Introduction
Natural products, particularly those that bind to
microtubules, are important in the development of anticancer
therapeutic agents (1). Diosmetin
(Dio) is a flavone compound in various dietary sources, including
oregano, citrus fruits, and it may also be extracted from certain
medicinal herbs, such as Rosa agrestis Savi (Rosaceae),
Chrysanthemum morifolium (Asteraceae) and Dianthus
versicolor Fisch (Caryophyllaceae) (2,3).
Flavonoids are considered be associated with a variety of
beneficial effects, including antioxidant activity (4), which protect tissues against
oxidative stress and associated pathologies, such as inflammation
(5).
Dio is used in traditional Mongolian medicine to
treat liver diseases (6).
Hepatocellular carcinoma (HCC) is a global health problem (7) and therapeutic strategies for HCC are
predominantly focused on chemotherapy, for example, the alkylating
agent cisplatin or the topoisomerase inhibitor doxorubicin
(8).
Dio exerts synergistic cytostatic effects in HepG2
cells via cytochrome P450, family 1 (CYP1)-catalyzed metabolism,
activation of c-Jun N-terminal kinase (JNK)/extracellular
signal-regulated kinase (ERK) and tumor protein p53
(p53)/cyclin-dependent kinase inhibitor 1A upregulation (3). The differential expression of CYP1
enzymes in cancer cells has been proposed to be a potential
therapeutic target and the CYP1 family has been implicated in
carcinogenesis (9). It was
reported that Dio was metabolized to luteolin via an aromatic
demethylation reaction on the B-ring by CYP1 member A1 (CYP1A1),
CYP1 member B1 (CYP1B1) and the hepatic isozyme CYP1 member A2
(CYP1A2). CYP1A1 and CYP1A2 also produce additional unidentified
metabolites in breast adenocarcinoma cells (10). A previous study has investigated
the metabolism of the flavonoids using recombinant CYP1A1, CYP1B1
and CYP1A2 enzymes, an investigated their anti-proliferative
activity in the MDA-MB-468 and MCF-7 human breast adenocarcinoma
cell lines and the MCF-10A normal breast cell line (11).
Transforming growth factor-β (TGF-β) is, like
activins, inhibins and bone morphogenetic proteins, an important
polypeptide growth factors (12).
The majority of human tumors, including melanoma, secrete large
quantities of TGF-β, which directly influences the microenvironment
and promote tumor growth, peritumoral angiogenesis and
dissemination (13). Furthermore,
TGF-β may increase the motility and invasion of certain cancer
cells (14). TGF-β exerts its
effects via TGF-β receptor type I (TβRI) and type II (TβRII)
receptors. The activated TβRI initiates an intracellular signaling
pathway by phosphorylating the receptor-regulated Smads (R-Smads),
which include Smad2 and Smad3. Activated R-Smads form heteromeric
complexes with Smad4, which build up in the nucleus and regulate
the transcription of target genes (15). p53 is a tumor suppressor that
affects genomic stability and triggers apoptosis following cellular
exposure to a variety of stressors (16). p53 also promotes transcription and
may regulate the transcription and expression of a range of target
genes, which leads to cell cycle arrest and apoptosis. These target
genes include B-cell lymphoma 2 (Bcl-2) and BCL2-associated X
protein (Bax) expression (17).
Thus, the aim of the present study was to investigate the
association between TGF-β and Dio-induced cell apoptosis in HepG2
cells.
Materials and methods
Compounds and reagents
Diosmetin
(C16H12O6; Fig. 1A) was purchased from Sigma-Aldrich
(St. Louis, MO, USA). The original concentration of Dio stored at
−20°C was 10 mg/ml. TGF-β human recombinant was purchased from
Prospec-Tany TechnoGene, Ltd. (East Brunswick, NJ, USA) and
anti-human antibodies against p53, Bcl-2, Bax, TGF-β, TβRII, Smad3,
phosphorylated (p)-Smad2/3 and GADPH were all purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Horseradish
peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG)
secondary antibody was obtained from EarthOx Life Sciences, LLC
(Millbrae, CA, USA). Cell Counting Kit-8 (CCK8) and
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT) were purchased from Beyotime Institute of Biotechnology
(Haimen, China).
Cell culture and Dio treatment
HepG-2 cells were provided by the Affiliated
Hospital of Guangdong Medical College (Zhanjiang, China). The cells
were maintained in a humidified atmosphere of 5% CO2 at
37°C, and cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc., USA) supplemented with 10% (v/v) fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
and 100 U/ml streptomycin. HepG2 cells were grown in standard
media, and when 60–70% confluent, the cells were treated with
different concentrations of Dio (5, 10 and 15 µg/ml) or
TGF-β protein/Dio (10 µg/ml) for 24 h. Images were captured
by microscopy (magnification, ×100).
Annexin V/propidium iodide (PI) double
staining
Apoptotic cells (2×104 cells) were
quantified with an Annexin V-fluorescein isothiocyanate (FITC)/PI
kit (BD Biosciences, Franklin Gardens, NJ, USA) and detected with
the FACSCalibur™ flow cytometer (BD Biosciences). Data were
analyzed with Modfit 3.2 and BD FACSDiva 6.1.3 software (BD
Biosciences). Briefly, cells were pretreated with 5, 10, 15 and 20
µg/ml Dio for 24 h and washed with phosphate-buffered saline
(PBS), and then cells were collected and resuspended in binding
buffer [10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
(pH 7.5), 2.5 mM CaCI2 and 140 mM NaCI]. Cells were
incubated with Annexin V-FITC and PI for 15 min in the dark, prior
to flow cytometric analysis. Annexin V-positive cells indicated
early stage apoptosis, whereas Annexin V and PI-positive cells
indicated late stage apoptosis.
MTT and CCK8 analysis
HepG2 cell density was adjusted to 2×104
cells/100 µl, and the cells were seeded into 96-well plates
and placed in an incubator overnight (37°C in 5% CO2) to
allow for attachment and recovery. MTT and CCK8 analyses were
performed separately. Briefly, cells were pretreated with 5, 10, 15
and 20 µg/ml Dio for 24 h. A total of 20 µl MTT
solution (5 mg/ml in PBS) solution was transferred to each well to
yield a final 120 µl/well and to separate wells a total of
10 µl CCK8 (5 mg/ml in PBS) was transferred. The plates were
incubated for 4 h at 37°C in 5% CO2 and the absorbance
was recorded using the EnSpire™ 2300 Multilabel Plate Reader
(PerkinElmer, Inc., Waltham, MA, USA) at wavelengths of 595 nm and
450 nm, respectively. The half maximal inhibitory concentration
(IC50) of Dio was calculated using software (Shmm,
1.0.0.0).
Western blotting
Cells were collected and lysed in lysis buffer [100
mM Tris-HCI (pH 6.8), 4% (m/v) sodium dodecylsulfonate (SDS), 20%
(v/v) glycerol, 200 mM 2-mercaptoethanol, 1 mM phenylmethyl
sulfonylfluoride and 1 g/ml aprotinin] for 30 min on ice. The
lysates were separated using centrifugation at 4°C for 15 min at
3,913 × g. The total protein concentration in the supernatants was
detected using the BCA Protein assay kit (Beyotime Institute of
Biotechnology). Proteins (20 µg) were separated using 8–15%
SDS-PAGE and subsequently transferred to nitrocellulose membranes.
These were saturated with 5% milk in Tris-buffered saline and 1%
Tween-20 (TBST) and incubated with the following primary antibodies
in a diluent overnight at 4°C: p53 (9282), Bcl-2 (2876), Bax
(2772), TGF-β (3711), TGF-βRII (11888), Smad3 (9513), p-Smad2/3
(8828) and GAPDH (2118) (all 1:1,000; Cell Signaling Technology).
Membranes were washed three times with TBST and incubated with
HRP-conjugated goat anti-rabbit IgG (E030120-01; EarthOx Life
Sciences) for 1 h at room temperature, followed by washing four
times with TBST. An enhanced chemiluminescence kit (GE Healthcare
Life Sciences, Chalfont, UK) was added to the membrane and
detection was performed using an Odyssey® CLx Infrared
Imaging system (LI-COR Biosciences, Lincoln, NE, USA).
Immunofluorescence (IF)
Cells were seeded into 6-well plates were pretreated
with 10 µg/ml Dio for 24 h. All floating and attached cells
were harvested and fixed with ice-cold 4% formaldehyde for 10 min
and then washed with ice-cold PBS. Cells were permeabilized with
0.3% Triton X-100, washed with ice-cold PBS and stained with the
following primary antibodies against: p53 (9282), p-p53(Ser15)
(9284), p-p53(Ser46) (2521), p-p53(Ser20) (9287), p-p53(Thr81)
(2676), p-p53(Ser37) (9289), p-p53(Thr18) (2529) and p-p53(Ser33)
(2526) (all 1:500; Cell Signaling Technology, Inc.). Cells were
subsequently incubated with Alexa Fluor® secondary
antibodies (Invitrogen; Thermo Fisher Scientific, Inc.). Following
staining with 4′,6-diamidino-2-phenylindole (DAPI), cells and
nuclei were observed with a fluorescence microscope (Leica TCS
SP5II, Leica Microsystems GmbH, Germany) at wavelengths of 520±20
nm and blue fluorescence at 460 nm.
Statistical analysis
Data from the present study were analyzed using
GraphPad Prism 5 (GraphPad Software, Inc. La Jolla, CA, USA). Data
are presented as the mean ± standard deviation from triplicate
experiments unless otherwise stated. Statistical differences were
assessed using the Student's t-test and *P<0.05 was
considered to indicate a statistically significant difference.
Results
Dio inhibits cell proliferation and
promotes cell apoptosis
It was demonstrated that untreated HepG2 cells grew
well and were observed to have with normal skeletons, whereas cells
treated with Dio were distorted and a number of them became round
and floating. The number of normal cells was reduced, and sloughed
cells were increased in a concentration-dependent manner (Fig. 1B). MTT and CCK8 analysis was used
to evaluate the inhibitory effects of Dio in HepG2 cells. Results
from the present study demonstrate that Dio inhibits cell
proliferation in HepG2 cells in a concentration-dependent manner
(Fig. 1C). Cell apoptosis was
detected by flow cytometry with the results indicating that Dio may
induce cell apoptosis in a concentration-dependent manner (Fig. 1D). In the early stages of
apoptosis, cells were Annexin V-positive (Q4), whereas Annexin V
and PI-positive cells were considered to be in the late stage of
apoptosis (Q2). The IC50 of Dio was determined to be
12.0 µg/ml.
TGF-β is important in Dio-triggered
apoptosis
To investigate whether cell growth reversed
following recombinant TGF-β administration, HepG2 cells were
treated with 10 µg/ml Dio and different concentrations of
TGF-β and images were captured using microscopy (magnification,
×100; Fig. 2A and B). Cells were
treated with or without Dio (10 µg/ml) and with or without
TGF-β (2 ng/ml) for 24 h. The MTT assay was performed to detect the
proliferation inhibition rate of the cells (Fig. 2C), and the inhibition rate of cells
treated with Dio (10 µg/ml) and TGF-β (2 ng/ml) was comapred
with Dio treatment, however, higher than that of TGF-β
treatment.
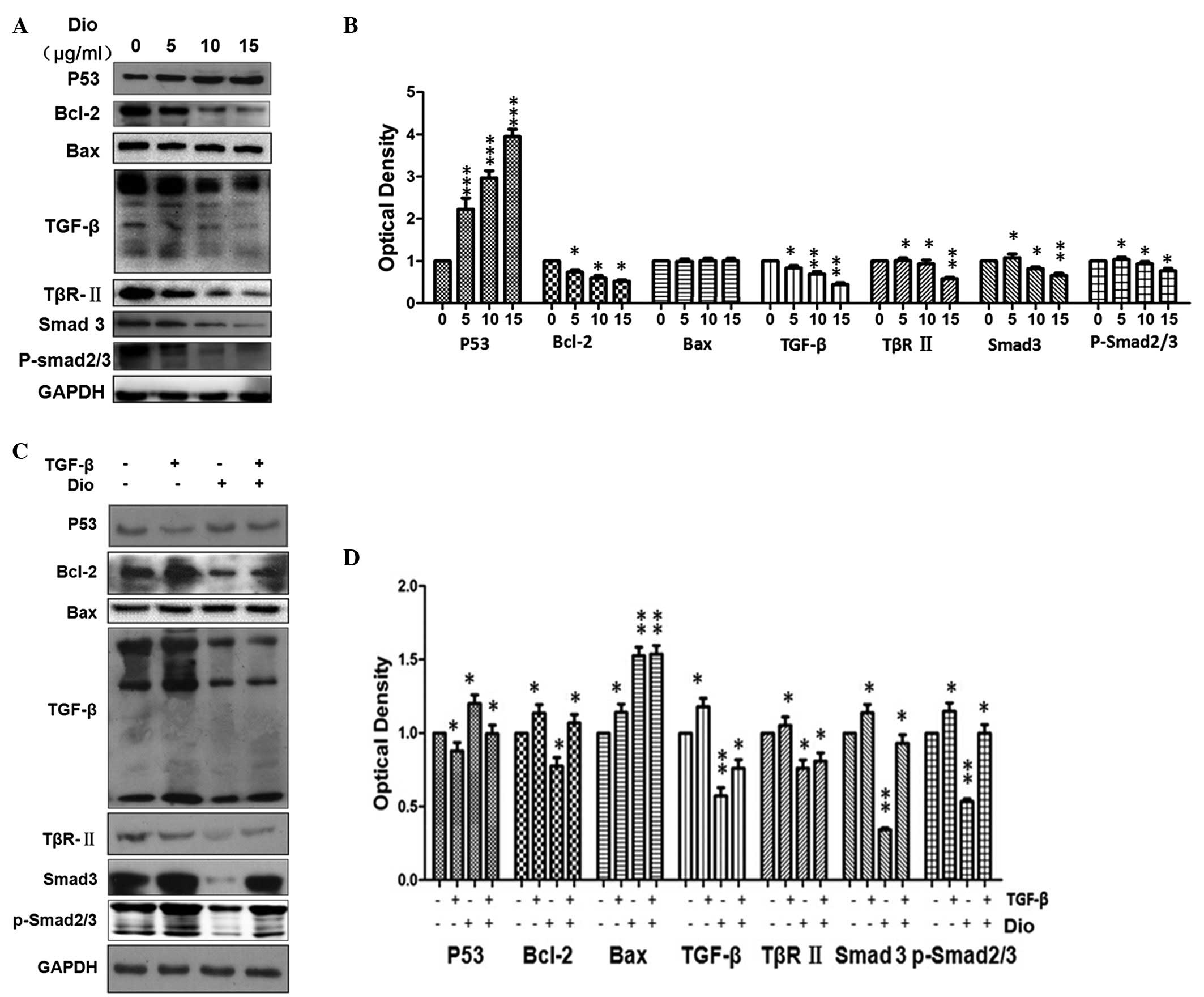
Dio regulates expression levels of
apoptosis-associated proteins and TGF-β signaling pathway
protein
As cell apoptosis is associated with the
Bcl-2-associated mitochondria-dependent apoptosis signaling pathway
(18), the present study further
investigated the link between p53 and Bcl-2 in HepG-2 cells treated
with Dio (Fig. 3). Cells were
exposed to 0,5,10 or 15 µg/ml Dio for 24 h and the
expression levels of p53 were demonstrated to be upregulated in a
dose-dependent manner (P<0.001), while Bcl-2 was downregulated
in a dose-dependent manner (P<0.01). Protein expression levels
of TGF-β, TβRII, Smad3 and p-Smad2/3 were reduced, also in a
dose-dependent manner (P<0.05; Fig.
3A). Bax protein expression levels show no change compared with
the control. The results demonstrated that cell growth was
partially reversed following treatment with recombinant TGF-β.
Correspondingly, Bcl-2, TGF-β, TβRII, Smad3 and P-Smad2/3 increased
when stimulated by TGF-β compared with the control. Furthermore,
p53 protein expression levels decreased when stimulated by TGF-β
compared with the control and Bax protein expression levels showed
no change when compared with the control. p53 protein expression
levels decreased when with TGF-β protein and Dio, compared with Dio
treatment alone, while protein expression levels of Bcl-2, TGF-β,
TβRII, Smad3 and p-Smad2/3 (Fig.
3C).
Dio induces apoptosis by enhancing p53
expression and phosphorylation at Ser15, 33 and 37
p53 is important in the cellular response to DNA
damage and other genomic aberrations. Activation of p53 may lead to
cell cycle arrest and DNA repair, or apoptosis (19). Bcl-2 protein expression levels were
reduced and total p53 expression was increased in HepG2 cells
treated with Dio for 24 h. Using IF microscopy, nuclear
accumulation of p-p53 (at Ser15, 33 and 37) was observed (Fig. 4). It was observed that following
Dio treatment for 24 h, p53 phosphorylation increased at Ser15, 33
and 37, however, no marked increase was detected at Ser20, 46,
Try18 or 81.
Discussion
Natural products have been widely used in the
development of anticancer therapeutic agents, although the
underlying mechanisms of cancer cell suppression remain to be
elucidated (20). Previous studies
into Dio-induced cell apoptosis predominantly focused on cytochrome
P450 (11,21). Dio exerts cytostatic effects in
MDA-MB 468 cells, due to CYP1A1 and CYP1B1-catalyzed conversion to
the flavone luteolin and CYP1 enzymes increase the
antiproliferative activity of dietary flavonoids in breast cancer
cells (9,11). Cyp26 b1 regulates retinoic
acid-dependent signals in T cells and its expression is inhibited
by transforming growth factor-β (22). To increase understanding of TGF-β
signal participation, HepG2 cells were treated with different
concentrations of Dio and cell growth was observed. Cell growth was
inhibited and the expression levels of apoptosis-associated and
TGF-β signaling pathway-associated proteins was altered. However,
following TGF-β administration, cell apoptosis was partly reversed.
TGF-β signaling pathway-associated proteins were also regulated.
Notably, Dio reduced TβRII expression levels, however, addition of
exogenous TGF-β protein regulated Dio-induced inhibition of cell
proliferation and apoptosis, which indicates that TGF-β signaling
pathway is key in Dio-induced cancer cell apoptosis. The present
study hypothesized that exogenous TGF-β protein compensates for
decrease in receptor expression levels. TGF-β and TGF-β receptor
binding initiates a range of cellular responses via binding to and
activation of specific cell surface receptors with intrinsic
serine/threonine kinase activity. These activated TGF-β receptors
stimulate the phosphorylation of R-Smad proteins, which have been
identified as important in regulating the expression levels of
extracellular matrix proteins via the TGF-β signaling pathway
(23,24).
p53 uses various cellular inputs to regulate
apoptosis, proliferation and differentiation (25,26).
p53 has also been associated with cancer cell metastasis,
metabolism and small G protein signal transduction. p53 can induce
apoptosis in response to multiple stimuli via cell growth arrest,
apoptosis and senescence (16,27).
As a transcriptional factor, p53 regulates transcription and
expression of a variety of target genes, such as Bcl-2 and Bax,
ultimately leading to cell cycle arrest and apoptosis (19,28).
The present study demonstrated that Dio significantly upregulates
p53 expression and increases the ratio of Bax/Bcl-2 proteins in
cells treated with Dio, which suggests p53 and Bax/Bcl-2 proteins
are key in Dio-induced cell apoptosis. Furthermore, Dio increases
intracellular p53 protein expression level, particularly the p-p53
at Ser15, 33 and 37. p53 may be phosphorylated by ataxia
telangiectasia mutated, ataxia telangiectasia and Rad3 related, and
DNA-depended protein kinase. Phosphorylation impairs the ability of
MDM2 proto-oncogene to bind p53, promoting accumulation and
activation of p53 in response to DNA damage. Exogenous TGF-β partly
reverses Dio-induced cell apoptosis in HepG2 cells, which
demonstrates that TGF-β/TβRII signaling pathway may be an important
target for therapeutic agents based on Dio-induced cell apoptosis
in HepG2 cells.
Acknowledgments
The present study was supported in part by grants
from the Zhanjiang 2013 Annual Financial Capital Competitive
Project Science and Technology Project, Zhanjiang Key Laboratory of
Hepatobiliary Diseases (grant no. 2013A402-4), Zhanjiang 2014
Annual Financial Capital Competitive Project Science and Technology
Project (grant no. 2014A01029), the Start Project of Doctor
Scientific Research Funds, Affiliated Hospital of Guangdong Medical
College (grant no. B2012039).
References
|
1
|
Yue QX, Liu X and Guo DA:
Microtubule-binding natural products for cancer therapy. Planta
Med. 76:1037–1043. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bitis L, Kultur S, Melikoglu G, Ozsoy N
and Can A: Flavonoids and antioxidant activity of Rosa agrestis
leaves. Nat Prod Res. 24:580–589. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Androutsopoulos VP and Spandidos DA: The
flavonoids diosmetin and luteolin exert synergistic cytostatic
effects in human hepatoma HepG2 cells via CYP1A-catalyzed
metabolism, activation of JNK and ERK and P53/P21 up-regulation. J
Nutr Biochem. 24:496–504. 2013. View Article : Google Scholar
|
|
4
|
Rice-Evans CA, Miller NJ and Paganga G:
Structure-antioxidant activity relationships of flavonoids and
phenolic acids. Free Radic Biol Med. 20:933–956. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quintieri L, Bortolozzo S, Stragliotto S,
Moro S, Pavanetto M, Nassi A, Palatini P and Floreani M: Flavonoids
diosmetin and hesperetin are potent inhibitors of cytochrome P450
2C9-mediated drug metabolism in vitro. Drug Metab Pharmacokinet.
25:466–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Obmann A, Werner I, Presser A, Zehl M,
Swoboda Z, Purevsuren S, Narantuya S, Kletter C and Glasl S:
Flavonoid C-and O-glycosides from the Mongolian medicinal plant
Dianthus versicolor Fisch. Carbohydr Res. 346:1868–1875. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang JD and Roberts LR: Hepatocellular
carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 7:448–458.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Poon RT and Borys N: Lyso-thermosensitive
liposomal doxorubicin: An adjuvant to increase the cure rate of
radiofrequency ablation in liver cancer. Future Oncol. 7:937–945.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Androutsopoulos VP, Mahale S, Arroo RR and
Potter G: Anticancer effects of the flavonoid diosmetin on cell
cycle progression and proliferation of MDA-MB 468 breast cancer
cells due to CYP1 activation. Oncol Rep. 21:1525–1528.
2009.PubMed/NCBI
|
|
10
|
Androutsopoulos V, Wilsher N, Arroo RR and
Potter GA: Bioactivation of the phytoestrogen diosmetin by CYP1
cytochromes P450. Cancer Lett. 274:54–60. 2009. View Article : Google Scholar
|
|
11
|
Androutsopoulos VP, Ruparelia K, Arroo RR,
Tsatsakis AM and Spandidos DA: CYP1-mediated antiproliferative
activity of dietary flavonoids in MDA-MB-468 breast cancer cells.
Toxicology. 264:162–170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Drabsch Y and ten Dijke P: TGF-β signaling
in breast cancer cell invasion and bone metastasis. J Mammary Gland
Biol Neoplasia. 16:97–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Busse A and Keilholz U: Role of TGF-β in
melanoma. Curr Pharm Biotechnol. 12:2165–2175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heldin CH, Landström M and Moustakas A:
Mechanism of TGF-beta signaling to growth arrest, apoptosis, and
epithelial-mesenchymal transition. Curr Opin Cell Biol. 21:166–176.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meulmeester E and ten Dijke P: The dynamic
roles of TGF-β in cancer. J Pathol. 223:205–218. 2011. View Article : Google Scholar
|
|
16
|
Zhang Q, Liu J, Liu B, Xia J, Chen N, Chen
X, Cao Y, Zhang C, Lu C, Li M and Zhu R: Dihydromyricetin promotes
hepato-cellular carcinoma regression via a p53 activation-dependent
mechanism. Sci Rep. 4:46282014.
|
|
17
|
Wu S, Liu B, Zhang Q, Liu J, Zhou W, Wang
C, Li M, Bao S and Zhu R: Dihydromyricetin reduced Bcl-2 expression
via p53 in human hepatoma HepG2 cells. PloS One. 8:e768862013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar
|
|
19
|
Brady CA, Jiang D, Mello SS, Johnson TM,
Jarvis LA, Kozak MM, Kenzelmann Broz D, Basak S, Park EJ,
McLaughlin ME, et al: Distinct p53 transcriptional programs dictate
acute DNA-damage responses and tumor suppression. Cell.
145:571–583. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harvey AL: Natural products in drug
discovery. Drug Discov Today. 13:894–901. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ciolino HP, Wang TT and Yeh GC: Diosmin
and diosmetin are agonists of the aryl hydrocarbon receptor that
differentially affect cytochrome P450 1A1 activity. Cancer Res.
58:2754–2760. 1998.PubMed/NCBI
|
|
22
|
Takeuchi H, Yokota A, Ohoka Y and Iwata M:
Cyp26b1 regulates retinoic acid-dependent signals in T cells and
its expression is inhibited by transforming growth factor-β. PloS
One. 6:e160892011. View Article : Google Scholar
|
|
23
|
Nagaraj NS and Datta PK: Targeting the
transforming growth factor-beta signaling pathway in human cancer.
Expert Opin Investig Drugs. 19:77–91. 2010. View Article : Google Scholar
|
|
24
|
Perrot CY, Javelaud D and Mauviel A:
Insights into the transforming growth factor-β signaling pathway in
cutaneous melanoma. Ann Dermatol. 25:135–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Speidel D: Transcription-independent p53
apoptosis: An alternative route to death. Trends Cell Biol.
20:14–24. 2010. View Article : Google Scholar
|
|
26
|
Javelaud D, Alexaki VI, Dennler S,
Mohammad KS, Guise TA and Mauviel A: TGF-β/SMAD/GLI2 signaling axis
in cancer progression and metastasis. Cancer Res. 71:5606–5610.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee JH, Gaddameedhi S, Ozturk N, Ye R and
Sancar A: DNA damage-specific control of cell death by cryptochrome
in p53-mutant ras-transformed cells. Cancer Res. 73:785–791. 2013.
View Article : Google Scholar
|
|
28
|
Burmakin M, Shi Y, Hedström E, Kogner P
and Selivanova G: Dual targeting of wild-type and mutant p53 by
small molecule RITA results in the inhibition of N-Myc and key
survival oncogenes and kills neuroblastoma cells in vivo and in
vitro. Clin Cancer Res. 19:5092–5103. 2013. View Article : Google Scholar : PubMed/NCBI
|