Introduction
Obesity, whose incidence is rapidly growing in
developed societies, is a risk factor involved in a number of
severe diseases, including diabetes, heart conditions and different
cancer types (1,2). A variety of physical techniques,
including ultrasound, laser and electrical and/or magnetic stimuli,
ranging from direct current (DC) and low or ultralow frequencies to
high frequencies or radiofrequencies (RF), have been assayed in
antiobesity treatments (3–5).
Concerning RF, treatments with electromagnetic
fields or electric currents within the 100 kHz-3 GHz frequency
spectrum, which induces tissue heating through molecular friction,
have been reported effective in therapies for adipose tissue
reduction (6–9). The antiadipogenic or lipolytic
effects of such electrothermal therapies, which are dependent on
the signal frequency and power, have received support from in
vivo and in vitro experimental studies (10–12).
Specifically, in capacitive-resistive electric transfer (CRET)
therapy, the target tissues are heated through exposure to 448 kHz
sine wave currents. The non-invasive therapy is applied manually,
exerting pressure with capacitive or resistive electrodes over the
skin, so that the underlying target tissues receive two
simultaneous stimuli: One thermal, electrically-induced, and one
mechanical (8). Previous
experimental studies applying 448 kHz CRET currents at densities of
400–800 µA/mm2 that increased the temperature of
the culture medium up to 42°C, induced a significant reduction in
intracellular lipid depot in OP9 mouse preadipocytes differentiated
into mature adipocytes (13).
These above results supported the hypothesis that
the antiadipogenic or lipolytic effects of CRET treatment are due
to the cellular or tissular response to the electrically-induced
hyperthermia. However, previous studies by our group have shown
that in vitro exposure to CRET currents in the 448–570 kHz
range significantly affects essential cellular functions, including
the control of proliferation in different human cell types, even
when the currents are administered at subthermal doses (14–19).
Specifically, when applied at the frequency of 448 kHz, the
subthermal stimulus significantly affected the cell cycle and
proliferation of adipose-derived human stem cells (ADSC) through
upregulation of proliferating cell nuclear antigen and
extracellular signal-regulated kinases 1 and 2 (ERK1/2). These
results revealed that, at least at the cellular level, CRET
currents can exert an electrostimulatory action that is independent
of the electrically-induced hyperthermia. Therefore, the aim of the
present study was to determine if, besides the antiadipogenic
effects induced by thermal treatment with 448 kHz CRET (13), electric stimulation with the same
signal can also interfere, by itself and under conditions of
normothermia, with the adipocytic differentiation. The present
study investigated the effect of in vitro exposure to short
pulses of CRET current at 448 kHz, administered at a subthermal
density of 50 µA/mm2, on the early adipogenic
differentiation of human ADSC. The present study was focused
primarily on the comparative analysis of fatty acid content in ADSC
exposed or sham-exposed to CRET at early stages of
chemically-induced adipogenic differentiation. The results revealed
a significant decrease in fatty acids in the CRET exposed samples.
The present study subsequently delved into the molecular basis of
the observed response. Therefore, the action of CRET on the enzyme,
mitogen-activated protein kinase kinases 1 and 2 (MEK1/2), and on
the transcription factor, nuclear peroxisome proliferator-activated
receptor (PPAR)γ, both of which serve important roles in the
regulation of adipogenesis (20–22).
Additionally, since PPARγ directly activates a number of genes
involved in lipid synthesis and/or storage in adipocytes, the
action of CRET on the expression levels of selected genes was
assessed in ADSC at the early stages of adipogenic differentiation.
Throughout, PPARγ refers to the expression of PPAR protein, whereas
PPARG refers to PPAR gene expression.
Materials and methods
Cell culture
The stromal-vascular fraction of adipose tissue is
the source of both new adipocytes in vivo (23) and mesenchymal cells in
vitro, termed ADSC (24,25).
Human ADSC were our biological model of choice for two fundamental
reasons: i) ADSC are responsive to subthermal treatment with CRET
(15); ii) ADSC are an optimal
model for investigating phenomena involved in adipogenic processes.
The use, as an alternative model, of immortalized preadipocytes
from human origin was unsuitable for the present study, due to the
genetic alterations typical of such established cell lines. ADSC
were isolated from subcutaneous adipose tissue surgically obtained
from healthy donors (2 men and 2 women; 29–69-years-old). All
participants provided written informed consent to participate in
the present study. Protocols for informed consent, and for
collection and processing of the samples met the ethical standards
applicable in the European Union, and were evaluated and approved
by the Ethics Committee for Clinical Trials of the Ramón y Cajal
University Hospital (Madrid, Spain). The isolation protocol was
performed, as described previously (15). Briefly, ADSC were isolated from
0.5–1 cm3 pieces of fat, free of blood vessel debris and
fibrotic tissue, sliced into 1–2 mm3 fragments. These
fragments were digested with 1 mg/ml collagenase A (Roche Applied
Science, Basel, Switzerland) for 40 min at 37°C. The digested
tissue was dissociated using a P1000 pipette with filtered tips.
The resulting cell dispersion was subsequently centrifuged at 300 ×
g for 5 min at room temperature to isolate the vascular-stromal
fraction. The resulting pellet was resuspended in culture medium
(MesenPro-RS medium; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), supplemented with 1% glutamine (Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin-streptomycin (Gibco;
Thermo Fisher Scientific, Inc.), and seeded into a 75
cm2 T-flask. After 24 h incubation at 37°C in an
atmosphere containing 5% CO2 and 100% humidity, the
flask was rinsed twice with Hank's balanced salt solution (HBSS;
Gibco; Thermo Fisher Scientific, Inc.) and fresh MesenPro was
added. After 4 days, the culture medium was renewed, and following
another 3 days, when confluent, the cells were subcultured. For
subculturing, the cells were detached using 0.05% trypsin plus
0.02% ethylenediaminetetraacetic acid (EDTA; Sigma-Aldrich, St.
Louis, MO, USA) in HBSS and seeded into a new flask at a density of
670 cells/cm2. All experiments were performed with cells
between passages 3 and 7, which were seeded into 60 mm Petri dishes
at a density of 2,270 cells/cm2. Plating was performed
directly on the dish surface, with the exception of
immunofluorescence studies, in which the cells were seeded onto
glass coverslips placed inside the dish.
Adipogenic differentiation of ADSC
After 4 days of growing in Petri dishes, the
cultures were incubated in adipogenic differentiation medium
composed of high-glucose Dulbecco's modified Eagle's medium (Thermo
Fisher Scientific Inc.), supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific Inc.), 1% glutamine and 1%
penicillin-streptomycin (Gibco; Thermo Fisher Scientific Inc.), 250
µM 3-isobutyl-1-metylxanthine (Gibco; Thermo Fisher
Scientific Inc.), 200 µM indomethacin (Sigma-Aldrich), 10
µg/ml insulin (Sigma-Aldrich) and 1 µM dexamethasone
(Sigma-Aldrich). The cultures were maintained in this medium for
periods of 2–9 days, with the medium being renewed every 3–4 days.
The samples were CRET- or sham-exposed during the last 48 h of the
adipogenic treatment. Therefore, the electric stimulation was
applied to cells at early or intermediate stages of the adipogenic
differentiation. This procedure was adopted on the basis of pilot
data indicating that early adipogenic phases can be particularly
sensitive to subthermal treatment with CRET, whereas mature
adipocytes are susceptible to thermal doses of CRET (13).
CRET exposure
The exposure system was described previously
(14,18). Briefly, the exposure to electric
current was performed by means of pairs of sterile stainless steel
electrodes designed ad hoc for in vitro stimulation, which
were fitted inside all Petri dishes, CRET-exposed and sham-exposed.
Only cells grown on the rectangular area located within the
electrode gap were used in the present study, with the cells on the
remaining surface being discarded. For CRET exposure, the electrode
pairs were connected in series to a signal generator (INDIBA Activ
HCR 902; INDIBA®, Barcelona, Spain). For sham-exposure,
the electrode pairs inserted in control dishes were also connected
to the generator, however, were not energized. The stimulation
pattern consisted of 5 min pulses of 448 kHz, sine wave current at
a subthermal density of 50 µA/mm2, separated by 4
h interpulse lapses, for a total period of 48 h. Such exposure
parameters have been previously shown by our group to affect human
cell proliferation (14–19). During the 48 h treatment interval,
the CRET- and sham-exposed cultures were grown in two separate,
identical CO2 incubators (Thermo Fisher Scientific,
Inc.). Stimulation parameters, as well as atmospheric conditions
inside the incubators (37°C, 90% humidity and 5% CO2)
were constantly monitored. The electromagnetic environment inside
the incubators was monitored using specific magnetometers for three
frequency ranges of interest: Static, power frequency and RF. The
recorded values coincided with those reported in previous studies
(15) and corresponded to field
levels typically found in laboratory environments.
Oil red O staining and quantification of
lipid content
To assess the adipogenic differentiation of ADSC,
the quantity of fatty acids synthesized by cultures grown for 2 or
9 days in differentiating medium were quantified and compared with
those in samples maintained in basal medium for the same intervals
(n=4 dishes/condition). After 48 h of CRET- or sham-exposure during
the last two days of incubation in the presence of the
corresponding media, the samples were washed with
phosphate-buffered saline (PBS) and were fixed in 4%
paraformaldehyde at 4°C for 20 min. The cells were subsequently
permeabilized with 60% isopropanol for 3 min and stained with Oil
Red O (Sigma-Aldrich) for 30 min. The stained fatty acids were
extracted by stirring of the samples in 99% isopropanol for 5 min,
and the fatty acid content was assessed by spectrophotometry at 510
nm using a CE 2021 spectrophotometer (Cecil Instruments Ltd.,
Cambridge, UK).
Immunoblotting
ADSC cultured in adipogenic medium for 2 or 9 days
were CRET- or sham-exposed for the last 48 h of incubation,
following the above described protocol. The cells growing on the
dish surface between the electrode pairs were harvested in PBS and
centrifuged at 200 × g for 5 min. The pellet was lysed for 45 min
at 4°C in a buffer containing 10 mM Tris-HCl, 10 µM KCl, 1
mM dithiothreitol, 1 mM EDTA, 1 mM PMSF, 10 mg/ml leupeptin, 5
µg/ml pepstatin, 100 mM NaF, 20 mM β-glycerophosphate, 20 mM
sodium molybdate, 0.5% Triton X-100 and 0.1% sodium dodecyl sulfate
(SDS). The lysates were centrifuged at 12,000 × g for 15 min at 4°C
and the protein concentration in the supernatant was determined
through Bradford's method. Sample protein concentrations were
estimated from a standard curve using serial dilutions of bovine
serum albumin ranging from 1–20 µg/ml and Coomassie
Brilliant Blue G-250 working solution. Final concentrations in the
Bradford reagent were 0.4% Coomassie Brilliant Blue G-250 (w/v),
0.2% ethanol (v/v) and 0.4% phosphoric acid (v/v; all
Sigma-Aldrich). Protein concentrations were measured at 595 nm
using a CE 2021 spectrophotometer. Proteins were separated by
SDS-polyacrylamide gel electrophoresis and transferred to
Odyssey® nitrocellulose membranes (LI-COR Biosciences,
Nebraska, USA) using a semi-dry transfer method (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The membranes were blocked
in PBS containing 5% non-fat dry milk for 1 h at room temperature.
The membranes were subsequently incubated overnight at 4°C in
antibodies against rabbit monoclonal anti-human anti-PPARγ (81B8)
(1:1,000; 2443), rabbit monoclonal anti-human anti-phosphorylated
(p-)MEK1/2 (1:1,000; 9154; both Cell Signaling Technology, Inc.,
Danvers, MA, USA) and mouse monoclonal anti-human anti-β-actin
(1:5,000; A5441; Sigma-Aldrich) as a loading control, in the
blocking buffer. PPARγ antibody enables detection of the two PPARγ
isoforms of interest for the present study: PPARγ1 and PPARγ2.
Following primary antibody incubation, the membranes were washed
four times with PBS-Tween. Membranes were subsequently incubated
for 1 h at room temperature with IRDye 800CW-conjugated goat
polyclonal anti-rabbit immunoglobulin (Ig)G (1:10,000; 926.32211)
and with IRdye 680LT-conjugated goat polyclonal anti-mouse IgG
(1:15,000; 926.68020; both LI-COR Biosciences). The fluorescent
intensity of the blots was measured with a LI-COR Odyssey scanner
(LI-COR Biosciences) and evaluated using Quantity One software,
version 4.6.7 (Bio-Rad Laboratories, Inc.).
Immunofluorescence
Cultures between passages 3 and 5 were seeded onto
coverslips and incubated in differentiation medium for 2–9 days.
The cells were CRET- or sham-stimulated during the last 48 h of
differentiation. The cells were fixed with 4% paraformaldehyde for
20 min at 4°C, and permeabilized with ethanol/acetic acid at −20°C
for 20 min. Afterwards, the cells were incubated overnight at 4°C
with mouse monoclonal anti-human anti-PPARγ antibody (1:50;
sc-81152 Santa Cruz Biotechnologies, Inc., Santa Cruz, CA, USA) and
fluorescently stained with anti-mouse IgG conjugated to Alexa Fluor
488 (1:500; A27012; Molecular Probes; Thermo Fisher Scientific,
Inc.) for 1 h at room temperature. The cell nuclei were
counterstained with bisBenzimide H 33258 (Sigma-Aldrich) at a
concentration of 1×10−5 M.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA from ADSC was extracted with TRI
Reagent (Sigma-Aldrich), according to the manufacturer's protocol.
The cells were homogenized in 1 ml TRI Reagent containing 1
µl glycogen (20 mg/ml; Sigma-Aldrich) as a carrier for
nucleic acid precipitation. A total of 500 ng total RNA was used to
generate cDNA by RT using a Primer Script RT™ reagent kit (Takara
Bio Inc., Shiga, Japan). RT-qPCR amplification was performed using
the SYBR Green I Master kit and LightCycler 480 II (Roche Applied
Science). The initial denaturation step was 95°C for 5 min,
followed by 45 cycles of amplification at 95°C for 10 sec, 60°C for
15 sec, and 72°C for 15 sec. The melting curves were evaluated and
the PCR reaction products were separated on a 2% agarose gels and
stained with ethidium bromide to confirm the presence of a single
product. The efficiency of the reaction was evaluated by amplifying
serial dilutions of cDNA (1:10, 1:100, 1:1,000, and 1:10,000). The
association between the threshold cycle (Ct) and the log [RNA] was
linear (−3.6< slope <−3.2). The relative quantities of target
genes were normalized against the expression of the housekeeping
gene, ribosomal protein large P0, according to the ΔCt method
(26). The primers used in RT-qPCR
are shown Table I.
 | Table ISequence of primers used in reverse
transcription-quantitative polymerase chain reaction. |
Table I
Sequence of primers used in reverse
transcription-quantitative polymerase chain reaction.
| Primer | GeneBank ID | Sequence (5′→3′) |
|---|
| RPLP0 | NM_001002 | Forward:
CCTCATATCCGGGGGAATGTG |
| | Reverse:
GCAGCAGCTGGCACCTTATTG |
| PPARG1 | NM_138712 | Forward:
AAGGCCATTTTCTCAAACGA |
| | Reverse:
AGGAGTGGGAGTGGTCTTCC |
| PPARG2 | NM_138712 | Forward:
CCATGCTGTTATGGGTGAAA |
| | Reverse:
TCAAAGGAGTGGGAGTGGTC |
| FABP4 | NM_001442 | Forward:
AGCACCATAACCTTAGATGGGG |
| | Reverse:
CGTGGAAGTGACGCCTTTCA |
| SCD | NM_005063 | Forward:
TCTAGCTCCTATACCACCACCA |
| | Reverse:
TGTCGTCTTCCAAGTAGAGGG |
| PLIN | NM_002666 | Forward:
GTGGAGTACCTCCTCCCTG |
| | Reverse:
GGTGTATCGAGAGAGGGTGTT |
| ANGPTL4 | NM_139314 | Forward:
GGCTCAGTGGACTTCAACCG |
| | Reverse:
CCGTGATGCTATGCACCTTCT |
| SREBP1c | NM_001005291 | Forward:
ACCGACATCGAAGGTGAAGT |
| | Reverse:
AGCATGTCTTCGAAAGTGCA |
| FASN | NM_004104 | Forward:
TACGTACTGGCCTACACCCAGA |
| | Reverse:
TGAACTGCTGCACGAAGAAGCATAT |
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. Unless stated otherwise, differences between treated
and control samples were analyzed by two-tailed unpaired Student's
t-test, using Graph-Pad Prism 6.01 software (GraphPad Software, San
Diego, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
CRET effect on lipid content
Quantitative analysis of Oil Red O staining revealed
that intermittent exposure to CRET during the last 48 h of
incubation significantly reduced cell lipid content with respect to
sham-exposed controls, in samples grown in differentiating medium
for 2 days (61.32±15.05% below controls; P<0.05) or 9 days
(8.48±2.37% below controls; P<0.05) (data not shown).
CRET effects on the expression of
PPARγ
From the above results, and since the
ligand-activated transcription factor PPARγ is crucial in lipid
metabolism and in adipocyte differentiation, the potential effects
of CRET on the expression of PPARγ was assessed by immunoblotting
analysis. As expected, samples incubated in basal medium expressed
no PPARγ at either at 2 or 9 days of incubation (Fig. 1). The increased expression of PPARγ
induced by the adipogenic medium was unaffected by CRET when the
electric stimulus was applied during the first two days of
differentiation. By contrast, when applied from incubation day 7 to
9, CRET significantly reduced the expression of PPARγ, 17% below
the sham-exposed, differentiated controls. These results indicated
that CRET affected the expression of PPARγ at intermediate
adipogenic phases, however, not at the earliest differentiation
stages.
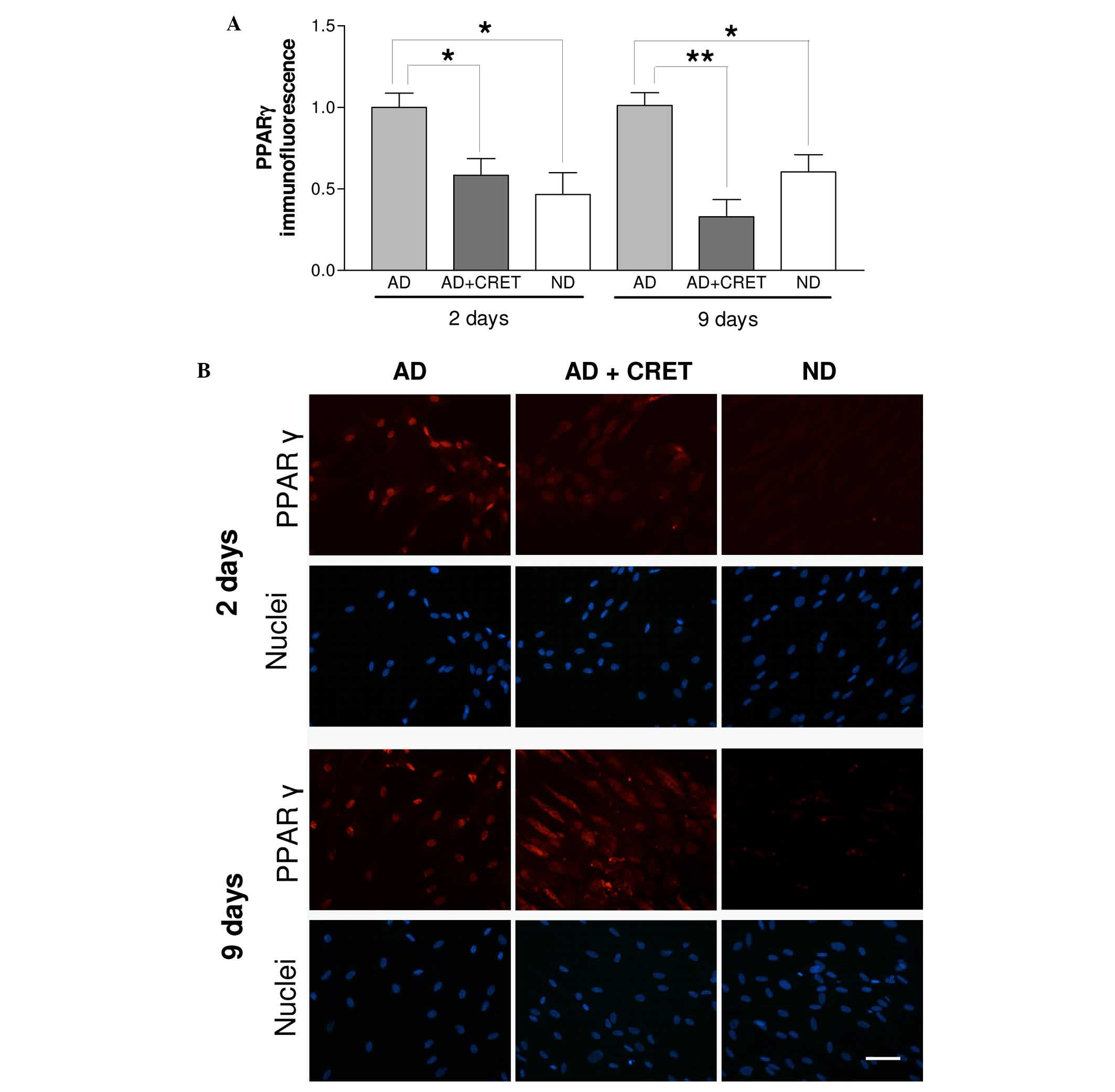
Effects of CRET on the nuclear expression
and intracellular location of PPARγ
The number of cells exhibiting nuclear expression of
PPARγ in CRET-exposed samples was significantly reduced with
respect to sham exposed, differentiated controls, both in samples
with 2 or 9 days of incubation in differentiating medium, (42 and
67% reduction, respectively; Fig.
2A). In addition, in samples with 9 days of differentiation and
treated with CRET, the immunostaining revealed a predominantly
cytoplasmic location of PPARγ, which is in contrast with the
preferential nuclear location of PPARγ in sham-exposed
differentiated controls and in undifferentiated cells (Fig. 2B). The electric stimulation induced
no such response in samples with 2 days of adipogenic
differentiation.
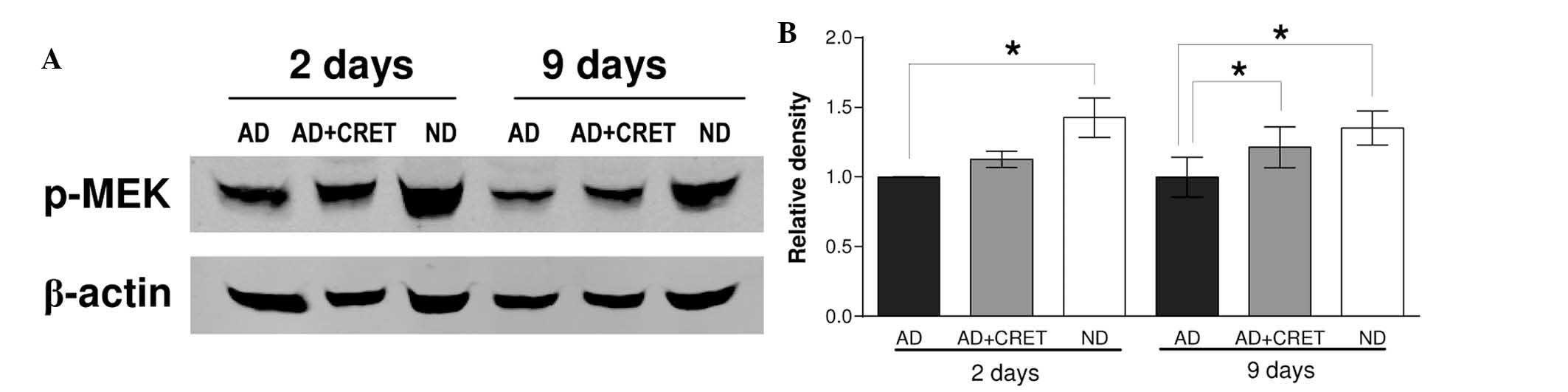
Effect of CRET on the expression of
p-MEK
It has been previously reported that mitogenic
stimuli can induce increased nuclear expression of MEK1, whose
interaction with PPARγ results in the inactivation and
translocation of PPARγ to the cytoplasm (20–22).
The possibility that CRET exposure can induce changes in the
expression of p-MEK that are associated with the observed
cytoplasmic expression of PPARγ was investigated by immunoblotting
of the expression of p-MEK. The results revealed that in the
absence of electrical stimulation, incubation in adipogenic medium
for 2 or 9 days induced a statistically significant decrease in the
expression of p-MEK (42 and 35%, respectively, below
non-differentiated controls; Fig.
3). In differentiated cultures, CRET stimulation induced
overexpression of p-MEK with respect to the sham-exposed controls
(12 and 21% at 2 and 9 days, respectively), the effect being
statistically significant in samples differentiated for 9 days.
Effects of CRET on the regulation of
genes intervening in adipocyte differentiation
As expected, incubation for 2 or 9 days in the
presence of adipogenic medium significantly increased the mRNA
expression of PPARG and of the other selected genes intervening in
lipid storage and PPARγ activation (data not shown). The results in
Fig. 4 demonstrated that when
applied during the two initial days of differentiation, CRET
exposure failed to induce significant changes in the mRNA
expression levels of the studied genes. By contrast, when the
electric stimulus was administered from day 7–9 of adipogenesis,
the mRNA expression of PPARG1 was significantly reduced by 9%, with
respect to sham-exposed controls. A similar trend, though not
significant statistically, was also observed for the expression of
PPARG2. In concomitance with the reduced mRNA expression of PPARG1,
CRET also induced significant decreases in the mRNA expression of
genes located downstream of PPARG, including perilipin (18%),
angiopoietin-like (ANGPTL)4 (20%) and fatty acid synthase (FASN;
11%).
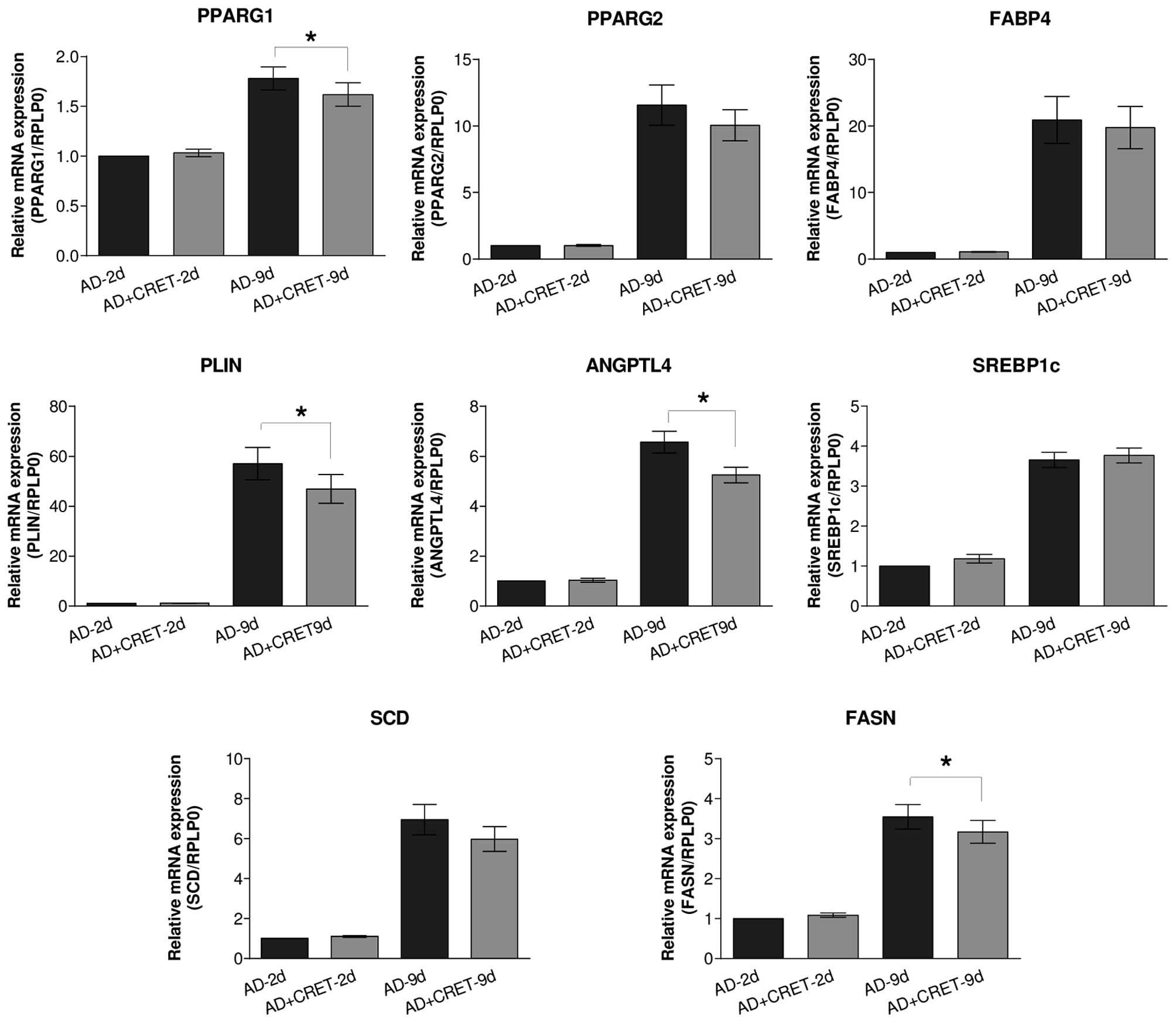 | Figure 4Analysis of mRNA expression levels of
multiple proteins. Reverse transcription-quantitative polymerase
chain reaction analysis of the effects of CRET stimulation on the
expression levels of PPARG1, PPARG2, FABP4, PLIN, ANGPTL4, SREBP1c,
SCD and FASN. The data are presented as the mean ± standard
deviation of six replicates per experimental time, with 12 samples
per replicate, normalized over samples differentiated for 2 days
and sham-exposed to CRET (*P<0.05 by Paired t-test).
PPARG, peroxisome proliferator-activated receptor gamma; FABP,
fatty acid binding protein; PLIN, perilipin; ANGPTL,
angiopoietin-like; SREBP, sterol regulatory element-binding
protein; SCD, stearoyl-CoA desaturase; FASN, fatty acid synthase;
CRET, capacitive-resistive electric transfer; AD, control samples
incubated in adipogenic medium for 2 or 9 days and sham-exposed to
CRET; AD + CRET, samples grown in adipogenic medium and exposed to
CRET during the final 48 h; ND: samples grown in basal,
non-differentiating medium. |
Discussion
ADSC can differentiate to form mature adipocytes,
maintaining this ability throughout life. Since restrictive dieting
is known to induce a reduction in size, but not in number, of
adipocytes in adipose tissue, it has been postulated that
cytoplasmic accumulation of fat during adipogenic differentiation
is crucial in human obesity (27).
Therefore, the experimental study of the effects of antiobesity
therapies on the regulation of the adipogenic differentiation can
provide relevant information for optimizing the effectiveness of
therapies, including those applying electrothermal currents. For
instance, when applied at thermal doses, stimulation with 448 kHz
CRET has been reported to cause a significant decrease in the
quantity of fatty deposits in OP9 mouse stromal cells in advanced
stages of differentiation (13).
In the present study, exposure to short, subthermal pulses of 448
kHz CRET currents resulted in a significant and consistent
reduction of lipid content in human ADSC at the early stages of the
adipogenic differentiation. Taken together, these results suggested
that, when administered simultaneously, the thermal stimulus,
together with mechanical pressure applied in the CRET therapy, the
electric stimulus is able to significantly reduce the large
quantities of lipids present in advanced stages of adipogenesis.
Additionally, subthermal electric stimulation can induce, by itself
and in the absence of the thermal factor, an antiadipogenic action
in the earlier stages of adipogenesis.
The present study hypothesized that the early
antiadipogenic action of the subthermal stimulus is exerted through
a chain of responses at the molecular level, rather than through a
more direct effect of emptying of the lipid vesicles. In order to
investigate the processes potentially involved in the subthermal
effects, the protein and mRNA expression levels of PPARγ, the key
nuclear transcription factor in adipogenesis control, were
determined. Immunoblotting assay failed to detect significant
changes with respect to the controls in the expression of PPARγ in
the samples at the earlier stages, grown in differentiation medium
for 2 days only and exposed simultaneously to electric stimulation.
By contrast, in samples differentiated for 9 days, the subthermal
treatment induced a significant decrease in PPARγ with respect to
sham exposed controls. On the other hand, an immunofluorescence
assay revealed that CRET treatment significantly decreased the
number of cells displaying nuclear PPARγ, both in samples
differentiated for 2 or 9 days, the latter showing a predominantly
cytoplasmic location of PPARγ (Fig.
2B).
Although little is known about its intracellular
distribution, experimental evidence locates PPARγ in the nucleus of
the quiescent cells, where it exerts its predominant function as a
transcription factor for adipogenesis. On this basis, the apparent
discordance between the responses obtained at 2 days in the
immunoblot vs. immunofluorescence assays, may be attributable to
the fact that immunoblotting quantifies the total PPARγ protein in
the sample, regardless of its cellular location. By contrast,
immunofluorescence exclusively quantifies PPARγ that, being present
in the cell nucleus, acts as an adipogenic transcription factor.
Therefore, the mentioned discordance would not occur if only the
nuclear, active PPARγ protein was susceptible to the electric
stimulus. As a whole, these results were coherent with the observed
reduced lipid content in CRET-treated ADSC and indicate that such
an effect can be mediated by electrically-induced changes in the
expression of PPARγ.
It has been reported that when the cell receives a
mitogenic stimulus, p-MEK, the kinase enzyme of the MAPK-ERK1/2
pathway, is overexpressed and binds physically to PPARγ, causing
its translocation from the nucleus to the cytoplasm (20–22)
and preventing PPARγ from exerting its function as an activator of
adipogenic effector genes. To elucidate whether this is a potential
mechanism involved in the observed translocation of PPARγ to the
cytoplasm of the samples differentiated for 9 days and treated with
CRET, the expression of p-MEK was analyzed. While in
undifferentiated samples immunoblotting revealed a high expression
of p-MEK, typical of proliferating cultures, the differentiation
medium induced significant low expression of p-MEK (Fig. 3). Compared with differentiated,
sham-exposed controls, CRET treatment induced the overexpression of
p-MEK, although this effect only reached statistical significance
in cultures with 9 days of differentiation. These results suggested
that this overexpression of p-MEK may result in the downregulation
of the genomic activity of PPARG. Therefore, the 448 kHz electric
signal may be interpreted by the cell as a mitogenic stimulus,
which induces the overexpression of p-MEK. With regards to this,
previous studies by our group have shown that 448 kHz CRET can
stimulate mitosis in undifferentiated mesenchymal ADSC by
activating the expression of p-ERK, another member of the same MAPK
proliferative pathway (15). In
cultures undergoing adipogenic differentiation, early activation of
this pathway may not result in changes in cell proliferation,
however, only in the translocation of PPARγ towards the cytoplasm,
and subsequent hindering of the adipogenic differentiation. In
other words, CRET stimulation may result in at least one
non-genomic interaction of PPARγ with different protein partners
(e.g. those associated with the cytoskeleton, lipid droplets and
kinases), leading to alternative cytoplasmic signaling, which would
result in partial inhibition of early adipogenic
differentiation.
Regarding the influence of CRET on the expression of
genes involved in the adipogenic metabolism and differentiation, no
significant changes were detected in the gene expression levels in
ADSC differentiated for 2 days and treated with CRET. By contrast,
in samples differentiated for 9 days, CRET caused decreased
expression of PPARG1, ANGPTL4, perilipin and FASN, without
significantly affecting the other genes analyzed (Fig. 4). These results revealed that CRET
stimulation not only induces decreased protein expression of PPARγ,
but also results in the downregulation of PPARG, which leads to the
reduced expression of a number of genes whose transcription
directly requires PPARγ protein, including ANGPTL4 and perilipin,
or indirectly, including FASN (28). ANGPTL4 is known to irreversibly
inactivate lipoprotein lipase (LPL) (29). Therefore, it was hypothesized that
the decreased expression of this gene may result in increased
lipolytic activity of LPL. Perilipin is a protein covering lipid
vesicles, protecting them against the action of cytosolic lipases
(30). Therefore, decreased gene
expression of perilipin in response to CRET may promote
mobilization of intracellular reservoirs of triacylglycerol.
Finally, FASN is a multi-enzyme protein that, in the presence of
NADPH, catalyzes the synthesis of palmitate from acetyl-CoA and
malonyl-CoA to long chain saturated fatty acids (31). Therefore, the CRET-induced decrease
in FASN expression may also contribute to reduced quantities of
intracellular lipids. Taken together, these results are consistent
with those from the other tests performed in the present study, and
indicated that the hindering effects of CRET on early adipogenic
stages would be mediated, at least in part, by electrically induced
low expression of genes involved in the control of the synthesis
and mobilization of fatty acids during adipogenesis.
In conclusion, the 448 kHz CRET therapy
simultaneously applies three stimuli: Electrical, thermal and
mechanical, which may act in synergy in antiobesity treatments. The
present study identified and described potential molecular
mechanisms through which the assayed 448 kHz electric signal can,
by itself and in the absence of the accompanying thermal and
mechanical components of the CRET treatments, interfere with
processes controlling the synthesis and mobilization of fat at
early adipogenic stages. The affected processes included the
activation of MEK1/2, reduced expression of PPARγ protein and
downregulation of the gene expression levels of PPARG1, perilipin,
ANGPTL4 and fatty acid synthase. The possibility cannot be
disregarded that these effects also contributed to the
antiadipogenic action exerted on mature adipocytes by thermal
treatment with CRET (13), even if
the extent of such potential contribution remains to be
determined.
Acknowledgments
The authors would like to thank Dr María Antonia
Martínez-Pascual and Mrs. Lorena Crespo-Toro (both IRYCIS,
University Hospital Ramón y Cajal) for their valuable technical
contribution. This work was financially supported by the Fundación
para la Investigación Biomédica del Hospital Ramón y Cajal (no.
2012/0032).
References
|
1
|
Hurt RT, Kulisek C, Buchanan LA and
McClave SA: The obesity epidemic: Challenges, health initiatives,
and implications for gastroenterologists. Gastroenterol Hepatol
(NY). 6:780–792. 2010.
|
|
2
|
Lavie CJ, Milani RV and Ventura HO:
Obesity and cardiovascular disease: Risk factor, paradox, and
impact of weight loss. J Am Coll Cardiol. 53:1925–1932. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abraham MT and Mashkevich G: Monopolar
radiofrequency skin tightening. Facial Plast Surg Clin North Am.
15:169–177. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Belenky I, Margulis A, Elman M, Bar-Yosef
U and Paun SD: Exploring channeling optimized radiofrequency
energy: A review of radiofrequency history and applications in
esthetic fields. Adv Ther. 29:249–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mulholland RS, Paul MD and Chalfoun C:
Noninvasive body contouring with radiofrequency, ultrasound,
cryolipolysis, and low-level laser therapy. Clin Plast Surg.
38:503–520. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alexiades-Armenakas M, Dover JS and Arndt
KA: Unipolar radiofrequency treatment to improve the appearance of
cellulite. J Cosmet Laser Ther. 10:148–153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brightman L, Weiss E, Chapas AM, Karen J,
Hale E, Bernstein L and Geronemus RG: Improvement in arm and
post-partum abdominal and flank subcutaneous fat deposits and skin
laxity using a bipolar radiofrequency, infrared, vacuum and
mechanical massage device. Lasers Surg Med. 41:791–798. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Valentim da Silva RM, Barichello PA,
Medeiros ML, de Mendonça WC, Dantas JS, Ronzio OA, Froes PM and
Galadari H: Effect of capacitive radiofrequency on the fibrosis of
patients with cellulite. Dermatol Res Pract.
2013:7158292013.PubMed/NCBI
|
|
9
|
van der Lugt C, Romero C, Ancona D,
Al-Zarouni M, Perera J and Trelles MA: A multicenter study of
cellulite treatment with a variable emission radio frequency
system. Dermatol Ther. 22:74–84. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boisnic S, Divaris M, Nelson AA, Gharavi
NM and Lask GP: A clinical and biological evaluation of a novel,
noninvasive radio-frequency device for the long-term reduction of
adipose tissue. Lasers Surg Med. 46:94–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamida ZH, Comtois AS, Portmann M, Boucher
JP and Savard R: Effect of electrical stimulation on lipolysis of
human white adipocytes. Appl Physiol Nutr Metab. 36:271–275. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Trelles MA, van der Lugt C, Mordon S, Ribé
A and Al-Zarouni M: Histological findings in adipocytes when
cellulite is treated with a variable-emission radiofrequency
system. Lasers Med Sci. 25:191–195. 2010. View Article : Google Scholar
|
|
13
|
Kato S, Saitoh Y and Miwa N: Repressive
effects of a capacitive-resistive electric transfer (CRet)
hyperthermic apparatus combined with provitamin C on intracellular
lipid-droplets formation in adipocytes. Int J Hyperthermia.
29:30–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hernandez-Bule ML, Cid MA, Trillo MA, Leal
J and Ubeda A: Cytostatic response of HepG2 to 0.57 MHz electric
currents mediated by changes in cell cycle control proteins. Int J
Oncol. 37:1399–1405. 2010.PubMed/NCBI
|
|
15
|
Hernández-Bule ML, Paíno CL, Trillo MÁ and
Úbeda A: Electric stimulation at 448 kHz promotes proliferation of
human mesenchymal stem cells. Cell Physiol Biochem. 34:1741–1755.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hernández-Bule ML, Roldán E, Matilla J,
Trillo MA and Ubeda A: Radiofrequency currents exert cytotoxic
effects in NB69 human neuroblastoma cells but not in peripheral
blood mononuclear cells. Int J Oncol. 41:1251–1259. 2012.PubMed/NCBI
|
|
17
|
Hernández-Bule ML, Trillo MA, Bazán E,
Martinez-Pascual MA, Leal J and Ubeda A: Nonthermal levels of
electric currents applied in capacitive electric transfer therapy
provokes partial cytotoxic effects in human neuroblastoma cultures.
Neurocirugia (Astur). 15:366–371. 2004. View Article : Google Scholar
|
|
18
|
Hernández-Bule ML, Trillo MA, Cid MA, Leal
J and Ubeda A: In vitro exposure to 0.57-MHz electric currents
exerts cytostatic effects in HepG2 human hepatocarcinoma cells. Int
J Oncol. 30:583–592. 2007.PubMed/NCBI
|
|
19
|
Hernández-Bule ML, Trillo MÁ and Úbeda A:
Molecular mechanisms underlying antiproliferative and
differentiating responses of hepatocarcinoma cells to subthermal
electric stimulation. PLoS One. 9:e846362014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burgermeister E, Chuderland D, Hanoch T,
Meyer M, Liscovitch M and Seger R: Interaction with MEK causes
nuclear export and downregulation of peroxisome
proliferator-activated receptor gamma. Mol Cell Biol. 27:803–817.
2007. View Article : Google Scholar :
|
|
21
|
Burgermeister E and Seger R: MAPK kinases
as nucleo-cytoplasmic shuttles for PPARgamma. Cell Cycle.
6:1539–1548. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Burgermeister E and Seger R: PPARgamma and
MEK interactions in cancer. PPAR Res. 2008:3094692008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hollenberg CH and Vost A: Regulation of
DNA synthesis in fat cells and stromal elements from rat adipose
tissue. J Clin Invest. 47:2485–2498. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Van RL, Bayliss CE and Roncari DA:
Cytological and enzymological characterization of adult human
adipocyte precursors in culture. J Clin Invest. 58:699–704. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zannettino AC, Paton S, Arthur A, Khor F,
Itescu S, Gimble JM and Gronthos S: Multipotential human
adipose-derived stromal stem cells exhibit a perivascular phenotype
in vitro and in vivo. J Cell Physiol. 214:413–421. 2008. View Article : Google Scholar
|
|
26
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cristancho AG and Lazar MA: Forming
functional fat: A growing understanding of adipocyte
differentiation. Nat Rev Mol Cell Biol. 12:722–734. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lowe CE, O'Rahilly S and Rochford JJ:
Adipogenesis at a glance. J Cell Sci. 124:2681–2686. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mattijssen F and Kersten S: Regulation of
triglyceride metabolism by Angiopoietin-like proteins. Biochim
Biophys Acta. 1821:782–789. 2012. View Article : Google Scholar
|
|
30
|
Brasaemle DL, Subramanian V, Garcia A,
Marcinkiewicz A and Rothenberg A: Perilipin A and the control of
triacylglycerol metabolism. Mol Cell Biochem. 326:15–21. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jayakumar A, Tai MH, Huang WY, al-Feel W,
Hsu M, Abu-Elheiga L, Chirala SS and Wakil SJ: Human fatty acid
synthase: Properties and molecular cloning. Proc Natl Acad Sci USA.
92:8695–8699. 1995. View Article : Google Scholar : PubMed/NCBI
|