Introduction
Asthma is a chronic airway inflammatory disease,
which is associated with various cell and cellular components. The
majority of asthma occurs in the developing world and it often
begins in childhood. The rates of asthma have markedly increased
since the 1960s, in 2013 there were 242 million asthma patients,
and it resulted in ~489,000 deaths (1,2). It
is characterized by inflammation, eosinophilia and mucus
hypersecretion in the airways and lungs. Goblet cells may
contribute to the immunoglobulin E secretion and airway
hyperresponsiveness (AHR) (3). It
is reported that eosinophils, mast cells and lymphocytes also
contribute to asthma (4–7). Previous studies indicated that
cytokines, such as interleukin (IL)-4, IL-5 and IL-13 produced by
type 2 T helper (Th) cells could regulate eosinophilia
in the airways (8–10). Conversely, eosinophils may produce
cytokines and chemokines, which could contribute to the development
of asthma (11,12). Therefore, cytokines and other
mediators are considered to be particularly important in the
treatment of asthma, and regulation of cytokines and chemokines may
contribute to the treatment of asthma (13–16).
Furthermore, cytokine and chemokine regulation may improve the
efficacy of clinical treatment methods (17,18).
Oridonin (Fig. 1),
an extract from the traditional Chinese medicinal herb, Xihuangcao
is widely used in China (19). It
has been shown to exert various pharmaceutical effects, such as
anti-tumor (20),
anti-proliferation (21),
anti-inflammatory and antiviral effects. In addition, Oridonin may
be administered for the treatment of upper respiratory tract
infection (22). In China Oridonin
has been administered for the treatment of inflammatory diseases
for hundreds of years and has become one of the most popular herbs
used clinically (23). Recent
studies have indicated that Oridonin exhibits significant
immunosuppressive effects in mice and modulates the
Th1/Th2 balance in in vitro studies
with rats (24,25). However, whether Oridonin could be
used in the treatment of asthma, and the underlying mechanisms of
its anti-inflammatory activity in mice and rats remain unknown.
In the present study, an ovalbumin (OVA)-induced
asthma mouse model was used to evaluate the anti-asthmatic effect
of Oridonin and to elucidate the mechanism of its anti-inflammatory
effect. In addition, the in vitro effects of Oridonin on
Th1/Th2 cytokine balance in mice were
assessed.
Materials and methods
Oridonin
Oridonin (purity, >98.0%; Fig. 1) was purchased from Zelang Medical
Technology Co. Ltd. (Nanjing, China). Oridonin was dissolved in
dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA). In
the cell assay-based studies, the final DMSO concentration was
<0.01%.
Animals
A total of 32 male BALB/c mice (age, 4–6 weeks) were
purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai,
China). The mice were maintained under specific pathogen-free
conditions for at least 7 days prior to the ex vivo and
in vivo studies. The mice had access to food and water ad
libitum, and were maintained at 20–26°C under a 12-h light/dark
cycle. The mice were divided into 4 groups of 8, as follows: i)
phosphate-buffered saline (PBS; Invitrogen; Thermo Fisher
Scientific, Inc.) group; ii) OVA group; iii) Oridonin-L; and iv)
Oridonin-H. All animal studies were performed according to the
Guide for the Care and Use of Laboratory Animals (26).
Lymphocyte preparation
The BALB/c mice were sacrificed with carbon dioxide
and their spleens were harvested. The spleens were sliced into
small pieces and pulverized using a syringe. The cell suspension in
RPMI 1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was filtered through a 70-µm cell strainer
(BD Biosciences, Franklin Lakes, NJ, USA). Red blood cells were
lysed using 10 mM EDTA (Invitrogen; Thermo Fisher Scientific,
Inc.), as previously described (27). The cells were then washed with PBS
and re-suspended in PBS. NycoPrep™ 1.077A (Axis-Shield PoC, Oslo,
Norway) was used to isolate the lymphocytes from the spleen cells
as previously described (25).
Finally, the lymphocytes were cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum. The primary mouse spleen
cells were stimulated with 5 µg/ml concanavalin A
(Sigma-Aldrich) prior to Oridonin treatment.
Enzyme-linked immunosorbent assay
(ELISA)
Supernatant and bronchoalveolar lavage (BAL) fluid
from the different groups was harvested by flushing the lung with
0.5 ml ice-cold PBS, following sacrifice with carbon dioxide, as
described below and stored at −80°C. Cytokine and chemokine levels
in the cell supernatant or BAL fluid were measured by ELISA assay.
The ELISA kits were purchased from R&D Systems, Inc.
(Minneapolis, MN, USA).
Mouse asthma model
On day 0 and 14, mice (8 mice/group) were injected
with 20 µg OVA (Sigma-Aldrich). On day 28, 29 and 30, the
mice were administrated with Oridonin (10 or 20 mg/kg) or vehicle
(0.5% sodium salt of carboxy methyl cellulose), 1 h after
administration the mice were challenged with OVA (1%). The
administration doses were established according to a previous study
(24).
Measurement of AHR
AHR was evaluated in the Oridonin-treated or vehicle
group mice by whole-body plethysmography 24 h after the final OVA
challenge. The mice were placed in separate chambers and exposed to
20, 40, 60 and 80 mg/ml aerosolized methacholine solution
(Sigma-Aldrich). The bronchoconstriction was then measured for 5
min. The highest Penh value obtained in each group was expressed as
a basal Penh value compared with the PBS challenge, which served as
the control.
BAL
Twenty-four-hours subsequent to the final aerosol
OVA challenge, the BALB/c mice were sacrificed with carbon dioxide
and the trachea of the mice were incised. Ice-cold PBS was injected
into the lungs of the mice through the trachea and the BAL fluid
was subsequently harvested. Each mouse was injected with PBS three
times. Cytospin slides (Thermo Fisher Scientific, Inc.) were
stained in a modified Wright stain (Sigma-Aldrich); the number of
inflammatory corpuscles and total cells were counted using a
hemocytometer (Countess II FL Automated Cell Counter; Thermo Fisher
Scientific, Inc.).
Histologic examination
All the mice were sacrificed with carbon dioxide and
the lungs were harvested. The lungs were infused, via the trachea,
with formalin (Sigma-Aldrich) overnight. The tissues were then
paraffinized (Sigma-Aldrich) and sliced into 7-µm sections.
Hematoxylin and eosin (H&E; Beyotime Institute of
Biotechnology) staining was performed to evaluate eosinophil
infiltration and periodic acid-Schiff staining was performed to
measure mucus production. Quantitative analysis of eosinophil
infiltration and mucus production was performed as previously
described (28). The scoring
system for cell infiltration was: 0, no cells; 1, few cells; 2, a
ring of cells that were one cell layer deep; 3, a ring of cells 2–4
cell layers deep; 4, a ring of cells >4 cells deep. Goblet cell
hyperplasia in the airway epithelium was quantified based on a
five-point system: 0, no goblet cells; 1, <25% of the
epithelium; 2, 25–50% of the epithelium; 3, 50–75% of the
epithelium; 4, >75% of the epithelium.
Statistical analysis
Data were presented as means ± standard deviation
for the in vitro experiments and means ± standard error of
the mean for in vivo study. Statistical analysis was
performed with the Student's t-test using SPSS 14.0 (SPSS, Inc.,
Chicago, IL, USA) to determine the differences between groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Oridonin treatment modulated the
Th1/Th2 balance in mice
A previous study indicated that Oridonin regulated
the Th1/Th2 balance in rats (25). The present study aimed to
investigate whether Oridonin modulates the
Th1/Th2 cytokine balance in mice. Our
preliminary experiment indicated that Oridonin dose-dependently
decreased the viability of the primary mice spleen cells, and that
the half maximal inhibitory concentration is ~37 mM (data not
shown). In the present study primary mice spleen cells were
cultured, stimulated with concanavalin A and treated with Oridonin
(12.5 or 25 mM). The supernatant was then harvested for ELISA. The
results indicated that Oridonin treatment significantly decreased
the Th1 cytokine level [interferon (IFN)-γ and IL-2;
P<0.001] in a dose-dependent manner, and significantly increased
the Th2 cytokine level (transforming growth factor;
TGF-β and IL-10; P<0.001), also in a dose-dependent manner, in
the mice spleen cell culture supernatant (Fig. 2). Thus, the results indicate that
Oridonin regulates the Th1/Th2 cytokine
balance in mice.
Effects of Oridonin on AHR
Previous studies have indicated that asthma is
associated with the Th1/Th2 balance (29), and that Oridonin may modulate the
rat Th1/Th2 balance in vitro (25). The present study demonstrated that
Oridonin modulated the mice Th1/Th2 balance
in vitro, therefore, the effect of Oridonin was investigated
in a mouse model of asthma. Asthma is defined as excessive
narrowing of the airways when challenged with contractile agonists;
OVA is a contractile agonist. To investigate the anti-asthmatic
effect of Oridonin on asthma in mice, a mouse model of asthma was
established with methacholine to induce the AHR in OVA-challenged
mice, and AHR was measured. Mice that had been sensitized with OVA
continuously for three days were stimulated with methacholine to
induce AHR. The control group was stimulated with PBS. The results
indicate that Oridonin significantly inhibited methacholine-induced
AHR when compared with the vehicle group (P<0.05; Fig. 3).
Effects of Oridonin on eosinophil
recruitment
Eosinophil recruitment is an important
characteristic in asthma (30).
When challenged with OVA, numerous leukocytes were recruited in the
lung (31), therefore, the effect
of Oridonin on eosinophil recruitment was investigated in BAL fluid
of OVA-challenged mice. The OVA-challenged mice lungs were flushed
with cold PBS 48 h after the last challenge and cells in the BAL
fluid were counted. The results indicated that Oridonin
significantly decrease the number of eosinophils, neutrophils and
total cells in BAL fluid when compared with the control group
(P<0.05). The effects of Oridonin on the number of macrophages
and lymphocytes were marginal (Fig.
4).
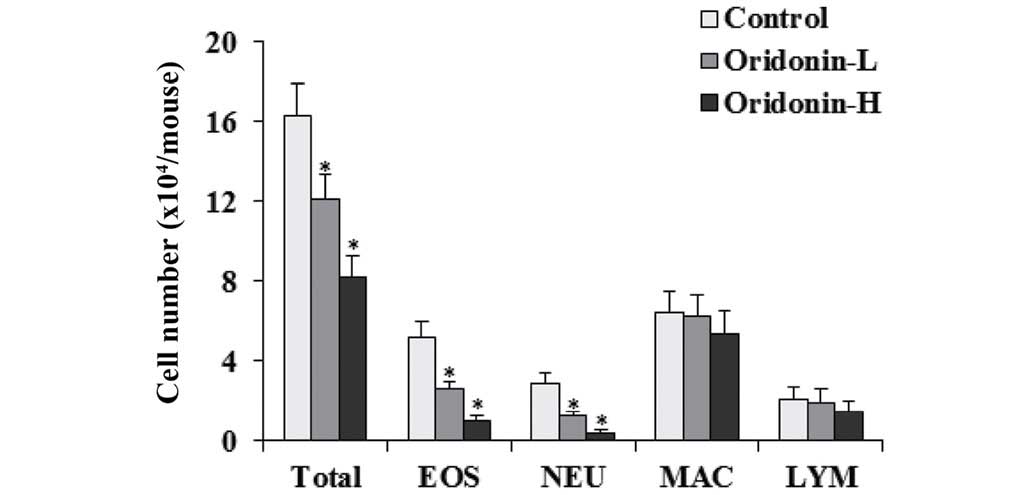 | Figure 4Effects of Oridonin on BAL fluid cell
infiltration. BAL fluids were collected 24 h after the final OVA
challenge. Differential cell count was performed on a minimum of
500 cells to identify EOS, NEU, MAC, and LYM. Control,
OVA-challenged mice; Oridonin-L, asthmatic mice treated with
Oridonin (10 mg/kg); Oridonin-H, asthmatic mice treated with
Oridonin (20 mg/kg). Values are expressed as the mean ± standard
error of the mean. *P<0.05 vs. the control group.
BAL, bronchoalveolar lavage; EOS, eosinophil; NEU, neutrophil; MAC,
macrophage; LYM, lymphocyte; OVA, ovalbumin. |
Effect of Oridonin on cytokine levels in
BAL fluid
Humoral immune response usually causes the allergic
reaction, when exposed to an allergen the immune cells secrete a
series of cytokines and these cytokines, such as IL-4 and IL-13
contribute to the allergic condition (32). Kim et al (33) indicated that the imbalance of IL-5,
eotaxin and IFN-γ was associated with asthma. Therefore, the
present study analyzed the effect of Oridonin on cytokine levels in
the BAL fluid of mice. The lungs of OVA-challenged mice were
lavaged with cold PBS 48 h after the final challenge and the BAL
fluid was collected. The ELISA assay was performed to evaluate the
level of the associated cytokines in the mice BAL fluid according
to the manufacturer's instructions. The findings demonstrated that
the level of IL-4, IL-13, IL-5 and eotaxin increased significantly
in the BAL fluid of the OVA-challenged mice (P<0.05). The
results indicated that Oridonin treatment significantly inhibited
the IL-4, IL-13, IL-5 and eotaxin levels in BAL fluid, but exerted
a marginal effect on IFN-γ (Fig.
5). These results indicate that Oridonin modulated the level of
cytokines produced by immune cells in asthmatic mice.
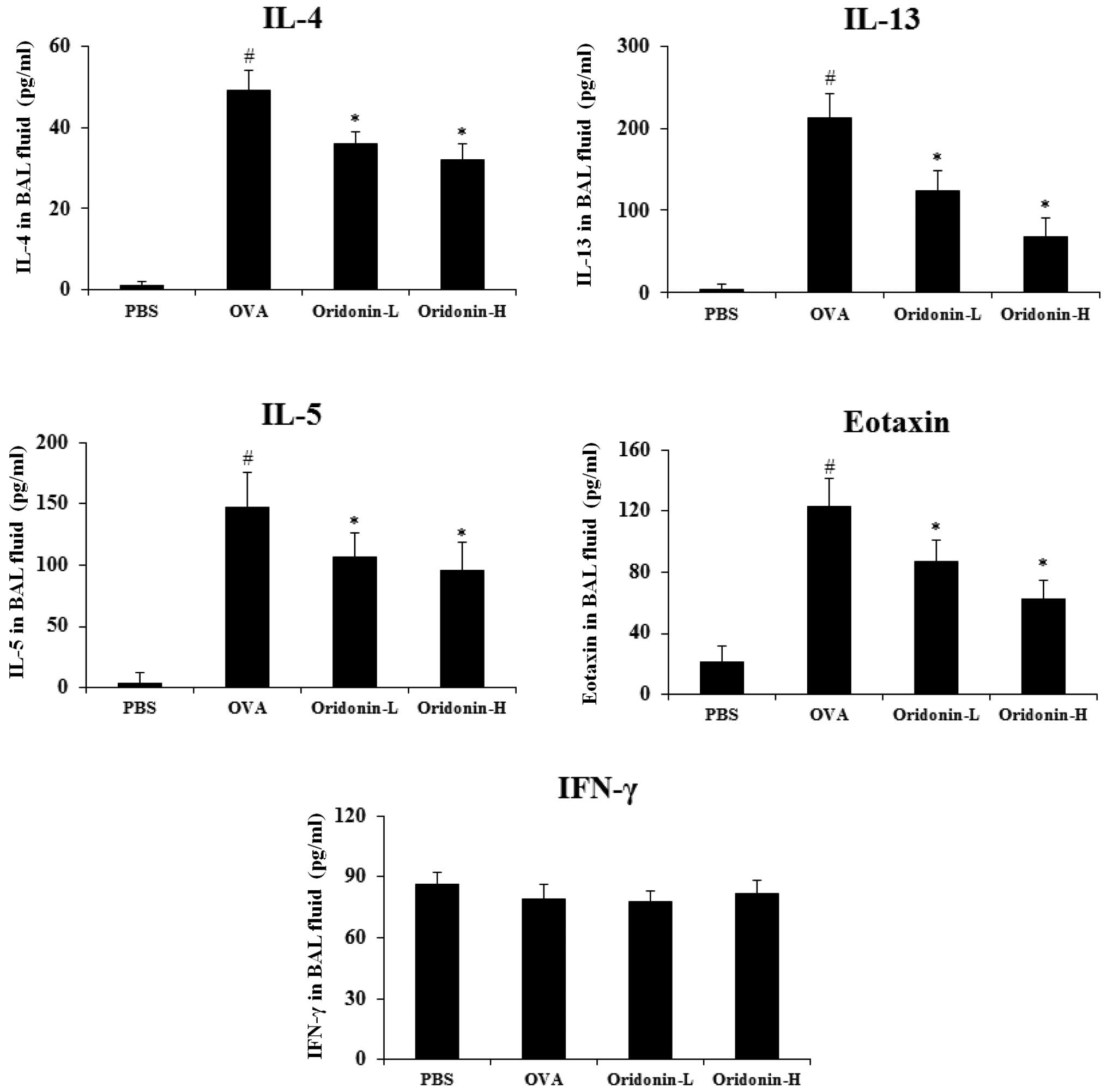 | Figure 5Effects of Oridonin on cytokine and
chemokine levels in BAL fluid. BAL fluids were collected 2 h after
the final OVA challenge. Levels of IL-4, IL-5, IL-13, eotaxin and
IFN-γ were analyzed using enzyme-linked immunosorbent assay. PBS,
PBS-challenged mice; OVA, OVA-challenged mice; Oridonin-L,
asthmatic mice treated with Oridonin (10 mg/kg); Oridonin-H,
asthmatic mice treated with Oridonin (20 mg/kg). Values are
expressed as the mean ± standard error of the mean.
#P<0.05 vs. PBS group; *P<0.05 vs. OVA
group. IL, interleukin; IFN, interferon; PBS, phosphate-buffered
saline; OVA, ovalbumin; BAL, bronchoalveolar lavage. |
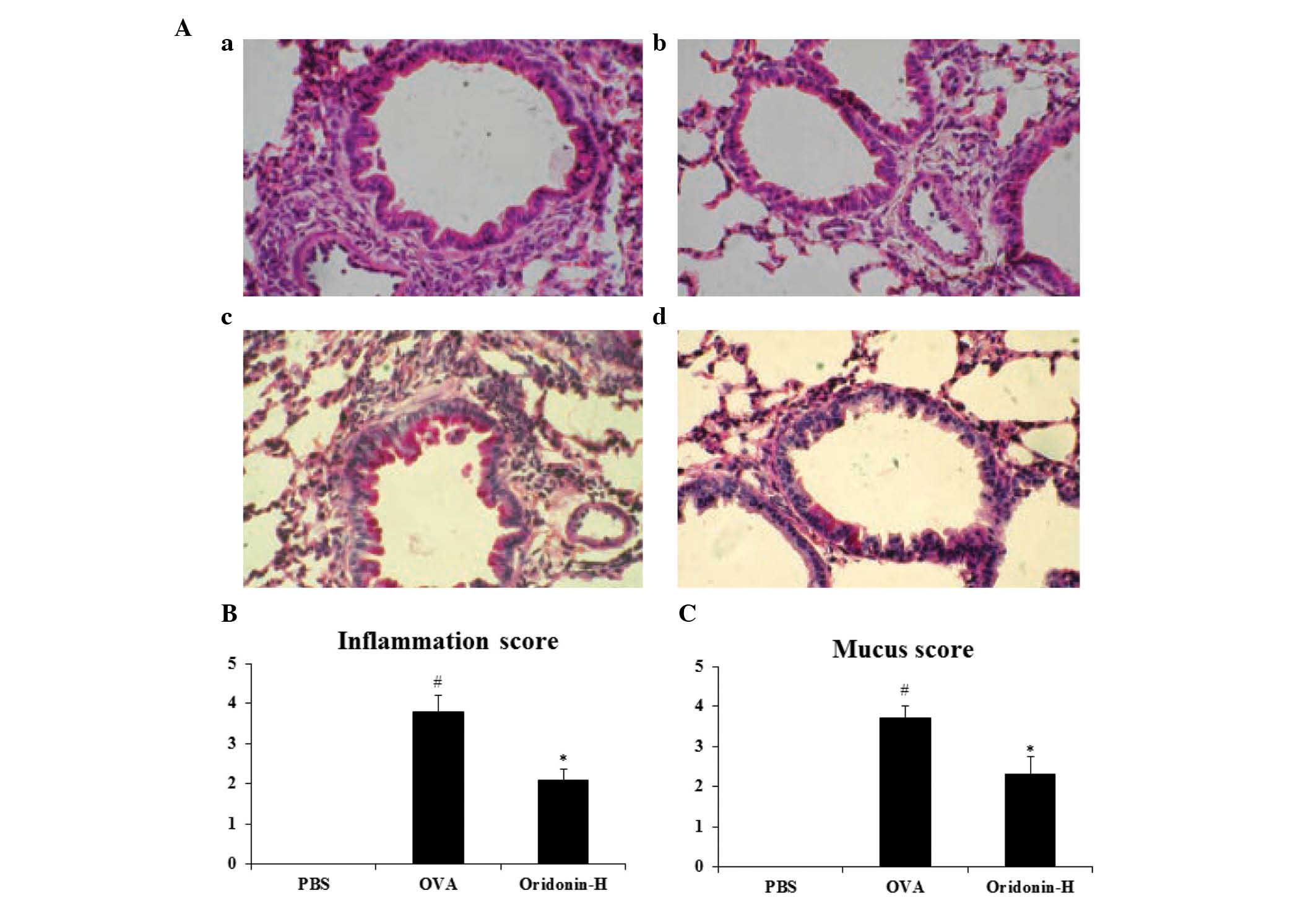
Effects of Oridonin on eosinophil
infiltration and mucus production
Significant migration of inflammatory cells
(eosinophils) to peribronchiolar tissues was observed in the mice
that had been challenged with OVA (31). Therefore, the effect of Oridonin on
the eosinophil infiltration and mucus production was investigated
in the lungs of asthmatic mice. Twenty-four hours after the last
OVA challenge, mice were sacrificed and their lungs were harvested.
Lung tissues were stored in formalin and histologically examined by
a pathologist (Fig. 6A). The
histopathological examination results indicated that following
challenge with OVA, the eosinophil infiltration and mucus
production were significantly increased compared to the PBS group
(P<0.05). However, following administration of oridonin the
inflammation score decreased from 3.8 to 2.1 (P<0.05) and the
mucus score decreased from 3.7 to 2.3 (P<0.05). These results
indicate that Oridonin significantly inhibited eosinophil
infiltration (Fig. 6B) and mucus
production (Fig. 6C) in the
asthmatic lung when compared with that of the control group.
Discussion
Asthma is a chronic disease of the airways. It is
characterized by airway inflammation, the increased secretion of
mucus and AHR (34). Previous
studies have indicated that immune cells (T, B, and mast cells and
eosinophils), cytokines and chemokines contribute to inflammatory
responses (34,35). T helper cell type 2 produces
Th2 cytokines, which are associated with the maturation
of B cells. Th2 cytokines include IL-4, IL-5, and IL-13
and these cytokines are important in humoral immune responses.
Furthermore, chemokines are essential for eosinophil infiltration
into lung tissues. Therefore modulation of cytokine and chemokine
balance may be a potential therapeutic strategy for asthma.
Oridonin is a chemical compound extracted from the
traditional Chinese medicinal herb, Xihuangcao, which is a popular
herbal medicine in China (19).
Previous studies have indicated that Oridonin may cure lymphoid
malignancies (36), leukemia
(37) and auto immune disease
(24). However, to the best of our
knowledge, the effects of Oridonin on asthma have not been
reported.
A previous study indicated that Oridonin regulated
the Th1/Th2 balance in rats (25); in the present study, it was
demonstrated that Oridonin also modulated
Th1/Th2 balance in mice. Therefore, Oridonin
was evaluated in vivo in a mouse asthma model to assess the
anti-asthmatic effects. Our acute toxicity study indicated that
when administrated with 50 mg/kg Oridonin, the mice survived and no
loss of body weight was observed (data not shown). The results
indicated that Oridonin significantly decreased the AHR in
asthmatic mice and decreased the eosinophil, neutrophil and total
cell number in BAL fluid. To further investigate the potential
mechanisms of action of Oridonin, cytokine and chemokine production
was assessed in the BAL fluid of mice.
The levels of Th2 cytokines and
chemokines increased in the OVA-challenged mice. IL-5 is important
in the survival and recruitment of eosinophils; while IL-13 is
significant in AHR, eosinophilic infiltration and mucus secretion
(35,38). In addition, chemokines contribute
to eosinophil migration in the airways, with Regulated on
Activation, Normal T Cell Expressed and Secreted and eotaxin as the
two most important chemokines. Adhesion molecules are also involved
in eosinophil migration (39).
These factors, Th2 cytokines, chemokines and adhesion
molecules, may contribute to AHR in asthma together (40). The present study indicates that
Oridonin significantly inhibited the IL-4, IL-13, IL-5 and eotaxin
levels in BAL fluid. These results indicate that the regulation of
cytokine balance may contribute to asthma in mice. However, the
underlying mechanisms remain unclear and further investigation is
required.
In conclusion, the present study demonstrates that
Oridonin regulated the cytokine balance in OVA-challenged mice,
inhibited AHR and reduced lung eosinophilia, mucus hypersecretion
in asthmatic mice. These findings suggest that Oridonin may serve
as a potential novel compound for the treatment of asthma.
Acknowledgments
The present study was supported by the Innovation
and Development Fund for Scientific Research Institution of
Xinjiang Uyghur Autonomous Region: The Construction of Traditional
Chinese Medicine Base for Transformation and Prevention of
Respiratory System Disease in Xinjiang (grant. no. 2015008).
References
|
1
|
Global Burden of Disease Study 2013
Collaborators: Global, regional, and national incidence,
prevalence, and years lived with disability for 301 acute and
chronic diseases and injuries in 188 countries, 1990–2013: A
systematic analysis for the Global Burden of Disease Study 2013.
Lancet. 386:743–800. 2015. View Article : Google Scholar
|
|
2
|
GBD 2013 Mortality and Causes of Death
Collaborators: Global, regional, and national age-sex specific
all-cause and cause-specific mortality for 240 causes of death,
1990–2013: a systematic analysis for the Global Burden of Disease
Study 2013. Lancet. 385:117–171. 2015. View Article : Google Scholar
|
|
3
|
Elias JA, Lee CG, Zheng T, Ma B, Homer RJ
and Zhu Z: New insights into the pathogenesis of asthma. J Clin
Invest. 111:291–297. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Robinson DS, Hamid Q, Ying S, Tsicopoulos
A, Barkans J, Bentley AM, Corrigan C, Durham SR and Kay AB:
Predominant TH2-like bronchoalveolar T-lymphocyte population in
atopic asthma. N Engl J Med. 326:298–304. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okayama Y, Ra C and Saito H: Role of mast
cells in airway remodeling. Curr Opin Immunol. 19:687–693. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Holgate ST: Epithelium dysfunction in
asthma. J Allergy Clin Immunol. 120:1233–1244; quiz 1245–1246.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McFadden ER Jr: Acute severe asthma. Am J
Respir Crit Care Med. 168:740–759. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sinigaglia F and D'Ambrosio D: Regulation
of helper T cell differentiation and recruitment in airway
inflammation. Am J Respir Crit Care Med. 162:S157–S160. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Broide DH: Immunologic and inflammatory
mechanisms that drive asthma progression to remodeling. J Allergy
Clin Immunol. 121:560–570; quiz 571–572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cho JY, Miller M, Baek KJ, Han JW, Nayar
J, Lee SY, McElwain K, McElwain S, Friedman S and Broide DH:
Inhibition of airway remodeling in IL-5-deficient mice. J Clin
Invest. 113:551–560. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pascual RM and Peters SP: Airway
remodeling contributes to the progressive loss of lung function in
asthma: An overview. J Allergy Clin Immunol. 116:477–486; quiz 487.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Flood-Page P, Menzies-Gow A, Phipps S,
Ying S, Wangoo A, Ludwig MS, Barnes N, Robinson D and Kay AB:
Anti-IL-5 treatment reduces deposition of ECM proteins in the
bronchial subepithelial basement membrane of mild atopic
asthmatics. J Clin Invest. 112:1029–1036. PubMed/NCBI
|
|
13
|
Panettieri RA Jr: Cellular and molecular
mechanisms regulating airway smooth muscle proliferation and cell
adhesion molecule expression. Am J Respir Crit Care Med.
158:S133–S140. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeffery PK: Remodeling in asthma and
chronic obstructive lung disease. Am J Respir Crit Care Med.
164:S28–S38. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wenzel S: Severe asthma in adults. Am J
Respir Crit Care Med. 172:149–160. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tagaya E and Tamaoki J: Mechanisms of
airway remodeling in asthma. Allergol Int. 56:331–340. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mauad T, Bel EH and Sterk PJ: Asthma
therapy and airway remodeling. J Allergy Clin Immunol.
120:997–1009; quiz 1010–1011. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vanacker NJ, Palmans E, Kips JC and
Pauwels RA: Fluticasone inhibits but does not reverse
allergen-induced structural airway changes. Am J Respir Crit Care
Med. 163:674–679. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu JJ, Huang RW, Lin DJ, Wu XY, Peng J,
Pan XL, Song YQ, Lin Q, Hou M, Wang DN, et al: Oridonin-induced
apoptosis in leukemia K562 cells and its mechanism. Neoplasma.
52:225–230. 2005.PubMed/NCBI
|
|
20
|
Chen S, Gao J, Halicka HD, Huang X,
Traganos F and Darzynkiewicz Z: The cytostatic and cytotoxic
effects of oridonin (Rubescenin), a diterpenoid from Rabdosia
rubescens, on tumor cells of different lineage. Int J Oncol.
26:579–588. 2005.PubMed/NCBI
|
|
21
|
Ren KK, Wang HZ, Xie LP, Chen DW, Liu X,
Sun J, Nie YC and Zhang RQ: The effects of oridonin on cell growth,
cell cycle, cell migration and differentiation in melanoma cells. J
Ethnopharmacol. 103:176–180. 2006. View Article : Google Scholar
|
|
22
|
Liu JJ, Wu XY, Peng J, Pan XL and Lu HL:
Antiproliferation effects of oridonin on HL-60 cells. Ann Hematol.
83:691–695. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu Y, Xue Y, Wang Y, Feng D, Lin S and Xu
L: Multiple-modulation effects of Oridonin on the production of
proinflammatory cytokines and neurotrophic factors in LPS-activated
microglia. Int Immunopharmacol. 9:360–365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu J, Yang F, Zhang Y and Li J: Studies
on the cell-immunosuppressive mechanism of Oridonin from Isodon
serra. Int Immunopharmacol. 7:945–954. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu AP, Du JM, Li JY and Liu JW: Oridonin
promotes CD4+/CD25+ Treg differentiation, modulates Th1/Th2 balance
and induces HO-1 in rat splenic lymphocytes. Inflamm Res.
57:163–170. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Committee for the Update of the Guide for
the Care and Use of Laboratory Animals: Guide for the Care and Use
of Laboratory Animals. 8th edition. National Academies Press;
Washington, D.C: 2011
|
|
27
|
Xue H, Guo H, Li YC and Hao ZM: Heme
oxygenase-1 induction by hemin protects liver cells from
ischemia/reperfusion injury in cirrhotic rats. World J
Gastroenterol. 13:5384–5390. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Myou S, Leff AR, Myo S, Boetticher E, Tong
J, Meliton AY, Liu J, Munoz NM and Zhu X: Blockade of inflammation
and airway hyperresponsiveness in immune-sensitized mice by
dominant-negative phosphoinositide 3-kinase-TAT. J Exp Med.
198:1573–1582. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hansbro PM, Kaiko GE and Foster PS:
Cytokine/anti-cytokine therapy-novel treatments for asthma? Br J
Pharmacol. 163:81–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Possa SS, Leick EA, Prado CM, Martins MA
and Tibério IFLC: Eosinophilic inflammation in allergic asthma.
Front Pharmacol. 4:462013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Duan W, Chan JH, Wong CH, Leung BP and
Wong WS: Anti-inflammatory effects of mitogen-activated protein
kinase kinase inhibitor U0126 in an asthma mouse model. J Immunol.
172:7053–7059. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abbas AK, Murphy KM and Sher A: Functional
diversity of helper T lymphocytes. Nature. 383:787–793. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim CK, Kita H, Callaway Z, Kim HB, Choi
J, Fujisawa T, Shin BM and Koh YY: The roles of a Th2 cytokine and
CC chemokine in children with stable asthma: Potential implication
in eosinophil degranulation. Pediatr Allergy Immunol. 21:e697–e704.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Busse WW and Lemanske RF Jr: Asthma. N
Engl J Med. 344:350–362. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Herrick CA and Bottomly K: To respond or
not to respond: T cells in allergic asthma. Nat Rev Immunol.
3:405–412. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ikezoe T, Yang Y, Bandobashi K, Saito T,
Takemoto S, Machida H, Togitani K, Koeffler HP and Taguchi H:
Oridonin, a diterpenoid purified from Rabdosia rubescens, inhibits
the proliferation of cells from lymphoid malignancies in
association with blockade of the NF-kappaB signal pathways. Mol
Cancer Ther. 4:578–586. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou GB, Kang H, Wang L, Gao L, Liu P, Xie
J, Zhang FX, Weng XQ, Shen ZX, Chen J, et al: Oridonin, a
diterpenoid extracted from medicinal herbs, targets AML1-ETO fusion
protein and shows potent antitumor activity with low adverse
effects on t(8; 21) leukemia in vitro and in vivo. Blood.
109:3441–3450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wynn TA: IL-13 effector functions. Annu
Rev Immunol. 21:425–456. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lukacs NW: Role of chemokines in the
pathogenesis of asthma. Nat Rev Immunol. 1:108–116. 2001.
View Article : Google Scholar
|
|
40
|
Wills-Karp M: Immunologic basis of
antigen-induced airway hyperresponsiveness. Annu Rev Immunol.
17:255–281. 1999. View Article : Google Scholar : PubMed/NCBI
|