14.4 (12–19.3) | 0.001 |
| SAP-SV | 13.3
(10.4–15.4) | 16.7 (13–20.9) | 0.001 |
| SAP-CV | 9.1 (7.2–11.2) | 9.9 (8–13.7) | 0.200 |
| DAP-SD | 8.3 (7.2–10.5) | 9.8 (8.4–12.7) | 0.014 |
| DAP-SV | 9.7 (8–12.4) | 12 (10.7–15.6) | 0.003 |
| DAP-CV | 11.1 (7–14.5) | 12.6
(8.1–16.2) | 0.210 |
C, BP variability
vs. NIHSS at 7 days
|
|---|
| Variability | NIHSS≤3 | NIHSS>3 | P |
|---|
| SAP-SD | 12.6 (9.9–16) | 13.7
(12.5–19.4) | 0.020 |
| SAP-SV | 13.1 (10.8–18) | 15.6 (13.3–22) | 0.012 |
| SAP-CV | 9.3 (7.2–11.8) | 9.9 (8.4–12) | 0.300 |
| DAP-SD | 8.4 (7.3–10.6) | 10.1
(8.3–11.9) | 0.017 |
| DAP-SV | 10.6 (8.2–12) | 12.5 (9.9–16) | 0.013 |
| DAP-CV | 11.7
(7.8–14.4) | 12.6
(8.1–16.2) | 0.360 |
D, BP variability
vs. infarct volume growth
|
|---|
| Variability | No growth | Growth | P |
|---|
| SAP-SD | 12.6
(10.4–15.8) | 14.2
(12.3–21.1) | 0.020 |
| SAP-SV | 13.8
(12.3–16.8) | 16.3
(12.3–22.9) | 0.100 |
| SAP-CV | 8.9 (7.3–10.7) | 10.7 (9–14) | 0.020 |
| DAP-SD | 8.3 (7.5–9.9) | 11 (8.5–12.6) | 0.006 |
| DAP-SV | 10.6 (8.5–12) | 13.3
(10.4–16.3) | 0.004 |
| DAP-CV | 11.2
(6.9–14.3) | 14.2 (9–17.6) | 0.050 |
NOx levels were significantly lower in
stroke patients compared with healthy controls at day 1 and 2
In healthy control subjects, NOx levels were 15.3
µM (12.1–17.9). These levels were significantly higher in
women than in men (P=0.02; data not shown). In stroke patients, NOx
levels measured on day 1 and day 2 of their hospital stay were
significantly lower (P=0.008) than those obtained in healthy
subjects. At day 7, NOx levels were significantly higher than at
day 1 (P<0.001) and day 2 (P<0.01), but indistinguishable
from healthy controls (Fig. 2A).
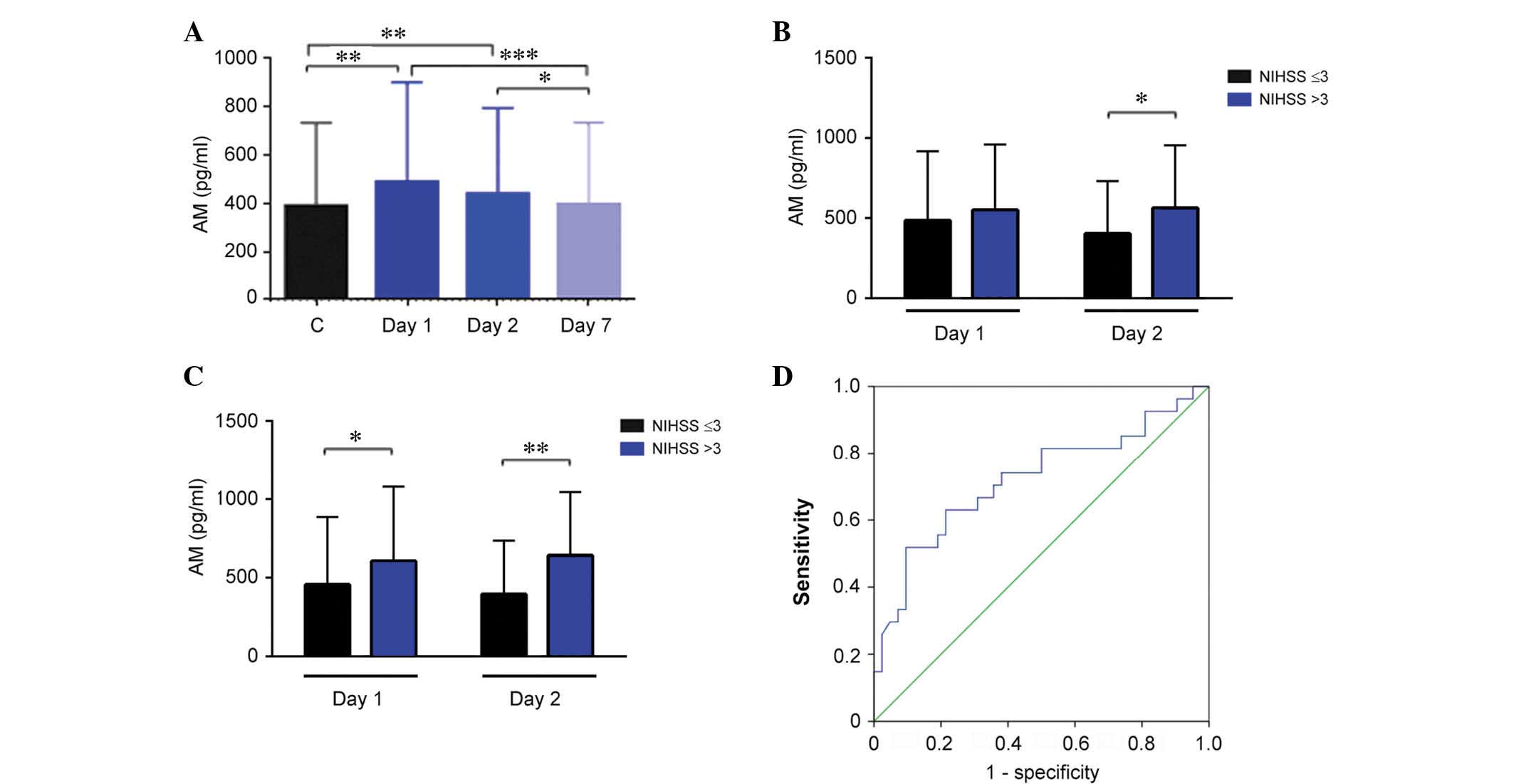
In stroke patients, no differences were observed with gender.
 | Figure 2Evolution of NOx levels and their
association with NIHSS and blood pressure. (A) The amount of
circulating NOx was measured in healthy controls and stroke
patients at days 1, 2, and 7. (B) Patients with higher NOx levels
measured at day 2 had a lower NIHSS score at day 1. (C) When NOx
data were divided in tertiles, patients in the third (highest)
tertile had lower DAP at day 2 and 3. (D) Also, patients with
higher SAP (third tertile) at admission had the lowest levels of
circulating NOx. Bars represent the mean ± standard deviation for
DAP and the median ± interquartile range for NO.
*P<0.05; **P<0.01;
***P<0.001. NIHSS, National Institutes of Health
Stroke Scale; NO, nitric oxide; NOx, nitrate/nitrite and S-nitroso
compounds; DAP, diastolic arterial pressure; SAP, systolic arterial
pressure; C, control; T1, tertile 1; T2, tertile 2; T3, tertile
3. |
When NOx levels were compared with neurological
severity, it was observed that patients with an NIHSS ≤3 at day 1
had significantly higher values of NOx on day 2 (Fig. 2B; P=0.02). There was also an
association with BP parameters. Patients in the third tertile of
NOx levels at day 1 had a significantly lower DAP on days 2
(P=0.009) and 3 (P=0.02; Fig. 2C).
Patients in the third tertile of SAP at admission had significantly
lower (P=0.01) NOx levels than other patients in the second and
first tertile (Fig. 2D).
AM levels were significantly higher than
in healthy controls at day 1 and 2
Healthy control volunteers had a median AM value of
389.7 pg/ml (343.9–475.9). No differences were observed for either
gender or age groups. In stroke patients, AM levels were
significantly higher than in healthy controls at day 1 (P=0.003)
and 2 (P=0.005), however, these values were indistinguishable from
controls at day 7 (Fig. 3A). Among
patients, women had significantly higher AM levels than men
(P=0.02; data not shown). Notably, patients that had undergone
treatment with antiaggregants or statins previous to the stroke had
significantly lower levels of AM as measured at day 2 (P=0.04 and
P=0.001, respectively; data not shown). Patients with a history of
hypertension had similar levels of AM to the other stroke patients,
however, their AM concentration decline from day 1 to 7 was
significantly steeper than in other patients (P=0.005; data not
shown).
There was no association between AM levels and
neurological severity as measured using the NIHSS at admission.
However, patients with a high NIHSS score (>3) at day 1 had
significantly higher AM levels at day 2 (Fig. 3B; P=0.014). A similar association
was observed with NIHSS values measured at day 7 and AM values
obtained on day 1 and day 2 of treatment (Fig. 3C; P=0.012 and P=0.002,
respectively). These data suggested that AM levels measured on day
2 may predict neurological severity at day 7. To investigate this
hypothesis, a ROC curve analysis was performed and it was
demonstrated that the optimal threshold for AM levels was 522.13
pg/ml. This value renders an area under the curve of 0.721 (95%
confidence interval, 0.590–0.852), a sensitivity of 70%, and a
specificity of 60% (Fig. 3D).
Previous treatment with antihypertensives
and antiaggregants was protective against infarct volume
growth
Infarct volume growth was defined as the difference
between the infarct volume measured by MRI at day 7 and the initial
volume measured on day 1 of treatment. Patients were classified as
having infarct volume growth when they exhibited a ≥20% increase,
26 patients (37.1%) experienced such growth while 44 (62.9%) did
not. Demographic, clinical, and analytical characteristics of the
patients were examined to investigate whether any had predictive
value over the potential infarct volume growth as measured at day 7
(Table III). Notably, among the
reported risk factors, only a previous treatment with
antihypertensives or antiaggregants resulted in a clear protection
against infarct volume growth (P=0.04 and P=0.01, respectively).
There was a linear association between NIHSS scores (>3), either
at admission or at 24 h, and volume growth. There was also an
association with elevated diastolic blood pressure but not with the
systolic component. In addition, patients with occlusion of a large
vessel were more likely to experience infarct growth (P=0.02). The
association with NOx was notable as there was no association with
specific NOx levels but with the increment in NOx concentration
from day 1 to 7 (P=0.03). No association was observed with AM
levels.
 | Table IIIUnivariate analysis of potential
predictors of infarct volume growth. |
Table III
Univariate analysis of potential
predictors of infarct volume growth.
| Paramete | Growth
| P-value |
|---|
| No | Yes |
|---|
| All patients
(n=70) | 44 (62.9%) | 26 (37.1%) | |
| Gender (men) | 28 (71.8%) | 11 (28.2%) | 0.080 |
| Age | 74.79±10,13 | 70.92±14.71 | 0.170 |
| Hypertension | 29 (61.7%) | 18 (38.3%) | 0.700 |
| Diabetes | 16 (76.2%) | 5 (23.8%) | 0.100 |
| Dyslipidemia | 23 (67.6%) | 11 (32.4%) | 0.410 |
| Ischemic
cardiopathy | 6 (75.0%) | 2 (25.0%) | 0.430 |
| Atrial
fibrillation | 8 (47.1%) | 9 (52.9%) | 0.410 |
| Previous
stroke | 10 (76.9%) | 3 (23.1%) | 0.220 |
| Previous
treatments | | | |
|
Antihypertensives | 34 (70.8%) | 14 (29.2%) | 0.040 |
| Statins | 20 (71.4%) | 8 (28.6%) | 0.200 |
|
Antiaggregants | 19 (82.6%) | 5 (14.4%) | 0.010 |
|
Anticoagulants | 5 (55.6%) | 4 (44.4%) | 0.600 |
| Basal NIHSS | 2 (1–5) | 6 (2–11) | 0.007 |
| 24 h NIHSS | 1 (0–3) | 4 (1–5) | 0.003 |
| Basal glycemia
(mg/dl) | 105.18±38.15 | 110.42±29.34 | 0.050 |
| 72 h glycemia | 116.05±34.12 | 113.06±26.33 | 0.620 |
| Hyperthermia | 7 (43.8%) | 9 (56.3%) | 0.070 |
| SAP at ER | 157.2±25.5 | 167±36.0 | 0.200 |
| DAP at ER | 81.3±15.2 | 91.5±22.3 | 0.046 |
| TOAST | | | 0.710 |
|
Atherothrombotic | 17 (70.8%) | 7 (29.2%) | |
| Cardioembolic | 15 (62.5%) | 9 (34.5%) | |
| Lacunar | 5 (50.0%) | 5 (50.0%) | |
| Other strokes | 7 (58.3%) | 5 (41.7%) | |
| Large vessel
occlusion | 5 (35.7%) | 9 (64.3%) | 0.020 |
| Basal infarct vol.
(cm3) | 2.8 (0.6–0.5) | 3.9 (1.1–28.2) | 0.050 |
| Day 1 NOx
(µM) | 9.3 (8–14.6) | 9.1 (5.9–14.4) | 0.300 |
| Day 2 NOx | 10.6
(7.6–13.6) | 9.9 (6.9–14) | 0.300 |
| NOx increase (day
7-day 1) | 5 (1.9–12.2) | 1.2 (0–7.6) | 0.030 |
| Day 1 AM
(pg/ml) | 497.8
(442–647.4) | 565.9
(403.5–637.6) | 0.610 |
| Day 2 AM | 428.5
(355.6–571.4) | 440.8
(352.6–708.3) | 0.520 |
| AM increase (day
7-day 1) | −125.6
(−216.8–(−)34.9) | −151.9
(−228.6–(−)30) | 0.700 |
Multiparametric analysis predicted male
gender and increase in NOx levels from day 1 to day 2 improved
prognosis
Subsequent to conducting univariate analysis,
multivariate logistic regressions were performed to identify
independent predictors of clinical outcomes. These outcomes were
the growth of the infarct volume (day 7 - day 1), the NIHSS scores
measured at day 7 and at 3 months after the onset of the condition,
and the Rankin scale, also measured at 3 months (Table IV). As predictors of infarct
volume growth it was demonstrated that pretreatment with
antiaggregant therapeutic agents protects patients, whereas a high
NIHSS score taken at 24 h, the confirmation of the occlusion of a
large vessel, and the steep increase on NOx levels provide a poorer
prognosis. For neurological severity measured at day 7, it was
observed that male gender and the increase in NOx levels from day 1
to day 2 are protective, whereas a high NIHSS score taken at 24 h,
confirmation of the occlusion of a large vessel, and the increase
of AM levels from day 1 to day 2 are markers of a poorer prognosis.
For the neurological severity measured at 3 months the increase in
NOx levels from day 1 to day 2 is a positive marker for improved
recovery, whereas a high NIHSS score at 24 h and increased AM
levels are negative predictors. As measured by functional prognosis
at 3 months, male gender and a high increase in NOx levels from day
1 to day 2 are reliable predictors for a good prognosis (Table IV).
 | Table IVMultiparametric analysis
(multivariate logistic regression) identifying independent
predictors for each of the clinically meaningful parameters. |
Table IV
Multiparametric analysis
(multivariate logistic regression) identifying independent
predictors for each of the clinically meaningful parameters.
| Parameter | OR | 95% CI | P-value |
|---|
| Infarct volume
growth | | | |
|
Antiaggregants | 0.12 | 0.019–0.799 | 0.030 |
| 24 h NIHSS | 7.50 | 1.01–56.07 | 0.040 |
| Large vessel
occlusion | 9.36 | 1.23–71.11 | 0.030 |
| NOx increase (day
7-day 2) | 35.3 | 2.8–439.6 | 0.006 |
| Neurological
severity (NIHSS) at 7 days | | | |
| Gender (male) | 0.02 | 0.003–0.25 | 0.002 |
| NOx increase (day
2-day 1) | 0.92 | 0.85–0.99 | 0.040 |
| AM (day 2) | 8.07 | 1.45–44.7 | 0.020 |
| 24 h NIHSS | 8.37 | 1.62–43.1 | 0.010 |
| Large vessel
occlusion | 80.43 | 5.38–120.38 | 0.001 |
| Neurological
severity (NIHSS) at 3 months | | | |
| NOx increase (day
2-day 1) | 0.90 | 0.82–0.99 | 0.030 |
| 24 h NIHSS | 4.90 | 1.14–20.83 | 0.030 |
| AM increase (day
2-day 1) | 5.46 | 1.25–23.94 | 0.020 |
| Functional
prognosis (Rankin) at 3 months | | | |
| Gender (male) | 0.10 | 0.017–0.59 | 0.030 |
| NOx increase (day
2-day 1) | 0.91 | 0.84–0.99 | 0.040 |
Discussion
The present study has identified that different
parameters associated with blood pressure and circulating levels of
NOx and AM may act as predictors of clinical outcome in acute
ischemic stroke patients.
Previous treatments and infarct volume
growth
It was observed in the present study that patients
that had been treated with either antihypertensive or platelet
antiaggregant therapeutic agents prior to stroke onset were
protected against the growth of the infarct volume. It has been
demonstrated that in-hospital treatment with these therapeutic
agents may explain the decrease in mortality from ischemic stroke
(27), and that taking these
therapeutic agents prior to stroke onset may have a marked positive
prognosis (28,29). Data from the present study confirms
these previous observations.
BP
In the patients investigated in the present study,
women had a higher SAP than men at admission. This gender bias has
been widely reported, despite men and women having similar risk
factors (30). This difference
between genders and the commonly high values in BP were
progressively controlled during day 1 and 2 of stay in the stroke
unit. The success of stroke units is a consequence of the control
of all physiological factors that may influence the evolution of
the ischemic lesion, referred to as non-pharmacological
neuroprotection (31), which
includes monitoring BP. Patients with higher BP presented higher
values of neurological severity measured at 24 h, suggesting that
lowering BP may be a good strategy to reduce brain injury. However,
the scientific literature is divided on this issue. The majority of
observational studies associate high BP with poor clinical outcomes
(32,33). By contrast, other reports show
improved clinical evolution with higher BP levels (34), and low values of BP have been also
associated with a poor prognosis and higher mortality (35). A previous study has suggested that
a U-shaped association may be present, with high BP values inducing
brain edema and hemorrhagic transformation and low BP values
contributing to the transformation of the penumbra into infarcted
area (36). Previous
interventional studies using antihypertensive therapeutic agents
suggest that reducing BP during the acute phase is safe and may
reduce mortality at 3 months (37,38),
whereas other previous studies have demonstrated either no benefit
(39) or a small increase in early
adverse events (40). A recent
review, which combines data from 26 articles and 17,011 patients,
concluded that there is no evidence that reducing BP during the
acute phase of the stroke may save lives or reduce disability
(41).
However, the variability of DAP and SAP values
during the acute phase may provide more information. In the present
study, an increased BP variability was observed in women, in
patients with higher NIHSS scores, and in patients where infarct
volume growth was reported. This is in agreement with previous
reports where BP variability has been associated with poor clinical
outcome (26,42,43).
NOx
The present study has demonstrated that peripheral
concentrations of NOx in stroke patients are lower than in the
healthy control population. Previous studies disagree on whether
peripheral NOx increases or decreases following stroke. Certain
previous studies agree with the results from the present study
indicating lower NOx levels in patients (44,45),
whereas other studies have described elevated levels compared with
healthy controls (46,47). This discrepancy may be due to
different methods of measuring NOx or to variations in the
L-arginine pathway, as a result of endothelial dysfunction, leading
to elevated levels of symmetric and asymmetric dimethylarginine.
These metabolites are elevated in stroke patients and result in
reduced production of NO (48).
Depending on the quantity of these metabolites, the final levels of
NOx in the blood may vary. In addition, the potential contributions
of NOS-independent sources of NO must be considered (49). In the patients investigated in the
current study, during the first week of the follow-up period, NOx
levels returned to normal, suggesting a progressive recovery of
homeostasis. This elevation of NOx levels post-stroke is in
agreement with previous studies (14).
The present study demonstrated that elevations of
NOx levels may be beneficial or detrimental for the patient,
depending on when they occur. Elevation of NOx from day 1 to day 2
was demonstrated to be protective and it predicted a positive
outcome at 7 days and 3 months, for neurological recovery and
functional dexterity. However, an elevation of NOx from day 2 to
day 7 predicted growth of the infarct volume. This dual behavior
may be associated with the type of NOS isoform that is activated.
The initial elevation of NOx production may be associated with the
endothelial NOS, an isoform that has been demonstrated to be
neuroprotective via exerting an effect on vasodilatation,
inhibition of platelet aggregation, and induction of angiogenesis
(50). The elevation occurring
from day 2 to 7 may be more associated with the activation of the
inducible NOS isoform, the activity of which is initiated at ~12 h
after ischemic onset and continues for up to 8 days later (51). This isoform is considered damaging
to the surrounding tissue due to the unregulated large quantities
of NO produced (7).
As expected, an inverse association between NOx
levels and BP was observed, confirming the vasodilatatory effect of
NO in a clinical setting, as has been thoroughly reported in
experimental models (7,9).
AM
The present study observed that stroke patients had
significantly higher values of plasma AM than healthy individuals,
this is in agreement with previous literature (20,52).
By day 7, AM levels returned to normal, suggesting a progressive
resolution of the pathology. A high AM level at day 2 or an
increase in AM levels from day 1 to day 2 was associated with
increased neurological severity at day 7 and at 3 months after the
stroke. This was confirmed with a ROC curve analysis, where high AM
values predicted a poorer outcome. A previous study has
demonstrated that the AM level in the blood of ischemic stroke
patients may be also predictive of 3-month mortality and
unfavorable outcomes (52),
suggesting AM is a negative predictor of clinical performance.
In experimental animals, it has been identified that
eliminating AM from the central nervous system results in larger
infarcts following acute ischemic stroke (21) and that injecting AM reduces infarct
volume (53). These studies
indicated the neuroprotective effects of AM are due to its
vasodilatatory, pro-angiogenic, and anti-apoptotic effects.
However, another previous study indicated intracerebroventricular
injection of AM resulted in an increase of ischemic injury
(54). This detrimental effect of
AM may be mediated by its modulatory action on the immune system
(55), which is important for
stroke resolution (56).
Overexpression of AM has been detected in leukocytes of stroke
patients and this higher expression is associated with stroke
severity (57). The clinical
observations suggest that, during human stroke, AM levels may be a
consequence of stroke severity, despite the neuroprotective effects
of AM on the infarcted tissue.
The current study observed that patients taking
antiaggregants or statins prior to stroke onset had lower levels of
AM measured at day 2. Statins are well known for reducing
cholesterol levels, however, they also exert pleiotropic actions,
including antioxidative and cellular protective effects. These
characteristics suggest that statins may provide novel therapeutic
approaches for various neurological disorders, including stroke
(58). There is little information
regarding the association between AM and statins. Using AM
heterozygous knockout mice, Yamamoto et al (59) demonstrated that statins
significantly inhibited fibrosis and apoptosis while inducing
angiogenesis in a model of heart fibrosis. The underlying mechanism
for the protective action of statins in stroke may include a
reduction of AM levels.
In conclusion, BP variability and temporal profiles
of NOx and AM levels have been demonstrated as predictors of
clinical outcomes in stroke patients, as measured by infarct volume
growth, neurological NIHSS scales at 7 days and 3 months, and
functional prognosis at 3 months. Development of rapid tests for
evaluating NO and AM levels may be useful for predicting patient
outcome, for developing personalized therapeutic strategies, and
for stratifying stroke patients in clinical trials.
Acknowledgments
The authors would like to thank Dr. Enrique
Ramalle-Gómara (Epidemiology Health Prevention Service, Logroño,
Spain) for his help with the statistical analysis, Dr. Gemma
Quincoces (Nuclear Medicine, University of Navarra, Pamplona,
Spain) for her help with the γ-counter, and Ms. Judit Narro (Center
for Biomedical Research of La Rioja, Logroño, Spain) for her
excellent technical assistance. The present study was financed by
Fundación Rioja Salud.
Abbreviations:
|
AM
|
adrenomedullin
|
|
BP
|
blood pressure
|
|
CV
|
coefficient of variation
|
|
DAP
|
diastolic blood pressure
|
|
MRI
|
magnetic resonance imaging
|
|
NO
|
nitric oxide
|
|
NOS
|
NO synthase
|
|
NOx
|
nitrate/nitrite and S-nitroso
compounds
|
|
ROC
|
receiving operating characteristic
|
|
SAP
|
systolic arterial pressure
|
|
SD
|
standard deviation
|
|
SV
|
successive variation
|
References
|
1
|
Albers GW, Caplan LR, Easton JD, Fayad PB,
Mohr JP, Saver JL and Sherman DG; TIA Working Group: Transient
ischemic attack-proposal for a new definition. N Engl J Med.
347:1713–1716. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Albers GW: Acute cerebrovascular syndrome:
Time for new terminology for acute brain ischemia. Nat Clin Pract
Cardiovasc Med. 3:5212006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mendis S, Davis S and Norrving B:
Organizational update: The world health organization global status
report on noncommunicable diseases 2014; one more landmark step in
the combat against stroke and vascular disease. Stroke.
46:e121–e122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sarikaya H, Ferro J and Arnold M: Stroke
prevention-medical and lifestyle measures. Eur Neurol. 73:150–157.
2015. View Article : Google Scholar
|
|
5
|
Jickling GC and Sharp FR: Biomarker panels
in ischemic stroke. Stroke. 46:915–920. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Z, Lin Y, Liu Y, Chen Y, Wang B, Li
C, Yan S, Wang Y and Zhao W: Serum uric acid levels and outcomes
after acute ischemic stroke. Mol Neurobiol. 2015.Epub ahead of
print.
|
|
7
|
Rodrigo J, Fernández AP, Serrano J,
Peinado MA and Martínez A: The role of free radicals in cerebral
hypoxia and ischemia. Free Radic Biol Med. 39:26–50. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Terpolilli NA, Moskowitz MA and Plesnila
N: Nitric oxide: Considerations for the treatment of ischemic
stroke. J Cereb Blood Flow Metab. 32:1332–1346. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garry PS, Ezra M, Rowland MJ, Westbrook J
and Pattinson KT: The role of the nitric oxide pathway in brain
injury and its treatment-from bench to bedside. Exp Neurol.
263:235–243. 2015. View Article : Google Scholar
|
|
10
|
Ho JJ, Man HS and Marsden PA: Nitric oxide
signaling in hypoxia. J Mol Med (Berl). 90:217–231. 2012.
View Article : Google Scholar
|
|
11
|
Ito Y, Ohkubo T, Asano Y, Hattori K,
Shimazu T, Yamazato M, Nagoya H, Kato Y and Araki N: Nitric oxide
production during cerebral ischemia and reperfusion in eNOS- and
nNOS-knockout mice. Curr Neurovasc Res. 7:23–31. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martínez-Murillo R, Fernández AP, Serrano
J, Rodrigo J, Salas E, Mourelle M and Martínez A: The nitric oxide
donor LA 419 decreases brain damage in a focal ischemia model.
Neurosci Lett. 415:149–153. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Willmot M, Gray L, Gibson C, Murphy S and
Bath PM: A systematic review of nitric oxide donors and L-arginine
in experimental stroke; effects on infarct size and cerebral blood
flow. Nitric Oxide. 12:141–149. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paspalj D, Nikic P, Savic M, Djuric D,
Simanic I, Zivkovic V, Jeremic N, Srejovic I and Jakovljevic V:
Redox status in acute ischemic stroke: Correlation with clinical
outcome. Mol Cell Biochem. 406:75–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Serrano J, Uttenthal LO, Martínez A,
Fernández AP, Martínez de Velasco J, Alonso D, Bentura ML,
Santacana M, Gallardo JR, Martínez-Murillo R, et al: Distribution
of adrenomedullin-like immunoreactivity in the rat central nervous
system by light and electron microscopy. Brain Res. 853:245–268.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
López J and Martínez A: Cell and molecular
biology of the multifunctional peptide, adrenomedullin. Int Rev
Cytol. 221:1–92. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Garayoa M, Martínez A, Lee S, Pío R, An
WG, Neckers L, Trepel J, Montuenga LM, Ryan H, Johnson R, et al:
Hypoxia-inducible factor-1 (HIF-1) up-regulates adrenomedullin
expression in human tumor cell lines during oxygen deprivation: A
possible promotion mechanism of carcinogenesis. Mol Endocrinol.
14:848–862. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Serrano J, Alonso D, Encinas JM, Lopez JC,
Fernandez AP, Castro-Blanco S, Fernández-Vizarra P, Richart A,
Bentura ML, Santacana M, et al: Adrenomedullin expression is
up-regulated by ischemia-reperfusion in the cerebral cortex of the
adult rat. Neuroscience. 109:717–731. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Serrano J, Fernández AP, Sánchez J,
Rodrigo J and Martínez A: Adrenomedullin expression is up-regulated
by acute hypobaric hypoxia in the cerebral cortex of the adult rat.
Brain Pathol. 18:434–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Somay G, Halac GU, Uslu E and Aydin S:
Plasma adrenomedullin in acute ischemic stroke. Neurosciences
(Riyadh). 12:351–353. 2007.
|
|
21
|
Hurtado O, Serrano J, Sobrado M, Fernández
AP, Lizasoain I, Martínez-Murillo R, Moro MA and Martínez A: Lack
of adrenomedullin, but not complement factor H, results in larger
infarct size and more extensive brain damage in a focal ischemia
model. Neuroscience. 171:885–892. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Duangjit S, Muangpaisan W,
Chotinaiwattarakul W and Dharmasaroja P: Functional recovery at 3
months in stroke patients not receiving thrombolytic therapy: The
comparison between patients arriving earlier and later than 4.5 h.
J Stroke Cerebrovasc Dis. 23:91–98. 2014. View Article : Google Scholar
|
|
23
|
Berzina G, Sveen U, Paanalahti M and
Sunnerhagen KS: Analysing the modified rankin scale using concepts
of the international classification of functioning, disability and
health. Eur J Phys Rehabil Med. 2015.Epub ahead of print.
PubMed/NCBI
|
|
24
|
Blanco JR, Jarrin I, Martinez A, Siles E,
Larrayoz IM, Cañuelo A, Gutierrez F, Gonzalez-Garcia J, Vidal F and
Moreno S: CoRIS-Biobanco: Shorter telomere length predicts poorer
immunological recovery in virologically suppressed HIV-1-infected
patients treated with combined antiretroviral therapy. J Acquir
Immune Defic Syndr. 68:21–29. 2015. View Article : Google Scholar
|
|
25
|
Martínez A, Elsasser TH, Bhathena SJ, Pío
R, Buchanan TA, Macri CJ and Cuttitta F: Is adrenomedullin a causal
agent in some cases of type 2 diabetes? Peptides. 20:1471–1478.
1999. View Article : Google Scholar
|
|
26
|
Geeganage C, Tracy M, England T, Sare G,
Moulin T, Woimant F, Christensen H, De Deyn PP, Leys D, O'Neill D,
et al: Relationship between baseline blood pressure parameters
(including mean pressure, pulse pressure and variability), and
early outcome after stroke: Data from the tinzaparin in acute
ischaemic stroke trial (TAIST). Stroke. 42:491–493. 2011.
View Article : Google Scholar
|
|
27
|
Minnerup J, Wersching H, Unrath M and
Berger K: Explaining the decrease of in-hospital mortality from
ischemic stroke. PLoS One. 10:e01314732015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pikija S, Trkulja V, Malojcic B,
Mutzenbach JS and Sellner J: A high burden of ischemic stroke in
regions of Eastern/Central Europe is largely due to modifiable risk
factors. Curr Neurovasc Res. 12:341–352. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morales Vidal SG and Ruland S: Platelet
antiaggregants in stroke prevention. Neurol Clin. 31:633–657. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hiramoto JS, Katz R, Weisman S and Conte
M: Gender-specific risk factors for peripheral artery disease in a
voluntary screening population. J Am Heart Assoc. 3:e0006512014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stroke Unit Trialists' Collaboration:
Organised inpatient (stroke unit) care for stroke. Cochrane
Database Syst Rev. 9:CD0001972013.PubMed/NCBI
|
|
32
|
Ishitsuka K, Kamouchi M, Hata J, Fukuda K,
Matsuo R, Kuroda J, Ago T, Kuwashiro T, Sugimori H, Nakane H, et
al: High blood pressure after acute ischemic stroke is associated
with poor clinical outcomes: Fukuoka stroke registry. Hypertension.
63:54–60. 2014. View Article : Google Scholar
|
|
33
|
Tziomalos K, Giampatzis V, Bouziana SD,
Spanou M, Papadopoulou M, Kostaki S, Dourliou V, Papagianni M,
Savopoulos C and Hatzitolios AI: Elevated diastolic but not
systolic blood pressure increases mortality risk in hypertensive
but not normotensive patients with acute ischemic stroke. Am J
Hypertens. 28:765–771. 2015. View Article : Google Scholar
|
|
34
|
Yong M, Diener HC, Kaste M and Mau J:
Characteristics of blood pressure profiles as predictors of
long-term outcome after acute ischemic stroke. Stroke.
36:2619–2625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wohlfahrt P, Krajcoviechova A, Jozifova M,
Mayer O, Vanek J, Filipovsky J and Cifkova R: Low blood pressure
during the acute period of ischemic stroke is associated with
decreased survival. J Hypertens. 33:339–345. 2015. View Article : Google Scholar
|
|
36
|
Vemmos KN, Tsivgoulis G, Spengos K,
Zakopoulos N, Synetos A, Manios E, Konstantopoulou P and Mavrikakis
M: U-shaped relationship between mortality and admission blood
pressure in patients with acute stroke. J Intern Med. 255:257–265.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bath PM, Martin RH, Palesch Y, Cotton D,
Yusuf S, Sacco R, Diener HC, Toni D, Estol C and Roberts R; PRoFESS
Study Group: Effect of telmisartan on functional outcome,
recurrence and blood pressure in patients with acute mild ischemic
stroke: A PRoFESS subgroup analysis. Stroke. 40:3541–3546. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Potter J, Mistri A, Brodie F, Chernova J,
Wilson E, Jagger C, James M, Ford G and Robinson T: Controlling
hypertension and hypotension immediately post stroke (CHHIPS)-a
randomised controlled trial. Health Technol Assess. 13:iiiix–xi.
2009. View Article : Google Scholar
|
|
39
|
Sandset EC, Bath PM, Boysen G, Jatuzis D,
Kõrv J, Lüders S, Murray GD, Richter PS, Roine RO, Terént A, et al:
The angiotensin-receptor blocker candesartan for treatment of acute
stroke (SCAST): A randomised, placebo-controlled, double-blind
trial. Lancet. 377:741–750. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sandset EC, Murray GD, Bath PM, Kjeldsen
SE and Berge E; Scandinavian Candesartan Acute Stroke Trial (SCAST)
Study Group: Relation between change in blood pressure in acute
stroke and risk of early adverse events and poor outcome. Stroke.
43:2108–2114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bath PM and Krishnan K: Interventions for
deliberately altering blood pressure in acute stroke. Cochrane
Database Syst Rev. 10:CD0000392014.PubMed/NCBI
|
|
42
|
Stead LG, Gilmore RM, Vedula KC, Weaver
AL, Decker WW and Brown RD Jr: Impact of acute blood pressure
variability on ischemic stroke outcome. Neurology. 66:1878–1881.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fukuda K, Kai H, Kamouchi M, Hata J, Ago
T, Nakane H, Imaizumi T and Kitazono T; FSR Investigators; steering
committee of the Fukuoka Stroke Registry included: Day-by-day blood
pressure variability and functional outcome after acute ischemic
stroke: Fukuoka stroke registry. Stroke. 46:1832–1839. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rashid PA, Whitehurst A, Lawson N and Bath
PM: Plasma nitric oxide (nitrate/nitrite) levels in acute stroke
and their relationship with severity and outcome. J Stroke
Cerebrovasc Dis. 12:82–87. 2003. View Article : Google Scholar
|
|
45
|
Abdullah A, Ssefer V, Ertugrul U, Osman E,
Esref A, Ugur CM, Adalet A, Yavuz Y, Faysal E and Nebahat T:
Evaluation of serum oxidant/antioxidant balance in patients with
acute stroke. J Pak Med Assoc. 63:590–593. 2013.PubMed/NCBI
|
|
46
|
Cure MC, Tufekci A, Cure E, Kirbas S,
Ogullar S, Kirbas A, Unal H, Yuce S and Cakmak S: Low-density
lipoprotein subfraction, carotid artery intima-media thickness,
nitric oxide and tumor necrosis factor alpha are associated with
newly diagnosed ischemic stroke. Ann Indian Acad Neurol.
16:498–503. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gonullu H, Aslan M, Karadas S, Kati C,
Duran L, Milanlioglu A, Aydin MN and Demir H: Serum prolidase
enzyme activity and oxidative stress levels in patients with acute
hemorrhagic stroke. Scand J Clin Lab Invest. 74:199–205. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Molnar T, Pusch G, Papp V, Feher G,
Szapary L, Biri B, Nagy L, Keki S and Illes Z: The L-arginine
pathway in acute ischemic stroke and severe carotid stenosis:
Temporal profiles and association with biomarkers and outcome. J
Stroke Cerebrovasc Dis. 23:2206–2214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Salom MG, Arregui B, Carbonell LF, Ruiz F,
González-Mora JL and Fenoy FJ: Renal ischemia induces an increase
in nitric oxide levels from tissue stores. Am J Physiol Regul
Integr Comp Physiol. 289:R1459–R1466. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cui X, Chopp M, Zacharek A, Zhang C,
Roberts C and Chen J: Role of endothelial nitric oxide synthetase
in arteriogenesis after stroke in mice. Neuroscience. 159:744–750.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Khan M, Sekhon B, Giri S, Jatana M, Gilg
AG, Ayasolla K, Elango C, Singh AK and Singh I:
S-Nitrosoglutathione reduces inflammation and protects brain
against focal cerebral ischemia in a rat model of experimental
stroke. J Cereb Blood Flow Metab. 25:177–192. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang H, Tang B, Yin CG, Chen Y, Meng QL,
Jiang L, Wang WP and Niu GZ: Plasma adrenomedullin levels are
associated with long-term outcomes of acute ischemic stroke.
Peptides. 52:44–48. 2014. View Article : Google Scholar
|
|
53
|
Xia CF, Yin H, Borlongan CV, Chao J and
Chao L: Postischemic infusion of adrenomedullin protects against
ischemic stroke by inhibiting apoptosis and promoting angiogenesis.
Exp Neurol. 197:521–530. 2006. View Article : Google Scholar
|
|
54
|
Wang X, Yue TL, Barone FC, White RF, Clark
RK, Willette RN, Sulpizio AC, Aiyar NV, Ruffolo RR Jr and
Feuerstein GZ: Discovery of adrenomedullin in rat ischemic cortex
and evidence for its role in exacerbating focal brain ischemic
damage. Proc Natl Acad Sci USA. 92:11480–11484. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pedreño M, Morell M, Robledo G,
Souza-Moreira L, Forte-Lago I, Caro M, O'Valle F, Ganea D and
Gonzalez-Rey E: Adrenomedullin protects from experimental
autoimmune encephalomyelitis at multiple levels. Brain Behav Immun.
37:152–163. 2014. View Article : Google Scholar :
|
|
56
|
Ma S, Zhao H, Ji X and Luo Y: Peripheral
to central: Organ interactions in stroke pathophysiology. Exp
Neurol. 272:41–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu J, Yan J, Greer JM, Read SJ, Henderson
RD, Rose SE, Coulthard A and McCombe PA: Correlation of
adrenomedullin gene expression in peripheral blood leukocytes with
severity of ischemic stroke. Int J Neurosci. 124:271–280. 2014.
View Article : Google Scholar
|
|
58
|
Malfitano AM, Marasco G, Proto MC, Laezza
C, Gazzerro P and Bifulco M: Statins in neurological disorders: An
overview and update. Pharmacol Res. 88:74–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yamamoto C, Fukuda N, Jumabay M, Saito K,
Matsumoto T, Ueno T, Soma M, Matsumoto K and Shimosawa T:
Protective effects of statin on cardiac fibrosis and apoptosis in
adrenomedullin-knockout mice treated with angiotensin II and high
salt loading. Hypertens Res. 34:348–353. 2011. View Article : Google Scholar
|