Introduction
Diseases of the oral cavity include dental caries,
periodontitis, cervical abrasion and halitosis (1). Periodontitis, a bacteria-induced
chronic inflammatory disease, is recognized as the most common
cause of tooth loss in humans (2,3).
Gram-negative bacteria, including Porphyromonas gingivalis,
Prevotella intermedia, Fusobacterium nucleatum and
Aggregatibacter actinomycetemcomitans are major pathogens
involved in periodontitis. At the early stage of infection, these
bacteria activate host innate immune responses, which result in the
recruitment of neutrophils, macrophages and lymphocytes to the site
of infection. The progression to chronic inflammation leads to the
formation of a periodontal pocket, ulceration of the gingival
surface, destruction of the periodontal ligament and the alveolar
bone, and finally tooth loss (4,5).
Therefore, it is important to inhibit inflammation in periodontal
tissues to maintain tooth health. Recently, there has been an
increased interest in the field of herbal medicine as a source of
novel therapeutic agents that exhibit anti-inflammatory properties
for use in the treatment of periodontal diseases (6).
Withania somnifera is a member of the
Solanaceae or nightshade family and its major components are
alkaloids and withanolides. The latter consist of a steroid
backbone bound to a lactone or one of its derivatives and exert
prominent anti-inflammatory activities (7). Withaferin A (WA) is a representative
withanolide and has been widely investigated for its
anti-inflammatory and anti-cancer effects. A previous study
demonstrated that WA reduces lipopolysaccharide (LPS)-induced
inducible nitric oxide synthase (iNOS) expression and nitric oxide
(NO) production by downregulating Akt and nuclear factor (NF)-κB
activation in a macrophage cell line (8). In addition, WA inhibits the
constitutive or induced expression of inflammatory mediators, such
as cytokines and adhesion molecules (such as intercellular adhesion
molecule 1 and vascular cell adhesion protein), in various cells,
including epithelial cells (9–12),
suggesting that WA has anti-inflammatory effects in a wide range of
host cells.
As representative innate immune cells, macrophages
are specialized phagocytes and are responsible for the control of
the growth of invading bacteria. They can produce inflammatory
mediators, such as cytokines, chemokines or NO, via pattern
recognition receptor- (PRR-) mediated signaling. Toll like
receptors (TLRs) are a representative family of PRRs and are
characterized by a cytosolic effector Toll/interleukin (IL)-1R
homology (TIR) domain and extracellular leucine-rich repeats (LRRs)
that are responsible for the recognition of microbial molecules
(13). Several studies have shown
that TLR2 and TLR4 are involved in cellular immune responses to
periodontal pathogens (14–16).
However, thus far the effect of WA on TLR expression in host cells
remains unknown. In the present study, the inhibitory effect of WA
was examined on the inflammatory responses of macrophages in
response to two periodontal pathogens, F. nucleatum and
A. actinomycetemcomitans.
Materials and methods
Animals
Wild-type male C57BL/6 mice (8 weeks old; 21–23 g
body weight) were purchased from Koatech (Pyeongtaek, Korea). The
animals were housed in an animal room at a constant temperature
(22–24°C) and light-dark cycle with 14 h of light and 10 h dark.
Food and water were available ad libitum. Mice were
acclimatized to the laboratory room for 1–3 weeks prior to the
experiment. Mice were sacrificed by cervical dislocation and their
femur and tibia were used to prepare macrophages. Animal studies
were approved and conducted according to the regulations of the
Institutional Animal Care and Use Committee at Konyang University
(Daejeon, Korea).
Bacterial culture
F. nucleatum (25586; American Type Culture
Collection, Mannasas, VA, USA) and A. actinomycetemcomitans
(43718; American Type Culture Collection) were purchased from the
American Type Culture Collection (Manassas, VA, USA). Bacteria were
grown on brain heart infusion (BHI) broth containing hemin (5
mg/ml) and vitamin K (10 mg/ml) at 37°C under anaerobic conditions.
Bacteria were allowed to grow to optical density (OD)600 = 0.6,
which corresponds to ~109 CFU/ml of viable bacteria as
determined by serial dilution and plate counts, and frozen aliquots
were stored at −80°C. For bacterial infection, aliquots were thawed
and diluted to the desired concentration in phosphate-buffered
saline or media.
Preparation and stimulation of murine
macrophages
Bone marrow-derived macrophages (BMDMs) were
prepared as previously described (17). Bone marrow cells were cultured with
Iscove's modified Dulbecco's medium (IMDM; Welgene, Gyeongsan,
Korea) containing 30% L929 cell culture supernatant (KCTC,
Jeongeup, Korea), 1X minimum essential medium, non-essential amino
acids, 1 mM sodium pyruvate, 10% fetal bovine serum and 100 U/ml
penicillin/streptomycin (all purchased from Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 6 days. The cells were
seeded in 48-well plates at a concentration of 2×105
cells/well or in 6-well plates at a concentration of
2×106 cells/well, and incubated in a 5% CO2
incubator at 37°C. The day after seeding, the cells were infected
with F. nucleatum and A. actinomycetemcomitans at the
indicated multiplicity of infection (MOI; presented as
macrophage/bacterium ratios) in the absence or presence of WA
(50–1,000 nM, Sigma-Aldrich, St. Louis, MO, USA). Culture
supernatants were collected 6 h after infection for further
analysis.
Measurement of cytokines
The concentrations of IL-6 and tumor necrosis factor
(TNF)-α in culture supernatants from F. nucleatum- and A.
actinomycetemcomitans-infected BMDMs were determined using a
commercial enzyme-linked immunosorbent assay (ELISA) kit (R&D
Systems, Inc., Minneapolis, MN, USA).
Measurement of nitric oxide
The NO synthase activity in the culture supernatant
of infected cells was determined by measuring the NO accumulation
by the Griess reaction as previously described (18).
Immunoblotting
BMDMs were infected with F. nucleatum or
A. actinomycetemcomitans at MOI 10 with or without
pretreatment of WA (250 nM) for 2 h, and were lysed at the
indicated time points (0, 15, 30 and 60 min). The cells were lysed
in a buffer containing 1% Nonidet-P40 supplemented with a complete
protease inhibitor cocktail (Roche Diagnostics Deutschland GmbH,
Mannheim, Germany) and 2 mM dithiothreitol (Sigma-Aldrich). The
extracted protein concentration was measured using a Bio-Rad
Protein Assay Dye Reagent Concentrate (cat. no. 500-0006; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Samples of protein (30
µg) were cooled on ice following incubation at 95–100°C for
10 min. Lysates were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to
polyvinylidene fluoride membranes by electroblotting. The membranes
were blocked by incubation with 5% skimmed milk for 1 h at room
temperature. The following primary antibodies were incubated with
the membrane overnight at 4°C: Rabbit anti-human poly clonal phos
phorylated IκB-α (1:1,000; cat. no. 5209; Cell Signaling
Technology, Inc., Danvers, MA, USA); rabbit anti-human polyclonal
phos phorylated (p)-c-Jun N-terminal kinase (JNK; 1:1,000; cat. no.
9251; Cell Signaling Technology, Inc.); rabbit anti-human
polyclonal p-p38 antibody (1:1,000; cat. no. sc-101759; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA); mouse anti-human monoclonal
p-ERK antibody (1:1,000; cat. no. sc-7383; Santa Cruz
Biotechnology, Inc.); rabbit anti-human polyclonal ERK antibody
(1:1,000; cat. no. sc-94; Santa Cruz Biotechnology, Inc.); rabbit
anti-human polyclonal anti-β-actin antibody (1:2,000; cat. no.
sc-130656; Santa Cruz Biotechnology, Inc.); anti-iNOS (1:1,000;
cat. no. ab15323; Abcam, Cambridge, MA, USA); anti-TLR2 (1:1,000;
cat. no. IMG-319; Novus Biologicals, LLC., Littleton, CO, USA); and
anti-TLR4 (1:1,000; cat. no. ab13556; Abcam). The membrane was then
rinsed with Tris-buffered saline with Tween 20 (TBST) three times,
each time for 10 min. The membrane was then incubated with
secondary horseradish peroxidase-conju gated goat anti-rabbit IgG
(1:4,000; sc-2301; Santa Cruz Biotechnology, Inc.) or goat
anti-mouse IgG (1:2,000; cat. no. 2031; Santa Cruz Biotechnology,
Inc.) antibodies for 2 h at room temperature. The membrane was then
washed three time with TBST for 10 min. Proteins were detected with
SuperSignal West Pico Chemiluminescent Substrate (Gibco; Thermo
Fisher Scientific, Inc.). Images of the blots were captured on
CP-BU new film (Agfa HealthCare, Mortsel, Belgium) using an
Automatic X-ray film processor (JP-33; JPI Healthcare, Seoul,
Korea).
Statistical analysis
The differences among the mean values of different
groups were assessed. Data are expressed as the mean ± standard
deviation. Statistical calculations were performed using one-way
analysis of variance followed by the Tukey post test using GraphPad
Prism version 5.00 (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
WA reduces the production of IL-6 and
TNF-α by macrophages in response to F. nucleatum and A.
actinomycetemcomitans
To determine the inhibitory effect of WA on cytokine
production by macrophages, BMDMs were pretreated with various doses
of WA for 2 h and subsequently infected with F. nucleatum
and A. actinomycetemcomitans for 6 h. ELISA results showed
that treatment with F. nucleatum and A.
actinomycetemcomitans led to a substantial production of IL-6
and TNF-α by the cells, which was inhibited by WA in a
dose-dependent manner (Fig.
1).
NF-κB and MAPK activation is inhibited by
WA in macrophages in response to F. nucleatum and A.
actinomycetemcomitans
Our previous study demonstrated that NF-κB and MAPKs
(p38, ERK, and JNK) are important for F. nucleatum-induced
production of IL-6 and TNF-α (15). Induction of cytokine production by
A. actinomycetemcomitans is also dependent on NF-κB and p38
MAPK signaling (15). Accordingly,
it was examined whether WA affects F. nucleatum- and A.
actinomycetemcomitans-mediated activation of NF-κB and MAPKs in
BMDMs. F. nucleatum-induced phosphorylation of IκB-α and ERK
was detected 15, 30, and 60 min after infection, whereas p38 and
JNK were phosphorylated at 30 min post-infection (Fig. 2A). This phosphorylation of IκB-α,
p38, ERK and JNK was impaired by WA treatment (Fig. 2A). In addition, A.
actinomycetemcomitans led to activation of NF-κB and MAPKs in
macrophages starting 15 min after infection (Fig. 2B). A.
actinomycetemcomitans-induced ERK phosphorylation was delayed
by WA, and was only detected 60 min after infection (Fig. 2B). A.
actinomycetemcomitans-induced phosphorylation of IκB-α and p38
was markedly inhibited by WA treatment at all time points tested
(Fig. 2B). By contrast, WA
treatment only led a marginal decrease in JNK phosphorylation in
macrophages in response to A. actinomycetemcomitans 30 min
after infection (Fig. 2B).
 | Figure 2WA inhibits the activation of NF-κB
and MAPKs in BMDMs in response to F. nucleatum and A.
actinomycetemcomitans. BMDMs were infected with (A) F.
nucleatum (F. n) or (B) A. actinomycetemcomitans (A. a)
at a multiplicity of infection of 10 with or without pretreatment
of WA (250 nM) for 2 h and cellular protein was extracted at the
indicated time points. Phosphorylation of IκB-α, p38, JNK and ERK
was examined by western blotting. Primary antibodies against the
regular form of ERK and β-actin were used to confirm the loaded
protein amounts. The results shown are from one representative
experiment of two independent experiments performed. NF-κB, nuclear
factor-κB; MAPKs, mitogen-activated protein kinases; BMDMs, bone
marrow-derived macrophages; WA, withaferin A; IκB-α, JNK, c-Jun
N-terminal kinases; ERK, extracellular signal-regulated kinases;
p-, phosphorylated. |
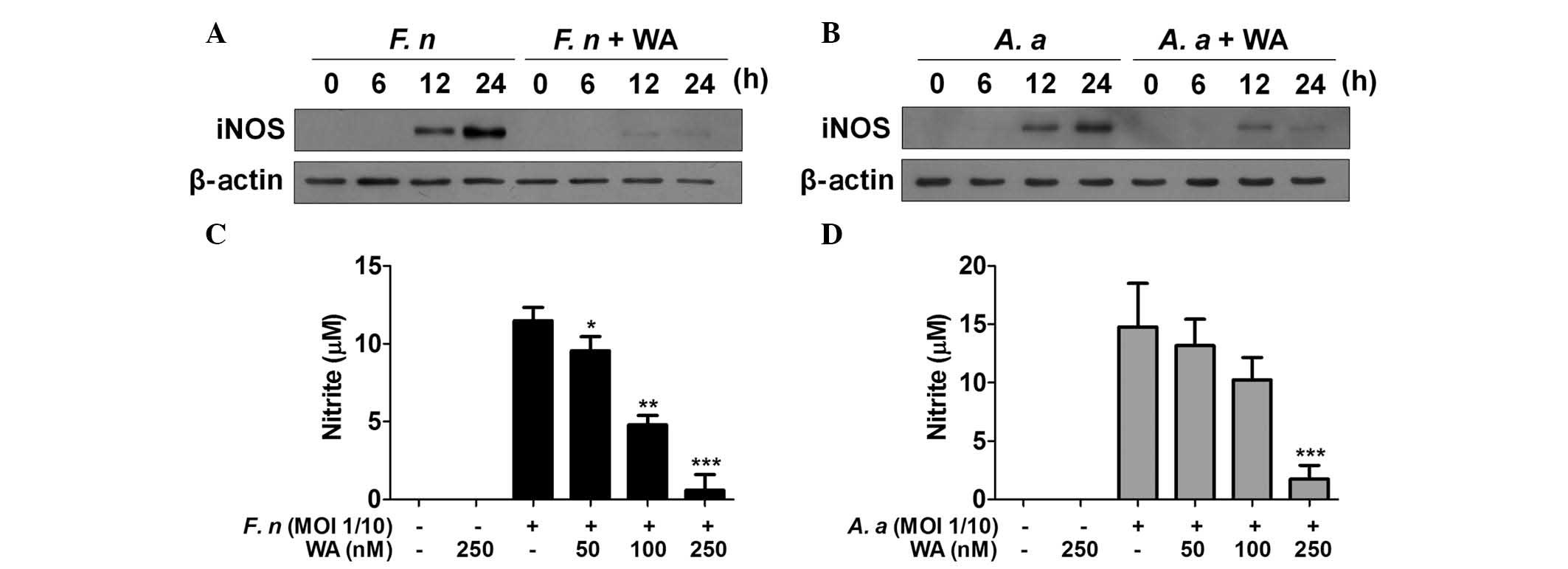
WA inhibits iNOS expression and NO
production in macrophages in response to F. nucleatum and A.
actinomycetemcomitans
NO is a critical factor for the control of bacterial
growth and iNOS is a key enzyme catalyzing the NO production from
L-arginine. Periodontal pathogens can stimulate macrophages to
produce NO (19,20) and a selective iNOS inhibitor,
mercaptoethylguanidine, prevents bone destruction in
ligature-induced rodent periodontitis (21). Accordingly, it was examined whether
WA had an inhibitory effect on iNOS expression and NO production
induced by F. nucleatum and A. actinomycetemcomitans
in macrophages. Western blot analysis demonstrated the presence of
iNOS protein in F. nucleatum-infected macrophages 12 and 24
h after infection, which was mostly impaired by WA treatment
(Fig. 3A). A.
actinomycetemcomitans also induced iNOS expression with similar
kinetics to F. nucleatum (Fig.
3B). WA inhibited A. actinomycetemcomitans-induced
expression of iNOS at 24 h, while it had little effect on iNOS
expression at 12 h (Fig. 3B). The
level of NO in the culture supernatants of BMDMs infected with
F. nucleatum or A. actinomycetemcomitans was detected
in the absence or presence of WA. However, NO was undetectable
under these conditions regardless of bacterial infection or WA
treatment (data not shown), even though F. nucleatum and
A. actinomycetemcomitans could induce expression of iNOS in
macrophages. Therefore, the experimental design was altered and
cells were also treated with interferon-γ, which is known to
enhance NO production in macrophages (22). The results showed that F.
nucleatum and A. actinomycetemcomitans induced NO
production in BMDMs, which was inhibited by WA in a dose-dependent
manner (Fig. 3C and D). These
findings indicate that WA may effectively inhibit NO production
induced by periodontal pathogens in macrophages.
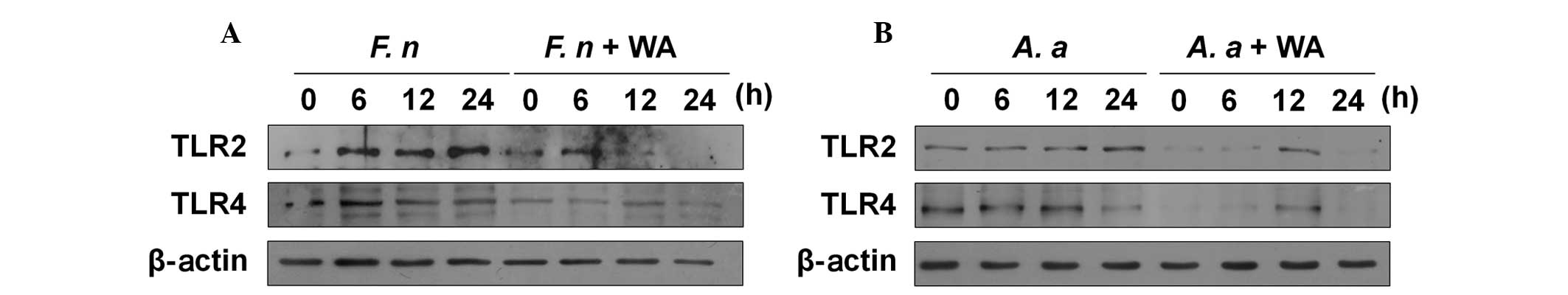
WA reduces the expression of TLR2 and
TLR4 in macrophages in response to F. nucleatum and A.
actinomycetemcomitans
TLR2 and TLR4 are involved in the production of IL-6
and TNF-α in macrophages in response to F. nucleatum and
A. actinomycetemcomitans (15). Therefore, this study aimed to
determine whether WA affects the expression of TLR2 and TLR4 in
macrophages. F. nucleatum increased the protein expression
of TLR2 and TLR4 in macrophages 6 h after infection, and the TLR2
expression level remained increased at 24 h (Fig. 4A). Notably, WA reduced the
endogenous and induced expression of TLR2 and TLR4 in F.
nucleatum-infected macrophages (Fig. 4A). A. actinomycetemcomitans
also marginally increased the TLR2 expression in macrophages at 12
and 24 h after infection, whereas the TLR4 expression level was
decreased in the cells at 24 h (Fig.
4B). Likewise, WA inhibited both TLR2 and TLR4 expression in
A. actinomycetemcomitans-infected macrophages (Fig. 4B). These findings suggest that WA
may exert its anti-inflammatory effect in macrophages in response
to F. nucleatum and A. actinomycetemcomitans by
inhibiting TLR-mediated signaling.
Discussion
Periodontal destruction is the result of the complex
interplay between pathogenic bacteria and the host immune responses
(23). Bacterial components, such
as LPS, and toxic products of periodontal pathogens trigger host
immune responses, which results in the destruction of the
periodontal tissue. Traditional therapeutics controlling
periodontal diseases include dental cleaning, subgingival
scaling/root planning and the use of antibiotics. These treatments
can reduce the levels of pathogenic bacteria in periodontal pockets
(24). In addition to the use of
antibiotics, there has been an increased interest in the use of
natural products as adjuncts to manage inflammatory disorders,
including periodontitis, due to their anti-inflammatory properties
(6).
In the present study, the anti-inflammatory effects
of WA on F. nucleatum- and A.
actinomycetemcomitans-infected macrophages were investigated.
The results showed that WA has inhibitory effects on cytokine
production, the activation of NF-κB and MAPKs, and NO production in
macrophages in response to the two types of bacteria. This suggests
that WA can be used as a natural preventive and therapeutic agent
for periodontitis. It is well-known that WA has NF-κB inhibiting
activity in a wide range of cell types in response to various
stimuli (7). However, the effect
of WA on MAPK activation appears to be dependent on the cell type
and stimuli. WA alone has been shown to activate p38, ERK and JNK
MAPKs in U937 human leukemic cells with different kinetics
(25). In addition, WA was shown
to induce the phosphorylation of p38 in MCF-7 breast cancer cells
within 3 h of treatment (26). In
K562 human erythromyeloblastoid leukemia cells, WA induced
activation of ERK, although p38 activation was not affected
(27). By contrast, WA inhibited
TNF-α-induced activation of ERK in human pulmonary epithelial
cells, although it did not alter the activation of p38 and JNK
(28). In the present study, WA
could almost completely inhibit the activation of NF-κB and MAPKs
in macrophages in response to F. nucleatum and A.
actinomycetemcomitans, although its inhibitory effect on A.
actinomycetemcomitans-induced JNK phosphorylation was not
identified to be significant. It remains to be elucidated whether
the inhibitory effect of WA on MAPKs is specific to periodontal
pathogens and/or macrophages.
TLR2 and TLR4 appears to be critical for periodontal
pathogen-induced immune responses although there is controversy
regarding the bacterial preparation used and the cell type tested
(14–16). In a study using sonicated bacteria,
A. actinomycetemcomitans induced IL-8 production in HEK293 cells
via TLR2 and TLR4, whereas F. nucleatum elicited IL-8
production exclusively via a TLR2-dependent pathway (14). In human periodontal ligament cells,
TLR2 and TLR4 are essential for the F. nucleatum-induced
production of cytokines (16).
Likewise, in macrophages, double deficiency of TLR2 and TLR4 leads
to decreased production of IL-6 and TNF-α, and delayed IκB-α
degradation in response to F. nucleatum and A.
actinomycetemcomitans (15).
In the present study, it was demonstrated for the first time that
WA inhibits endogenous and induced expression of TLR2 and TLR4 in
macrophages, indicating that WA can regulate TLR-mediated
signaling. In fact, in the present study, an MOI of 1/10 was used
to stimulate macrophages. This dose of F. nucleatum and
A. actinomycetemcomitans could induce the production of
substantial levels of TNF-α in TLR2/4 double-deficient macrophages
(15) and a high dose of WA (250
nM) completely inhibited TNF-α production in this study. This
suggests that WA may inhibit other signaling proteins involved in
F. nucleatum and A. actinomycetemcomitans-induced
production of cytokines in addition to TLR2 and TLR4. Previously it
was demonstrated that endosomal TLRs are important for cytokine
production in TLR2/4 double-deficient macrophages (15), suggesting that WA likely exerts its
anti-inflammatory effects by direct inhibition of multiple TLRs. In
addition to TLRs, Nod-like receptors (NLRs) participate in the
bacteria-induced immune responses in host cells. The first
identified NLRs, Nod1 and Nod2, recognize the peptidoglycan motifs
mesodiaminophimelic acid and muramyl dipeptide, respectively
(29). Periodontal pathogens,
including F. nucleatum and A. actinomycetemcomitans,
are known to stimulate Nod1- and Nod2, and their peptidoglycans
induce NF-κB activation in Nod1- and Nod2-transfected HEK293 cells
(30). A previous study revealed
that vimentin, an intermediate filament protein, is an important
regulator of Nod2 function (31).
WA has been shown to disrupt the interaction between vimentin and
Nod2, and inhibit Nod2-dependent NF-κB activation (31). Although the role of Nod2 in the
cytokine production by macrophages in response to F.
nucleatum and A. actinomycetemcomitans remains unknown,
it would be useful to clarify whether WA regulates the
Nod2-mediated immune response in macrophages in response to
periodontal pathogens.
In conclusion, it was demonstrated that WA has
inhibitory effects on F. nucleatum- and A.
actinomycetemcomitans-induced immune responses in macrophages
by downregulating TLR signaling. These results indicate that WA may
have potential as a novel therapeutic and preventive agent for
periodontitis.
Acknowledgments
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Science ICT & Future Planning
(grant nos. 2012R1A1A2041944 and 2014R1A4A1005309).
References
|
1
|
Costalonga M and Herzberg MC: The oral
microbiome and the immunobiology of periodontal disease and caries.
Immunol Lett. 162:22–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gjermo P, Rösing CK, Susin C and Oppermann
R: Periodontal diseases in Central and South America. Periodontol
2000. 29:70–78. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Albandar JM: Epidemiology and risk factors
of periodontal diseases. Dent Clin North Am. 49:517–532. v–vi.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jotwani R and Cutler CW: Adult
periodontitis - specific bacterial infection or chronic
inflammation? J Med Microbiol. 47:187–188. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Darveau RP, Tanner A and Page RC: The
microbial challenge in periodontitis. Periodontology 2000.
14:12–32. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Palaska I, Papathanasiou E and Theoharides
TC: Use of poly-phenols in periodontal inflammation. Eur J
Pharmacol. 720:77–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vanden Berghe W, Sabbe L, Kaileh M,
Haegeman G and Heyninck K: Molecular insight in the multifunctional
activities of Withaferin A. Biochem Pharmacol. 84:1282–1291. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oh JH, Lee TJ, Park JW and Kwon TK:
Withaferin A inhibits iNOS expression and nitric oxide production
by Akt inactivation and down-regulating LPS-induced activity of
NF-kappaB in RAW 264.7 cells. Eur J Pharmacol. 599:11–17. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maitra R, Porter MA, Huang S and Gilmour
BP: Inhibition of NFkappaB by the natural product Withaferin A in
cellular models of Cystic Fibrosis inflammation. J Inflamm (Lond).
6:152009. View Article : Google Scholar
|
|
10
|
Mohan R, Hammers HJ, Bargagna-Mohan P,
Zhan XH, Herbstritt CJ, Ruiz A, Zhang L, Hanson AD, Conner BP,
Rougas J and Pribluda VS: Withaferin A is a potent inhibitor of
angiogenesis. Angiogenesis. 7:115–122. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vyas AR and Singh SV: Molecular targets
and mechanisms of cancer prevention and treatment by withaferin a,
a naturally occurring steroidal lactone. AAPS J. 16:1–10. 2014.
View Article : Google Scholar :
|
|
12
|
Hahm ER and Singh SV: Withaferin A-induced
apoptosis in human breast cancer cells is associated with
suppression of inhibitor of apoptosis family protein expression.
Cancer Lett. 334:101–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kawai T and Akira S: The role of
pattern-recognition receptors in innate immunity: update on
Toll-like receptors. Nat Immunol. 11:373–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kikkert R, Laine ML, Aarden LA and van
Winkelhoff AJ: Activation of toll-like receptors 2 and 4 by
gram-negative periodontal bacteria. Oral Microbiol Immunol.
22:145–151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park SR, Kim DJ, Han SH, Kang MJ, Lee JY,
Jeong YJ, Lee SJ, Kim TH, Ahn SG, Yoon JH and Park JH: Diverse
Toll-like receptors mediate cytokine production by Fusobacterium
nucleatum and Aggregatibacter actinomycetemcomitans in macrophages.
Infect Immun. 82:1914–1920. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun Y, Shu R, Li CL and Zhang MZ:
Gram-negative periodontal bacteria induce the activation of
Toll-like receptors 2 and 4, and cytokine production in human
periodontal ligament cells. J Periodontol. 81:1488–1496. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Celada AGP, Rinderknecht E and Schreiber
RD: Evidence for a gamma-interferon receptor that regulates
macrophage tumoricidal activity. J Exp Med. 160:55–74. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Green LC, Wagner DA, Glogowski J, Skipper
PL, Wishnok JS and Tannenbaum SR: Analysis of nitrate, nitrite, and
[15N] nitrate in biological fluids. Anal Biochem. 126:131–138.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Frolov I, Houri-Hadad Y, Soskolne A and
Shapira L: In vivo exposure to Porphyromonas gingivalis
up-regulates nitric oxide but suppresses tumour necrosis
factor-alpha production by cultured macrophages. Immunology.
93:323–328. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blix IJ and Helgeland K: LPS from
Actinobacillus actinomycetemcomitans and production of nitric oxide
in murine macrophages J774. Eur J Oral Sci. 106:576–581. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lohinai Z, Benedek P, Fehér E, Györfi A,
Rosivall L, Fazekas A, Salzman AL and Szabó C: Protective effects
of mercaptoethylguanidine, a selective inhibitor of inducible
nitric oxide synthase, in ligature-induced periodontitis in the
rat. Brit J Pharmacol. 123:353–360. 1998. View Article : Google Scholar
|
|
22
|
Totemeyer S, Sheppard M, Lloyd A, Roper D,
Dowson C, Underhill D, Murray P, Maskell D and Bryant C: IFN-gamma
enhances production of nitric oxide from macrophages via a
mechanism that depends on nucleotide oligomerization domain-2. J
Immunol. 176:4804–4810. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Benakanakere M and Kinane DF: Innate
cellular responses to the periodontal biofilm. Front Oral Biol.
15:41–55. 2012. View Article : Google Scholar
|
|
24
|
Heitz-Mayfield LJ, Trombelli L, Heitz F,
Needleman I and Moles D: A systematic review of the effect of
surgical debridement vs non-surgical debridement for the treatment
of chronic periodontitis. J Clin Periodontol. 29(Suppl 3): 92–102;
discussion 160–162. 2002. View Article : Google Scholar
|
|
25
|
Oh JH, Lee TJ, Kim SH, Choi YH, Lee SH,
Lee JM, Kim YH, Park JW and Kwon TK: Induction of apoptosis by
withaferin A in human leukemia U937 cells through down-regulation
of Akt phosphorylation. Apoptosis. 13:1494–1504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang X, Mukerji R, Samadi AK and Cohen
MS: Down-regulation of estrogen receptor-alpha and rearranged
during transfection tyrosine kinase is associated with with-aferin
a-induced apoptosis in MCF-7 breast cancer cells. BMC Complement
Altern Med. 11:842011. View Article : Google Scholar
|
|
27
|
Suttana W, Mankhetkorn S, Poompimon W,
Palagani A, Zhokhov S, Gerlo S, Haegeman G and Berghe WV:
Differential chemosensitization of P-glycoprotein overexpressing
K562/Adr cells by withaferin A and Siamois polyphenols. Mol Cancer.
9:992010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oh JH and Kwon TK: Withaferin A inhibits
tumor necrosis factor-alpha-induced expression of cell adhesion
molecules by inactivation of Akt and NF-kappaB in human pulmonary
epithelial cells. Int Immunopharmacol. 9:614–619. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Caruso R, Warner N, Inohara N and Núñez G:
NOD1 and NOD2: Signaling, host defense, and inflammatory disease.
Immunity. 41:898–908. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okugawa T, Kaneko T, Yoshimura A,
Silverman N and Hara Y: NOD1 and NOD2 mediate sensing of
periodontal pathogens. J Dent Res. 89:186–191. 2010. View Article : Google Scholar
|
|
31
|
Stevens C, Henderson P, Nimmo ER, Soares
DC, Dogan B, Simpson KW, Barrett JC; International Inflammatory
Bowel Disease Genetics Consortium; Wilson DC and Satsangi J: The
intermediate filament protein, vimentin, is a regulator of NOD2
activity. Gut. 62:695–707. 2013. View Article : Google Scholar
|