Introduction
The rise in global obesity rates over the last three
decades has been substantial and widespread, becoming a major
public health epidemic in developed and developing countries
(1–3). Obesity is also well-recognized as a
risk factor associated with multiple pain syndromes, including
lower back pain, tension-type headaches and migraines,
fibromyalgia, abdominal pain and chronic widespread pain (4–7).
These pain conditions are not solely due to mechanical stress on
the joints due to increased load, other factors have been suggested
to be involved in the progression of chronic pain in obesity.
Evidence from experimental studies has demonstrated
that obesity is associated with increased peripheral inflammation
and pain sensitivity in response to inflammatory stimulation
(8–10). The spinal cord is the predominant
relay station in the neural communication between inflamed areas
and the central nervous system (CNS) (11). Obesity has been shown to induce the
downregulation of spinal peroxisome proliferator-activated receptor
(PPAR)α, which sensitizes peripheral inflammatory stimulation by
increasing the inflammatory response in the spinal cord,
contributing to augmented peripheral inflammation and inflammatory
hyperalgesia (12). Thus,
upregulating PPARα in the spinal cord may offer a novel therapeutic
strategy for preventing augmented peripheral inflammation and
inflammatory hyperalgesia in obesity.
Ursolic acid (UA), a natural pentacyclic
triterpenoid carboxylic acid, is the major component of several
traditional medicine herbs and has multiple biological activities,
including the regulation of lipid and glucose metabolism (13,14),
in addition to anti-oxidative, antimutagenic, anticarcinogenic,
antimicrobial and anti-atherosclerotic effects (15–17).
UA also exhibits analgesic and anti-inflammatory activities in
normal animals (18,19), however, the molecular mechanism,
and whether UA has beneficial effects on inflammatory hyperalgesia
in obesity remain to be elucidated. Previous studies have
demonstrated that UA increases the expression and activity of PPARα
in the liver of obese animals (13,14),
and UA has been shown to cross the blood brain barrier (20,21).
The present study was performed to test the hypothesis that UA
prevents increased peripheral inflammation and inflammatory
hyperalgesia in obesity by restoring downregulated spinal PPARα.
For this purpose, a diet-induced rat model of obesity was used,
which has been considered to be the most relevant model with regard
to human obesity (22).
Materials and methods
Animals
Adult male Sprague-Dawley rats weighing 85±3 g were
purchased from the Beijing Laboratory Animal Research Center
(Beijing, China). They were housed in a room maintained at 23–25°C
on a 12-h-light/dark cycle, and were provided with rat chow and
water ad libitum. The study was approved by the ethics
committee of Shandong University (Shandong, China), and all animal
experiments were performed in accordance with the guidelines of the
Institutional Animal Care and Use Committee of Shandong
University.
Experimental protocol
Following 1 week of adaptation to their housing
environment, the rats were randomly divided into the following
groups (n=18 in each group): i) High-fat diet (HFD) control group,
comprising HFD-fed rats without treatment; ii) HFD+UA
(Sigma-Aldrich, St. Louis, MO, USA), comprising HFD-fed rats
treated with UA; iii) HFD+UA+PPARα small interfering (si)RNA,
comprising HFD-fed rats treated with UA and PPARα siRNA; iv)
HFD+UA+scrambled siRNA, comprising HFD-fed rats treated with UA and
scrambled siRNA; v) low-fat diet (LFD), comprising LFD-fed rats
without treatment.
The rats, which were assigned to the HFD or LFD
groups were fed a HFD (45% kcal as fat; Research Diets, New
Brunswick, NJ, USA) or a LFD (10% kcal as fat) for 12 weeks,
respectively. UA (250 mg/kg/day) was orally administered for 8
weeks, starting from week 4, when the HFD-fed rats had become
significantly heavier, compared with the LFD-fed rats (12). The dose of UA was based on a
previous study (14). PPARα siRNA
(2 μg/10 μl) or scrambled siRNA was intrathecally
injected at 10 weeks, as previously described (12). Body weights were measured weekly.
At the end of the experiments, 12 weeks post-HFD or LFD feeding),
six rats from each group were sacrificed by decapitation using a
guillotine (Kent Scientific Corporation, Torrington, CT, USA) and
5-ml blood samples were collected for the measurement of metabolic
parameters, and the spinal cord was removed for assessment of the
expression levels of PPARα and inflammatory mediators, and the
activity of nuclear factor (NF)-κB at baseline. Other rats (n=12
rats in each group) received a subcutaneous injection of 3%
carrageenan (50 μl; Sigma-Aldrich) into the mid-plantar
region of the right hindpaw, as previously described (12). The thermal and mechanical responses
of the right hindpaw, and the paw volumes of the right and left
hindpaws were then measured at baseline, and at 2, 4, 6, 8 and 24 h
following carrageenan injection. At 6 h post-carrageenan injection,
six rats from each group were sacrificed by decapitation using a
guillotine to assess the expression levels of PPARα and
inflammatory mediators, and the activity of NF-κB in the spinal
cord in response to carrageenan injection. The remaining rats were
sacrificed 24 h after carrageenan injection by decapitation using a
guillotine.
Intrathecal injection of siRNA
PPARα siRNA (Shanghai GenePharma Co., Ltd.,
Shanghai, China) was constructed using IMG-800 vector
(pSuppressorNeo; Imgenex, San Diego, CA, USA), as previously
described (12). The siRNA target
sequence used for the construction of the PPARα plasmid vector was:
5′-TCGAGTGTGATCGAAGCTGCAAGATGGAATTCGATCTTGCAGCTTCGATCACACTTTTT-3′.
The PPARα siRNA or scrambled siRNA (Shanghai GenePharma) was
suspended in serum-free media and intrathecally injected in a
volume of 10 μl using a 25-μl Hamilton microsyringe
(Hamilton Co., Reno, NV, USA) fitted with a 27-gauge needle. The
needle was inserted into the subarachnoid space through the
intervertebral foramen. The correct placement of the needle was
verified by a tail or foot flick response.
Hindpaw withdrawal responses to thermal
and mechanical stimulation
Prior to the assessment of response to thermal and
mechanical stimulation, the animals underwent acclimatization to
the assessment environment for 1 week by placing them in the
thermal and mechanical assessment boxes for 30–60 min twice daily.
The hindpaw withdrawal responses to thermal stimulation were
assessed using a plantar test instrument (#7370; Ugo Basile;
Comerio, VA, Italy), as previously described (12). The thermal stimulation was
performed using an infrared beam, which was directed onto the
plantar surface of the hindpaw, and the latency to paw withdrawal
was recorded. At each time point (0, 2, 4, 6, 8 and 24 h after the
carrageenan injection), three measurements were performed and the
average value was calculated. A cut-off value of 25 sec was applied
in the absence of a response to prevent tissue damage. The hindpaw
withdrawal responses to mechanical stimulation were assessed using
a dynamic plantar esthesiometer (# 37400; Ugo Basile), which
consisted of a filament, which touched the plantar surface of the
hindpaw and began to exert an upwards force until the paw was
withdrawn. A cut-off of 50 g was applied to prevent tissue
damage.
Assessment of paw edema
Paw edema was measured using a plethysmometer
(#7140; Ugo Basile), as described previously (12). The paw volume was determined based
on the displacement of water when the paw was submerged into the
water. The volumes of the left and the right paws were measured,
and the changes in paw volume were calculated as the percentage
difference in volume between the ipsilateral and contralateral
paw.
Western blot analysis
Nuclear and cytoplasmic fractions were extracted
from the spinal cord using a Nuclear Extract kit (cat. no. 40010;
Active Motif, Carlsbad, CA, USA). For the assessment of
inflammatory mediators, protein was isolated from the spinal cord
using cell lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM
EDTA-Na2, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium
pyrophosphate, 1 mM β-glycerophosphate, 1 mM
Na3VO4 and 1 μg/ml leupeptin; Cell
Signaling Technology, Inc., Beverly, MA, USA). The protein
concentration was determined by a Bradford assay (Bio-Rad Labs
Informatics Division, Philadelphia, PA, USA). Briefly, dye reagent
was prepared (dilution, 1 part dye reagent concentrate with 4 part
double-distilled water). The bovine serum albumin (BSA) protein
standard was prepared by making serial dilutions from a stock
solution. 10 μl BSA protein standards and samples were added
and a blank of double-distilled water was used. After incubation
for 5 min at room temperature, the absorbance was measured at 595
nm on a microplate reader (EL311S; Bio-Tek Instruments, Winooski,
VT, USA). The optical density readings were taken and a standard
curve was generated. The concentrations of samples were obtained
based on the standard curve.
Nuclear protein levels of PPARα and NF-κB p65,
protein levels of interleukin (IL)-1β, tumor necrosis factor
(TNF)-α, cyclooxygenase (COX)-2 and inducible nitric oxide synthase
(iNOS), and cytoplasmic protein levels of inhibitor of NF-κBα
(IκBα), were detected using western blot analysis. Briefly, 20
μg protein was loaded onto 10% SDS-polyacrylamide gel
(Bio-Rad Labs Informatics Division). Following electrophoresis (200
V for 35 min), proteins were transferred to a polyvinylidene
fluoride membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The membrane was blocked with Tris-buffered saline and Tween-20
(Bio-Rad Labs Informatics Division) with 5% skimmed milk, and
incubated overnight at 4°C with the following primary antibodies
(all purchased from Santa Cruz Biotechnology, Inc., Dallas, TX,
USA): Rabbit polyclonal anti-human IL-1β (cat. no. sc-7884; 1:200);
goat polyclonal anti-human TNF-α (cat. no. sc-1350; 1:400); goat
polyclonal anti-human COX-2 (cat. no. sc-23983; 1:400); rabbit
polyclonal anti-mouse iNOS (cat. no. sc-650; 1:200); rabbit
polyclonal anti-human NF-κB p65 (cat. no. sc-109; 1:200); rabbit
polyclonal anti-human IκBα (cat. no. sc-847; 1:300); rabbit
polyclonal anti-human PPAR-α (cat. no. sc-9000; 1:200); and goat
polyclonal anti-human β-actin (cat. no. sc-1615; 1:1,000). After
washing three times, the membrane was incubated for 1 h at room
temperature with the following secondary antibodies (all purchased
from Santa Cruz Biotechnology, Inc.): Goat anti-rabbit horseradish
peroxidase conjugated secondary antibody (cat. no. sc-2030;
1:5,000) or donkey anti-goat horseradish peroxidase conjugated
secondary antibody (cat. no. sc-2020; 1:5,000). After washing three
times, the signals of the detected proteins were visualized using
an enhanced chemiluminescence reaction system (EMD Millipore,
Billerica, MA, USA). The density of the bands was analyzed using
National Institutes of Health ImageJ software (version 1.48; NIH,
Bethesda, MD, USA) and all data were normalized to β-actin.
PPARα and NF-κB p65 activity
The DNA binding activity of PPARα and NF-κB p65 in
the spinal nuclear extraction were measured using Transcription
Factor Assay kits (cat. no. ab13112; Abcam, Cambridge, MA, USA and
Active Motif), according to the manufacturer's protocols.
Biochemical measurements
Whole blood samples were centrifuged at 2,100 × g
for 15 min at 4°C and the plasma from each blood sample was
collected. The plasma levels of glucose, total cholesterol and
triglyceride were measured using a Roche Hitachi 911 Chemistry
Analyzer (Hitachi Inc.,Pleasanton, CA, USA). The plasma levels of
insulin, leptin and adiponectin were analyzed using Invitrogen rat
ELISA kits (cat. nos. ERINS, KRC2281 and KRP0041, respectively;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocols.
Statistical analysis
GraphPad Prism 6 (GraphPad Software, Inc., La Jolla,
CA, USA) was used for data analysis. Data are presented as the mean
± standard error of the mean. The differences between groups were
analyzed by two-way analysis of variance, followed by Bonferroni
post-hoc tests for multiple comparisons. P<0.05 was considered
to indicate a statistically significant difference.
Results
Effects of UA on body weight and
metabolic parameters
As shown in Fig. 1,
body weights were significantly higher throughout the investigation
in all HFD groups, compared with those in the LFD group, beginning
at week 4. No differences in body weight were observed among the
four HFD groups at 4 weeks. Compared with the HFD control rats, the
HFD+UA rats exhibited significantly reduced body weight gain at 12
weeks. The intrathecal injections of PPARα siRNA or scrambled siRNA
had no effects on body weight gain in the HFD+UA rats.
 | Figure 1Effects of UA on body weight gain and
metabolic parameters in HFD-induced obese rats. UA was orally
administered for 8 weeks, starting 4 weeks following provision of
the respective diets. PPARα siRNA or Sc siRNA was intrathecally
injected at week 10. (A) Body weights and plasma levels of (B)
glucose, (C) insulin, (D) triglycerides, (E) cholesterol, (F)
leptin and (G) adiponectin were examined. Data are presented as the
mean ± standard error of the mean. *P<0.05 vs. LFD;
†P<0.05, vs. HFD control. UA, ursolic acid; HFD,
high-fat diet; LFD, low-fat diet; Sc, scrambled; siRNA, small
interfering RNA; PPARα, peroxisome proliferator-activated receptor
α. |
At 12 weeks, the plasma levels of insulin, leptin
and cholesterol were higher, and the plasma levels of adiponectin
were lower in the HFD control rats, compared with those in the LFD
rats. Compared with the HFD control rats, the HFD+UA rats exhibited
significant improvements in all the above parameters. The
intrathecal injections of PPARα siRNA and scrambled siRNA did not
alter these metabolic parameters in the HFD+UA rats. No differences
in the plasma levels of glucose or triglycerides were observed
between the experimental groups.
Effects of UA on thermal and mechanical
hyperalgesia and paw edema
No differences were observed in thermal
hyperalgesia, mechanical allodynia or paw edema between the
experimental groups at baseline (prior to carrageenan injection),
as shown in Fig. 2. Following
carrageenan injection, all groups of rats exhibited significant
thermal hyperalgesia, mechanical allodynia and paw edema in the
injected paw. The maximum decreases in thermal response latency and
mechanical response threshold, and maximum increase in paw volume,
were observed in the carrageenan-injected paw at 6 h in the LFD
rats, but at 4 h in the HFD control rats. Compared with the LFD
rats, the HFD control rats had more pronounced thermal hyperalgesia
between 4 and 8 h following carrageenan injection (Fig. 2A). Compared with the HFD control
rats, the HFD+UA rats exhibited significantly attenuated thermal
hyperalgesia. Of note, the intrathecal injection of PPARα siRNA,
but not of scrambled siRNA, completely eliminated the beneficial
effect of UA on thermal hyperalgesia in the HFD+UA rats in response
to carrageenan injection. No significant differences were observed
in the mechanical response thresholds between the experimental
groups throughout the experimental period (Fig. 2B). Compared with the LFD rats, the
HFD control rats exhibited increased paw edema between 2 and 24 h
following carrageenan injection (Fig.
2C). The HFD+UA rats exhibited significantly reduced paw edema,
compared with the HFD control rats. The improvement in paw edema
observed in the HFD+UA rats was completely abrogated by the
intrathecal injection of PPARα siRNA, but not scrambled siRNA.
 | Figure 2Effects of UA on thermal and
mechanical hyperalgesia and paw edema. Effects of UA on (A) paw
withdrawal latency, (B) paw withdrawal threshold and (C) paw volume
at baseline, and 2, 4, 6, 8 and 24 h post-carrageenan injection.
Data are presented as the mean ± standard error of the mean.
*P<0.05, vs. LFD. UA, ursolic acid; HFD, high-fat
diet; LFD, low-fat diet; Sc, scrambled; siRNA, small interfering
RNA; PPARα, peroxisome proliferator-activated receptor α. |
Effects of UA on the inflammatory
response in the spinal cord
As the inflammatory response in the spinal cord has
been shown to be associated with peripheral inflammation and pain
sensitivity, the present study examined the effect of systemic
administration of UA on the expression levels of inflammatory
mediators and activation of NF-kB, a key mediator of inflammation,
in the spinal cord at baseline and 6 h post-carrageenan injection.
The time points selected were based on previous studies showing
that the acute phase of the inflammatory response and hyperalgesia
(0–6 h) is characterized by central sensitization, mediated
predominantly by prostanoids, the products of COX-2 (11,23).
No differences were observed in the expression
levels of the IL-1β, TNF-α, COX-2 or iNOS inflammatory mediators
(Fig. 3), the activity of NF-κB
p65 or the expression of IκBα (Fig.
4) in the spinal cord between the experimental groups at
baseline. At 6 h post-carrageenan injection, all groups of rats
exhibited markedly increased expression levels of inflammatory
mediators, augmented expression and activity of NF-κB p65, and
reduced expression of IκBα in the spinal cord, compared with
respective baseline values. Notably, the HFD control rats exhibited
a more pronounced inflammatory response, which was prevented in the
HFD+UA rats. The decreased inflammatory response in the spinal cord
of the HFD+UA rats was reversed by intrathecal injection of PPARα
siRNA, but not scrambled siRNA.
 | Figure 3Effects of UA on expression levels of
inflammatory mediators in the spinal cord at baseline and 6 h
following CAR injection. (A) Representative western blots from each
group are shown. The expression levels of (B) IL-1β, (C) TNF-α; (D)
COX-2 and (E) iNOS were quantified. Data were corrected by β-actin.
Data are presented as the mean ± standard error of the mean.
*P<0.05, vs. respective baseline;
†P<0.05, vs. LFD under the same condition. UA,
ursolic acid; HFD, high-fat diet; LFD, low-fat diet; CAR,
carrageenan; Sc, scrambled; siRNA, small interfering RNA; IL-1β,
interleukin-1β; TNF-α, tumor necrosis factor-α; COX-2,
cyclooxygenase-2; iNOS, inducible nitric oxide synthase; PPARα,
peroxisome proliferator-activated receptor α. |
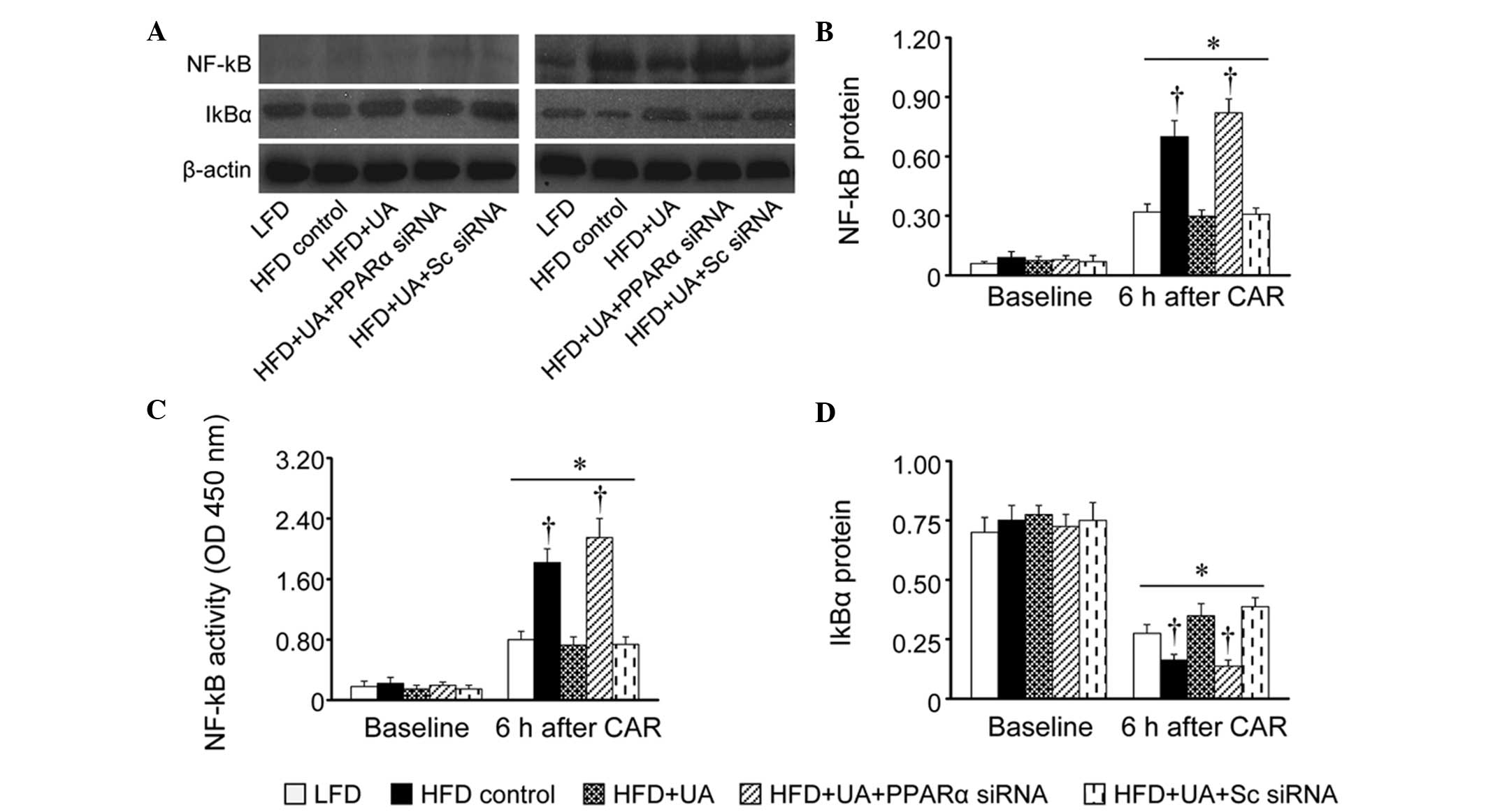 | Figure 4Effects of UA on the expression and
activity of NF-κB p65, and expression of IκBα in the spinal cord at
baseline and 6 h following CAR injection. (A) Representative
western blots from each group are shown. The (B) expression and
activity (C) of NF-κB p65, and (D) expression of IκBα were
quantified and corrected by β-actin. Data are presented as the mean
± standard error of the mean. *P<0.05, vs. respective
baseline; †P<0.05, vs. LFD under the same condition.
UA, ursolic acid; HFD, high-fat diet; LFD, low-fat diet; CAR,
carrageenan; Sc, scrambled; siRNA, small interfering RNA; NF-κB,
nuclear factor-κB; PPARα, peroxisome proliferator-activated
receptor α; IκBα, inhibitor of NF-κB; OD, optical density. |
Effects of UA on the expression and
activity of PPARα in the spinal cord
To further investigate the molecular mechanisms by
which UA attenuates exaggerated inflammatory response in the spinal
cord, and prevents augmented peripheral inflammation and
inflammatory hyperalgesia in obesity, the present study measured
the expression and activity of PPARα in the spinal cord.
At 12 weeks, the HFD control rats exhibited
significant decreases in the expression and activity of PPARα at
baseline, compared with the LFD rats (Fig. 5). Of note, the decreases in the
spinal expression and activity of PPARα were normalized in the
HFD+UA rats. However, intrathecal injection of PPARα siRNA in the
HFD+UA rats reduced the spinal expression and activity of PPARα to
the same extent as in the HFD control rats. Intrathecal injection
of scrambled siRNA in the HFD+UA rats had no effects on the
expression and activity of PPARα.
 | Figure 5Effects of UA on PPARα. The (A)
expression levels and (B) activity of PPARα in the spinal cord were
determined at baseline and 6 h following CAR injection. Western
blot data were corrected by β-actin. Data are presented as the mean
± standard error of the mean. *P<0.05, vs. respective
baseline; †P<0.05, vs. LFD under the same condition.
UA, ursolic acid; HFD, high-fat diet; LFD, low-fat diet; CAR,
carrageenan; Sc, scrambled; siRNA, small interfering RNA; PPARα,
peroxisome proliferator-activated receptor α; OD, optical
density. |
Carrageenan injection caused significant reductions
in the spinal expression and activity of PPARα in all groups,
compared with their baseline values. Of note, the spinal expression
and activity of PPARα remained significantly higher in the LFD
rats, HFD+UA rats and HFD+UA rats treated with scrambled siRNA,
compared with the other two groups.
Discussion
The present study reported the novel finding that
the systemic administration of UA restored the HFD-induced
downregulation of spinal PPARα, which resulted in inhibition of the
exaggerated expression of inflammatory mediators in the spinal cord
in response to peripheral inflammatory challenge, and the
prevention of augmented peripheral pain sensitivity and paw edema
in the HFD-induced obese rats. To the best of our knowledge, the
present study is the first to report that UA exerted beneficial
effects on peripheral inflammation and inflammatory hyperalgesia in
obesity via the activation of PPARα in the spinal cord.
Inflammatory mediators in the CNS have been
implicated in the pathogenesis of peripheral inflammation and
inflammatory hyperalgesia (10).
Peripheral injury induces the increased expression of central
inflammatory mediators, particularly COX-2 and iNOS, which are
involved in inflammatory signaling to the CNS (11,24,25).
Increased expression of COX-2 in the CNS promotes the synthesis and
release of prostaglandin E2, contributing to the severity of the
inflammatory and peripheral pain responses (24). Carrageenan injection in the paws of
rodents can rapidly induce the expression of the proinflammatory
cytokines, COX-2 and iNOS, in the spinal cord and other regions of
the CNS (24). Emerging evidence
suggests that the expression levels of inflammatory mediators in
the CNS in response to peripheral inflammatory challenge can be
mediated by PPARα, a ligand-activated transcription factor which
belongs to the nuclear receptor superfamily (26) and is expressed in the CNS,
including the brain and spinal cord (11,23,27).
PPARα is important in regulating lipid metabolism and adipocyte
differentiation (28,29). In addition, PPARα is crucial in the
regulation of inflammatory responses (30,31).
Previous experimental investigation has demonstrated that
HFD-induced obesity causes decreases in the expression and activity
of PPARy in the spinal cord, which results in the exaggerated
expression of inflammatory mediators in response to carrageenan
injection in the paw, contributing to augmented peripheral
inflammation and inflammatory hyperalgesia (12). UA, a natural pentacyclic
trit-erpenoid and a major bioactive compound in several medicinal
herbs, has been shown to increase the expression of PPARα in the
liver in obese animals (14). UA
also crosses the blood brain barrier (20,21).
In the present study, it was found that the systemic administration
of UA for 8 weeks, commencing following a 4-week-period of feeding
with a HFD, significantly prevented the carrageenan-induced
augmentation of peripheral inflammation and inflammatory
hyperalgesia in obese rats, as evidenced by reduced paw edema and
increased thermal latency. Molecular investigations revealed that
UA restored obesity-induced reductions in the baseline expression
and activity of PPARα in the spinal cord, which led to decreased
expression levels of inflammatory mediators and the activity of
NF-kB in response to peripheral carrageenan injection. Notably, the
expression levels of inflammatory mediators in the spinal cord at
baseline were similar among the groups, although the baseline
expression and activity of PPARα were decreased in the HFD control
rats, and were normalized in the HFD+UA rats. Following carrageenan
injection, the expression levels of inflammatory mediators in the
spinal cord were significantly higher in the HFD control rats,
compared with the LFD rats, but were attenuated in the HFD+UA rats.
These observations suggested that changes in the spinal expression
and activity of PPARα may not affect the expression of inflammatory
mediators under normal conditions. However, the decreased spinal
expression and activity of PPARα at baseline may increase
susceptibility to peripheral inflammatory stimulation, causing an
exaggerated inflammatory response in the spinal cord.
In the present study, it was observed that the
abnormal metabolic parameters in obese rats were ameliorated by the
systemic administration of UA. These results are consistent with
findings of others that UA improves metabolic disorders in
HFD-induced obese animals (13,14).
However, the restoration of spinal PPARα by UA may not be entirely
attributed to improvement in metabolic disorders, as UA partially
improved, but did not normalize, the metabolic parameters in the
obese animals. Furthermore, knockdown of spinal PPARα in the HFD+UA
rats by the intrathecal injection of PPARα siRNA did not alter the
UA-induced improvement in metabolic parameters, but reversed the
UA-induced decrease in the expression levels of spinal inflammatory
mediators, and eliminated the beneficial effects of UA on
peripheral inflammation and inflammatory hyperalgesia. These
results indicated that the systemic administration of UA inhibited
the exaggerated inflammatory responses in the spinal cord in
response to peripheral inflammatory stimulation by upregulating
spinal PPARα, thereby preventing augmented peripheral inflammation
and inflammatory hyperalgesia.
PPARα has been suggested to control inflammatory
gene expression by modulating the activity of the NF-κB
transcription factor. The expression of a variety of inflammatory
genes is driven by NF-κB, the transcriptional activity of which is
regulated by the inhibitory protein, IκBα, in the cytoplasm
(32,33). Multiple stimuli can rapidly induce
the degradation of IκBα, resulting in translocation of the NF-κB
complex to the nucleus, where it activates gene transcription
(32). The knockout of PPARα has
been shown to increase NF-κB transcriptional activity via
downregulation of the cytoplasmic inhibitor, IκBα (34), whereas the activation of PPARα
inhibits NF-κB transcriptional activity via the upregulation of
IκBα (35). The results of the
present study showed that the systemic administration of UA in
obese rats inhibited the augmentation of the expression and
activity of NF-κB p65 in the spinal cord, which was associated with
increased expression of IκBα in the cytoplasm. These results
suggested that the restoration of spinal PPARα via systemic
administration of UA prevented the exaggerated inflammatory
response to peripheral inflammatory stimulation by suppressing
spinal NF-κB activity. Of note, a previous study demonstrated that
the systemic administration of UA attenuates the
D-Galactose-induced inflammatory response in the CNS by inhibiting
central NF-κB activity (36).
In conclusion, the present study demonstrated that
the systemic administration of UA inhibited the exaggerated spinal
cord inflammatory response to peripheral inflammatory stimulation
in HFD-induced obesity. This occurred via the restoration of the
downregulated expression of spinal PPARα, thereby preventing
augmented peripheral inflammation and inflammatory hyperalgesia.
These observations suggested that UA may provide a potential
therapeutic option for the prevention of increased inflammatory
pain in obese patients.
Acknowledgments
This study was supported by the Youth Science
Foundation of Shandong Province, China (grant no. Y2013020046).
References
|
1
|
Prentice AM: The emerging epidemic of
obesity in developing countries. Int J Epidemiol. 35:93–99. 2006.
View Article : Google Scholar
|
|
2
|
Hurt RT, Kulisek C, Buchanan LA and
McClave SA: The obesity epidemic: Challenges, health initiatives,
and implications for gastroenterologists. Gastroenterol Hepatol
(NY). 6:780–792. 2010.
|
|
3
|
Jankun J, Al-Senaidy A and
Skrzypczak-Jankun E: Can inactivators of plasminogen activator
inhibitor alleviate the burden of obesity and diabetes? (Review)
Int J Mol Med. 29:3–11. 2012.
|
|
4
|
Marcus DA: Obesity and the impact of
chronic pain. Clin J Pain. 20:186–191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hitt HC, McMillen RC, Thornton-Neaves T,
Koch K and Cosby AG: Comorbidity of obesity and pain in a general
population: Results from the southern pain prevalence study. J
Pain. 8:430–436. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
D'Arcy Y: Managing pain in obese patients.
Nurse Pract. 36:28–32; quiz 33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patterson RE, Frank LL, Kristal AR and
White E: A comprehensive examination of health conditions
associated with obesity in older adults. Am J Prev Med. 27:385–390.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roane DS and Porter JR: Nociception and
opioid-induced analgesia in lean (Fa/-) and obese (fa/fa) Zucker
rats. Physiol Behav. 38:215–218. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Croci T and Zarini E: Effect of the
cannabinoid CB1 receptor antagonist rimonabant on nociceptive
responses and adjuvant-induced arthritis in obese and lean rats. Br
J Pharmacol. 150:559–566. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iannitti T, Graham A and Dolan S:
Increased central and peripheral inflammation and inflammatory
hyperalgesia in Zucker rat model of leptin receptor deficiency and
genetic obesity. Exp Physiol. 97:1236–1245. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
D'Agostino G, La Rana G, Russo R, Sasso O,
Iacono A, Esposito E, Raso GM, Cuzzocrea S, Lo Verme J, Piomelli D,
et al: Acute intracerebroventricular administration of
palmitoylethanolamide, an endogenous peroxisome
proliferator-activated receptor-alpha agonist, modulates
carrageenan-induced paw edema in mice. J Pharmacol Exp Ther.
322:1137–1143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang J, Zhang Q, Zhao L, Li D, Fu Z and
Liang L: Down-regulation of PPARα in the spinal cord contributes to
augmented peripheral inflammation and inflammatory hyperalgesia in
diet-induced obese rats. Neuroscience. 278:165–178. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jia Y, Bhuiyan MJ, Jun HJ, Lee JH, Hoang
MH, Lee HJ, Kim N, Lee D, Hwang KY, Hwang BY, et al: Ursolic acid
is a PPAR-α agonist that regulates hepatic lipid metabolism. Bioorg
Med Chem Lett. 21:5876–5880. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li S, Liao X, Meng F, Wang Y, Sun Z, Guo
F, Li X, Meng M, Li Y and Sun C: Therapeutic role of ursolic acid
on ameliorating hepatic steatosis and improving metabolic disorders
in high-fat diet-induced non-alcoholic fatty liver disease rats.
PLoS One. 9:e867242014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J: Pharmacology of oleanolic acid and
ursolic acid. J Ethnopharmacol. 49:57–68. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ikeda Y, Murakami A and Ohigashi H:
Ursolic acid: An anti- and pro-inflammatory triterpenoid. Mol Nutr
Food Res. 52:26–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim M, Sung B, Kang YJ, Kim DH, Lee Y,
Hwang SY, Yoon JH, Yoo MA, Kim CM, Chung HY and Kim ND: The
combination of ursolic acid and leucine potentiates the
differentiation of C2C12 murine myoblasts through the mTOR
signaling pathway. Int J Mol Med. 35:755–762. 2015.
|
|
18
|
Vasconcelos MA, Royo VA, Ferreira DS,
Crotti AE, Andrade e Silva ML, Carvalho JC, Bastos JK and Cunha WR:
In vivo analgesic and anti-inflammatory activities of ursolic acid
and oleanoic acid from Miconia albicans (Melastomataceae). Z
Naturforsch C. 61:477–482. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Y, Li JS, Zhang X, Wu YJ, Huang K and
Zheng L: Ursolic acid inhibits early lesions of diabetic
nephropathy. Int J Mol Med. 26:565–570. 2010.PubMed/NCBI
|
|
20
|
Tsai SJ and Yin MC: Antioxidative and
anti-inflammatory protection of oleanolic acid and ursolic acid in
PC12 cells. J Food Sci. 73:H174–H178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martin R, Carvalho-Tavares J, Hernández M,
Arnés M, Ruiz-Gutierrez V and Nieto ML: Beneficial actions of
oleanolic acid in an experimental model of multiple sclerosis: A
potential therapeutic role. Biochem Pharmacol. 79:198–208. 2010.
View Article : Google Scholar
|
|
22
|
Madsen AN, Hansen G, Paulsen SJ,
Lykkegaard K, Tang-Christensen M, Hansen HS, Levin BE, Larsen PJ,
Knudsen LB, Fosgerau K and Vrang N: Long-term characterization of
the diet-induced obese and diet-resistant rat model: A polygenetic
rat model mimicking the human obesity syndrome. J Endocrinol.
206:287–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
D'Agostino G, La Rana G, Russo R, Sasso O,
Iacono A, Esposito E, Mattace Raso G, Cuzzocrea S, Loverme J,
Piomelli D, et al: Central administration of palmitoylethanolamide
reduces hyper-algesia in mice via inhibition of NF-kappaB nuclear
signalling in dorsal root ganglia. Eur J Pharmacol. 613:54–59.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ichitani Y, Shi T, Haeggstrom JZ,
Samuelsson B and Hökfelt T: Increased levels of cyclooxygenase-2
mRNA in the rat spinal cord after peripheral inflammation: An in
situ hybridization study. Neuroreport. 8:2949–2952. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Samad T and Abdi S: Cyclooxygenase-2 and
antagonists in pain management. Curr Opin Anaesthesiol. 14:527–532.
2001. View Article : Google Scholar
|
|
26
|
Kota BP, Huang TH and Roufogalis BD: An
overview on biological mechanisms of PPARs. Pharmacol Res.
51:85–94. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moreno S, Farioli-Vecchioli S and Cerù MP:
Immunolocalization of peroxisome proliferator-activated receptors
and retinoid X receptors in the adult rat CNS. Neuroscience.
123:131–145. 2004. View Article : Google Scholar
|
|
28
|
Berger J and Moller DE: The mechanisms of
action of PPARs. Annu Rev Med. 53:409–435. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Filip-Ciubotaru F, Foia L, Manciuc C and
Grigore C: PPARs: Structure, mechanisms of action and control. Note
I. Rev Med Chir Soc Med Nat Iasi. 115:477–484. 2011.In Romanian.
PubMed/NCBI
|
|
30
|
Duval C, Fruchart JC and Staels B: PPAR
alpha, fibrates, lipid metabolism and inflammation. Arch Mal Coeur
Vaiss. 97:665–672. 2004.PubMed/NCBI
|
|
31
|
Cuzzocrea S, Mazzon E, Di Paola R, Peli A,
Bonato A, Britti D, Genovese T, Muià C, Crisafulli C and Caputi AP:
The role of the peroxisome proliferator-activated receptor-alpha
(PPAR-alpha) in the regulation of acute inflammation. J Leukoc
Biol. 79:999–1010. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Auphan N, DiDonato JA, Rosette C, Helmberg
A and Karin M: Immunosuppression by glucocorticoids: Inhibition of
NF-kappa B activity through induction of I kappa B synthesis.
Science. 270:286–290. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
George A, Marziniak M, Schäfers M, Toyka
KV and Sommer C: Thalidomide treatment in chronic constrictive
neuropathy decreases endoneurial tumor necrosis factor-alpha,
increases interleukin-10 and has long-term effects on spinal cord
dorsal horn met-enkephalin. Pain. 88:267–275. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao W, Iskandar S, Kooshki M, Sharpe JG,
Payne V and Robbins ME: Knocking out peroxisome
proliferator-activated receptor (PPAR) alpha inhibits
radiation-induced apoptosis in the mouse kidney through activation
of NF-kappaB and increased expression of IAPs. Radiat Res.
167:581–591. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vanden Berghe W, Vermeulen L, Delerive P,
De Bosscher K, Staels B and Haegeman G: A paradigm for gene
regulation: Inflammation, NF-kappaB and PPAR. Adv Exp Med Biol.
544:181–196. 2003. View Article : Google Scholar
|
|
36
|
Lu J, Wu DM, Zheng YL, Hu B, Zhang ZF, Ye
Q, Liu CM, Shan Q and Wang YJ: Ursolic acid attenuates
D-galactose-induced inflammatory response in mouse prefrontal
cortex through inhibiting AGEs/RAGE/NF-κB pathway activation. Cereb
Cortex. 20:2540–2548. 2010. View Article : Google Scholar : PubMed/NCBI
|



















