Introduction
Cancer remains a serious disease, although its
treatment options are well established. Chemotherapy is frequently
used to treat cancer, and well-designed schedules for drug
administrations are effective in treating cancer with fewer
side-effects. However, certain cancer cells acquire a
chemotherapy-resistant phenotype, resulting in difficulties in
treatment (1–6). As the chemotherapy-resistant
phenotype is tightly linked to the mechanism of
epithelial-to-mesenchymal transition, chemotherapy-resistant cancer
cells metastasize to distant organs (4–6).
Therefore, the treatment of primary cancer cells and residual
chemotherapy-resistant cancer cell populations are required to
completely prevent cancer recurrence and metastasis (4–7).
Rubus coreanus Miquel (RCM), also known as
Korean black raspberry, has long been used as a food and
traditional medicine in Korea, Japan and China. Previous studies
have reported that RCM is effective in the prevention and treatment
of certain diseases, including cancer and inflammatory diseases
(8–16). In cancer, RCM has been shown to
repress the in vitro metastatic abilities of prostate cancer
cells and cause apoptotic cell death (9,15).
However, the effect of RCM in other types of cancer has not been
fully investigated, even in in vitro experimental settings,
nor has the effect of RCM against anticancer drug-resistant cancer
cells. Therefore, the role of RCM in cancer remains to be fully
elucidated. In addition, although ellagic acid and quercetin,
compounds found in RCM extract, are known to have anticancer
properties (17–19), their effects in
chemotherapy-resistant cancer cells remain to be elucidated.
The present study aimed to investigate the effect of
RCM on chemotherapy-resistant cancer cells. Doxorubicin-resistant
NCI/ADR-RES ovarian cancer cells were used, as these cells are a
well-established in vitro model system for investigations on
chemotherapy-resistance (20). The
current study hypothesized that RCM is effective in the treatment
of chemotherapy-resistant cancer.
Materials and methods
Cell culture and drug preparation
The doxorubicin-resistant human ovarian cancer cell
line, NCI/ADR-RES, was provided by Dr Hwa Jeong Lee (Ewha Womans
University, Seoul, Korea). The NCI/ADR-RES cells were cultured in
RPMI 1640 (WelGENE, Inc., Daegu, Korea) with 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
1% antibiotics-antimycotic solution (WelGENE, Inc.) in a humidified
atmosphere with 5% CO2 at 37°C. The RCM extract was
prepared by Hanpoong Pharmaceutical Company (Jeonju, Korea). The
RCM was dissolved in 30% ethanol. Ellagic acid and quercetin were
purchased from Tocris Biosciences (Boston, MA, USA) and
Sigma-Aldrich (St. Louis, MO, USA), respectively. Ellagic acid was
dissolved in distilled water at 25 mM, and quercetin was dissolved
in dimethyl sulfoxide (DMSO; Sigma-Aldrich) at 30 mg/ml.
Cell viability assay
An MTT assay was used to analyze the cell
viabilities. The cells were seeded in flat-bottomed 96-well
microplates at a density of 5×103 cells/well, and then
treated with 0, 12.5, 25, 50, 100 and 200 µg/ml RCM, with or
without doxorubicin (2 µg/ml; Sigma-Aldrich) for 72 h. Other
groups of cells were treated with ellagic acid or quercetin at
different concentrations (0, 10, 20 and 30 µg/ml) for 72 h.
MTT solution (Sigma-Aldrich) was added to the medium and incubated
at 37°C for 2 h. Insoluble formazan was dissolved with DMSO and
measured at 570 nm using a VersaMax ELISA microplate reader
(Molecular Devices, LLC, Sunnyvale, CA, USA). The data examined was
the average of three wells, and the experiment was independently
repeated three times.
Analysis of cell apoptosis using flow
cytometry
Annexin V detection assays were performed using
Annexin V, Alexa Fluor® 488 Conjugate (Molecular probes;
Thermo Fisher Scientific, Inc.) and 7-aminoactinomycin D
(Sigma-Aldrich). The NCI/ADR-RES cells were seeded at a density of
3×105 in a 60 mm plate. The cells were treated with RCM
at concentrations of 0, 50, 100 and 200 µg/ml for 24 h at
37°C, or were treated with the ellagic acid or quercetin compounds
at concentrations of 0, 10, 20 and 30 µg/ml. The cells were
pretreated with 50 µM of the JNK inhibitor, SP600125
(Sigma-Aldrich), for 1 h at 37°C. The cells were then washed with
phosphate-buffered saline (PBS), stained with Annexin V-fluorescein
isothiocyanate (Thermo Fisher Scientific, Inc.) and
7-aminoactinomycin D, and then analyzed using flow cytometry.
Western blot analysis
The NCI/ADR-RES cells were seeded at a density of
8.5×105 in a 100 mm plate. The NCI/ADR-RES cells were
treated with RCM (0, 12.5, 25, 50, 100, 200 µg/ml), ellagic
acid (0, 10, 20 and 30 µg/ml) or quercetin (0, 10, 20 and 30
µg/ml) for 24 h. Other groups of NCI/ADR-RES cells were
treated with SP600125 at 50 µM for 1 h, and then treated
with RCM (100 µg/ml), ellagic acid (10 µg/ml) or
quercetin (10 µg/ml) for 24 h at 37°C. Protein was obtained
following cell lysis with radioimmunoprecipitation assay buffer
(Biosesang, Inc., Seongnam, Korea). Subsequently, 15 µg of
protein was separated by standard 10 or 12% SDS-PAGE, and
transferred onto polyvinylidene fluoride membranes. Mouse
monoclonal anti-human B cell lymphoma-2 (Bcl-2; cat. no. sc-7382),
mouse monoclonal anti-human Bcl-2-associated X protein (BAX; cat.
no. sc-7480), goat polyclonal anti-human cleaved caspase 9 (cat.
no. sc-22182), rabbit polyclonal anti-human Raf-1 (cat. no.
sc-133), mouse monoclonal anti-human p-JNK (cat. no. sc-6254),
mouse monoclonal anti-human p-extracellular signal-regulated kinase
(ERK)2 (cat. no. sc-7383), mouse monoclonal anti-human ERK2 (cat.
no. sc-1647), goat poly-clonal anti-human mammalian target of
rapamycin (mTOR; cat. no. sc-1549), GSK3β and rabbit polyclonal
anti-human p53 (cat. no. 6243) antibodies were purchased from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) and used at a
dilution of 1:1,000. Rabbit monoclonal anti-human Fas (cat. no.
4233), rabbit monoclonal anti-human Trail (cat. no. 3219), rabbit
monoclonal anti-human cleaved caspase-8 (cat. no. 9496), rabbit
polyclonal anti-human cleaved caspase-3 (cat. no. 9661), rabbit
polyclonal anti-human poly (ADP-ribose) polymerase (PARP; cat. no.
9542), rabbit polyclonal anti-human p-cRaf (cat. no. 9421), mouse
monoclonal anti-human JNK (cat. no. 3708), rabbit polyclonal
anti-human AKT (cat. no. 9272), rabbit anti-human polyclonal
phosphorylated (p)-AKT (ser473; cat. no. 9271), rabbit polyclonal
anti-human p-mTOR (cat. no. 2971), rabbit polyclonal anti-human
p-GSK3β (cat. no. 9332) and rabbit polyclonal anti-human p-p53
(cat. no. 9284) antibodies were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA) and used at a dilution of
1:1,000. Mouse monoclonal anti-human cytochrome C antibody was
obtained from BD Biosciences (San Jose, CA, USA; cat. no. 556433)
and used at a dilution of 1:1,000. Mouse monoclonal anti-human
anti-α-tubulin antibody (cat. no. T5168) was obtained from
Sigma-Aldrich and used at a dilution of 1:100,000. The membranes
were incubated at 4°C overnight with specific primary antibodies,
and were washed three times in PBS with 0.01% Tween (Sigma-Aldrich;
PBST). The membranes were then incubated at room temperature for 1
h with horseradish peroxidase-conjugated goat anti-mouse (cat. no.
074-1806), goat anti-rabbit (cat. no. 474-1506) or rabbit anti-goat
(cat. no. 14-13-06) secondary antibodies (KPL, Inc., Gaithersburg,
MD, USA) used at dilutions of 1:10,000–1:50,000. Subsequently, the
membranes were washed with PBST three times and the protein bands
were visualized using an enhanced chemiluminescence system,
EZ-Western Lumi Pico (Dogen-Bio, Seoul, Korea) and exposed to X-ray
film (Agfa-Gevaert N.V., Mortsel, Belgium).
Statistical analysis
Microsoft Excel 2010 software (Microsoft
Corporation, Redmond, WA, USA) was used for statistical analysis.
Data are presented as the mean ± standard deviation of at least
three separate tests. The Student's t-test was used for
single-variable comparisons and P<0.05 was considered to
indicate a statistically significant difference.
Results
RCM causes apoptosis of
doxorubicin-resistant ovarian cancer cells
To examine the effect of RCM in
chemotherapy-resistant cancer, doxorubicin-resistant NCI/ADR-RES
ovarian cancer cells were treated with RCM extract at different
concentrations for 72 h and then subjected to cell viability
assays. RCM at concentrations of 12.5 and 25 µg/ml slightly
increased cell viability but cell viabiltiy was not affected at 50
µg/ml. RCM at 100 and 200 µg/ml significantly reduced
cell viability. No synergistic effect was observed when the cells
were co-treated with RCM and doxorubicin (Fig. 1A). Therefore, RCM inhibited the
viability of doxorubicin-resistant NCI/ADR-RES ovarian cancer
cells.
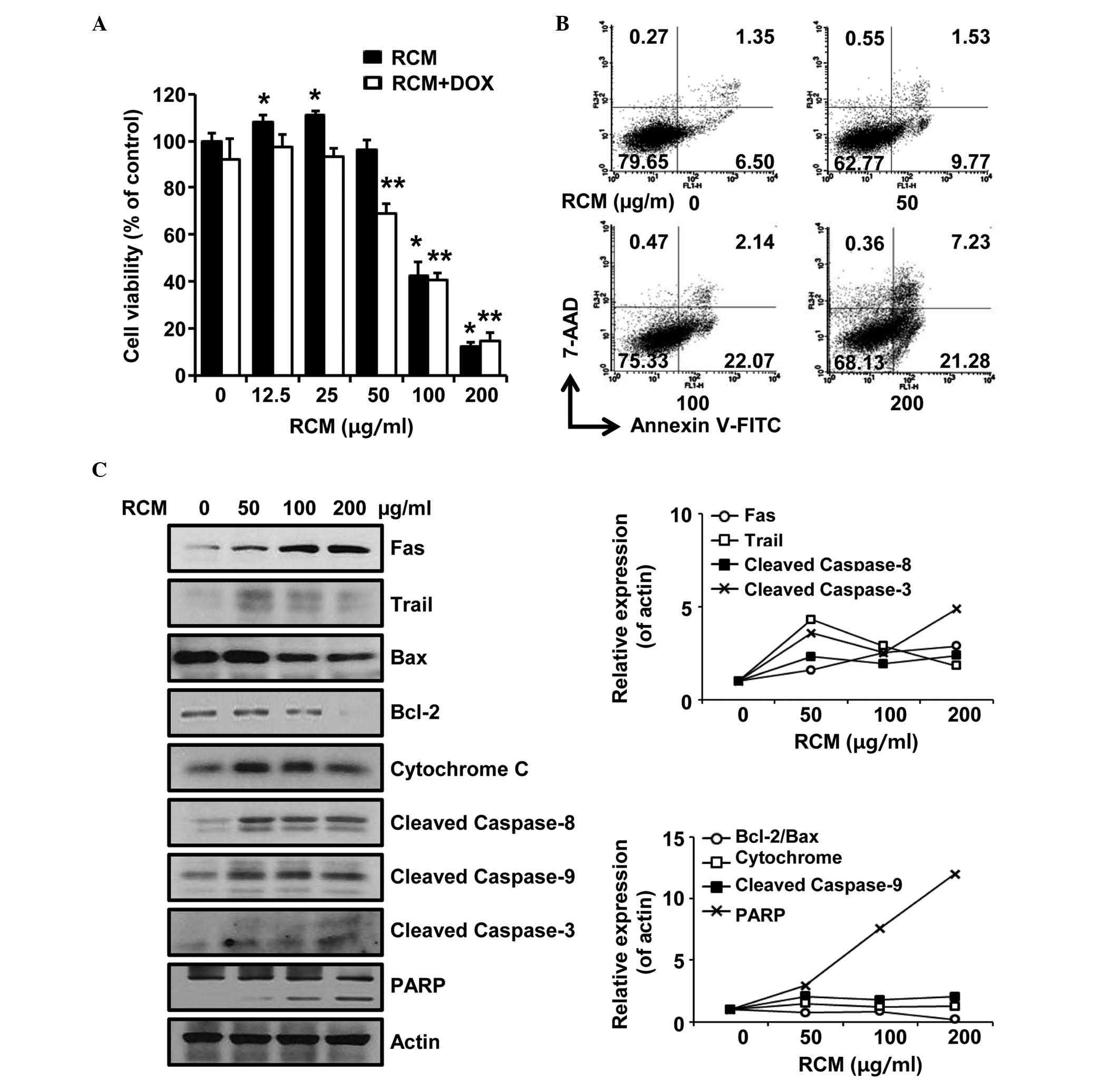 | Figure 1RCM induces apoptosis of NCI/ADR-RES
cells. (A) MTT assay. Cells were treated with the indicated
concentrations of RCM and 2 µg/ml of doxorubicin was added.
Viability assays were performed 72 h following treatment. RCM at
100–200 µg/ml significantly reduced cell viability.
Experiments were performed in triplicate and independently repeated
at least three times. Data are presented as the mean ± standard
deviation for triplicate experiments. *P<0.05
vs. RCM-untreated cells, **P<0.05 vs.
doxorubicin-treated cells.. (B) Cells were treated with the
indicated concentrations of RCM for 24 h and then subjected to
Annexin V assays. RCM increases the numbers of apoptotic cells. (C)
Cells were treated with RCM at the indicated concentrations for 24
h and then subjected to Western blot assays. Actin was detected as
an internal control. Left panel, RCM induced apoptosis-associated
proteins; right panel, data represent quantitative results for left
panel. RCM, Rubus coreanus Miquel; FITC, fluorescein
isothiocyanate; 7-AAD, 7-aminoactinomycin D; DOX, doxorubicin;
PARP, poly (ADP-ribose) polymerase; Bcl-2, B cell lymphoma-2; BAX,
Bcl-2-associated X protein. |
Subsequently, the present study examined whether RCM
caused apoptosis of the NCI/ADR-RES cells. In the Annexin V assays,
it was found that RCM increased the numbers of apoptotic cells
(Fig. 1B). Consistently, it was
found that RCM altered the expression levels of
apoptotic-associated proteins (Fig.
1C). Therefore, these data indicated that RCM caused apoptosis
of chemotherapy-resistant ovarian cancer cells in a dose-dependent
manner.
Ellagic acid and quercetin cause
apoptosis of doxorubicin-resistant ovarian cancer cells
As RCM induced the apoptosis of NCI/ADR-RES cells,
the present study further examined whether specific compounds found
in RCM also have effects on apoptosis. When the NCI/ADR-RES cells
were treated with the RCM-derived phytochemicals, ellagic acid or
quercetin, at different concentrations for 72 h, these compounds
were found to reduce cell viabilities (Fig. 2A). In addition, ellagic acid and
quercetin caused apoptotic cell death of the NCI/ADR-RES cells when
the cells were treated with either phytochemical for 24 h (Fig. 2B and C). Therefore, these data
suggested that the effects of RCM on NCI/ADR-RES cells was, in
part, due to the roles of these two compounds.
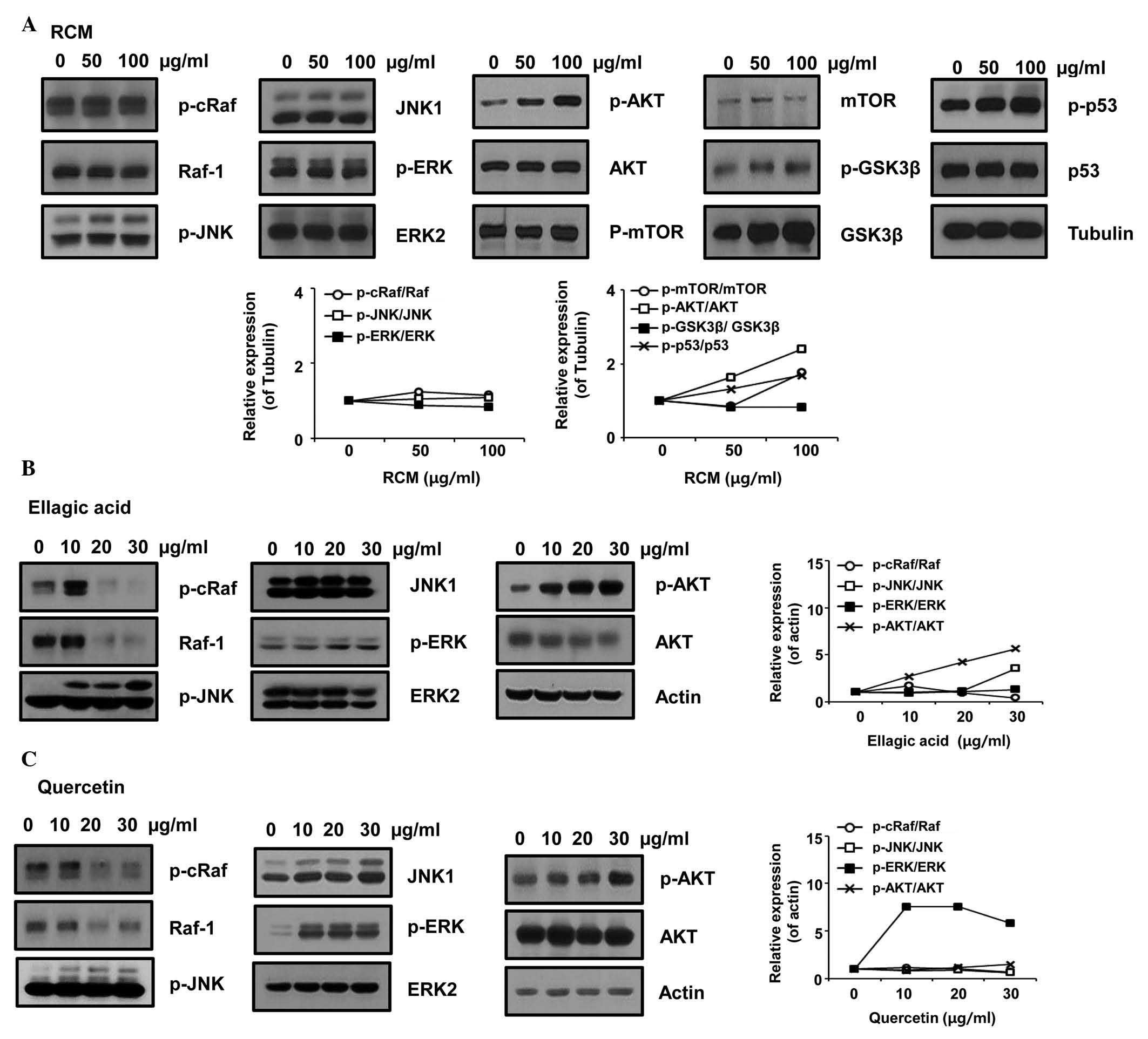
RCM induces the phosphorylation of JNK
and AKT
As RCM and its compounds induced the apoptosis of
NCI/ADR-RES cells, the present study further examined the effect of
RCM on intracellular signaling molecules. RCM, ellagic acid and
quercetin were observed to induce the phosphorylation of JNK and
AKT, whereas each compound decreased Raf phosphorylation. The
results also showed that ERK phosphorylation was not affected by
RCM and ellagic acid, however, it was significantly increased by
quercetin treatment (Fig. 3).
Therefore, these data indicated that RCM and its compounds may
cause apoptosis via JNK and/or AKT.
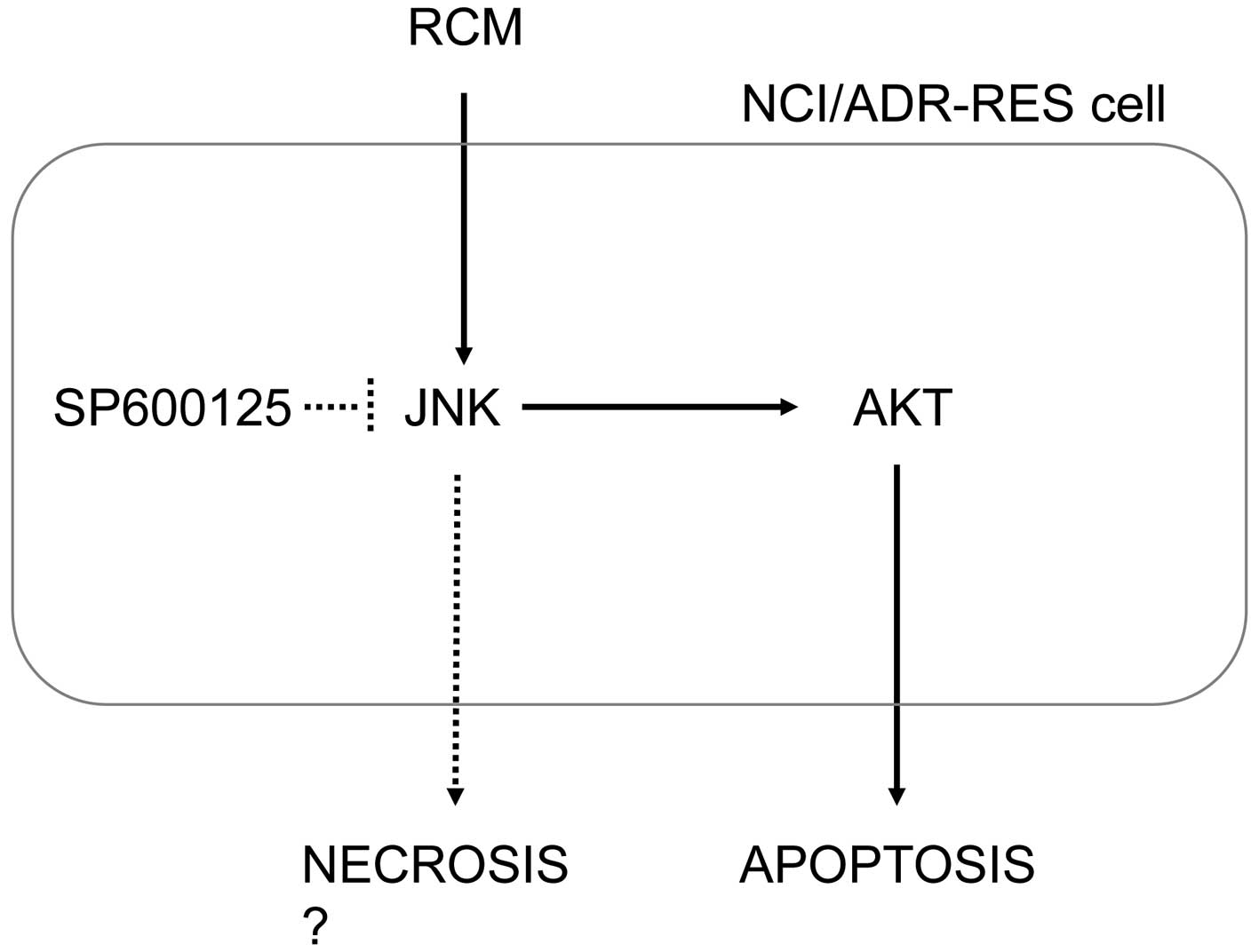
JNK is required for RCM-induced apoptosis
of NCI/ADR-RES cells
As RCM and its compounds were found to commonly
induce the phosphorylation of JNK and AKT, the present study
examined whether JNK was required for the effects of RCM on
NCI/ADR-RES cells. When the cells were pretreated with the JNK
inhibitor, SP600125, for 1 h and then treated with RCM, ellagic
acid or quercetin for another 24 h, JNK inhibition prevented
apoptosis, but increased the numbers of necrotic cells (Fig. 4A). Consistently, JNK inhibition
reduced PARP cleavage and inhibited the activation of caspases
(Fig. 4B). Therefore, JNK
phosphorylation appeared to be required for RCM-induced apoptotic
cell death, and the inhibition of JNK phosphorylation may switch
apoptosis to necrosis in RCM treatment.
 | Figure 4JNK inhibition inhibits RCM-induced
apoptosis. (A) Cells were treated with RCM, ellagic acid or
quercetin for 24 h and then subjected to Annexin V assays. Cell
numbers are marked in the quadrants of the graphs. JNK inhibition
prevented apoptosis by RCM, ellagic acid and quercetin, but
increased the numbers of necrotic cells. (B and C) Cells were
treated with the RCM, ellagic acid or quercetin, with or without
SP600125 for 24 h and then subjected to Western blot analysis. Top
panel, JNK inhibition reduced PARP cleavage, the activation of
caspases, and AKT phosphorylation. Bottom panel, data represent
quantitative results. RCM, Rubus coreanus Miquel; JNK, c-Jun
N-terminal kinase; FITC, fluorescein isothiocyanate; 7-ADD,
7-aminoactinomycin D' PARP, poly (ADP-ribose) polymerase; ERK2,
extracellular signal-regulated kinase 2; mTOR, mammalian target of
rapamycin; p-, phosphorylated. |
Subsequently, the present study examined the
association between JNK and AKT. As JNK phosphorylation was
inhibited, the levels of AKT phosphorylation were also reduced
(Fig. 4C). Therefore, these data
indicated that RCM-induced JNK phosphorylation mediated the
phosphorylation of AKT.
Discussion
In the present study, it was found that RCM and its
compounds, ellagic acid and quercetin, caused JNK-dependent
apoptotic cell death of doxorubicin-resistant NCI/ADR-RES ovarian
cancer cells. Although RCM and its compounds have been reported to
inhibit prostate cancer growth in vitro (9,15),
their inhibitory role in chemotherapy-resistant cancer has not been
reported.
Treatment with RCM led to apoptotic cell death of
the NCI/ADR-RES cells, which was found to occur in a dose-dependent
manner. NCI/ADR-RES cells express high levels of MDR1, thereby
acquiring a chemotherapy-resistant phenotype (20). RCM appeared to inhibit MDR1 efflux
and to regulate the expression of MDR1 (data not shown). However,
RCM showed no synergism with doxorubicin. In assessing the
potential synergistic effect, the highest dose of RCM (200
µg/ml) was effective in the inhibition of MDR1 efflux. As
this highest concentration of RCM caused marked apoptosis in the
experimental group, RCM-mediated apoptotic signaling was likely to
precede any synergistic effect with doxorubicin.
As RCM, ellagic acid and quercetin were observed to
inhibit cell viability to result in apoptosis, the present study
hypothesized that these compounds, in part, mediated the effect of
RCM on the NCI/ADR-RES cells. RCM and its compounds activated
caspase-8, caspase-9 and caspase-3, and reduced the level of BAX.
In addition, whereas ellagic acid did not affect the level of Fas,
RCM and quercetin increased Fas levels. The present study also
confirmed that RCM affected mitochondrial membrane potential (data
not shown). Thus, RCM appeared to activate the extrinsic and the
intrinsic apoptotic pathways. Furthermore, RCM led to the
accumulation of cells at the S phase (data not shown). Therefore,
it was hypothesized that RCM has pleiotropic roles in cancer
cells.
The present study also demonstrated that RCM,
ellagic acid and quercetin uniquely activated JNK and AKT, although
ellagic acid and quercetin differentially regulated Raf and ERK. In
addition, the JNK inhibitor, SP600125, reduced the apoptotic effect
of RCM. Thus, JNK mediated RCM-induced apoptosis. Consistently, the
JNK inhibitor inhibited the apoptotic effects of ellagic acid and
quercetin. Notably, it was further revealed that JNK inhibition
induced necrotic cell death upon treatment with RCM or its
compounds. Whereas the regulation of apoptosis and necrosis by JNK
remains to be fully elucidated, these data consistently showed that
JNK appeared to determine cell death, apoptosis and necrosis
(Fig. 5). In addition, JNK
inhibition decreased the phosphorylation of AKT induced by RCM and
its compounds, indicating that JNK is upstream of AKT. Accordingly,
AKT inhibition also reduced RCM-induced apoptotic cell death,
whereas the phosphorylation of JNK was not altered. In addition,
increased necrosis was also found in the cells treated with AKT
inhibitor (data not shown). A previous study revealed that JNK
directly interacts with AKT in the rat ventricular myocytes
(21). However, AKT phosphorylates
JNK, which inhibits apoptosis in 293 and HeLa cells (22). Therefore, although a direct
interaction between JNK and AKT was not examined, the present study
suggested that the JNK-AKT pathway is crucial for the apoptosis of
chemotherapy-resistant cancer cells.
In conclusion, the data obtained in the present
study consistently demonstrated that RCM induced NCI/ADR-RES cell
death. In addition, RCM-induced JNK activation determined the cell
death as either apoptosis or necrosis. Although further information
is required on the chemical compounds found in RCM extract, the
compounds examined in the present study, ellagic acid and
quercetin, were found to induce JNK-mediated apoptosis. Therefore,
the results of the present study suggested that RCM may be
beneficial for the treatment of chemotherapy-resistant cancer.
Acknowledgments
This study was supported by a grant from the Korean
Medicine R&D Project of the Ministry of Health and Welfare
(grant no. B120014). This is part of the master thesis of Ms. Min
Kyoung Kim.
References
|
1
|
Obenauf AC, Zou Y, Ji AL, Vanharanta S,
Shu W, Shi H, Kong X, Bosenberg MC, Wiesner T, Rosen N, et al:
Therapy-induced tumour secretomes promote resistance and tumour
progression. Nature. 520:368–372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fu F, Nowak MA and Bonhoeffer S: Spatial
heterogeneity in drug concentrations can facilitate the emergence
of resistance to cancer therapy. PLoS Comput Biol. 11:e10041422015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oshimori N, Oristian D and Fuchs E: TGF-β
promotes heterogeneity and drug resistance in squamous cell
carcinoma. Cell. 160:963–976. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borst P: Cancer drug pan-resistance:
Pumps, cancer stem cells, quiescence, epithelial to mesenchymal
transition, blocked cell death pathways, persisters or what? Open
Biol. 2:1200662012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meng F and Wu G: The rejuvenated scenario
of epithelial-mesenchymal transition (EMT) and cancer metastasis.
Cancer Metastasis Rev. 31:455–467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsumoto K, Onda T and Yaegashi N:
Pharmacotherapy for recurrent ovarian cancer: Current status and
future perspectives. Jpn J Clin Oncol. 45:408–410. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jung KA, Han D, Kwon EK, Lee CH and Kim
YE: Antifatigue effect of Rubus coreanus Miquel extract in mice. J
Med Food. 10:689–693. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim Y, Kim J, Lee SM, Lee HA, Park S, Kim
Y and Kim JH: Chemopreventive effects of Rubus coreanus Miquel on
prostate cancer. Biosci Biotechnol Biochem. 76:737–744. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi C, Lee H, Lim H, Park S, Lee J and Do
S: Effect of Rubus coreanus extracts on diabetic osteoporosis by
simultaneous regulation of osteoblasts and osteoclasts. Menopause.
19:1043–1051. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim SK, Kim H, Kim SA, Park HK and Kim W:
Anti-inflammatory and anti-superbacterial activity of polyphenols
isolated from black raspberry. Korean J Physiol Pharmacol.
17:73–79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Im SE, Nam TG, Lee H, Han MW, Heo HJ, Koo
SI, Lee CY and Kim DO: Anthocyanins in the ripe fruits of Rubus
coreanus Miquel and their protective effect on neuronal PC-12
cells. Food Chem. 139:604–610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chae HJ, Yim JE, Kim KA and Chyun JH:
Hepatoprotective effects of Rubus coreanus miquel concentrates on
liver injuries induced by carbon tetrachloride in rats. Nutr Res
Pract. 8:40–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi MR, Lee MY, Hong JE, Kim JE, Lee JY,
Kim TH, Chun JW, Shin HK and Kim EJ: Rubus coreanus Miquel
ameliorates scopolamine-induced memory impairments in ICR mice. J
Med Food. 17:1049–1056. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim Y, Lee SM and Kim JH: Unripe Rubus
coreanus Miquel suppresses migration and invasion of human prostate
cancer cells by reducing matrix metalloproteinase expression.
Biosci Biotechnol Biochem. 78:1402–1411. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shin JS, Cho EJ, Choi HE, Seo JH, An HJ,
Park HJ, Cho YW and Lee KT: Anti-inflammatory effect of a
standardized triterpenoid-rich fraction isolated from Rubus
coreanus on dextran sodium sulfate-induced acute colitis in mice
and LPS-induced macrophages. J Ethnopharmacol. 158:291–300. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Basu A, Nguyen A, Betts NM and Lyons TJ:
Strawberry as a functional food: An evidence-based review. Crit Rev
Food Sci Nutr. 54:790–806. 2014. View Article : Google Scholar
|
|
18
|
Zhang HM, Zhao L, Li H, Xu H, Chen WW and
Tao L: Research progress on the anticarcinogenic actions and
mechanisms of ellagic acid. Cancer Biol Med. 11:92–100.
2014.PubMed/NCBI
|
|
19
|
Miles SL, McFarland M and Niles RM:
Molecular and physiological actions of quercetin: Need for clinical
trials to assess its benefits in human disease. Nutr Rev.
72:720–734. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liscovitch M and Ravid D: A case study in
misidentification of cancer cell lines: MCF-7/AdrR cells
(re-designated NCI/ADR-RES) are derived from OVCAR-8 human ovarian
carcinoma cells. Cancer Lett. 245:350–352. 2007. View Article : Google Scholar
|
|
21
|
Golden HB, Watson LE, Nizamutdinov D, Feng
H, Gerilechaogetu F, Lal H, Verma SK, Mukhopadhyay S, Foster DM,
Dillmann WH and Dostal DE: Anthrax lethal toxin induces acute
diastolic dysfunction in rats through disruption of the
phospholamban signaling network. Int J Cardiol. 168:3884–3895.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim AH, Khursigara G, Sun X, Franke TF and
Chao MV: Akt phosphorylates and negatively regulates apoptosis
signal-regulating kinase 1. Mol Cell Biol. 21:893–901. 2001.
View Article : Google Scholar : PubMed/NCBI
|