Introduction
Inflammation is associated with a variety of
progressive diseases, including cancer, cardiovascular disease,
obesity and metabolic disorders, thus, to a certain extent,
eliminating or preventing inflammation is important to maintain
health (1). Inflammatory cells,
including macrophages, mononuclear phagocytes, eosinophils and
neutrophils, are activated during inflammation, and secrete high
levels of nitric oxide (NO), prostaglandin E2 (PGE2) and cytokines,
such as interleukin (IL)-1β, IL-6 and tumour necrosis factor-α
(TNF-α), resulting in cell and tissue damage (2,3).
Previous studies have demonstrated that macrophages perform an
important function in initiating and developing the inflammatory
process, as well as stimulating the excess production of
pro-inflammatory mediators. Activated macrophages produce a series
of pro-inflammatory mediators, including NO, PGE2, IL-1β, IL-6 and
TNF-α, which are important during the development of various
chronic diseases (3,4). NO and PGE2 are important
pro-inflammatory mediators regulated by inducible nitric oxide
synthase (iNOS) and cyclooxygenase-2 (COX-2), respectively. The
expression of pro-inflammatory mediators, including iNOS, COX-2,
TNF-α, IL-1β and IL-6, is regulated by the transcription factor
nuclear factor-κB (NF-κB) (5,6).
Canonical NF-κB exists in the cytoplasm as an inactive dimeric
protein composed of two subunits (P50 and P65) and is bound to
inhibitor of NF-κB (IκB) protein. When stimulated by other
endogenous inducers, including IL-1β and TNF-α, and exogenetic
factors, such as lipopolysaccharide (LPS), IκB is phosphorylated
and degraded. The released NF-κB dimer can then be translocated
from the cytoplasm to the nucleus when activated by p65
phosphorylation and regulate target gene transcription (7). Propagation of the inflammatory
process is regulated by multiple mechanisms. Upon LPS stimulation
of macrophages, the endotoxin (LPS) reaches specific toll-like
receptors on the cell membrane, inducing complex signalling
cascades and stimulation of the three mitogen-activated protein
kinase (MAPK) signalling pathways. The MAPK pathway is one of the
most widely studied intracellular signalling cascades. The pathway
is composed of p38MAPK, extracellular signal-regulated kinase 1/2
(ERK1/2) and c-JUN N-terminal kinase (JNK), all of which are
involved in NF-κB activation (7,8).
Thus, inhibiting the MAPK and NF-κB pathways to reduce the
production of pro-inflammatory cytokines is crucial for suppressing
the inflammatory response.
Several food-derived compounds have been
demonstrated to decrease inflammation associated with certain
diseases. Dietary antioxidants and flavonoids are abundant in
fruits and vegetables, and have important functions as
pharmacologically active compounds in Chinese herbs. The biological
activities of these compounds includes antioxidant,
anti-inflammatory, antiviral and anticarcinogenic effects (9,10).
These beneficial effects are partially due to the free radical
scavenging ability of the molecules, which suppresses the
production of inflammatory cytokines by interfering with
intracellular signalling pathways and modulating gene expression
(11,12). Quercetin (QE) is a common flavonol
present in a wide range of food resources, including fruits,
vegetables, tea, nuts, wine and seeds. QE is a potent bioactive
flavonoid with various beneficial effects on human health; it
promotes free radical scavenging, and demonstrates strong
antioxidant and anti-inflammatory activities (13–15).
Other functional food components that can ameliorate inflammation
include omega-3 long chain polyunsaturated fatty acids. Marine fish
oil is a major source of eicosapentaenoic acid (EPA) and
docosahexaenoic acid (DHA) (16).
These molecules provide various positive benefits to human health,
particularly in preventing cardiovascular and inflammatory
diseases. QE and DHA are present in a variety of foods as natural
compounds, and alternative DHA sources, including aquatic microbes
or transgenic crops, have been used to address fish stock
limitations and risks associated with ocean pollution (17). Thus, as these molecules are easily
consumed as food ingredients or functional food supplements in
combination, determining their synergistic, additive or
antagonistic effects may be important for developing novel food
products with beneficial effects on human health. The
anti-inflammatory capacities of QE and DHA have been investigated
individually but not in combination, and little is known regarding
the combinational anti-inflammatory effects of these compounds in
LPS-induced RAW264.7 murine macrophage cells. Several studies
indicate that DHA is more potent than EPA in reducing IL-1β and
IL-6 expression in LPS-activated macrophages (18). Thus, the anti-inflammatory effects
of QE and DHA, in combination and separately, must be
elucidated.
The present study aimed to assess whether QE and DHA
treatment of RAW264.7 cells can modulate the expression levels of
pro-inflammatory mediators (NO, iNOS, PGE2, COX-2, IL-1β, IL-6,
TNF-α) and key proteins (p50, p65, IκB, ERK, JNK, p38MAPK), as well
as the phosphorylation levels of proteins involved in the NF-κB and
MAPK signalling pathways. Additionally, the current study
investigated the synergistic anti-inflammatory effects of QE and
DHA on RAW264.7 cells stimulated with LPS to induce inflammatory
responses.
Materials and methods
Reagents
DHA (D2534; ≥98%), QE (Q4591; ≥98%),
dimethyl-sulphoxide (DMSO), LPS, Griess reagent, phosphate-buffered
saline (PBS),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltertra-zolium (MTT),
sodium nitrite and radioimmunoprecipitation assay (RIPA) buffer
were purchased from Sigma-Aldrich (St. Louis, MO, USA). Primary
antibodies against iNOS (rabbit polyclonal antibody; 1:400
dilution; cat. no. ab3523) and COX-2 (rabbit polyclonal antibody,
1:200 dilution; cat. no. ab15191) were purchased from Abcam Trading
Company (Shanghai, China). IκB (rabbit polyclonal antibody, 1:200
dilution; cat. no. sc847), phospho-(p-)-IκB (mouse monoclonal
antibody, 1:200 dilution; cat. no. sc8404) were obtained from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). p-ERK (rabbit
monoclonal antibody, 1:1,000 dilution; cat. no. 8544) and p-JNK
(rabbit monoclonal antibody, 1:800 dilution; cat. no. 4668) were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
p-p50 (rabbit polyclonal antibody, 1:700 dilution; cat. no.
BS4131), p-p38 (rabbit polyclonal antibody, 1:600 dilution; cat.
no. BS4635) and p-p65 (rabbit polyclonal antibody, 1:700 dilution;
cat. no. BS3556) were purchased from Bioworld Technology, Co., Ltd.
(Nanjing, China). and horseradish peroxidase (HRP)-conjugated
secondary antibodies were purchased from Cell Signalling
Technology, Inc. (Danvers, MA, USA). Enzyme-linked immunosorbent
assay (ELISA) kits for IL-1β, IL-6, TNF-α and PGE2 were obtained
from Nanjing Jiancheng Bioengineering Institute (Nanjing,
China).
Cell culture
RAW264.7 murine macrophage cells (Type Culture
Collection of the Chinese Academy of Sciences, Shanghai, China)
were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10% foetal
bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.), 2 mM
D-glutamine (Shanghai Hanhong Chemical Co., Ltd., Shanghai, China)
and antibiotics (100 U/ml penicillin and 100 µg/ml
streptomycin) (Dingguo Biotechnology Co., Ltd., Wuhan, China) at
37°C and 5% CO2 in a humidified incubator.
Cell viability assay
Cell viability was evaluated using an MTT assay.
RAW264.7 cells were seeded in 24-well plates at a density of
1×105 cells/well and incubated at 37°C for 24 h. The
cells were treated with DHA (30 and 60 µM), QE (20, 40 and
60 µM) or DHA + QE and then stimulated with LPS (100 ng/ml)
in medium or left untreated at 37°C for 24 h. MTT solution (~1 ml,
5 mg/ml) was added to each well and the plates were incubated at
37°C for 4 h. Blue formazan crystals were dissolved in DMSO and the
absorbance of the solution was measured at 570 nm using a
microplate reader (Multiskan FC; Thermo Fisher Scientific,
Inc.).
Nitrite assay
NO production (NOP) was measured as nitrite by
Griess reagent to evaluate the anti-inflammatory effects of DHA and
QE separately or in combination. Five conditions were examined, as
follows: Negative control (C−), medium only (DMEM, 10%
FBS, 2 mM D-glutamine and antibiotics); positive control
(C+), 100 ng/ml LPS; LPS plus DHA treatment (30 and 60
µM); LPS plus QE treatment (20, 40 and 60 µM); and
LPS plus DHA + QE treatment. The cells were cultured in 24-well
plates at 2×105 cells/well and grown to 80–90%
confluence. The medium was replaced with colourless serum-free DMEM
medium with antibiotics. The cells were then stimulated with LPS
(100 ng/ml), incubated with or without DHA, QE or DHA + QE, and
then cultured at 37°C for 24 h. The supernatant (100 µl) was
collected and mixed with 100 µl Griess reagent to produce a
final stable purple product. The absorbance of the mixture was
quantified by a microplate reader at 540 nm. Biological and
technical replicates were performed for all measurements in
triplicate. A standard sodium nitrite curve was established to
determine nitrite levels.
Measurement of pro-inflammatory cytokine
and PGE2 protein concentration levels
RAW264.7 cells were seeded in 24-well plates at a
density of 1×105 cells/well and grown to 80–90%
confluence. The medium was replaced with colorless serum-free DMEM
medium with antibiotics. The cells were then treated with one of
the following: Medium only (with vehicle); 100 ng/ml LPS; LPS plus
20 µM QE; LPS plus 30 µM DHA; or LPS plus QE + DHA.
The supernatant was collected after 24 h of treatment to evaluate
IL-1β, IL-6, TNF-α and PGE2 levels using ELISA kits, according to
the manufacturer's protocol.
Measurement of protein expression in
LPS-induced RAW264.7 cells by western blot analysis
Cells were washed with PBS then harvested and lysed
in RIPA buffer (Beyotime Institute of Biotechnology, Shanghai,
China), then 1 mM PMSF (Beyotime Biotechnology Inc., Shanghai,
China) was added into the lysate. The mixture was centrifuged at
4°C and 1,4000 × g for 5 min, the supernatant was collected for the
next step. The concentration of protein was quantified by a
Bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology). Total cell proteins (20 µg RAW264.7 lysates)
were separated by 10% sodium dodecyl sulfate-polyacrylamide gel
(Thermo Fisher Scientific, Inc.) electrophoresis and transferred to
polyvinylidene difluoride membranes (Merck Millipore, Darmstadt,
Germany). The membranes were blocked in Tris-buffered saline-Tween
20 solution (Dingguo Biotechnology Co., Ltd.) containing 5% non-fat
dry milk and incubated with specific antibodies overnight at 4°C.
Protein bands were visualized using an enhanced chemiluminescent
reagent (Thermo Fisher Scientific, Inc.) following incubation with
HRP-conjugated secondary antibodies for 2 h at room temperature.
The band intensity was quantified with BandScan 5.0 (Glyko, Novato,
CA, USA). Values of each sample were normalized to the fraction of
β-actin and all experiments were repeated 3 times.
Measurement of IκB, p65, p50, ERK1/2, JNK
and p38MAPK mRNA expression by semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from RAW264.7 cells using the
Qiagen RNeasy kit (Qiagen, Inc., Valencia, CA, USA), according to
the manufacturer's instructions. RNA samples (4.2 µg) were
treated with RNase-Free Recombinant DNase I (cat. no. #2270A,
Takara Bio Inc., Tokyo, Japan). cDNA was synthesized using the
Primer Script RT reagent kit according to the manufacturer's
instructions (Takara Co., Ltd., Tokyo, Japan). PCR was performed on
the cDNA with the appropriate sense and antisense primers, as
indicated in Table I (Nanjing
Genscript Biotechnology Co., Ltd., Nanjing, China). Each reaction
was performed in a total volume of 20 µl containing 2X 10
µl SYBR Green/Fluorescein qPCR Master mix (Fermentas; Thermo
Fisher Scientific, Inc.), 4 µl cDNA and 0.2 µmol/l of
each primer. PCR was conducted using an ABI, ViiA7 thermal cycler
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The cycling
conditions are as follows: 1 cycle of 50°C for 2 min;
pre-denaturation at 95°C for 10 min; 40 cycles of amplification
denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec and
elongation at 72°C for 12 sec, followed by a final extension
(denaturation at 95°C for 5 sec, annealing at 65°C for 1 min,
elongation at 97°C for 5 sec, and 50°C for cooling) in order to
generate a melting curve. The PCR products were separated using
electrophoresis on 1.4% agarose gels (Dingguo Biotechnology Co.,
Ltd.) and stained with ethidium bromide (Dingguo Biotechnology Co.,
Ltd.) for visualization. The relative expression of each gene was
calculated by the comparative Cq (cycle quantification or ΔΔCq)
method. The gene expression levels were normalized to the reference
gene (β-actin). Oligo dT primers, reverse transcriptase, RT buffer
and dNTPs used for reverse transcription (or the kit used).
 | Table IPrimers used to perform polymerase
chain reaction. |
Table I
Primers used to perform polymerase
chain reaction.
| Gene | Primer (5′-3′) |
|---|
| IκB |
| Sense |
GAACCTGAGGACGAGGACGAT |
| Antisense |
GTTGTCGGTTTTGGCTCCTGC |
| p65 |
| Sense |
CGGGATGGCTACTATGAGGCTGACC |
| Antisense |
GATTCGCTGGCTAATGGCTTGCT |
| p50 |
| Sense |
GTGATTTGTGCCAGCCAGGAAGC |
| Antisense |
TTCTTAACCCGAAGCCCTTGATT |
| p38 |
| Sense |
GGGACCTAAAGCCCAGCAACCT |
| Antisense |
CAGCCCACGGACCAAATATCCAC |
| ERK |
| Sense |
CATGGAGACGGACCTTTACAAGC |
| Antisense |
CACAAGTGGTGTTCAGCAGGAGG |
| JNK |
| Sense |
TCTCCAGCACCCATACATCAACG |
| Antisense |
GTTCCTCCAAATCCATTACCTCC |
| β-actin |
| Sense |
CACGATGGAGGGGCCGGACTCATC |
| Antisense |
TAAAGACCTCTATGCCAACACAGT |
Calculating the potentiating effects of
QE and DHA in combination
Enhancements of the effects of QE and DHA in
combination were evaluated according to the following previously
described method (16,19). Using NOP as an example, the maximum
inhibitory effects of compound A and B alone in a medium (expressed
as percentages) are calculated as follows: E(A) =%NOP in
C+ group − %NOP of compound A + standard error of the
mean (SEM); E(B) = %NOP in C+ group − %NOP of
compound B + SEM. The minimum inhibitory effects of the compounds
in combination in medium is calculated as follows: E(AB)
= %NOP in C+ group − %NOP of compound A and B - SEM.
Enhanced anti-inflammatory effects are observed when
E(AB) ≥ E(A) + E(B). Both QE and
DHA must have significant individual NOPs compared with the NOP
obtained when QE and DHA are combined. If either QE or DHA fails to
fulfil this criterion, the compounds are considered to have no
effect when combined. These criteria were also applied to determine
effects on protein phosphorylation and expression.
The Bliss independence model was used to evaluate
whether the enhanced effects in this study can be considered
synergistic, additive or antagonistic (16). This model is one of the most widely
adopted, recommended and accepted models for defining drug
interactions and is, thus, suitable for the present study. The
model equation is E(AB) = E(A) ×
E(B), where E(AB) is the effect of the
compounds in combination, and E(A) and E(B)
represent the individual effects of compounds A and B,
respectively. All equation results are expressed in fractions.
E(AB) < E(A) × E(B),
E(AB) = E(A) × E(B) and
E(AB) > E(A) × E(B) indicate
synergistic, additive and antagonistic effects, respectively.
Statistical analysis
Results are expressed as mean ± standard deviation,
and data were analyzed by one-way analysis of variance followed by
a Dunnett's test to evaluate statistical differences between groups
using SPSS 16.0 statistical software (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
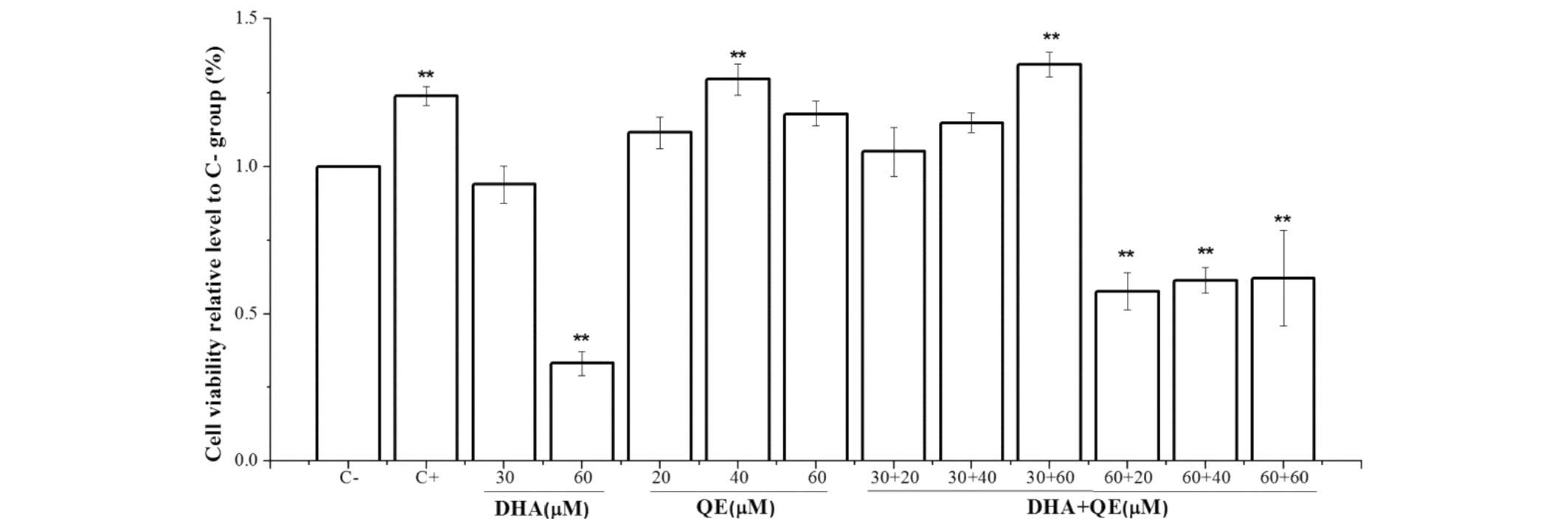
Cytotoxicity of DHA and QE on RAW264.7
cells
An MTT assay was performed to evaluate DHA and QE
toxicity on RAW264.7 cells. The DMSO concentration was controlled
within 0.01% in the culture medium to allow adequate dissolution
and did not affect the anti-inflammatory effects of DHA, QE and DHA
+ QE. The MTT assay (Fig. 1)
indicated that the viability of RAW264.7 macrophages was not
significantly changed under experimental conditions of 30 µM
DHA, 20 µM QE or 30 µM DHA + 20 µM QE compared
with the C− group. However, the higher concentration of
DHA (60 µM) and its combination with QE, as well as 40
µM QE alone, significantly decreased cell viability compared
with the C− group (P<0.01).
Effects of QE and DHA on NOP and iNOS
protein expression in LPS-stimulated RAW 264.7 cells
LPS, the major cell wall component in gram-negative
bacteria, can trigger inflammatory responses in macrophages. The
levels of several biomarkers, including NO, can be used to evaluate
the LPS-induced inflammatory response. RAW264.7 cells were
stimulated with LPS then incubated with various concentrations of
DHA (30 and 60 µM) and QE (20, 40 and 60 µM) alone or
in combination for 24 h to measure the effects of QE and DHA on NOP
suppression. The supernatant was collected to determine the NOP
levels.
Data for each group are calculated as the NO
expression level versus that of the C+ group. Then the
C+ group was set to 1, and the expression of each group
was relative to that of the C+ group. DHA (30 µM)
treatment significantly suppressed NOP compared with the
C+ group (30.4% NO reduction, P<0.05). QE (20
µM) similarly inhibited NOP compared with the C+
group (25.5% NO reduction, P<0.05). Combined treatment with the
molecules at the aforementioned concentrations exerted a stronger
suppressive effect than either DHA or QE individually (61.6% NO
reduction) (Fig. 2A). The Bliss
model, E(AB) = E(A) × E(B),
demonstrated that the E(AB) value (0.384) was less than
E(A) × E(B) (0.519; DHA × QE = 0.696 ×
0.745), indicating the synergistic effects of the two compounds.
The higher concentration of DHA (60 µM) and combination with
QE demonstrated an even greater inhibitory effect on NOP, however,
these concentrations reduced cell viability in the MTT assay
(Fig. 1). Thus, the present study
used the doses of 30 µM DHA and 20 µM QE to examine
their anti-inflammatory effect.
 | Figure 2Inhibitory effects of DHA and QE on
inflammatory mediators. (A) NO production was measured in
lipopolysaccharide (LPS)-stimulated RAW264.7 cells following
treatment with DHA (30 and 60 µM), QE (20, 40 and 60
µM) or DHA + QE. (B) iNOS protein expression levels, and (C)
IL-1β, (D) IL-6 and (E) TNF-α protein concentration levels were
measured in LPS-stimulated RAW264.7 cells treated with DHA (30
µM), QE (20 µM) or DHA + QE. Results are expressed as
percentage normalized to the C+ group. Values are
presented as the mean ± standard deviation of biological and
technical triplicates. *P<0.05 and
**P<0.01 vs. C+ group.
#P<0.05 and ##P<0.01 vs. DHA + QE.
Synergistic effects are indicated by the letter S. NO, nitric
oxide; C−, negative control; C+, positive
control; DHA, docosahexaenoic acid; QE, quercetin; IL, interleukin;
TNF-α, tumour necrosis factor-α. |
DHA + QE combination treatment exhibited
statistically significant suppression of LPS-induced iNOS protein
expression compared with the C+ group and the individual
drug treatments (P<0.01; Fig.
2B). However, an antagonistic effect was detected using the
Bliss model [E(AB) = 0.464; E(A) ×
E(B) = 0.418] (P=0.01; Fig.
2B). This effect may indicate that the combination of the two
inhibits NO expression through scavenging NO as well as inhibiting
the expression of iNOS.
Inhibitory effects of QE and DHA on the
expression of inflammatory mediators
Pro-inflammatory cytokines are critical markers of
inflammatory responses in LPS-stimulated macrophages. The
concentration levels of IL-1β, IL-6 and TNF-α were examined using
ELISA to evaluate the effects of DHA (30 µM) and QE (20
µM) on cytokine production. The results indicated that DHA +
QE in combination exert a stronger inhibitory effect on cytokine
production compared with DHA or QE alone (P<0.01; Fig. 2C–E). All inhibitory effects were
statistically significant and no enhancement of cytokine production
was observed.
The production of PGE2, another important
pro-inflammatory mediator, depends on COX-2 activity. The present
study examined the effect of QE and DHA, and their cooperation, on
PGE2 protein concentration and COX-2 protein expression. The
results demonstrated that DHA and QE significantly decreased PGE2
concentration levels compared with the C+ group
(P<0.01). Whilst QE and DHA individually significantly inhibited
PGE2 concentration levels, their use in combination further reduced
PGE2 concentration compared with individual treatment (P<0.01;
Fig. 3A). Synergistic effects were
determined using the Bliss model as E(AB) (0.576) was
less than E(A) × E(B) (0.632; DHA × QE =
0.752 × 0.840). Similarly, COX-2 protein expression was
significantly reduced in response to DHA + QE compared with
individual treatments [E(AB) = 0.524; E(A) ×
E(B) = 0.706], which further confirms the synergistic
effects of DHA and QE (Fig.
3B).
Effects of QE and DHA on the
phosphorylation of key proteins in LPS-induced RAW264.7 cells
RAW264.7 cells were stimulated with LPS and
incubated with DHA (30 µM), QE (20 µM) or DHA + QE
for 24 h. Phosphorylated protein expression levels were determined
by western blot analysis. Assays indicated that the inhibitory
effects of DHA+QE on p50, IκB and p65 phosphorylation were
increased when used in combination compared with individual use
(Fig. 4A and B). The
phosphorylation levels of p50, p65 and IκB were also significantly
increased following LPS induction (C+ group) in comparison with the
C-group. Following individual treatment with QE and DHA, the
phosphorylation levels of p50, p65 and IκB were decreased compared
with the C+ group (P=0.01). However, phosphorylation decreased
further to levels close to that in the C− group when cells were
treated with DHA+QE in combination. Synergistic effect was observed
when DHA and QE were combined to prevent the phosphorylation of p50
[E(AB) = 0.37; E(A) × E(B) = 0.46]
and p65 [E(AB) = 0.48; E(A) × E(B)
= 0.42]. By contrast, stimulation with LPS (C+ group)
significantly decreased the expression of IκB protein in comparison
with the C− group. IκB degradation was significantly
inhibited by DHA+QE. Furthermore, the effect of the drugs in
combination was greater then their individual use (Fig. 4C). According to the Bliss model, an
additive effect was detected when the drugs combined
[E(AB) = 0.30; E(A) × E(B) =
0.30). These findings suggest that IκB degradation and
phosphorylation are inhibited by DHA and QE.
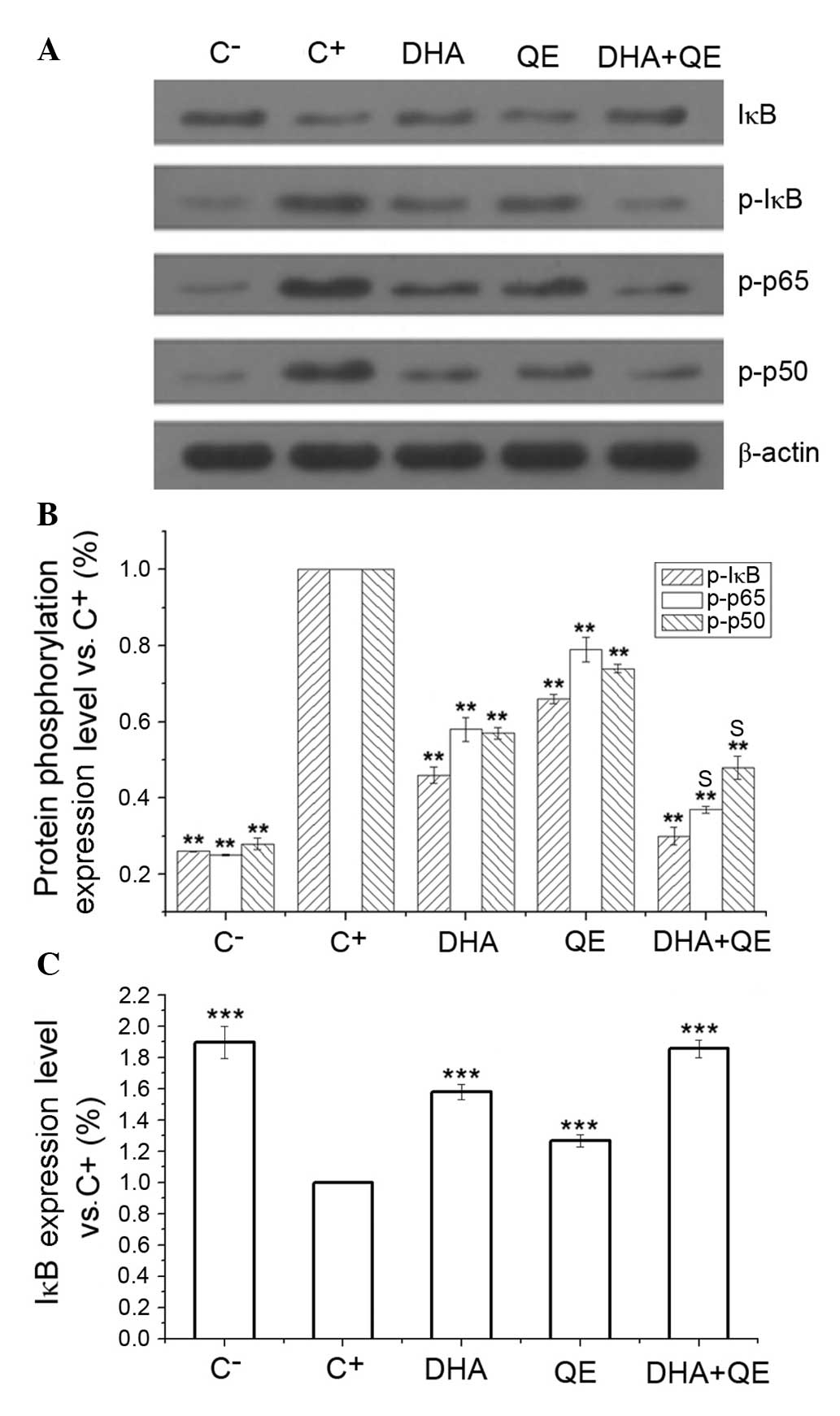 | Figure 4Effects of DHA and QE on IκB, p50 and
p65 phosphorylation and IκB degradation in lipopolysaccharide
(LPS)-induced RAW264.7 cells. RAW264.7 cells were stimulated with
LPS (100 ng/ml) and incubated with DHA (30 µM), QE (20
µM) or QE + DHA for 24 h. (A) The protein expression levels
of IκB, and phosphorylated IκB, p50 and p65 were determined by
western blot analysis. (B and C) Levels were quantified using
densitometry and each signal was normalized to the β-actin signal.
Results are expressed as percentages relative to the C+
group. Values are presented as the mean ± standard deviation of
biological and technical triplicates. **P<0.01 and
***P<0.001 vs. C+ group. Synergistic
effects are indicated by the letter S. C−, negative
control; C+, positive control; DHA, docosahexaenoic
acid; QE, quercetin; IκB, inhibitor of nuclear factor-κB; p-,
phospho-. |
The MAPK pathways are involved in the excess
production of pro-inflammatory cytokines during inflammation and
activate NF-κB in LPS-induced macrophages (2). The effect of QE and DHA on
LPS-stimulated phosphorylation of ERK, JNK and p38MAPK in RAW264.7
cells was examined to evaluate whether these molecules counteract
the effects of LPS at the molecular level. LPS treatment
(C+ group) significantly increased the phosphorylation
of ERK, JNK and p38MAPK. DHA and QE, alone and in combination,
reduced the phosphorylation of the MAPK pathway proteins compared
with LPS stimulation (C+ group; P<0.01). The effect
of DHA + QE treatment was more potent compared with QE or DHA
alone. Synergistic effects on ERK [E(AB) = 0.694;
E(A) × E(B) = 0.760] and JNK
[E(AB) = 0.628; E(A) × E(B) =
0.665] phosphorylation were detected when QE + DHA were used in
combination However, the combination exhibited an antagonistic
effect on p38 expression [E(AB) = 0.401; E(A)
× E(B) = 0.424] according to the Bliss model (Fig. 5).
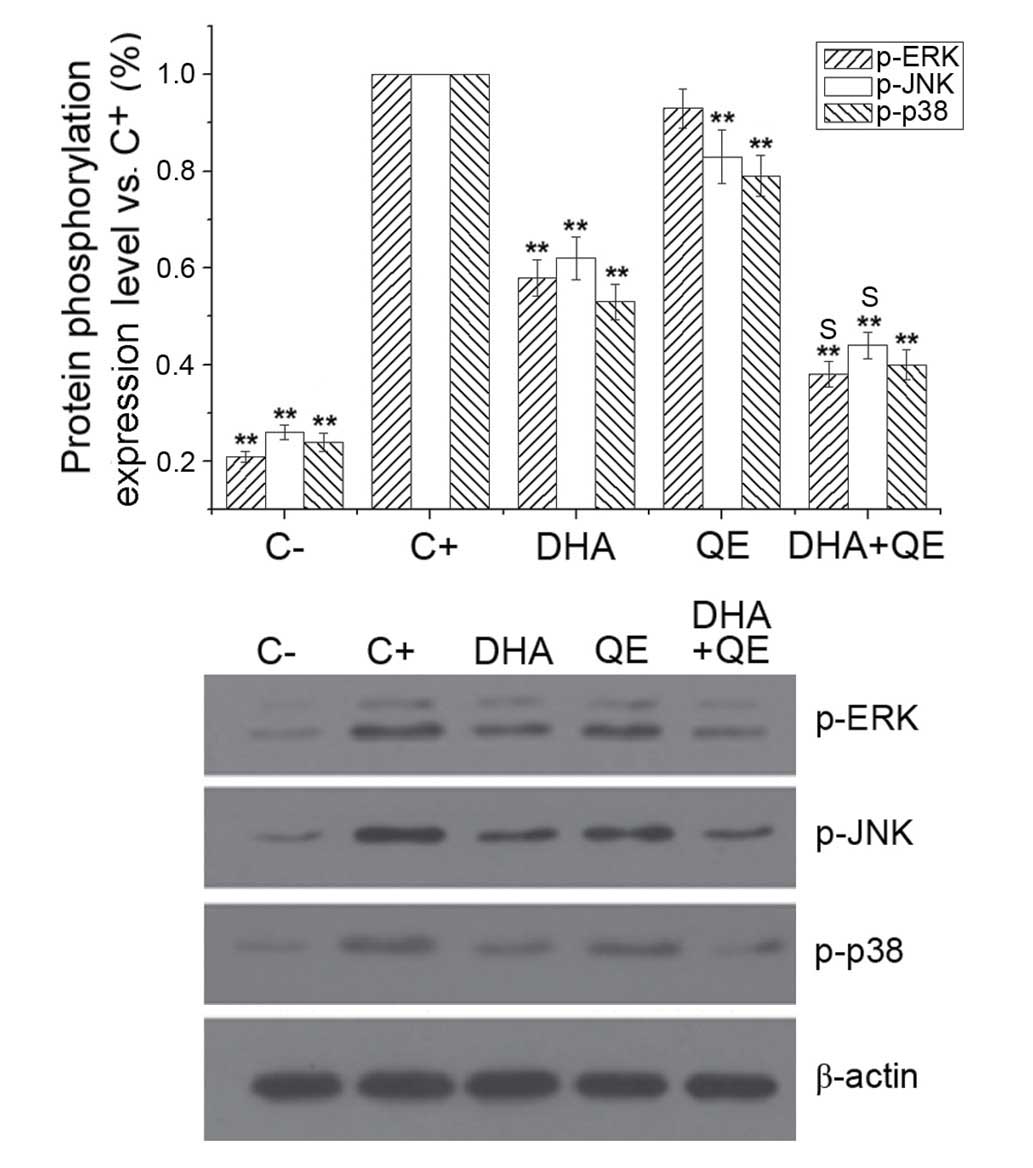 | Figure 5Effects of QE and DHA on ERK, JNK and
p38MAPK phosphorylation levels in lipopolysaccharide (LPS)-induced
RAW264.7 cells. RAW264.7 cells were stimulated with LPS (100 ng/ml)
and incubated with DHA (30 µM) QE (20 µM) or DHA + QE
for 24 h. Phosphorylation levels of ERK, JNK and p38MAPK were
determined by western blot analysis, and each signal was normalized
to the β-actin signal. Results are expressed as percentages of the
C+ group. Values are presented as the mean ± standard
deviation of biological and technical triplicates.
**P<0.01 vs. C+ group. Synergistic effects
are indicated by the letter S. C−, negative control;
C+, positive control; QE, quercetin; DHA,
docosahexaenoic acid; p-, phospho-; ERK, extracellular
signal-regulated kinase; JNK, c-JUN N-terminal kinase. |
Effects of DHA and QE on the mRNA
expression of key genes in LPS-induced RAW264.7 cells
RAW264.7 cells were stimulated with LPS (100 ng/ml)
for 24 h to induce the expression of genes regulating important
proteins involved in the NF-κB and MAPK signalling pathways. Cells
were then treated with DHA (30 µM), QE (20 µM) or DHA
+ QE to determine the effect of these molecules on IκB, p65, p50,
ERK1/2, JNK and p38MAPK mRNA expression (Fig. 6). mRNA expression levels that
exhibited significant differences compared with the C+
group were considered upregulated or downregulated, respectively.
RT-PCR demonstrated that p50, p65 (Fig. 6A), p38MAPK, ERK1/2 and JNK1/2 mRNA
expression levels (Fig. 6B) were
significantly increased in the C+ group compared with
the C− group. Individual QE or DHA treatment has no
effect on the mRNA expression levels of these genes, however the
combined use of DHA and QE significantly reduced the mRNA
expression of p50, p65, p38MAPK, ERK1/2 and JNK compared with the
C+ group treatment. However, according to the criterion
of the mathematical model mentioned above, the combination was
considered to have no effect. By contrast, LPS stimulation
(C+ group) decreased the mRNA expression of IκB in
comparison with the C− group, and DHA or QE treatment
increased the IκB levels (Fig.
6A). The DHA+QE combined treatment resulted in a stronger
upregulation of IκB mRNA expression compared with either compound
alone. Similarly, according to the criterion of the mathematical
model mentioned above, the combination was considered to have no
effect. This result may demonstrate that the inhibitory effect on
NF-κB and MAPK was through inhibiting the phosphorylation of p50,
p65, IκB, p38MAPK, ERK1/2 and JNK, which involves NF-κB and
MAPK.
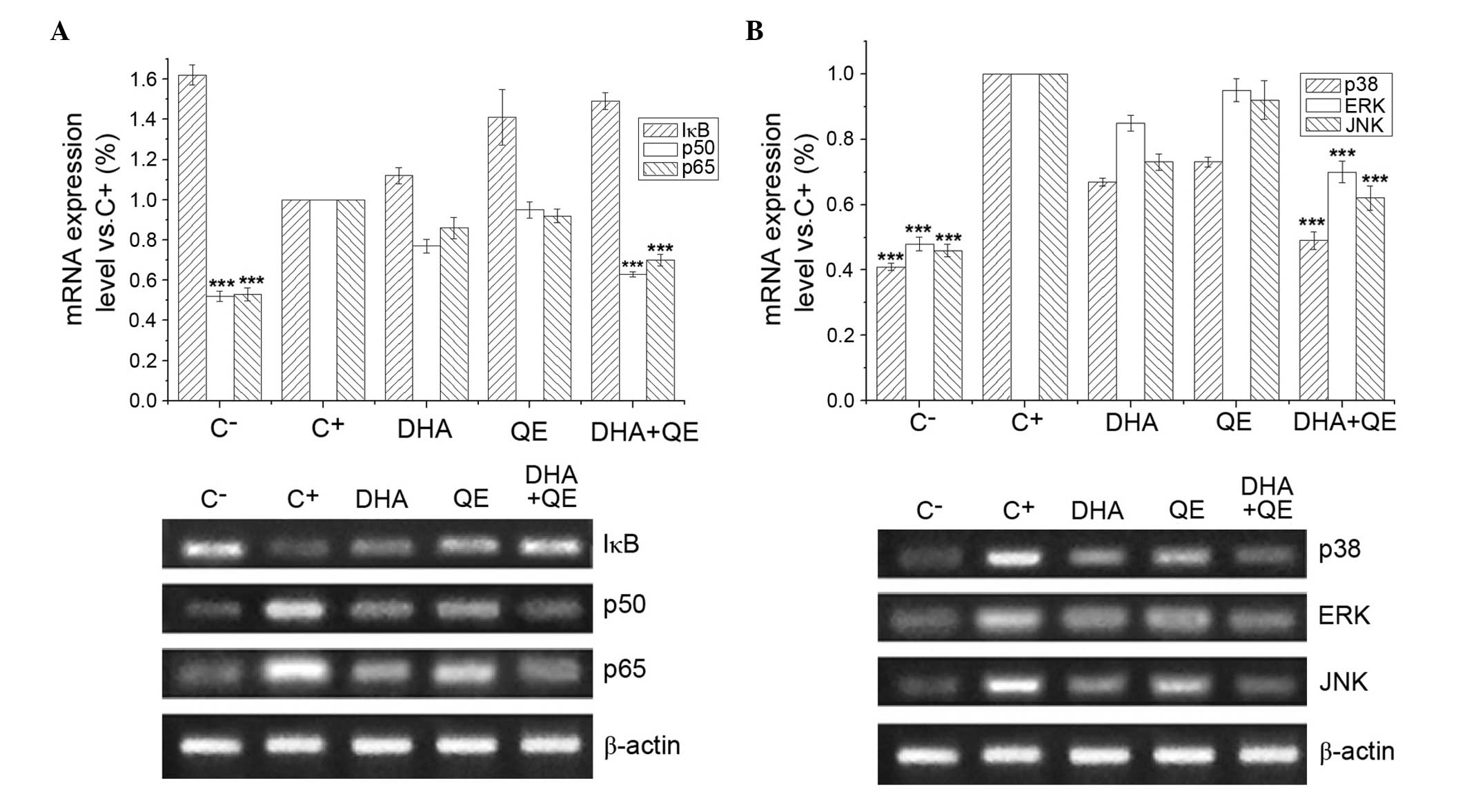 | Figure 6Effects of DHA and QE on IκB, p65,
p50, ERK1/2, JNK1/2 and p38MAPK mRNA expression levels. RAW264.7
cells were stimulated with lipopolysaccharide (LPS) and incubated
with DHA (30 µM), QE (20 µM) or DHA + QE for 20 h.
mRNA expression levels of (A) IκB, p65, p50, (B) ERK1/2, JNK1/2 and
p38MAPK were determined by reverse transcription-polymerase chain
reaction. Results are expressed as percentages of the C+
group. Values are presented as the mean ± standard deviation of
biological and technical triplicates. ***P<0.001 vs.
C+ group. C−, negative control;
C+, positive control; DHA, docosahexaenoic acid; QE,
quercetin; IκB, inhibitor of nuclear factor-κB; ERK, extracellular
signal-regulated kinase; JNK, c-JUN N-terminal kinase. |
Discussion
Understanding the advantageous effects of bioactive
compounds, and using this knowledge to improve and produce more
health-beneficial foods, is an important field in functional food
science (19,20). Such compounds can be investigated
using in vitro and in vivo models. The present study
presents initial in vitro experiments using two natural
bioactive molecules.
RAW264.7 murine macrophages are a commonly used cell
line for investigating inflammation due to their reproducible
response to LPS stimulation (19).
In the present study, murine RAW264.7 macrophages were stimulated
with LPS to induce an inflammatory response, and investigate the
anti-inflammatory effects of DHA and QE. The LPS concentration used
in the current study was lower and less aggressive than
concentrations used in previous studies (1 or 10 µg/ml)
(21,22). The LPS concentration used herein
was more appropriate for investigating the anti-inflammatory
effects of QE and DHA, as previous studies indicate that 100 ng/ml
LPS induces inflammation in macrophages at a level that is
sensitive to the anti-inflammatory effects of DHA (18,23).
As natural nutrients, the anti-inflammatory activity of DHA and QE
are less potent than those of other pharmaceutical agents,
therefore, the present study focused on anti-inflammatory compound
sensitivity at low levels of LPS stimulation. A previous study
reported that 48 subjects who consumed fish once or twice a week
presented plasma DHA and EPA levels of ~60 and 10 µg/ml, or
~182 and 33 µM, respectively (24). Thus, the DHA concentration used in
the present study is within the ranges of bioavailable levels
observed in plasma during previous in vivo studies. The
functions of QE, particularly its anti-inflammatory and
anti-obesity properties, have been extensively investigated. The
effects of QE in mice are well established, however, the activity
in human subjects is unclear (22). Poor in vivo results using QE
may be attributed to the limited aqueous solubility, stability and
cellular bioavailability of the compound (25). QE concentrations in human plasma
are generally in the low nanomolar range but may increase to the
micromolar range by supplementation (26). Several in vitro studies have
previously demonstrated that the anti-inflammatory and antioxidant
capacities of QE at concentrations of 2.5–100 µM are time-
and dose-dependent. Improved QE bioavailability has also been
observed through nano-crystallisation (22,25).
Thus, the concentrations of QE and DHA used in the present study
were selected based on previous in vitro and in vivo
models.
The aim of the present study was to evaluate the
anti-inflammatory effects of DHA and QE individually and in
combination. Previous studies demonstrated that QE has
anti-inflammatory effects in vitro and in vivo, and
that QE can suppress LPS-induced production of pro-inflammatory
cytokines (NO, PGE2, TNF-α, IL-1β and IL-6), modulate iNOS and
COX-2 synthesis, inhibit IκB kinase α and β, and stimulate IL-10
secretion and heme oxygenase-1 (HO-1) induction. QE has also be
reported to inhibit NF-κB, MAPKs, protein kinase B, Src, Janus
kinase 1, tyrosine kinase 2, and signal transducer and activator of
transcription 1 (27,28). The mechanisms of these
health-promoting effects are associated with QE regulation of
several important enzymes, cytokines, transcription factors and
antioxidant systems (29). DHA or
fish oil can modulate anti-inflammatory processes in macrophages
through G-protein-coupled receptors (GPR120 pathway), and regulate
inflammatory gene expression through decreased activation of NF-κB
and increased activation of peroxisome proliferator-activated
receptor-γ (30–32). In the present study, QE and DHA
individually reduced the levels of LPS-induced NO, iNOS, COX-2,
PGE2 and cytokines (IL-1β, IL-6 and TNF-α), and synergistic
inhibitory effects on NO, COX-2 and PGE2 levels were observed when
these molecules were combined. The findings of the present study
suggest that QE and DHA can inhibit the production of a variety of
inflammatory mediators. In particular, their use in combination
demonstrated significantly enhanced suppression of inflammatory
mediators compared with either compound alone. This result may be
attributed to the potency of DHA and QE as anti-inflammatory
agents, and their combination can affect a greater number of
proteins involved in signalling pathways to achieve enhanced
anti-inflammatory effects. Although understanding of the
anti-inflammatory mechanisms of action of QE and DHA in LPS-induced
RAW264.7 cells is limited, previous studies report that QE and DHA
diminish NO and PGE2 production via inhibition of iNOS and COX-2
protein expression (11,18). Previous research has consistently
demonstrated that DHA and QE decrease the production of
inflammatory cytokines (IL-1β, IL-6, TNF-α) and reduce their mRNA
expression (16,20,28).
Although the present study demonstrated that DHA and QE mediate
anti-inflammatory effects via proteins involved in the NF-κB
signalling pathway, further research is necessary to determine
whether the potential differential effects of DHA and QE occur
upstream of NF-κB. The inhibitory effect of DHA and QE on the
phosphorylation levels of p65, IκB and p50 was enhanced when the
compounds were used in combination. Previous reports and the
present study demonstrate that effect DHA/QE on NF-κB signalling in
macrophages is exerted by preventing LPS-induced phosphorylation of
IκB and p65 (23,33). In the present study, suppression of
LPS-induced NF-κB signalling was also observed at the mRNA level,
as DHA and QE combined treatment significantly reduced the mRNA
expression levels of p50 and p65. This finding demonstrates that
DHA and QE reduce NF-κB activation in LPS-induced macrophages.
MAPK proteins, which are activated by LPS in various
cell lines, are comprised of three subfamilies (ERK1/2, JNK1/2 and
p38MAPKs), and are involved in LPS-stimulated iNOS and COX-2
expression in macrophages. Activated MAPKs also regulate the
phosphorylation of numerous important signalling molecules
mediating cell inflammation, proliferation and apoptosis (34–36).
Combined DHA + QE treatment exerted a stronger inhibitory effect on
the phosphorylation levels of MAPK proteins compared with DHA or QE
alone. Whilst DHA alone markedly reduced the LPS-induced
phosphorylation of ERK, JNK and p38MAPK, QE alone did not markedly
inhibit ERK phosphorylation. Several studies have demonstrated that
DHA can modulate the MAPK signalling pathway in LPS-stimulated
macrophages (37,38). A previous investigation indicated
that QE can inhibit ERK1/2 and p38MAPK activation, but not JNK, in
LPS-induced RAW264.7 cells (39).
However, another study demonstrated that QE can suppress p38MAPK
and JNK but not ERK1/2 activation; this study also observed that
iNOS, COX-2 and HO-1 induction were suppressed by a p38 and JNK1/2
inhibitor but not an ERK inhibitor. The aforementioned findings
suggest that JNK and p38MAPK induce the activation of
anti-inflammatory (HO-1) and pro-inflammatory (iNOS and COX-2)
mediators in LPS-induced RAW264.7 cells (28). Thus, the results of the present
study are in agreement with the previous findings, demonstrating
that QE can effectively inhibit JNK and p38MAPK activation in the
MAPK signalling pathway. The results of the present study
demonstrate that DHA + QE treatment is effective, as this
combination can modulate more pathways than DHA or QE individually.
The synergistic effects of DHA and QE on the reduction of ERK and
JNK phosphorylation may complement their synergistic effects on the
suppression of NO, PGE2 and COX-2 levels. Previous reports
demonstrate that activated NF-κB has a half-life of <30 min.
Thus, maintenance of NF-κB activity depends on continuous
transcription and protein synthesis of the genes encoding NF-κB
proteins (40). The results of the
present study indicate that QE + DHA in combination significantly
decrease the mRNA expression levels of p50, p65, JNK and ERK1/2,
thus, inhibiting the inflammatory response.
In conclusion, combining DHA (30 µM) and QE
(20 µM) treatment enhances their anti-inflammatory effects
in LPS-induced RAW264.7 macrophages by decreasing the levels of
inflammatory mediators (NO, PGE2, iNOS and COX-2) and inflammatory
cytokines (IL-1β, IL-6 and TNF-α). This combination also exerts an
enhanced effect on the expression and phosphorylation of NF-κB and
MAPK signalling pathway proteins compared with their effects
individually. Future studies should investigate the
anti-inflammatory effects of different QE and DHA doses in
combination, as well as the use of the Loewe method to calculate
synergistic effects. Since the synergistic, additive and
antagonistic effect can be determined using different models, such
as the Loewe model, this may be useful to conduct in future
studies. The present study provides insight into the benefits of
foods and beverages containing these molecules. The findings of the
current study should be investigated further in future in
vivo studies by examining QE and DHA as nutritional supplements
to exert preventative or palliative effects on obesity,
atherosclerosis and cardiovascular diseases. The potential
mechanisms of the anti-inflammatory effects of DHA and QE in
LPS-stimulated RAW264.7 cells are summarized in Fig. 7.
Acknowledgments
The present study was supported by the Fundamental
Research Funds for the Central Universities (nos. 2013PY098 and
2014PY011).
Abbreviations:
|
DHA
|
docosahexaenoic acid
|
|
COX-2
|
cyclooxygenase-2
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
NF-κB
|
nuclear factor κB
|
|
IκB
|
inhibitor of NF-κB
|
|
IL
|
interleukin
|
|
iNOS
|
inducible nitric oxide synthase
|
|
JNK
|
c-JUN N-terminal kinase
|
|
LPS
|
lipopolysaccharide
|
|
MAPK
|
mitogen-activated protein kinase
|
|
NO
|
nitric oxide
|
|
NOP
|
NO production
|
References
|
1
|
Lorente-Cebrián S, Costa AG,
Navas-Carretero S, Zabala M, Martínez JA and Moreno-Aliaga MJ: Role
of omega-3 fatty acids in obesity, metabolic syndrome, and
cardiovascular diseases: A review of the evidence. J Physiol
Biochem. 69:633–651. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang D, Lin J, Chen Y, Liu S, Lu F, Chang
T, Wang M, Lin H and Chang Y: Suppressive effect of carotenoid
extract of Dunaliella salina alga on production of LPS-stimulated
pro-inflammatory mediators in RAW264.7 cells via NF-kB and JNK
inactivation. J Funct Foods. 5:607–615. 2013. View Article : Google Scholar
|
|
3
|
Pan MH, Lai CS and Ho CT:
Anti-inflammatory activity of natural dietary flavonoids. Food
Funct. 1:15–31. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hsu CL, Fang SC and Yen GC:
Anti-inflammatory effects of phenolic compounds isolated from the
flowers of Nymphaea mexicana Zucc. Food Funct. 4:1216–1222. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang N, Hauck C, Yum MY, Rizshsky L,
Widrlechner MP, McCoy JA, Murphy PA, Dixon PM, Nikolau BJ and Birt
DF: Rosmarinic acid in Prunella vulgaris ethanol extract inhibits
lipopolysaccharide-induced prostaglandin E2 and nitric oxide in RAW
264.7 mouse macrophages. J Agric Food Chem. 57:10579–10589. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lai C, Lai Y, Kuo D, Wu C, Ho C and Pan M:
Magnolol potently suppressed lipopolysaccharide-induced iNOS and
COX-2 expression via downregulating MAPK and NF-κB signaling
pathways. J Funct. 3:198–206. 2011.
|
|
7
|
Chiu FL and Lin JK: Tomatidine inhibits
iNOS and COX-2 through suppression of NF-κB and JNK pathways in
LPS-stimulated mouse macrophages. FEBS Lett. 582:2407–2412. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim KJ, Yoon KY and Lee BY: Low molecular
weight fucoidan from the sporophyll of Undaria pinnatifida
suppresses inflammation by promoting the inhibition of
mitogen-activated protein kinases and oxidative stress in RAW264.7
cells. Fitoterapia. 83:1628–1635. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Avior Y, Bomze D, Ramon O and Nahmias Y:
Flavonoids as dietary regulators of nuclear receptor activity. Food
Funct. 4:831–844. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahn J, Lee H, Kim S, Park J and Ha T: The
anti-obesity effect of quercetin is mediated by the AMPK and MAPK
signaling pathways. Biochem Biophys Res Commun. 373:545–549. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin HY, Juan SH, Shen SC, Hsu FL and Chen
YC: Inhibition of lipopolysaccharide-induced nitric oxide
production by flavonoids in RAW264.7 macrophages involves heme
oxygenase-1. Biochem Pharmacol. 66:1821–1832. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chow JM, Shen SC, Huan SK, Lin HY and Chen
YC: Quercetin, but not rutin and quercitrin, prevention of
H2O2-induced apoptosis via anti-oxidant
activity and heme oxygenase 1 gene expression in macrophages.
Biochem Pharmacol. 69:1839–1851. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Strobel P, Allard C, Perez-Acle T,
Calderon R, Aldunate R and Leighton F: Myricetin, quercetin and
catechin-gallate inhibit glucose uptake in isolated rat adipocytes.
Biochem J. 386:471–478. 2005. View Article : Google Scholar :
|
|
14
|
Das N, Sikder K, Bhattacharjee S, Majumdar
SB, Ghosh S, Majumdar S and Dey S: Quercetin alleviates
inflammation after short-term treatment in high-fat-fed mice. Food
Funct. 4:889–898. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Firdous AB, Sharmila G, Balakrishnan S,
RajaSingh P, Suganya S, Srinivasan N and Arunakaran J: Quercetin, a
natural dietary flavonoid, acts as a chemopreventive agent against
prostate cancer in an in vivo model by inhibiting the EGFR
signaling pathway. Food Funct. 5:2632–2645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pallarès V, Calay D, Cedó L, Castell-Auví
A, Raes M, Pinent M, Ardévol A, Arola L and Blay M: Enhanced
anti-inflammatory effect of resveratrol and EPA in treated
endotoxin-activated RAW 264.7 macrophages. Br J Nutr.
108:1562–1573. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Venegas-Calerón M, Sayanova O and Napier
JA: An alternative to fish oils: Metabolic engineering of oil-seed
crops to produce omega-3 long chain polyunsaturated fatty acids.
Prog Lipid Res. 49:108–119. 2010. View Article : Google Scholar
|
|
18
|
Mullen A, Loscher CE and Roche H:
Anti-inflammatory effects of EPA and DHA are dependent upon time
and dose-response elements associated with LPS stimulation in
THP-1-derived macrophages. J Nutr Biochem. 21:444–450. 2010.
View Article : Google Scholar
|
|
19
|
Pallarès V, Calay D, Cedó L, Castell-Auví
A, Raes M, Pinent M, Ardévol A, Arola L and Blay M: Additive,
antagonistic, and synergistic effects of procyanidins and
polyunsaturated fatty acids over inflammation in RAW 264.7
macrophages activated by lipopolysaccharide. Nutrition. 28:447–457.
2012. View Article : Google Scholar
|
|
20
|
Choi EY, Jin JY, Choi JI, Choi IS and Kim
SJ: DHA suppresses Prevotella intermedia lipopolysaccharide-induced
production of proinflammatory mediators in murine macrophages. Br J
Nutr. 111:1221–1230. 2014. View Article : Google Scholar
|
|
21
|
Manjeet KR and Ghosh B: Quercetin inhibits
LPS-induced nitric oxide and tumor necrosis factor-α production in
murine macrophages. Int J Immunopharmacol. 21:435–443. 1999.
View Article : Google Scholar
|
|
22
|
Boots AW, Haenen GR and Bast A: Health
effects of quercetin: From antioxidant to nutraceutical. Eur J
Pharmacol. 585:325–337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saw CL, Huang Y and Kong AN: Synergistic
anti-inflammatory effects of low doses of curcumin in combination
with polyunsaturated fatty acids: Docosahexaenoic acid or
eicosapentaenoic acid. Biochem Pharmacol. 79:421–430. 2010.
View Article : Google Scholar
|
|
24
|
Rusca A, Di Stefano AF, Doig MV, Scarsi C
and Perucca E: Relative bioavailability and pharmacokinetics of two
oral formulations of docosahexaenoic acid/eicosapentaenoic acid
after multiple-dose administration in healthy volunteers. Eur J
Clin Pharmacol. 65:503–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leiherer A, Mündlein A and Drexel H:
Phytochemicals and their impact on adipose tissue inflammation and
diabetes. Vascul Pharmacol. 58:3–20. 2013. View Article : Google Scholar
|
|
26
|
Sun M, Nie S, Pan X, Zhang R, Fan Z and
Wang S: Quercetin-nanostructured lipid carriers: Characteristics
and anti-breast cancer activities in vitro. Colloids Surf
Biointerfaces. 113:15–24. 2014. View Article : Google Scholar
|
|
27
|
Russo M, Spagnuolo C, Tedesco I, Bilotto S
and Russo GL: The flavonoid quercetin in disease prevention and
therapy: Facts and fancies. Biochem Pharmacol. 83:6–15. 2012.
View Article : Google Scholar
|
|
28
|
Endale M, Park SC, Kim S, Kim SH, Yang Y,
Cho JY and Rhee MH: Quercetin disrupts tyrosine-phosphorylated
phosphatidylinositol 3-kinase and myeloid differentiation factor-88
association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced
inflammatory mediators production in RAW 264.7 cells.
Immunobiology. 218:1452–1467. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gomes A, Fernandes E, Lima JL, Mira L and
Corvo ML: Molecular mechanisms of anti-inflammatory activity
mediated by flavonoids. Curr Med Chem. 15:1586–1605. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kalupahana NS, Claycombe KJ and
Moustaid-Moussa N: (n-3) Fatty acids alleviate adipose tissue
inflammation and insulin resistance: Mechanistic insights. Adv
Nutr. 2:304–316. 2011. View Article : Google Scholar :
|
|
31
|
Oh DY, Talukdar S, Bae EJ, Imamura T,
Morinaga H, Fan W, Li P, Lu WJ, Watkins SM and Olefsky JM: GPR120
is an omega-3 fatty acid receptor mediating potent
anti-inflammatory and insulin-sensitizing effects. Cell.
142:687–698. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wall R, Ross RP, Fitzgerald GF and Stanton
C: Fatty acids from fish: The anti-inflammatory potential of
long-chain omega-3 fatty acids. Nutr Rev. 68:280–289. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peet GW and Li J: IκB Kinases α and β show
a random sequential kinetic mechanism and are inhibited by
staurosporine and quercetin. J Biol Chem. 274:32655–32661. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feng D, Ling WH and Duan RD: Lycopene
suppresses LPS-induced NO and IL-6 production by inhibiting the
activation of ERK, p38MAPK, and NF-kappaB in macrophages. Inflamm
Res. 59:115–121. 2010. View Article : Google Scholar
|
|
35
|
Blüthgen N and Legewie S: Systems analysis
of MAPK signal transduction. Essays Biochem. 45:95–107. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Turjanski AG, Vaqué JP and Gutkind JS: MAP
kinases and the control of nuclear events. Oncogene. 26:3240–3253.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lo CJ, Chiu KC, Fu M, Chu A and Helton S:
Fish oil modulates macrophage P44/P42 mitogen-activated protein
kinase activity induced by lipopolysaccharide. JPEN J Parenter
Enteral Nutr. 24:159–163. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Moon Y and Pestka JJ:
Deoxynivalenol-induced mitogen-activated protein kinase
phosphorylation and IL-6 expression in mice suppressed by fish oil.
J Nutr Biochem. 14:717–726. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cho SY, Park SJ, Kwon MJ, Jeong TS, Bok
SH, Choi WY, Jeong WI, Ryu SY, Do SH, Lee CS, et al: Quercetin
suppresses proinflammatory cytokines production through MAP kinases
and NF-kappaB pathway in lipopolysaccharide-stimulated macrophage.
Mol Cell Biochem. 243:153–160. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hohmann HP, Remy R, Scheidereit C and van
Loon AP: Maintenance of NF-kappa B activity is dependent on protein
synthesis and the continuous presence of external stimuli. Mol Cell
Biol. 11:259–266. 1991. View Article : Google Scholar : PubMed/NCBI
|