Introduction
Laryngeal squamous cell carcinoma (LSCC) is one of
the most common head and neck cancers in the world and accounts for
almost 90% of all malignant laryngeal cancers (1). LSCC is accompanied by progressively
impaired laryngeal function that seriously affects the quality of
life of those affected by the disease (2,3).
Currently, the primary clinical interventions for LSCC are invasive
radiotherapy and surgery. Surgical procedures, such as a total
laryngectomy, are common, particularly in patients with advanced
LSCC (4). Over the previous
decades, novel therapeutic strategies have been developed and
introduced for the clinical treatment of LSCC; however, no
significant improvement in the prognosis of this disease has been
achieved (5). Thus, an
understanding of the molecular mechanisms underlying the pathology
of LSCC is crucial for the identification of novel therapeutic
targets for optimizing treatment.
Previous studies have suggested that there are
numerous microRNAs (miRNAs) with aberrant expression in LSCC
tissue, which may be potential therapeutic targets (6,7).
miRNAs are endogenous, single-stranded, non-coding RNAs, ~20
nucleotides long. Of the numerous LSCC-associated miRNAs that were
identified by miRNA microarray analysis, miR-21 was subsequently
found to be upregulated (7). In
addition, miR-21 was shown to function as a potent LSCC tumor
promoter by inhibiting cell apoptosis through the regulation of Ras
expression (8). miR-221 was
confirmed in vitro to induce the proliferation of laryngeal
cancer cells via targeting apoptotic protease activating factor-1
(Apaf-1) in in vitro studies (9). Bioinformatics analysis indicated that
the overexpression of the LSCC-associated miRNA miR-221 may
synergistically act with miR-21 in cell apoptosis (10). Additionally, experimental evidence
revealed that the phosphatase and tensin homolog (PTEN) gene was
associated with apoptosis in LSCC (11) and may be targeted by miR-21 and
miR-221 (12,13). This finding was consistent with the
observation of low expression of the PTEN gene and protein in a
HEp-2 LSCC cell line (11,14). PTEN is an important negative
regulatory factor of Akt (15),
and the activation of PTEN is associated with LSCC metastasis
(16). As a result, Akt negatively
controls the expression level of the tumor-suppressor gene tumor
protein p53 (TP53) in cancer cells (17). Low levels of p53, which is the
protein product of the TP53 gene, aid the growth of LSCC tumors, as
p53 is a common transcriptional activator of numerous pro-apoptotic
miRNAs, including miR-15a, miR-16-1, miR-26a, miR-34a, miR-143 and
miR-203 (18).
Overall, the accumulated data indicate that
simultaneous overexpression of miR-21 and miR-221 in LSCC may
aggregately weaken the negative regulation of Akt by PTEN,
resulting in the inefficiency of p53 to activate the transcription
of pro-apoptotic miRNAs. Based on this hypothesis, the present
study considered that co-inhibition of miR-21 and miR-221 may
contribute to a reversal of the imbalance between anti-apoptotic
and pro-apoptotic miRNAs established in LSCC. To investigate the
validity of the present hypothesis, an in vitro experiment
was performed, in which anti-miRNA oligonucleotide (AMO)-21 and
AMO-221 were co-transfected into the LSCC Hep-2 cell line. Cellular
apoptosis and the protein expression levels of PTEN and
phosphorylated-Akt (p-Akt) were monitored. The changes in the
cellular abundance of the aforementioned 6 pro-apoptotic miRNAs
were also investigated. The aim of the present study was to offer a
potential miRNA-based therapeutic method for the treatment of
LSCC.
Materials and methods
Cell culture and miRNA transfection
The LSCC Hep-2 cell line was purchased from the
Institute of Biochemistry and Cell Biology, Chinese Academy of
Science (Shanghai, China). The cells were maintained in Gibco
Dulbecco's modified Eagle's medium (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% Gibco fetal bovine serum
(FBS; Thermo Fisher Scientific, Inc.), 100µg/ml penicillin
(Beijing Leagene Biotech Co., Ltd., Beijing, China) and 100
µg/ml streptomycin (Beijing Leagene Biotech Co., Ltd.).
Subsequently, the cells were transferred to 96-well plates and
cultured in fresh medium, without antibiotics. For transfection,
cells were washed with serum-free medium once and then incubated
with 4 ml serum-free medium for 4–6 h. AMO-21 or AMO-221 and
Invitrogen Lipofectamine 2000 (Thermo Fisher Scientific, Inc.) were
separately mixed with 500 µl Gibco Opti-MEM I Reduced Serum
medium (Thermo Fisher Scientific, Inc.) for 5 min. Then, the two
mixtures were combined and incubated for 20 min at room
temperature. The Lipofectamine 2000 and AMO-21 or AMO-221 mixture
was added to the cells and incubated at 37°C for 6 h. Subsequently,
5 ml fresh medium containing 10% FBS was added to the flasks, and
the cells were maintained in the culture conditions until use.
Methyl thiazolyl tetrazolium (MTT)
assays
The colorimetric MTT assay was used to assess the
viability of cells cultured in 96-well culture plates. The dye
measures mitochondrial dehy-drogenase activity and utilizes the
fact that viable cells, rather than dead cells, reduce MTT.
Subsequent to AMO transfection, 5×103 cells were
incubated with 10 µl MTT (0.5 mg/ml) at 37°C for 4 h. The
purple formazan crystals were dissolved by the addition of 100
µl dimethyl sulfoxide (Tianjin Fu Chen Chemical Reagent
Factory, Tianjin, China). The absorbance was measured at a
wavelength of 490 nm using an EAR 340 AT Easy Microplate Reader
(SLT Lab-Instruments, SLT Labinstruments GmbH, Salzburg,
Austria).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Cellular expression levels of miR-15a, miR-16-1,
miR-26a, miR-34a, miR-143 and miR-203 were determined by RT-qPCR
(Table I). Total RNA (1 µg)
from each sample was used to generate complementary (c)DNA with
Moloney murine leukemia virus reverse transcriptase (Promega
Corporation, Madison, WI, USA). Subsequent to an initial
denaturation step at 95°C for 10 min using an Applied Biosystems
SYBR Green PCR Master Mix purchased from Thermo Fisher Scientific,
Inc., the reaction was subjected to 40 cycles of 95°C for 15 sec,
60°C for 30 sec and 72°C for 30 sec. An Applied Biosystems 7500
Fast Real-Time PCR Systems (Thermo Fisher Scientific, Inc.) was
used to perform cDNA amplification, including routine product
checking with the internal dissociation curve software. cDNA
transcript quantities were compared using the previously described
relative quantification method (19). The amount of detected miRNA was
normalized against the amount of the endogenous control, ubiquitin
6. The ratio of the relative value to the control sample was
provided as 2−ΔΔCq (19).
 | Table IPrimers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene name | Forward primer,
5′-3′ | Reverse primer,
5′-3′ |
|---|
| hsa-miR-15a | TAGCAGCACATAATGG | TGCGTGTCGTGGAGTC |
| hsa-miR-16-1 | TAGCAGCACGTAAATA | TGCGTGTCGTGGAGTC |
| hsa-miR-26a | TTCAAGTAATCCAGGA | TGCGTGTCGTGGAGTC |
| hsa-miR-34a | TGGCAGTGTCTTAGCT | TGCGTGTCGTGGAGTC |
| hsa-miR-143 | TGAGATGAAGCACTG | TGCGTGTCGTGGAGTC |
| hsa-miR-203 | GTGAAATGTTTAGGAC | TGCGTGTCGTGGAGTC |
| U6 |
GCTTCGGCAGCACATATACTAAAAT |
CGCTTCACGAATTTGCGTGTCAT |
Terminal
deoxynucleotidyl-transferase-mediated deoxynucleotide triphosphate
nick end labeling (TUNEL)-staining assays
Subsequent to washing 3 times with
phosphate-buffered saline (PBS; Beyotime Institute of
Biotechnology, Haimen, China), the cells were fixed with 4%
paraformaldehyde (Beijing Dingguo Changsheng Biotechnology Co.,
Ltd., Beijing, China) and permeabilized with 0.1% Triton X-100
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) and 0.1 mol/l sodium citrate buffer (Shanghai Haoran Bio
Technologies Co., Ltd., Shanghai, China). Subsequently, the In
Situ Cell Death Detection kit (Roche Diagnostics, Indianapolis,
IN, USA) was used to label apoptotic cells, and the nuclei were
stained with 4′,6-diamidino-2-phenylindole (Roche Molecular
Diagnostics, Pleasanton, CA, USA). The total number of cells and
the TUNEL-positive cells were counted using Image-Pro Plus software
(Media Cybernetics, Inc., Rockville, MD, USA) to calculate the
apoptosis rate, which was defined as the ratio of apoptotic cells
to total cells.
Western blot analysis
Total protein was extracted from the cells for
protein immunoblotting using a standard protocol. Briefly, 80
µg protein samples were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and blotted onto
nitrocellulose membranes. The blots were blocked with 5% non-fat
milk for 2 h at room temperature and probed with primary
antibodies, consisting of rabbit polyclonal anti-PTEN (dilution,
1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA; cat.
no. 9552), rabbit polyclonal anti-p-Akt (dilution, 1:1,000; Cell
Signaling Technology, Inc.; cat. no. 9271), rabbit polyclonal
anti-p53 (dilution, 1:1,000; Cell Signaling Technology, Inc.; cat.
no. 2527), rabbit polyclonal anti-β-actin (dilution, 1:1,000; BD
Biosciences, San Jose, CA, USA; cat. no. 612656) antibodies, in PBS
and incubated at 4°C overnight. Following overnight binding, the
membranes were washed with PBS-Triton X-100 and incubated for 1 h
at room temperature with secondary antibodies, as follows: Alexa
Fluor® 700-conjugated goat anti-mouse IgG (dilution, 1:8,000;
Invitrogen; Thermo Fisher Scientific, Inc.; cat. no. A-21036); and
IRDye ® 800CW goat anti-rabbit IgG (dilution, 1:8,000; LI-COR
Biosciences, Lincoln, NE, USA; cat. no. 926-32211). Finally, the
bands were collected using an Odyssey CLx Infrared Image System
fluorescent scanning system (LI-COR Biosciences) and quantified
with the Odyssey v1.2 software (LI-COR Biosciences) by measuring
intensity of the fluorescence as the area × optical density.
β-actin was used as an internal control. The results were expressed
as fold changes by normalizing the data to the control values.
Statistical analysis
All data were expressed as the mean ± standard
deviation. Statistical analyses, including an unpaired two-tailed
Student's t-test, and one-way analysis of variance followed by the
Bonferroni's multiple comparison post-hoc test, were conducted
using GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
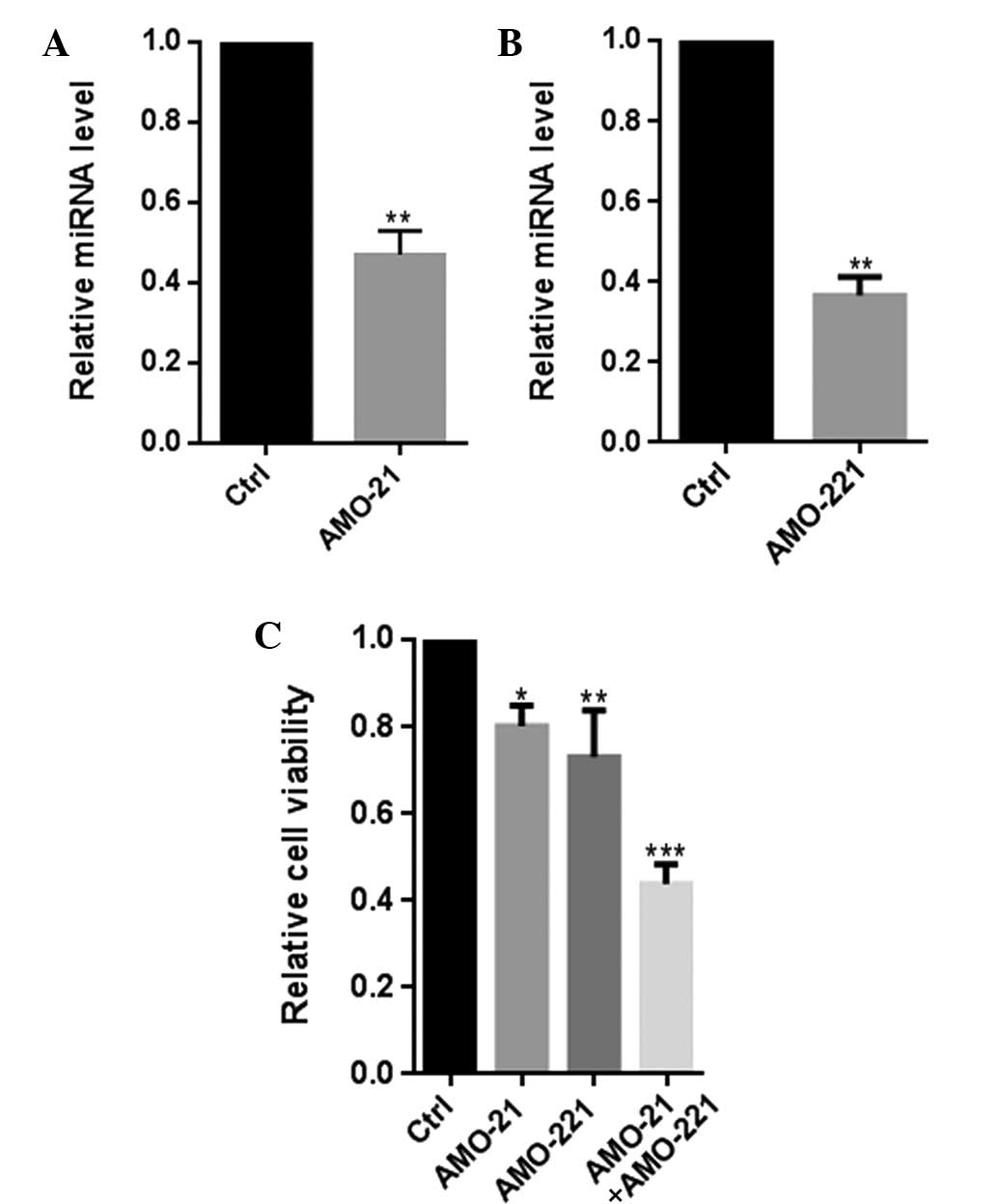
Co-transfection of AMO-21 and AMO-221
significantly decreased the cell viability of Hep-2 cells
Previously, it was confirmed that miR-21 and miR-221
were co-overexpressed in patients with LSCC (7,8). In
the present study, co-transfection of AMO-21 and AMO-221 were found
to clearly reduce the cellular expression of miR-21 and miR-221,
leading to a significant decline in the viability of Hep-2 cells,
(P<0.001; Fig. 1). Overall,
these results indicate a functional interaction between miR-21 and
miR-221 in their involvement in the pathology of LSCC.
The results of the MTT assays were
verified by observations from the TUNEL experiments
Joint application of AMO-21 and AMO-221 enhanced the
promotion of apoptotic alteration in the Hep-2 cells compared with
mono-transfection of either AMO at the same concentration (Fig. 2). Co-transfection caused an
increase of TUNEL-positive cells between 4% under normal conditions
and almost 50% post-transfection. Single transfections of AMO-21 or
AMO-221 resulted in an increase in TUNEL-positive cells between 4
and ~30%.
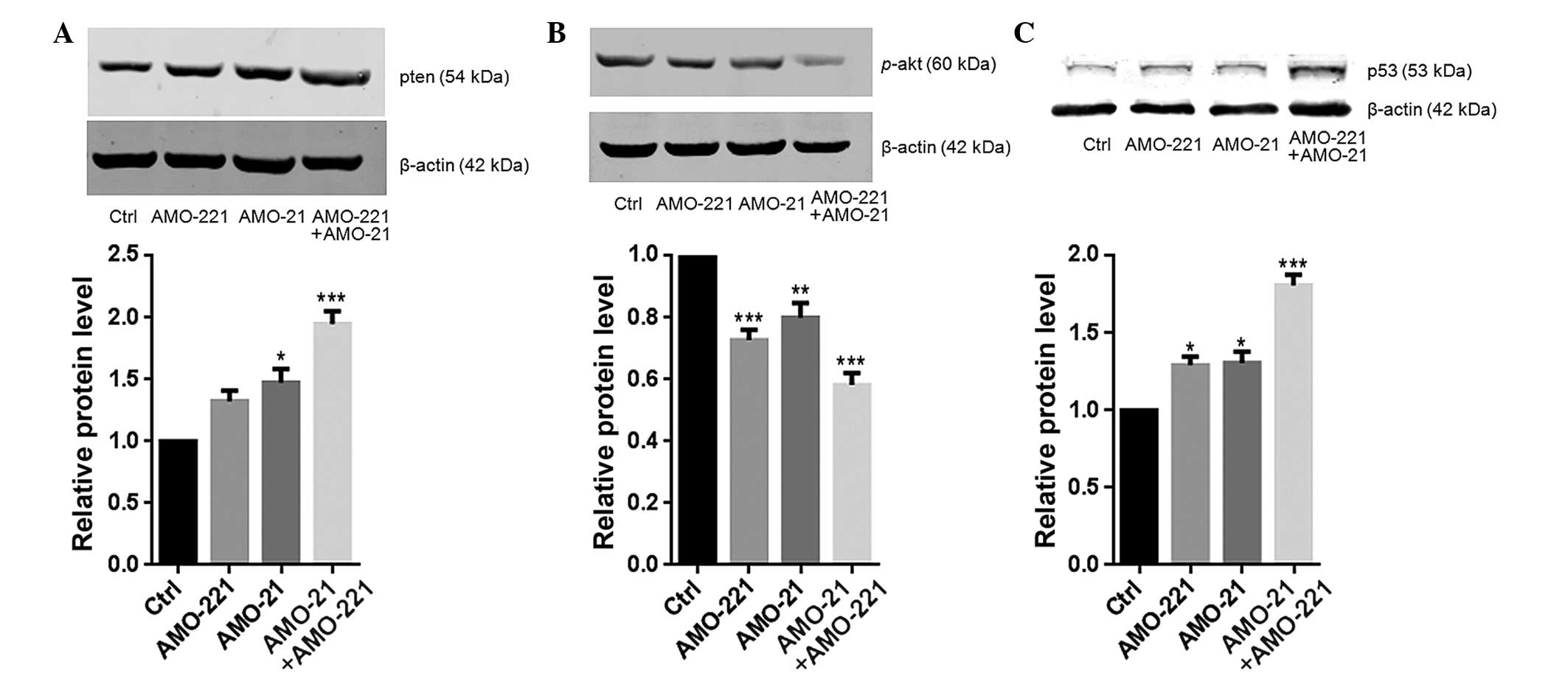
Co-transfection of AMO-21 and AMO-221
synergistically upregulated the expression of PTEN
Low expression of PTEN has been reported in the
laryngeal tissue of patients with LSCC (11,14).
The resulting weak negative regulation from PTEN may lead to
excessive activation of Akt (14).
In the present study, a low cellular content of PTEN and high
phosphorylation level of Akt were each observed in the Hep-2 cells
(Fig. 3A and B). PTEN is a common
target of miR-21 and miR-221 (11,12).
The co-transfection of 40 nM of AMO-21 and AMO-221 into Hep-2 cells
resulted in an enhanced total cellular concentration of PTEN
compared to the untransfected control cells (Fig.3A). Consistently, significant
weakening of Akt phosphorylation was observed in the cells that
were treated with AMO-21 and AMO-221 (P<0.001; Fig. 3B). As a result, this effect
eventually led to an almost 2-fold increase in p53 expression
compared with the control group (Fig.
3C). This finding is consistent with a previous study that
found Akt negatively controls p53 expression in cancer cells
(17).
AMO-21 and AMO-221 synergistically
enhanced expression of multiple p53-mediated pro-apoptotic
miRNAs
As an important transcription factor, p53 is
responsible for the transcription of multiple pro-apoptotic miRNAs,
including miR-15a, miR-16-1, miR-26a, miR-34a, miR-143 and miR-203
(18). In the present study,
RT-qPCR indicated that the expression of the 6 miRNAs was markedly
increased by the concurrent transfection of AMO-21 and AMO-221 in
Hep-2 cells (Fig. 4). Enforced
transcription of multiple pro-apoptotic miRNAs should be attributed
to a significant expression change in p53 (Fig. 3C), suggesting a synergistic effect
of AMO-21 and AMO-221 in re-adjusting the balance between
anti-apoptotic and pro-apoptotic miRNAs in Hep-2 cells.
Discussion
miRNAs have broad and important implications in
human cancer, which advocates the use of miRNAs as anti-cancer
therapeutic targets (20,21). As with numerous other fields of
cancer research, miRNA signatures and functions have been
persistently revealed in LSCC and associated cancers (6,7,22).
Typically, the majority of studies into miRNAs involved in the
progression of cancer focus on key individual intervening miRNAs.
However, off-target effects due to excessive dosage and
non-specific delivery, which is one of the inherent limitations of
this approach, should be considered (23). To solve or avoid this problem, an
alternative strategy is to combine multiple miRNAs, based on the
wide range of synergistic associations that have been suggested in
studies investigating miRNAs (24). Co-regulating one target gene or
even one biological module may lead to potent synergistic effects
by miRNA combinations at a low dose or concentration. The effects
shown by miR-10b and miR-21 in glioma and miR-14 and miR-145 in
bladder cancer demonstrate the validity of this approach (25,26).
In the present study, the potential synergistic
effects of another miRNA pair, miR-21 and miR-221, were
investigated in LSCC in vitro. These two miRNAs were each
confirmed to be overexpressed in human LSCC tissue (7), target PTEN in the PTEN-Akt signaling
pathway (12,13) and share multiple biological modules
(10). These findings strongly
suggest a possible synergistic interaction between miR-21 and
miR-221. Consistent with the reported accumulated data (10,12,13),
the present experiments demonstrated synergetic, rather than
cumulative, pro-apoptotic effects of co-inhibition with miR-21 and
miR-221 in Hep-2 cells. The expression of PTEN was notably
increased in the untransfected controls. In addition, a low level
of phosphorylation of Akt was observed. It has been well
established that PTEN is a pivotal negative regulatory factor of
Akt (13), whereas excessive
activation of Akt was found to be strongly associated with LSCC
metastasis (14). Overactivation
of Akt ultimately leads to an increase in the expression of the
tumor suppressor gene TP53 (15).
Briefly, decreased intensity of PTEN-Akt signaling reduced the
inhibitory role of PTEN-Akt signaling on this downstream p53
pathway. Thus, it was not notable that in the present study a clear
increase in the expression of p53 was found by blocking the
miRNA-mediated regulation of PTEN with co-transfection of AMO-21
and AMO-221.
Furthermore, the present findings suggest that p53
may play a central role in the balance of the function of
anti-apoptotic and pro-apoptotic miRNAs in Hep-2 cells.
Downregulation of anti-apoptotic or tumor-promoting miRNAs, such as
miR-21 and miR-221, led to the upregulation of the 6 pro-apoptotic
miRNAs tested in the present study, consisting of miR-15a,
miR-16-1, miR-26a, miR-34a, miR-143 and miR-203. This may also
result in the phenomenon of co-inhibition with miR-21 and miR-221
results in reduced cell viability and apoptosis in Hep-2 cells,
observed by MTT and TUNEL-staining assays. By co-regulating the
target gene PTEN and affecting the PTEN-Akt and p53 signaling
pathways, miR-21 and miR-221 exert functional control on the
cellular expression of these proteins with opposing functions and
promote cancer cell proliferation. By contrast, this specific
mechanism of a miRNA mutual interaction highlights the unique
advantage of the combination of AMO-21 and AMO-221 as a potential
treatment of LSCC, compared with the traditional strategy of
overexpressing a single pro-apoptotic miRNA or downregulating a
single tumor-promoting miRNA (22). Additionally, the joint application
of two AMOs, such as AMO-21 and AMO-221, overcomes the potential
issue of the non-specific effects of miRNA, because only low
concentrations or doses of the AMOs are required.
In conclusion, to the best of our knowledge, the
present in vitro study assessed for the first time the
potential synergistic pro-apoptotic effects of miR-21 and miR-221
in the LSCC Hep-2 cell line, by co-inhibition of the two
tumor-promoting miRNAs at a low intervention level. Novel insights
were also obtained for the mechanism underlying miRNA-miRNA
interactions in balancing and adjusting the cellular expression and
function of each interacting partner. The present findings indicate
that the combination of AMO-21 and AMO-221 should be considered to
possess therapeutic potential for future LSCC treatments. However,
additional in vivo functional experiments are required to
validate the present findings.
Acknowledgments
The present study was supported by grants from the
Foundation of Heilongjiang Provincial Health and Family Planning
Commission (grant no. 2014-309), the Talented Youth Fund of Harbin
Municipal Science and Technology Bureau (grant no. 2015RAQYJ076),
the Postdoctoral Fund (grant nos. LBH-Z12157 and 2014M551276), and
the National Science Foundation of China (grant nos. 81241085,
81372902 and 81402234).
References
|
1
|
Genden EM, Ferlito A, Silver CE, Jacobson
AS, Werner JA, Suárez C, Leemans CR, Bradley PJ and Rinaldo A:
Evolution of the management of laryngeal cancer. Oral Oncol.
43:431–439. 2007. View Article : Google Scholar
|
|
2
|
Marioni G, Marchese-Ragona R, Cartei G,
Marchesex F and Staffieri A: Current opinion in diagnosis and
treatment of laryngeal carcinoma. Cancer Treat Rev. 32:504–515.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng Y, Liu M, Li M, Zhang J, Ge J, Sun Y
and Tian L: The influence of the 'patient-to-patient model' on
swallowing problems in patients with supraglottic laryngeal cancer.
ORL J Otorhinolaryngol Relat Spec. 76:171–177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jenckel F and Knecht R: State of the art
in the treatment of laryngeal cancer. Anticancer Res. 33:4701–4710.
2013.PubMed/NCBI
|
|
5
|
Ma J, Liu Y, Huang XL, Zhang ZY, Myers JN,
Neskey DM and Zhong LP: Induction chemotherapy decreases the rate
of distant metastasis in patients with head and neck squamous cell
carcinoma but does not improve survival or locoregional control: A
meta-analysis. Oral Oncol. 48:1076–1084. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nohata N, Hanazawa T, Kinoshita T, Okamoto
Y and Seki N: MicroRNAs function as tumor suppressors or oncogenes:
Aberrant expression of microRNAs in head and neck squamous cell
carcinoma. Auris Nasus Larynx. 40:143–149. 2013. View Article : Google Scholar
|
|
7
|
Cao P, Zhou L, Zhang J, Zheng F, Wang H,
Ma D and Tian J: Comprehensive expression profiling of microRNAs in
laryngeal squamous cell carcinoma. Head Neck. 35:720–728. 2013.
View Article : Google Scholar
|
|
8
|
Ren J, Zhu D, Liu M, Sun Y and Tian L:
Downregulation of miR-21 modulates Ras expression to promote
apoptosis and suppress invasion of Laryngeal squamous cell
carcinoma. Eur J Cancer. 46:3409–3416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun X, Liu B, Zhao XD, Wang LY and Ji WY:
MicroRNA-221 accelerates the proliferation of laryngeal cancer cell
line Hep-2 by suppressing Apaf-1. Oncol Rep. 33:1221–1226.
2015.PubMed/NCBI
|
|
10
|
Zhu W, Zhao Y, Xu Y, Sun Y, Wang Z, Yuan W
and Du Z: Dissection of protein interactomics highlights microRNA
synergy. PLoS One. 8:e633422013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li D, Feng J, Wu T, Wang Y, Sun Y, Ren J
and Liu M: Long intergenic noncoding RNA HOTAIR is overexpressed
and regulates PTEN methylation in laryngeal squamous cell
carcinoma. Am J Pathol. 182:64–70. 2013. View Article : Google Scholar
|
|
12
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Garofalo M, Di Leva G, Romano G, Nuovo G,
Suh SS, Ngankeu A, Taccioli C, Pichiorri F, Alder H, Secchiero P,
et al: miR-221&222 regulate TRAIL resistance and enhance
tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell.
16:498–509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li R, Wang R, Zhai R and Dong Z: Targeted
inhibition of mammalian target of rapamycin (mTOR) signaling
pathway inhibits proliferation and induces apoptosis of laryngeal
carcinoma cells in vitro. Tumori. 97:781–786. 2011.
|
|
15
|
Paez J and Sellers WR: PI3K/PTEN/Akt
pathway. A critical mediator of oncogenic signaling. Cancer Treat
Res. 115:145–167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu C, Liu Y, Tan H, Li G, Su Z, Ren S, Zhu
G, Tian Y, Qiu Y and Zhang X: Metadherin regulates metastasis of
squamous cell carcinoma of the head and neck via Akt signalling
pathway-mediated epithelial-mesenchymal transition. Cancer Lett.
343:258–267. 2014. View Article : Google Scholar
|
|
17
|
Abraham AG and O'Neill E:
PI3K/Akt-mediated regulation of p53 in cancer. Biochem Soc Trans.
42:798–803. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suzuki HI, Yamagata K, Sugimoto K, Iwamoto
T, Kato S and Miyazono K: Modulation of microRNA processing by p53.
Nature. 460:529–533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
20
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in Cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Garzon R, Marcucci G and Croce CM:
Targeting microRNAs in cancer: Rationale, strategies and
challenges. Nat Rev Drug Discov. 9:775–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sethi N, Wright A, Wood H and Rabbitts P:
MicroRNAs and head and neck cancer: Reviewing the first decade of
research. Eur J Cancer. 50:2619–2635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh S, Narang AS and Mahato RI:
Subcellular fate and off-target effects of siRNA, shRNA and miRNA.
Pharm Res. 28:2996–3015. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu J, Li CX, Li YS, Lv JY, Ma Y, Shao TT,
Xu LD, Wang YY, Du L, Zhang YP, et al: MiRNA-miRNA synergistic
network: Construction via co-regulating functional modules and
disease miRNA topological features. Nucleic Acids Res. 39:825–836.
2011. View Article : Google Scholar :
|
|
25
|
Dong CG, Wu WK, Feng SY, Wang XJ, Shao JF
and Qiao J: Co-inhibition of microRNA-10b and microRNA-21 exerts
synergistic inhibition on the proliferation and invasion of human
glioma cells. Int J Oncol. 41:1005–1012. 2012.PubMed/NCBI
|
|
26
|
Noguchi S, Yasui Y, Iwasaki J, Kumazaki M,
Yamada N, Naito S and Akao Y: Replacement treatment with
microRNA-143 and -145 induces synergistic inhibition of the growth
of human bladder cancer cells by regulating PI3K/Akt and MAPK
signaling pathways. Cancer Lett. 328:353–361. 2013. View Article : Google Scholar
|