Introduction
Parkinson's disease (PD), a chronic and progressive
neurodegenerative movement disorder, is characterized by
progressive degeneration of dopaminergic neurons in the substantia
nigra pars compacta (SNc) (1). The
mechanisms of dopaminergic cell death have remained to be fully
elucidated. Lipid peroxidation, oxidative stress and mitochondrial
dysfunction are considered to mediate the progression of
dopaminergic neuron degeneration (2). As the etiology and pathogenesis of PD
have remained elusive, current therapies focus on the symptoms,
rather than the neurodegenerative progression of dopaminergic
neurons in SNc. Therefore, novel insight into the disease
mechanisms is urgently required. 6-Hydroxydopamine (6-OHDA), which
has been previously used to establish in vivo as well as
in vitro models of PD, is a neurotoxin mainly targeting
dopaminergic neurons (3,4). 6-OHDA exerts its effects via inducing
reactive oxygen species (ROS) overproduction and energy depletion
(1,5). PC12 cells are widely used for in
vitro studies exploring the mechanisms of neurodegenerative
diseases (6–8).
Seven members of the sirtuin family have been
associated with energy production, cell metabolism and DNA repair
in mammals. They are deacetylases acting on histones in the
presence of nicotinamide adenine dinucleotide (NAD+)
(9). Nicotinamide
phosphoribosyltransferase (NAMPT) is the rate-limiting enzyme in
the mammalian NAD+ salvage pathway for the conversion of
nicotinamide into nicotinamide mononucleotide (NMN), which is later
converted to NAD+ (10). NAMPT is thought to function in a
manner equivalent to that of pyrazinamidase/nicotinamidase 1 in
mammals (11), and increased
expression of NAMPT has been shown to positively regulate
NAD+ levels and enhance SIRT1 transcriptional regulatory
activity in mouse fibroblasts (12). The NAD+/NADH ratio is a
fundamental indicator of the cellular redox status (13) and ROS accumulation has been shown
to modify the activity of sirtuins (14). Furthermore, a previous study
demonstrated that NMN protected the rotenone-induced PC12 cells
from cell death by restoring the intracellular levels of
NAD+ and preventing ATP depletion (15).
SIRT1 has beneficial effects on numerous major
aging-associated pathologies, including diabetes (16), neurodegeneration (17), chronic heart failure (18) and cancer (19). Resveratrol treatment was shown to
attenuate cell stress caused by to caloric restriction via
activation of SIRT1 (20). SIRT1
also regulates an array of transcription factors, including nuclear
factor κB (21) and p53 (22). High levels of ROS induce the
expression of SIRT1 (23), which
in turn initiates DNA repair (24).
In the present study, the neuroprotective effects of
the NAMPT metabolite NAD+ against 6-OHDA-induced
neuro-toxicity in PC12 cells were elucidated. Furthermore, the
possible underlying mechanisms were examined by using an NAMPT
inhibitor and by assessing effects on indicators of oxidative
damage as well as SIRT1 activation.
Materials and methods
Cell culture and treatment
The PC12 rat adrenal pheochromocytoma cell line was
obtained from the Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China). PC12 cells were maintained in
Dulbecco's modified Eagle's medium supplemented with 5% (v/v) fetal
bovine serum (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), 10% heat-inactivated equine serum (Hyclone, Logan, UT,
USA), 100 U/ml penicillin and 0.1 mg/ml streptomycin
(Sigma-Aldrich, St Louis, MO, USA). Cells were incubated at 37°C in
a humidified atmosphere with 5% CO2. The medium was
replaced every two or three days and cells were routinely
subcultured at a 1:5 ratio at weekly intervals. For the
experiments, PC12 cells were pre-incubated with NMN (600
µM), FK866 (10 nm) or resveratrol (50 µM;
Sigma-Aldrich) for 2 h and then exposed to 6-OHDA for 24 h. The
control group was treated with an equivalent volume of dimethyl
sulfoxide (DMSO; final concentration, 0.1%; Sigma-Aldrich) added to
the medium.
Cell viability assay
Cell viability was estimated using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. In brief, PC12 cells were seeded in 96-well plates at 10,000
cells/well for 24 h. After 6-OHDA treatment for 24 h with or
without pre-incubation with NMN, FK866 or resveratrol, the cells
were incubated with 20 µl MTT solution (5 mg/ml;
Sigma-Aldrich) for 4 h. The culture medium was removed and the dark
blue formazan product was dissolved by adding 150 µl DMSO to
each well. The absorbance of each well was read at 570 nm using the
Rayto-RT 6000 enzyme-linked immunosorbent assay reader (Rayto Life
and Analytical Sciences, Guangdong, China), and the viability was
expressed as the percentage of the untreated cells.
NAD+/NADH and SIRT1 activity
assays
An NAD+/NADH quantification kit was used
to determine NAD+ and NADH levels (BioVision, Mountain
View, CA, USA) according to the manufacturer's instructions. To
assess SIRT1 activity, whole-cell extracts were prepared using a
mild lysis buffer plus protease inhibitor cocktail (Roche
Diagnostics, Basel, Switzerland). An Infinite M200 microplate
fluorimeter (Tecan, Hillsborough, NC, USA) was used to measure
SIRT1 activity.
Assessment of glutathione (GSH) and
malondialdehyde (MDA) levels
PC12 cells were washed with 1 ml phosphate-buffered
saline (PBS) three times and subsequently scraped off the bottom of
the flask in ice-cold PBS. Lysis buffer was then added to the
cells, followed by incubation for 40 min on ice. The lysates were
centrifuged at 10,000 × g for 10 min at 4°C. The supernatant was
collected and the protein content was determined using the
bicinchoninic acid (BCA) protein assay kit, followed by analysis of
the GSH and MDA content using a commercial colorimetric GSH and MDA
assay kit (Beyotime Institute of Biotechnology, Haimen, China).
Assessment of superoxide dismutase (SOD)
activity and lactate dehydrogenase (LDH)
According to the manufacturer's instructions, 100
µl culture supernatant was used to determine SOD activity
using a commercially available detection kit (Nanjing Jiancheng
Biochemical Reagent Co., Nanjing, China). For the LDH assay, 80
µl culture supernatant was collected and subjected to
analysis using an LDH assay kit (Nanjing KeyGen Biotech. Co., Ltd.,
Nanjing, China) according to the manufacturer's instructions.
Western blot analysis
Western blot analysis was performed according to the
protocol of a previous study (25). PC12 cells (106
cells/well) were seeded onto six-well plates and treated with
6-OHDA for 24 h with or without pre-incubation with FK866, NMN or
resveratrol. Subsequently, cells were lysed in
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology) containing protease inhibitor cocktail (Roche
Diagnostics) and then incubated on ice for 30 min. The cellular
debris was removed by centrifugation (14,000 × g for 10 min at 4°C)
and the protein concentration in the supernatant was determined
using a BCA protein assay kit. Equal amounts of protein (20–30
µg) were subjected to 10% sodium dodecyl sulfate
polyacrylamide gel electrophoresis and transferred onto a
polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). After blocking in 5% non-fat milk for 1 h at
room temperature, blots were incubated with rabbit monoclonal
anti-NAMPT (1:1,000; Abcam, Cambridge, MA, USA; cat. nos. ab45890),
anti-SIRT1 antibody (1:1,000; cat. no. ab32441) and mouse
monoclonal β-actin (1:1,000; cat. no. ab6276) overnight at 4°C.
Subsequent to three washes in Tris-buffered saline containing Tween
20, membranes were incubated with goat anti-rabbit horseradish
peroxidase-conjugated IgG or goat anti-mouse horseradish
peroxidase-conjugated IgG (1:5,000; Beyotime Institute of
Biotechnology; cat. nos. A0208 and A0216, respectively) for 1 h at
room temperature. Proteins were detected using the enhanced
chemiluminescence western blot detection kit (Amersham ECL plus
Western blotting detection system; GE Healthcare). The blots were
washed again and scanned. The proteins of interest were detected
using the Odyssey Western Detection system and quantified with the
Odyssey LI-COR System software (LI-COR Biosciences, Lincoln, NE,
USA).
Statistical analysis
Experiments were repeated at least three times with
consistent results. Values are expressed as the mean ± standard
error of the mean and one-way analysis of variance followed by
Tukey's multiple comparisons test was used for comparisons between
multiple groups. Statistical analysis was performed using GraphPad
Prism 6.0 (GraphPad Software, La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
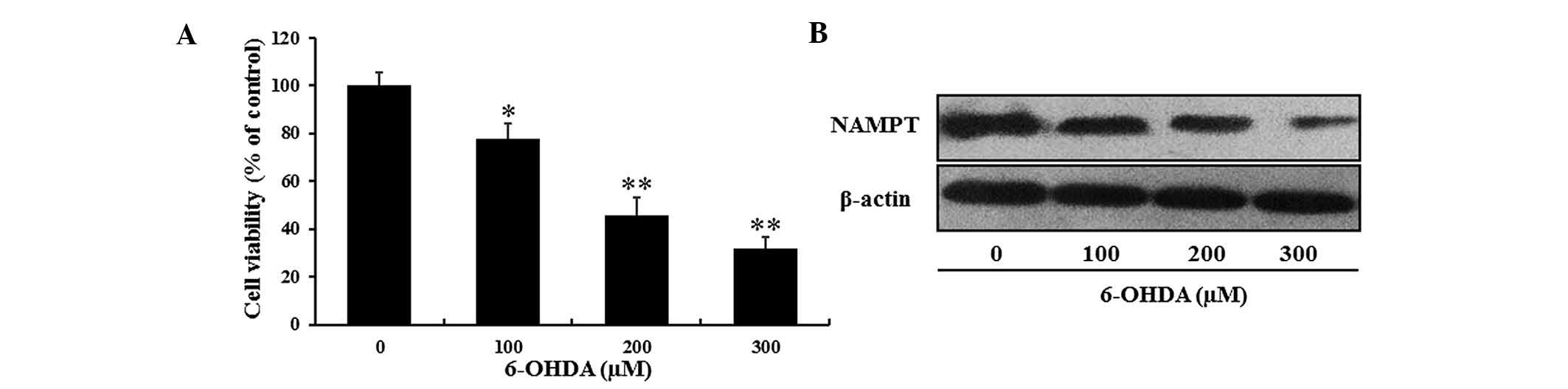
6-OHDA-induced decreases in PC12 cell
viability are accompanied with a reduction in NAMPT and
NAD+ levels
According to the protocols of previous studies
(8,26), the present study generated a
6-OHDA-induced in vitro model of neurodegeneration
resembling PD. PC12 cells were treated with various concentrations
of 6-OHDA for 24 h and the cell viability was determined using an
MTT assay. In accordance with the findings of the previous studies,
the viability of PC12 cells was decreased by 6-OHDA in a
dose-dependent manner. Only ~50% of PC12 cells survived after
exposure to 200 µM 6-OHDA for 24 h (Fig. 1A).
To investigate the effects of 6-OHDA-induced
neuronal cell death on NAMPT levels in PC12 cells, western blot
analysis was performed. Compared with the control group, NAMPT
expression was markedly decreased by 6-OHDA in a dose-dependent
manner (Fig. 1B).
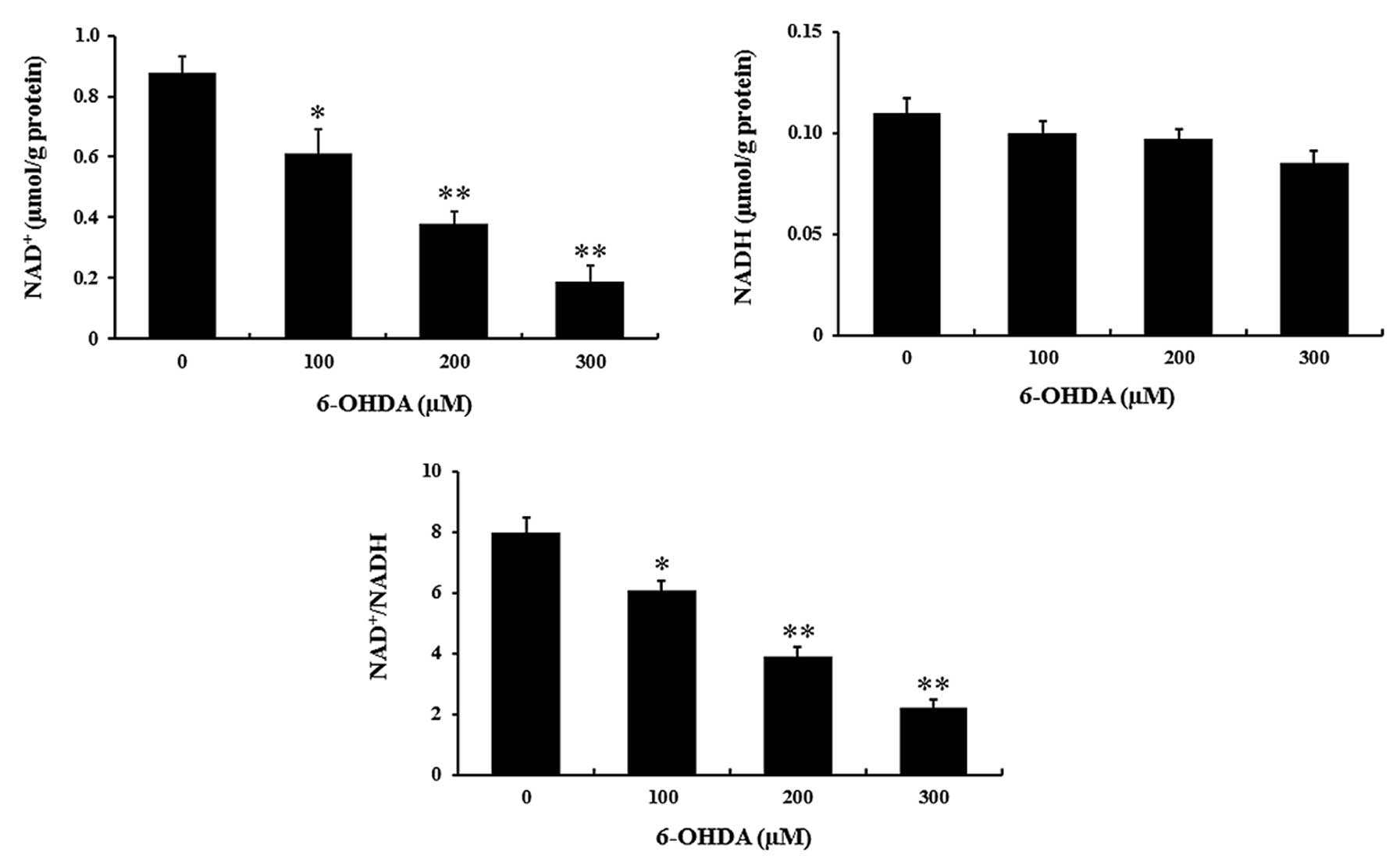
As studies have indicated that NAD+
levels are regulated by NAMPT in mammalian neurons, the present
study detected the concentration of NAD+ and NADH in
response to 6-OHDA treatment. Compared with that in the control
group, the NAD+ release was significantly decreased by
6-OHDA treatment (P<0.01) (Fig.
2), while NADH levels were not affected. Consequently, the
NAD+/NADH ratio was reduced by 6-OHDA in a
dose-dependent manner (P<0.01) (Fig. 2).
NMN protects PC12 cells against
6-OHDA-induced death, while NAMPT inhibition aggravates
6-OHDA-induced PC12 cell death
In order to determine the roles of NAMPT in the
molecular pathology of PD, PC12 cells were pre-treated with FK866,
a NAMPT-specific inhibitor, or NMN, the enzymatic product of NAMPT,
prior to induction with 6-OHDA. As shown in Fig. 3A, pre-incubation with FK866 (10 nM)
significantly decreased the viability of PC12 cells following
treatment with 6-OHDA from ~50 to 21% (P<0.01). Furthermore,
PC12 cells were pre-incubated with NMN (600 µM) followed by
incubation with 6-OHDA (200 µM) for 24 h (Fig. 3B). It was observed that NMN
markedly increased the viability of 6-OHDA-treated PC12 cells from
~45 to 74% (P<0.01). These results demonstrated that following
induction of PC12 cells with 6-OHDA, prior inhibition of NAMPT
activity decreased the cell viability, while NMN, the enzymatic
product of NAMPT, rescued cell viability.
NMN prevents 6-OHDA-induced oxidative
damage of PC12 cells and LDH release
Previous studies have demonstrated that oxidative
stress is as an important mediator in 6-OHDA-induced cell death
(27,28). To explore whether NMN protects PC12
cells against 6-OHDA induced cell death due to its anti-oxidant
capacity, the levels of GSH and SOD, which are the most common
indicators of anti-oxidant activity, were assessed in PC12 cells.
As a vital enzymatic anti-oxidant, SOD has an important role in the
clearance of ROS. As shown in Fig 4A
and B, treatment with 200 µM 6-OHDA significantly
reduced the intracellular concentration of GSH and SOD, which was
partially inhibited by treatment with 600 µM NMN
(P<0.05). Furthermore, the concentration of MDA, a lipid
oxidation biomarker, was assessed. After incubation with 6-OHDA for
24 h, the levels of MDA were significantly increased (P<0.01),
which was attenuated by NMN (600 µM) (P<0.01) (Fig. 4C). The release of LDH into the
culture medium due to plasma membrane damage is an indicator of
cell death. As shown in Fig. 4D,
6-OHDA markedly enhanced LDH release, which was significantly
attenuated by NMN (P<0.01). All of these results indicated that
NMN protects PC12 cells from 6-OHDA-induced death due to its
anti-oxidant activity.
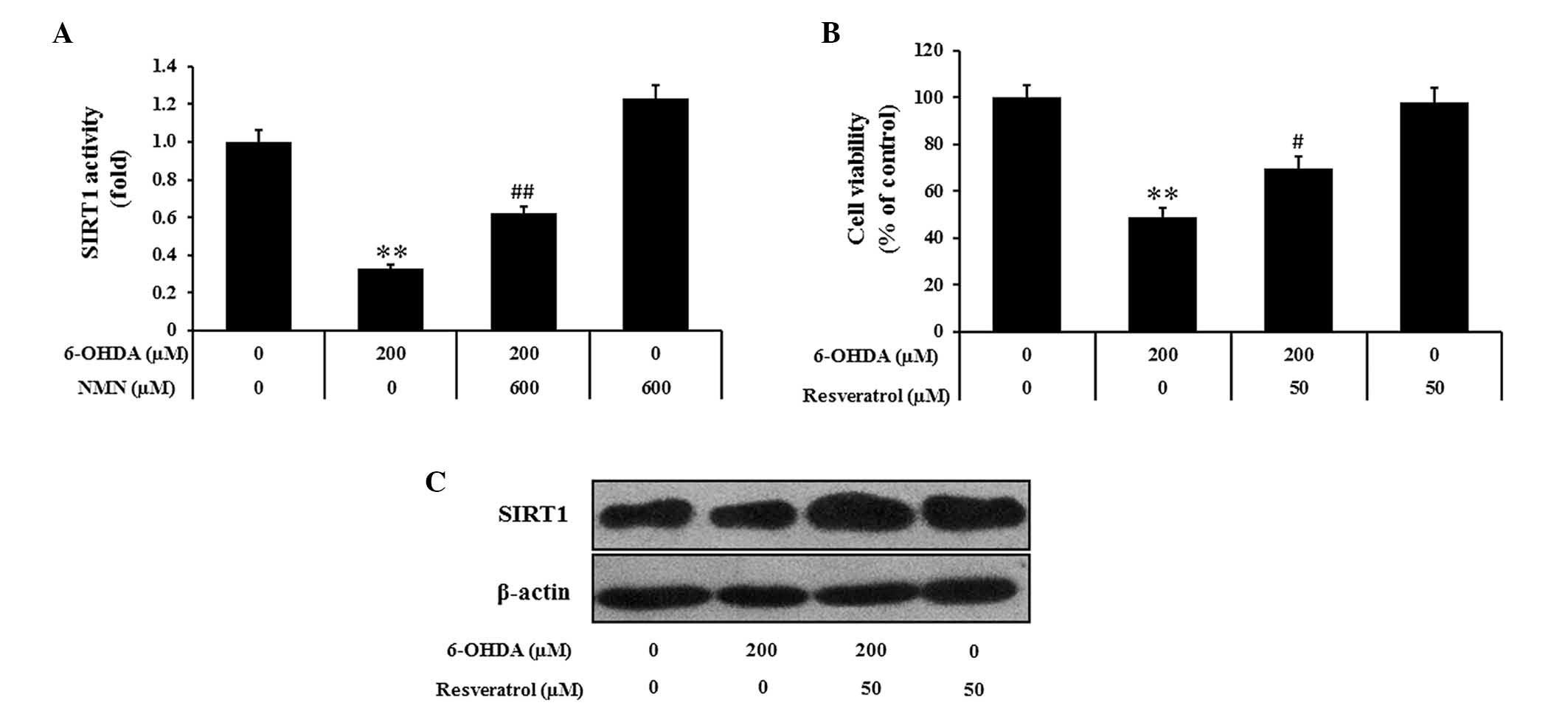
NMN may exert its neuroprotective effects
by activating SIRT1
Since SIRT1 is known to mediate several biological
effects of NAMPT, the present study further assessed whether the
protective effects of NMN against 6-OHDA-induced PC12 cell death
may be mediated via modulation of SIRT1 activity. Indeed, the
results indicated that 6-OHDA markedly decreased the activity of
SIRT1 compared to that in the control group, while NMN attenuated
this effect (P<0.01) (Fig. 5A).
The pharmacological activator resveratrol is known to exert
protective effects on PC12 cells against 6-OHDA-induced toxicity.
Pre-treatment with resveratrol (50 µM) markedly reduced the
inhibitory effects of 6-OHDA on cell viability (P<0.05)
(Fig. 5B). Furthermore, western
blot analysis showed that the 6-OHDA-induced reduction of SIRT1
expression was reversed by pre-treatment with resveratrol (Fig. 5C). All of these results indicated
that enhanced SIRT1 activity and increased SIRT1 expression are
associated with the protective effects of NMN against
6-OHDA-induced PC12 cell death.
Discussion
The present study demonstrated that NMN, the
enzymatic product of NAMPT, exerts protective effects against
6-OHDA-induced neurodegeneration in vitro. It was revealed
that following 6-OHDA treatment, PC12 cell viability was decreased,
NAMPT was downregulated, and the levels of NAD+ as well
as the NAD+/NADH ratio were significantly decreased.
Indicators of cell death and oxidative damage were increased, and
SIRT1 activation was decreased following treatment with 6-OHDA,
which was attenuated by treatment with NMN. Furthermore, the NAMPT
inhibitor FK866 was shown to aggravate the cytotoxic effects of
6-OHDA. The present study therefore indicated that decreases in
NAMPT/NAD+ and subsequent deactivation of SIRT1 may be implicated
in the molecular pathology of neurodegenerative disorders,
including PD.
The neurotoxin 6-OHDA induces dopaminergic neuronal
death and has been widely used to establish in vitro models
of PD (1). 6-OHDA exerts its toxic
effects via inducing ROS overproduction, which results in oxidative
stress and cell death, through the following pathways:
Extracellular auto-oxidation, monoamine oxidase-mediated
intracellular metabolism and mitochondrial respiratory chain
inhibition (29,30). PC12 cells have been widely used in
studies examining the molecular mechanisms of neurodegenerative
diseases (6,8). Therefore, 6-OHDA-treated PC12 cells
were used in the present study as a cell model to investigate the
implication of NAMPT in PD.
In the present study, 6-OHDA was shown to
concentration-dependently reduce NAD+ levels; this may
have been a consequence of the simultaneously observed reduction of
NAMPT, as NAMPT is required for the production of NAD+
(31). While NADH levels were not
affected by 6-OHDA, the NAD+/NADH ratio was decreased,
which may explain for the observed reduced cell survival rate due
to various stressors (32). In
addition, increases in NAMPT or NMN have been previously indicated
to markedly reduce cell death (31), which was consistent with the
observations of the present study that NMN enhanced the survival of
PC12 cells after incubation with 6-OHDA. Furthermore,
pre-incubation with the NAMPT inhibitor FK866 aggravated the
cytotoxic effects of 6-OHDA, which further suggested the
neuroprotective effects of NAMPT/NMN.
In neurodegenerative disorders, oxidative stress
originates from ROS overproduction and impaired anti-oxidative
defense is a major reason of neuronal death (33). The antioxidant defense system
consists of non-enzymatic anti-oxidants such as GSH and enzymes
such as SOD, which neutralize free radicals. The present study
revealed that, compared with those in the control group, the levels
of GSH and SOD were markedly reduced in 6-OHDA-treated PC12 cells,
which was attenuated by pre-treatment with NMN. These results
suggested that NMN, an enzymatic product of NAMPT, attenuated the
6-OHDA-induced increases in the levels of anti-oxidants. It is
therefore indicated that NAMPT may have an anti-oxidant role in
PC12 cells and may reduce the consumption of other anti-oxidants,
including GSH and SOD. Furthermore, the levels of MDA, a biomarker
of lipid oxidation, were markedly increased after 6-OHDA treatment
further confirming that 6-OHDA exerts its neurotoxic effects by
inducing oxidative stress in PC12 cells. However, pre-treatment
with NMN significantly reduced MDA levels in PC12 cells following
6-OHDA treatment, further indicating its anti-oxidant effects. In
brief, NAMPT could enhance the ability of scavenging anti-oxidant
action and the free radicals in by 6-OHDA induced PD cell
models.
SIRT1 is a unique protein deacetylator and is
closely linked to cellular survival due to its dependence on
NAD+ (34). In the
present study, it was demonstrated that 6-OHDA decreased SIRT1
activation in PC12 cells, which was attenuated by NMN. Furthermore,
the neuroprotective compound resveratrol inhibited 6-OHDA-induced
toxicity and increased the expression of SIRT1 in PC12 cells. These
results indicated that NAMPT/NMN may exert their
neuroprotective/anti-oxidant effects via activation of SIRT1.
In conclusion, the present study demonstrated that
NAMPT, a rate-limiting enzyme for mammalian NAD+,
markedly protected PC12 cells against 6-OHDA-induced oxidative
stress-associated cell death. The protective effects of NAMPT/NMN
may be attributed to, at least in part, their potent anti-oxidant
properties, as evidenced by the marked increases in GSH and SOD as
well as the reduction of MDA, through enhancing SIRT1 activity.
NAMPT and NAD as well as SIRT1 may therefore have a crucial role in
PD and other neurodegenerative disorders, and their upregulation
may represent a novel therapeutic strategy.
References
|
1
|
Beal MF: Experimental models of
Parkinson's disease. Nat Rev Neurosci. 2:325–334. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanrott K, Gudmunsen L, O'Neill MJ and
Wonnacott S: 6-hydroxydopamine-induced apoptosis is mediated via
extracellular auto-oxidation and caspase 3-dependent activation of
protein kinase Cdelta. J Biol Chem. 281:5373–5382. 2006. View Article : Google Scholar
|
|
3
|
Blandini F, Armentero MT and Martignoni E:
The 6-hydroxy-dopamine model: News from the past. Parkinsonism
Relat Disord. 14(Suppl 2): S124–S129. 2008. View Article : Google Scholar
|
|
4
|
Surendran S and Rajasankar S: Parkinson's
disease: Oxidative stress and therapeutic approaches. Neurol Sci.
31:531–540. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Soto-Otero R, Méndez-Alvarez E,
Hermida-Ameijeiras A, Muñoz-Patiño AM and Labandeira-Garcia JL:
Autoxidation and neurotoxicity of 6-hydroxydopamine in the presence
of some antioxidants. J Neurochem. 74:1605–1612. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han XH, Cheng MN, Chen L, Fang H, Wang LJ,
Li XT and Qu ZQ: 7,8-dihydroxyflavone protects PC12 cells against
6-hydroxydopamine-induced cell death through modulating PI3K/Akt
and JNK pathways. Neurosci Lett. 581:85–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu DP, Zhang K, Zhang ZJ, Sun YW, Guo BJ,
Wang YQ, Hoi PM, Han YF and Lee SM: A novel tetramethylpyrazine
bisnitrone (TN-2) protects against 6-hydroxyldopamine-induced
neurotoxicity via modulation of the NF-κB and the PKCα/PI3-K/Akt
pathways. Neurochem Int. 78:76–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang L, Wang R, Jin M, Huang Y, Liu A, Qin
J, Chen M, Wen S, Pi R and Shen W: Carvedilol attenuates
6-hydroxydo-pamine-induced cell death in PC12 Cells: Involvement of
Akt and Nrf2/ARE pathways. Neurochem Res. 39:1733–1740. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Imai SI, Armstrong CM, Kaeberlein M and
Guarente L: Transcriptional silencing and longevity protein Sir2 is
an NAD-dependent histone deacetylase. Nature. 403:795–800. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rongvaux A, Shea RJ, Mulks MH, Gigot D,
Urbain J, Leo O and Andris F: Pre-B-cell colony-enhancing factor,
whose expression is up-regulated in activated lymphocytes, is a
nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved
in NAD biosynthesis. Eur J Immunol. 32:3225–3234. 2002. View Article : Google Scholar
|
|
11
|
Revollo JR, Grimm AA and Imai S: The
regulation of nicotinamide adenine dinucleotide biosynthesis by
Nampt/PBEF/visfatin in mammals. Curr Opin Gastroenterol.
23:164–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Revollo JR, Grimm AA and Imai S: The NAD
biosynthesis pathway mediated by nicotinamide
phosphoribosyltransferase regulates Sir2 activity in mammalian
cells. J Biol Chem. 279:50754–50763. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ying W: NAD+/NADH and
NADP+/NADPH in cellular functions and cell death: Regulation and
biological consequences. Antioxid Redox Signal. 10:179–206. 2008.
View Article : Google Scholar
|
|
14
|
Furukawa A, Tada-Oikawa S, Kawanishi S and
Oikawa S: H2O2 accelerates cellular
senescence by accumulation of acetylated p53 via decrease in the
function of SIRT1 by NAD+ depletion. Cell Physiol
Biochem. 20:45–54. 2007.
|
|
15
|
Lu L, Tang L, Wei W, Hong Y, Chen H, Ying
W and Chen S: Nicotinamide mononucleotide improves energy activity
and survival rate in an in vitro model of Parkinson's disease. Exp
Ther Med. 8:943–950. 2014.PubMed/NCBI
|
|
16
|
Milne JC, Lambert PD, Schenk S, Carney DP,
Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, et al: Small
molecule activators of SIRT1 as therapeutics for the treatment of
type 2 diabetes. Nature. 450:712–716. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Araki T, Sasaki Y and Milbrandt J:
Increased nuclear NAD biosynthesis and SIRT1 activation prevent
axonal degeneration. Science. 305:1010–1013. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tanno M, Kuno A, Yano T, Miura T, Hisahara
S, Ishikawa S, Shimamoto K and Horio Y: Induction of manganese
superoxide dismutase by nuclear translocation and activation of
SIRT1 promotes cell survival in chronic heart failure. J Biol Chem.
285:8375–8382. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu T, Liu PY and Marshall GM: The
critical role of the class III histone deacetylase SIRT1 in cancer.
Cancer Res. 69:1702–1705. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cohen HY, Miller C, Bitterman KJ, Wall NR,
Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R and Sinclair
DA: Calorie restriction promotes mammalian cell survival by
inducing the SIRT1 deacetylase. Science. 305:390–392. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Salminen A, Huuskonen J, Ojala J,
Kauppinen A, Kaarniranta K and Suuronen T: Activation of innate
immunity system during aging: NF-kB signaling is the molecular
culprit of inflamm-aging. Ageing Res Rev. 7:83–105. 2008.
View Article : Google Scholar
|
|
22
|
Chen WY, Wang DH, Yen RC, Luo J, Gu W and
Baylin SB: Tumor suppressor HIC1 directly regulates SIRT1 to
modulate p53-dependent DNA-damage responses. Cell. 123:437–448.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hasegawa K, Wakino S, Yoshioka K,
Tatematsu S, Hara Y, Minakuchi H, Washida N, Tokuyama H, Hayashi K
and Itoh H: Sirt1 protects against oxidative stress-induced renal
tubular cell apoptosis by the bidirectional regulation of catalase
expression. Biochem Biophys Res Commun. 372:51–56. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jeong J, Juhn K, Lee H, Kim SH, Min BH,
Lee KM, Cho MH, Park GH and Lee KH: SIRT1 promotes DNA repair
activity and deacetylation of Ku70. Exp Mol Med. 39:8–13. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bu Q, Yang Y, Yan G, Hu Z, Hu C, Duan J,
Lv L, Zhou J, Zhao J, Shao X, et al: Proteomic analysis of the
nucleus accumbens in rhesus monkeys of morphine dependence and
withdrawal intervention. J Proteomics. 75:1330–1342. 2012.
View Article : Google Scholar
|
|
26
|
Zhao X, Zhai S, An MS, Wang YH, Yang YF,
Ge HQ, Liu JH and Pu XP: Neuroprotective effects of protocatechuic
aldehyde against neurotoxin-induced cellular and animal models of
Parkinson's disease. PloS One. 8:e782202013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blum D, Torch S, Lambeng N, Nissou M,
Benabid AL, Sadoul R and Verna JM: Molecular pathways involved in
the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the
apoptotic theory in Parkinson's disease. Prog Neurobiol.
65:135–172. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schober A: Classic toxin-induced animal
models of Parkinson's disease: 6-OHDA and MPTP. Cell Tissue Res.
318:215–224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saito Y, Nishio K, Ogawa Y, Kinumi T,
Yoshida Y, Masuo Y and Niki E: Molecular mechanisms of
6-hydroxydopamine-induced cytotoxicity in PC12 cells: Involvement
of hydrogen peroxide-dependent and-independent action. Free Radic
Biol Med. 42:675–685. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fujita H, Ogino T, Kobuchi H, Fujiwara T,
Yano H, Akiyama J, Utsumi K and Sasaki J: Cell-permeable cAMP
analog suppresses 6-hydroxydopamine-induced apoptosis in PC12 cells
through the activation of the Akt pathway. Brain Res. 1113:10–23.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang T and Sauve AA: NAD metabolism and
sirtuins: Metabolic regulation of protein deacetylation in stress
and toxicity. AAPS J. 8:E632–E643. 2006. View Article : Google Scholar
|
|
32
|
Bürkle A: Poly (ADP-ribose). The most
elaborate metabolite of NAD+. FEBS J. 272:4576–4589.
2005. View Article : Google Scholar
|
|
33
|
Halliwell B: Oxidative stress and
neurodegeneration: Where are we now? J Neurochem. 97:1634–1658.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang T and Kraus WL: SIRT1-dependent
regulation of chromatin and transcription: Linking NAD metabolism
and signaling to the control of cellular functions. Biochim Biophys
Acta. 1804:1666–1675. 2010. View Article : Google Scholar :
|