Introduction
Ongoing neurogenesis in adults is now an accepted
and well-characterized phenomenon in the mammalian brain. In the
subgranular zone (SGZ) of the dentate gyrus (DG) of the hippocampus
and the subventricular zone (SVZ) of the olfactory bulb, the adult
rodent brain regularly produces newly-formed cells that
differentiate into neurons, astrocytes and oligodendrocytes
(1). The process of adult
neurogenesis is influenced by numerous physiological and
pathological stimuli, including brain injury, neurotrophic factors
or chemokines, and the environment (2–5).
Environmental enrichment (EE) is defined as a
combination of 'complex inanimate objects and social stimulation'
(6). Compared with standard
housing conditions, EE provides enhanced stimulation of the
cognitive, sensory and motor systems in the brains of laboratory
animals (7–10). Current evidence demonstrates that
EE increases hippocampal neurogenesis, improves cognition and
promotes behavioral recovery following a brain injury in animals
(11–13). However, the effects of EE on
neurogenesis in the DG and SVZ remain to be fully resolved.
Furthermore, the mechanisms underlying EE exposure-induced
hippocampal neurogenesis remain to be elucidated.
Brain-derived neurotrophic factor (BDNF) is an
important neuronal growth factor in the brain that promotes
neuronal maturation and neurogenesis by activating cyclic adenosine
monophosphate (cAMP) response element-binding protein (CREB) and
protein kinase A (PKA) (14).
Increasing evidence suggests that BDNF and phosphorylated CREB
(pCREB) are key factors in the regulation of hippocampal
neurogenesis and improved cognition, which are observed following
EE (1,13,15–17).
Stromal cell-derived factor-1 (SDF-1) is a CXC chemokine produced
by bone marrow stromal cells. SDF-1 and its specific receptor,
C-X-C motif chemokine receptor 4 (CXCR4), which stimulates the
cAMP-mediated signaling pathway, are important in the regulation of
the proliferation and differentiation of neural precursors
(18–22).
In the present study, the impact of the provision of
daily EE for 30 days on neurogenesis in the DG and SVZ, as well as
on cognitive function in healthy, adult rats was examined. In
addition, the protein expression levels of BDNF, pCREB, PKA
catalytic subunit α (PKA C-α), SDF-1 and CXCR4 in the hippocampus
of rats housed in the presence or absence of EE were
determined.
Materials and methods
Animals and EE
Adult male Wistar rats (weight, 220–250 g) from the
Experimental Animal Center of China Medical University (Shenyang,
China) were randomly assigned to two groups: Standard environment
(SE; n=24) and EE (n=24). The EE animals were housed for 30 days in
a large cage (841×565×526 mm) containing a variety of toys,
including houses, mazes, wheels, chains, sinks, swings, ladders and
balls; the toys were changed once or twice per week. The SE group
was housed in standard vivarium cages (30×20×15 mm) without toys.
There were 3–4 rats in each standard cage and 8–12 rats in the EE
cage. All rats were housed under a temperature-regulated
environment with a 12-h light/dark cycle and free access to food
and water. All procedures were approved by the Institutional Animal
Care and Use Committee of China Medical University [Shenyang,
China; ref. SCXK (Liao) 2008-0005].
Morris water maze test
Spatial learning was analyzed with a match-to-place
version of the Morris water maze test on days 31–33, as described
previously (23). Following the
assessment (day 33), a probe trial of 60 sec in the absence of the
platform was performed to estimate the ability of the rats to
recall the location of the platform by measuring the number of
passings over its previous location. Swimming routes were monitored
with the SLY-WMS video tracking system (Beijing Sunny Instruments
Co., Ltd., Beijing, China).
5-Bromo-2-deoxyuridine (BrdU)
labeling
To examine the rate of cell differentiation and
survival, one cohort of rats (n=12 per group) was injected i.p.
with BrdU (100 mg/kg; Sigma-Aldrich, St. Louis, MO, USA) once a day
on days 11–15 and sacrificed on day 36. In order to investigate the
rate of cell proliferation, one cohort of rats (n=6 per group)
received injections of BrdU twice daily on days 34–35 prior to
sacrifice on day 36.
Tissue preparation
The rats were transcardially perfused with normal
saline and 4% paraformaldehyde under anesthesia, following which
the brains were removed immediately and postfixed in 4%
paraformaldehyde overnight at 4°C. Brains were then placed in a 30%
sucrose solution. When the tissues had sunk to the bottom of the
container, they were removed, placed in optimal cutting temperature
compound (Sakura Finetek USA, Inc., Torrance, CA, USA) and snap
frozen. A series of contiguous 40-µm-thick coronal sections
were cut on a cryotome (Leica Microsystems GmbH, Wetzlar, Germany).
A total of five coronal sections were selected from every sixth
section between bregma level −2.8 and −4.0 mm through the dorsal
hippocampus or between bregma level +0.96 and −0.24 mm through the
SVZ in each rat prior to immunostaining.
Immunofluorescence staining
As described previously (24), DNA was denatured and the
incorporated BrdU was detected immunologically using a sheep
anti-BrdU antibody (1:500; catalog no. ab1893; Abcam, Cambridge,
MA, USA). The following antibodies were used for phenotyping in
combination with anti-BrdU: Guinea pig anti-doublecortin (DCX;
1:1,000; catalog no. AB5910; EMD Millipore, Billerica, MA, USA),
mouse anti-neuronal nuclei (NeuN; 1:500; catalog no. MAB377;
Chemicon; EMD Millipore) or rabbit anti-glial fibrillary acidic
protein (GFAP; 1:1,000; catalog no. ab7260; Abcam). The sections
were incubated in the appropriate secondary antibodies: Alexa Fluor
594 donkey anti-sheep IgG (catalog no. A11016), Alexa Fluor 488
goat anti-guinea pig IgG (catalog no. A11073) or Alexa Fluor 488
goat anti-rabbit IgG (catalog no. A11034), all diluted 1:500 and
purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA),
prior to immunofluorescent assessment.
Quantification
Positively stained cells were counted by an
experimenter blinded to the details of the study. The
immunofluorescence images of BrdU-, BrdU/DCX-, BrdU/NeuN- and
BrdU/GFAP-positive cells in the DG, and BrdU- and BrdU/DCX-positive
cells in the SVZ were visualized on a fluorescent microscope with a
20× objective lens (Olympus Corporation, Tokyo, Japan). The numbers
of positive cells in five sections per rat were counted with ImageJ
software version 1.48 (National Institutes of Health, Bethesda, MD,
USA) and the mean number was calculated. Only cells in which a
BrdU-positive nucleus was co-expressed with DCX, NeuN or GFAP were
deemed to be newly-formed neurons, mature neurons or astrocytes,
respectively. For analysis of dendritic complexity, 18–20
DCX-positive cells from each section were randomly selected and
visualized using a confocal system (Leica SP2; Leica Microsystems
GmbH) with a multi-track configuration. Dendritic length was
assessed with FIJI (a distribution of ImageJ; www.fiji.sc/) using the Simple Neurite Tracer plugin.
Total dendritic length was assessed for each neuron by tracing main
dendritic processes extending from the soma and their branches.
Western blotting
Western blotting was performed on day 34 following
SE or EE. Hippocampal proteins of eight rats per group were
extracted using ice-cold radioimmunoprecipitation assay buffer
(Beyotime Institute of Biotechnology, Haimen, China), and protein
concentrations were measured using a standard Bradford assay
(Beyotime Institute of Biotechnology). Protein samples (40
µg) were separated on 10% SDS-PAGE gels at 80 V for 5 h and
transferred onto polyvinylidene difluoride (PVDF) membranes
(Beyotime Institute of Biotechnology). The PVDF membranes were
blocked with 5% bovine serum albumin (Sigma-Aldrich) for 2 h at
room temperature prior to antibody incubation. The following
primary antibodies were used: Rabbit anti-PKA C-α (1:1,000; catalog
no. 5842), rabbit anti-pCREB (1:1,000; catalog no. 9198) and rabbit
anti-CREB (1:1,000; catalog no. 9197), all Cell Signaling
Technology, Inc. (Danvers, MA, USA); rabbit anti-BDNF (1:500;
catalog no. ab108319), rabbit anti-SDF-1 (1:1,000; catalog no.
ab9797) and rabbit anti-CXCR4 (1:500; catalog no. ab197203), from
Abcam; and mouse anti-β-actin (1:1,000; catalog no. sc-47778; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA). The secondary
antibodies were horseradish peroxidase-conjugated goat anti-rabbit
IgG (1:2,000; catalog no. ZB2301; ZsBio, Beijing, China) and goat
anti-mouse IgG (1:2,000; catalog no. ZB2305; ZsBio). Proteins were
observed with an enhanced chemiluminescence reagent kit (Beyotime
Institute of Biotechnology). The densities of the protein signals
were quantified using Image-Pro Plus version 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Statistical analyses were performed using SPSS
software version 20 (IBM SPSS, Armonk, NY, USA). Data were analyzed
using the independent-samples t-test or repeated-measures
analysis of variance. Data are presented as the mean ± standard
error of the mean and P<0.05 was considered to indicate a
statistically significant difference.
Results
Quantifying the proliferation and
dendritic length of newly-formed neurons in the DG
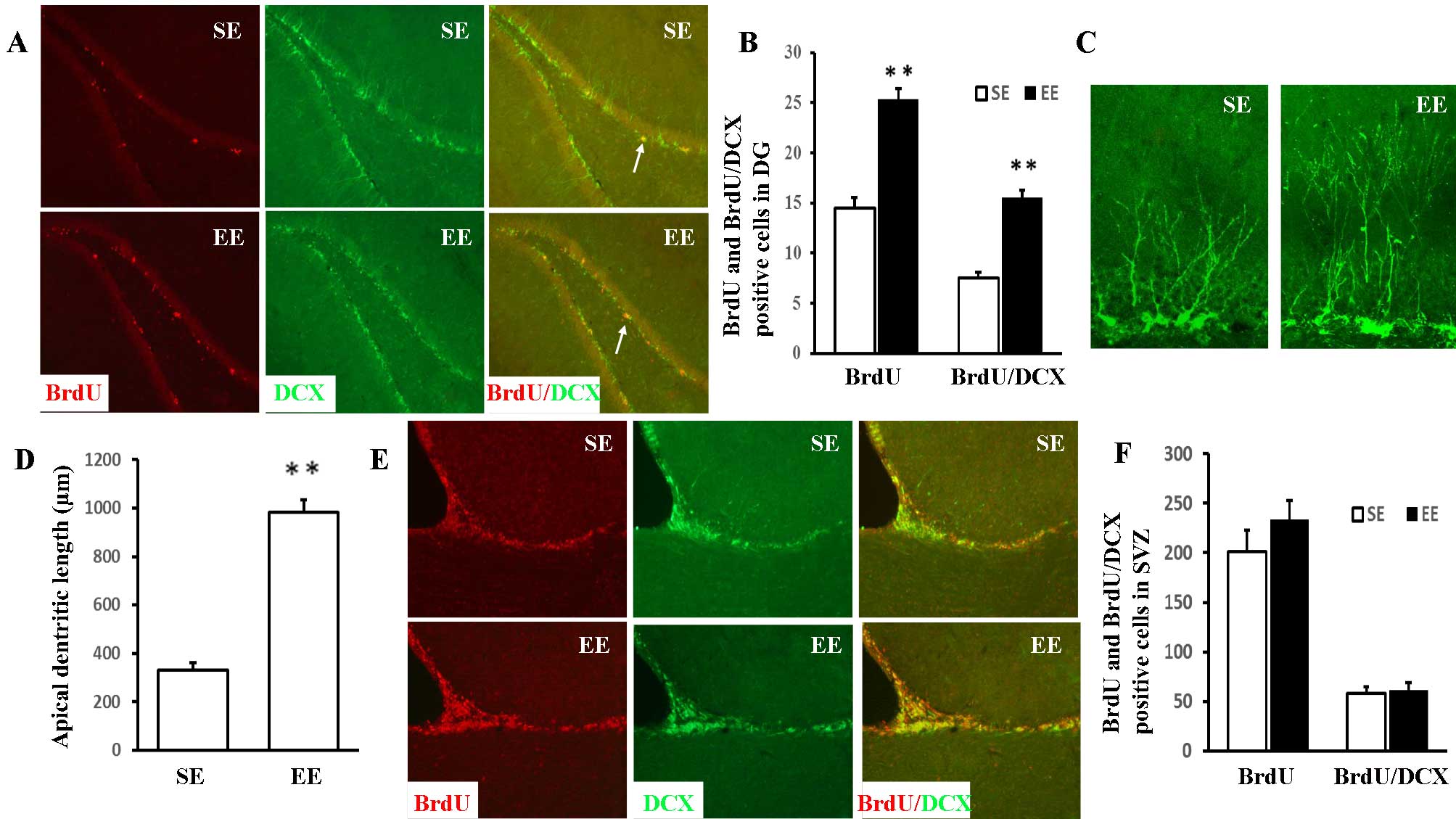
Double immunofluorescence staining of BrdU and DCX
(an immature neuron maker) was conducted to examine the identity of
the proliferating cells. In the DG, the numbers of BrdU-positive
and BrdU/DCX double-positive cells (Fig. 1A and B) were significantly
increased in EE compared with SE rats [25.34±1.05 vs. 14.51±1.06
(P<0.001) and 15.53±0.77 vs. 7.50±0.60 (P<0.001),
respectively]. The effect of EE on apical dendritic development was
assessed by measuring the total dendritic length of the
DCX-positive cells in the DG (Fig. 1C
and D). In contrast to the SE group (330.31±8.59 µm),
DCX-positive cells in the EE group exhibited a significant increase
in apical dendritic length (981.95±51.90 µm;
P<0.001).
Quantifying the proliferation of
newly-formed neurons in the SVZ
As presented in Fig. 1E
and F, no statistically significant differences were observed
between the EE and SE rats in the numbers of BrdU-positive and
BrdU/DCX double-positive cells in the SVZ [232.91±19.97 vs.
201.04±21.75 (P=0.299) and 60.93±3.07 vs. 58.29±2.43 (P=0.510),
respectively].
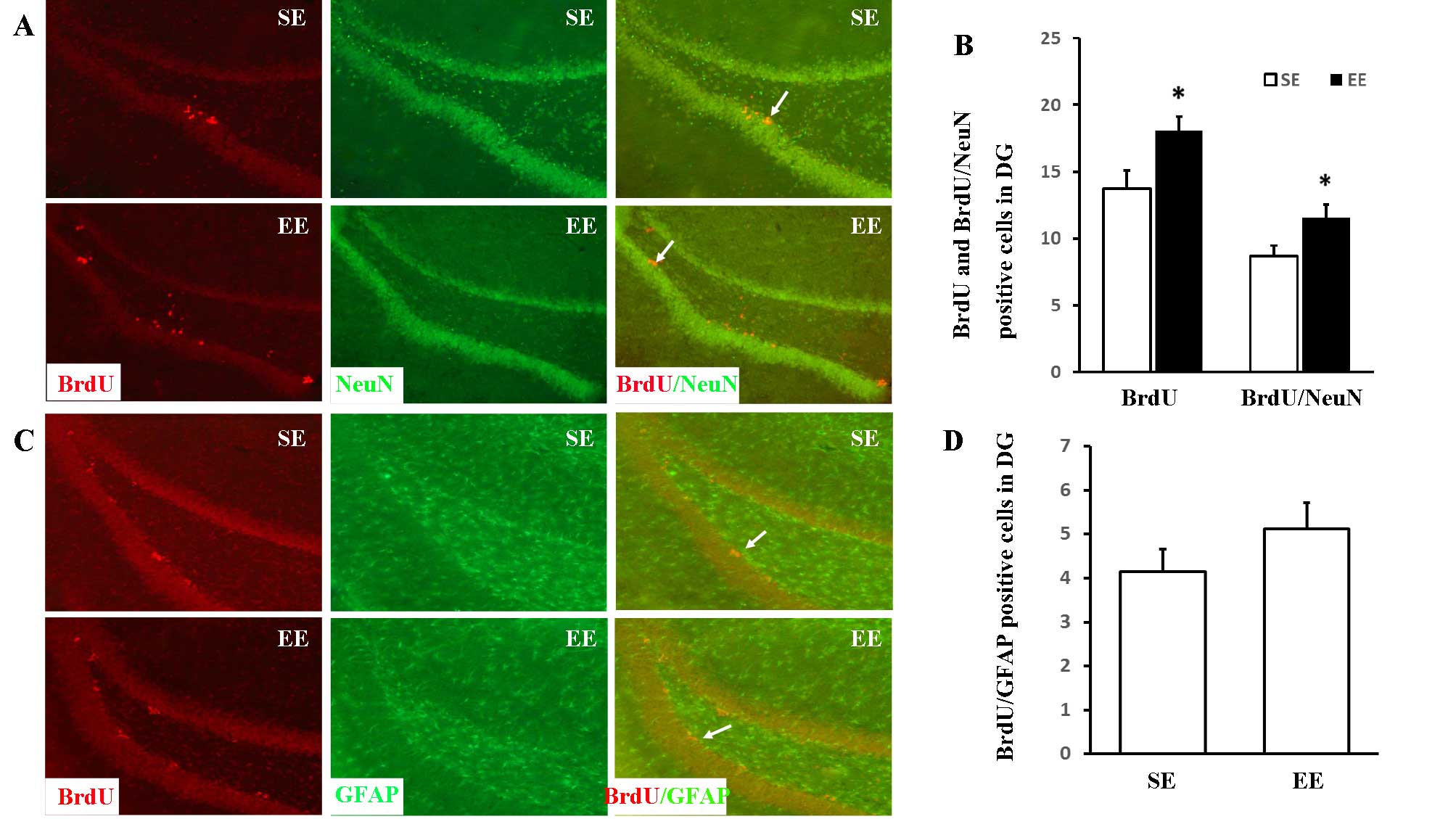
Quantification of the differentiation and
survival of the neuroblasts generated in the DG
The differentiation of newly-formed cells in the DG
was assessed by double-staining of BrdU and NeuN (a mature neuron
marker) or GFAP (an astrocyte marker). The long-term survival of
newly-formed neurons was determined by analysis of BrdU associated
with the neuronal marker, NeuN (Fig.
2A and B). The numbers of BrdU-positive and BrdU/NeuN
double-positive cells were markedly increased in the EE group in
comparison with the SE group [18.11±1.07 vs. 13.72±1.36 (P=0.025)
and 11.54±0.80 vs. 8.67±0.78 (P=0.016), respectively]. However, no
significant differences were observed between the numbers of
BrdU/GFAP double-positive cells (Fig.
2C and D) in the SE and EE groups (4.14±0.52 vs. 5.11±0.61;
P=0.206).
Protein expression levels of PKA C-α,
pCREB, BDNF, SDF-1 and CXCR4 in the hippocampus
To determine whether EE affected the protein
expression levels of PKA C-α, pCREB, BDNF, SDF-1 and CXCR4, western
blotting was conducted on day 34 (Fig.
3A). As presented in Fig. 3B,
western blotting identified a significant upregulation of pCREB,
BDNF, SDF-1 and CXCR4 expression in the hippocampus following EE;
however, there was no significant difference in the protein
expression level of PKA C-α between the two groups.
 | Figure 3Western blot analysis. (A)
Representative immunoblots of PKA C-α, BDNF, SDF-1, CXCR4, pCREB
and CREB in the hippocampus of rats in the SE and EE groups. (B)
Protein expression levels of pCREB, BDNF, SDF-1 and CXCR4 were
increased in the EE compared with the SE group, whereas no
significant difference was observed in the expression of PKA C-α.
The protein expression levels of PKA C-α, BDNF, SDF-1 and CXCR4
were normalized to β-actin expression, and pCREB was normalized to
CREB expression. *P<0.05 vs. SE; n=6 per group. PKA
C-α, protein kinase A catalytic subunit α; BDNF, brain-derived
neurotrophic factor; SDF-1, stromal cell-derived factor-1; CXCR4,
CXC chemokine receptor 4; CREB, cyclic adenosine monophosphate
response element-binding protein; pCREB, phosphorylated CREB; SE,
standard environment; EE, environmental enrichment. |
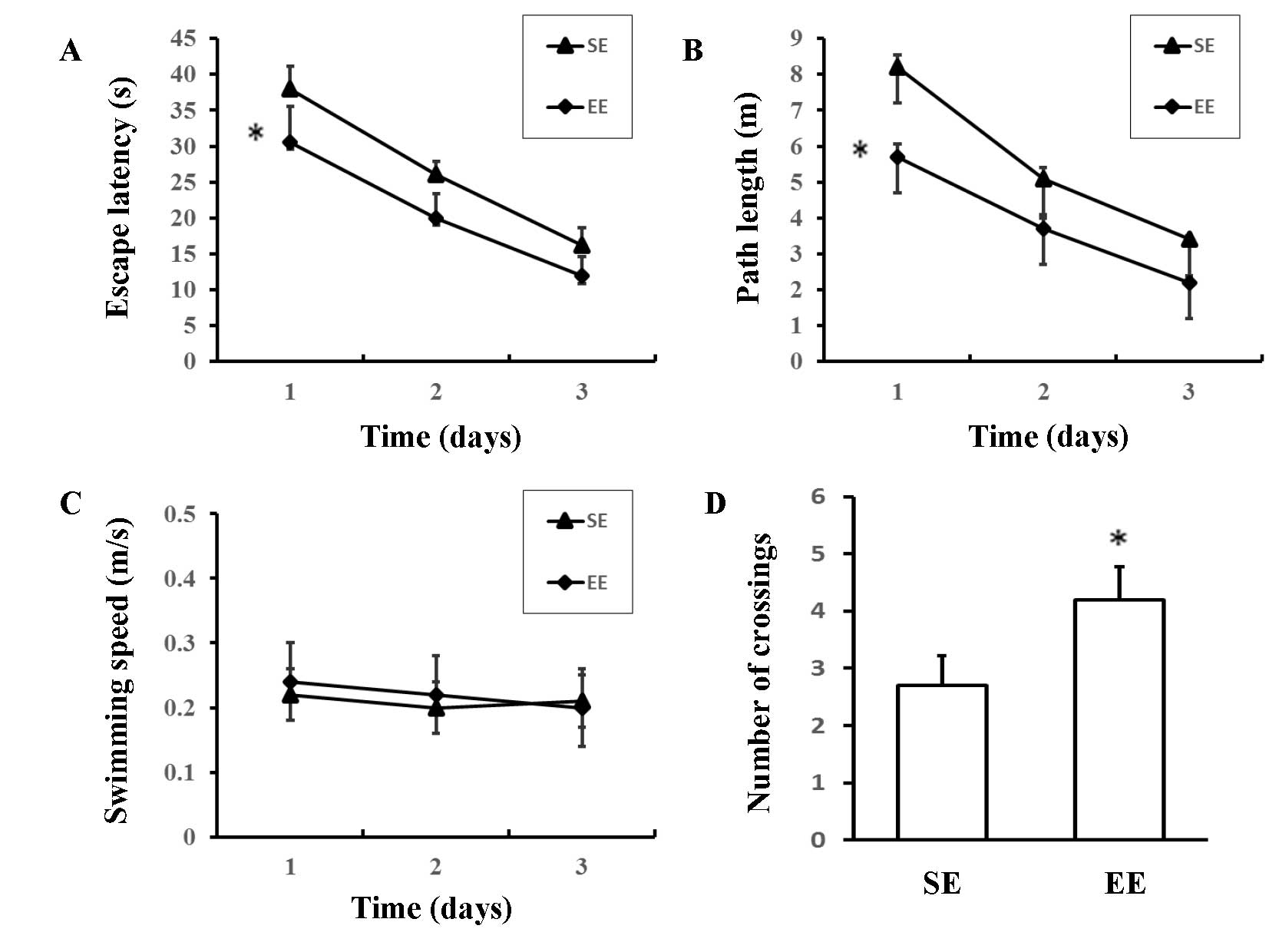
Cognitive function
The Morris water maze was used to assess spatial
learning in the rats. EE rats had shorter escape latencies [F
(1,18) =8.322; P=0.010; Fig. 4A] and path lengths [F (1,18)=6.976; P=0.017; Fig. 4B] compared with SE rats; however,
there were no significant differences in swimming speeds [F
(1,18) =0.132; P=0.721; Fig. 4C]. In the probe trial, there was a
significant increase in the number of crossings over the previous
position of the platform in the EE group compared with the SE group
(4.20±1.81 vs. 2.60±1.51; P=0.046; Fig. 4D). These results suggested that EE
rats demonstrated an improved capacity for spatial learning when
compared with the SE rats.
Discussion
The present study demonstrated that 30 days exposure
to EE increased the production and differentiation of newly-formed
neurons, as well as increasing the dendritic complexity of
DCX-positive cells in the DG of adult rats. These structural
alterations were accompanied by improved cognitive function in an
examination of spatial learning. By contrast, EE did not activate
neurogenesis in the SVZ. Furthermore, EE enhanced the protein
expression levels of BDNF, pCREB, SDF-1 and CXCR4 in the
hippocampus, which potentially contribute to the promotion of
hippocampal neurogenesis.
EE provides increased potential for physical
activity and complex social stimulation than is achieved under
standard conditions (8). In the
present study, EE consisted of a large cage with different toys,
including tunnels of various shapes for spatial exploration, a
running wheel, ladders and swings for motor stimulation, colored
balls for visual stimulation, wooden objects of various shapes and
texture for sensory perception, and a house for hiding. These toys
and their positions were changed once or twice every week to
provide a novel environment. In addition, the EE cage encouraged
social interaction, as larger numbers of rats (8–12 rats in the
present study) were housed together in wider and more spacious
cages with exploration chambers (25,26).
Currently, no accurate method to quantify environmental impact
exists. Xie et al (27)
hypothesized that enrichment-induced exercise was the common
downstream effect of all enrichment factors, including cage size,
the type and quantity of toys, the number of animals and the
duration of enrichment. The physical exercise of rats was recorded
and measured through the distance moved and velocity achieved, and
this was demonstrated to correlate with the degree of enrichment
and the enrichment impact. In addition, Walker and Mason (28) revealed that the degree of
enrichment-associated impact was reflected in the physical exercise
of animals stimulated by EE. Therefore, measuring the
enrichment-induced exercise of animals may allow quantification of
the environmental impact; therefore, this will be performed in any
of our future studies. In the present study, the abundant inanimate
and social stimulations had a distinct effect on physical exercise,
which is known to be beneficial to the central nervous system
(29), and the rats with EE
performed more enrichment-induced exercise compared with SE rats.
The results of the present study are consistent with previous
reports that adult rats exposed to EE exhibit improved cognitive
functions (12,30,31).
Although the underlying mechanisms remain to be elucidated, it is
possible that cognitive improvements associated with EE in the
normal brain are associated with the enhanced neural plasticity
occurring at various levels in the brain. The evidence for enhanced
neural plasticity includes neurogenesis, multiple structural
changes, increases in neurotrophic factors, and essential proteins
and genes involved in neuronal plasticity, as well as alterations
in neurotransmitters and receptors (11,12,32).
Previous reports have consistently demonstrated that
hippocampal neurogenesis is enhanced by EE. The newly-formed
neurons become integrated into the pre-existing hippocampal
circuitry and contribute to hippocampal function, at least to a
certain extent (12,33). However, whether EE alters the
differentiation and survival of newly-formed cells remains
controversial. Madronal et al (30) observed no differences between EE
and SE mice in the numbers of BrdU/NeuN double-positive cells in
the DG. By contrast, provision of EE to guinea pigs in the early
postnatal period increased cell proliferation and survival, with
greater numbers of cells exhibiting a neuronal phenotype compared
with controls (34). In the
present study, increased numbers of BrdU/NeuN double-positive cells
were observed in the DG of the EE, compared with the SE, group;
however, no differences were identified in the numbers of BrdU/GFAP
double-positive cells between the two groups. Taken together, these
results indicate that EE promoted the differentiation of
newly-formed cells into neurons rather than astrocytes and enhanced
the survival of these novel neurons in the DG.
Previous studies have reported that odor enrichment
i.e. an environment in which there are changing sources of odor
each day, effectively improved olfactory discrimination learning
and enhanced olfactory bulb neurogenesis (35–37).
The primary consequences of olfactory stimulation on neurogenesis
were the enhanced proliferation and survival of newly-formed cells
(35). In support of this, it has
been demonstrated, by comparing anosmic and wild-type mice, that a
specific sensory input is critical for the survival of newly-formed
lateral ventricle wall/olfactory bulb neurons (38). The marked differences between the
SVZ and DG regarding the spatial distribution of the components of
their neurogenesis systems may explain why neurogenesis in the SVZ
was unaffected by EE in the present study.
There is convincing evidence that PKA/CREB are
important in numerous features of neuronal plasticity, including
cell proliferation, differentiation, survival and learning
(39,40). Certain genes containing
cAMP-response element sequences in their promoter regions,
particularly BDNF, have been associated with promoting neurogenesis
and long-term memory (14).
Therefore, the roles of BDNF, pCREB and PKA as mediators of the
effects of EE on neurogenesis were evaluated in the present study.
It was observed that the activation of BDNF and pCREB protein may
link neurogenesis and cognition in EE rats. However, the effect of
EE on PKA-linked parameters remains to be elucidated. It has been
reported that EE modified the PKA-dependence of hippocampal
long-term potentiation and improved hippocampus-dependent memory
(41). By contrast, the provision
of EE for 30 days in the early postnatal period did not alter
immunoreactivity to PKA in the hippocampus (42). In the present study, no significant
alterations in PKA C-α protein expression levels were observed,
suggesting that the pCREB protein expression level increase
following EE may be due to signaling pathways that do not involve
PKA. Furthermore, it was demonstrated that provision of EE for 30
days enhanced the protein expression levels of SDF-1 and CXCR4 in
the hippocampus; previously, these factors have been implicated in
various processes of neurogenesis, including proliferation,
differentiation and survival, via stimulation of a cAMP-mediated
signaling pathway (43,44). In addition, EE may increase the
production of nerve growth factor (45) and induce the expression of the
neural cell adhesion molecule (46), which are associated with
neurogenesis in the DG. EE has been demonstrated to buffer
stress-induced damage in the hippocampus by enhancing the
expression of the gene encoding glucocorticoid receptors (47). Thus, it is likely that pCREB, BDNF
and SDF-1/CXCR4 are involved in mediating the neuroprotective
properties and cognitive effects associated with EE.
In conclusion, the results of the present study
demonstrate that EE treatment effectively improved cognitive
function and enhanced neurogenesis in the DG of adult rats, which
may explain certain effects of EE. The increased protein expression
levels of pCREB, BDNF and SDF-1/CXCR4 in the hippocampus may be
associated with these beneficial effects. These results may improve
understanding of the effects of environment on the brain and
provide a theoretical basis for promoting the use of EE in animal
facilities.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81372104), the
Natural Science Foundation of Liaoning Province (grant no.
201202276) and the Program for Liaoning Excellent Talents in
University (grant no. LR2013039).
References
|
1
|
Williamson LL, Chao A and Bilbo SD:
Environmental enrichment alters glial antigen expression and
neuroimmune function in the adult rat hippocampus. Brain Behav
Immun. 26:500–510. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scharfman H, Goodman J, Macleod A, Phani
S, Antonelli C and Croll S: Increased neurogenesis and the ectopic
granule cells after intrahippocampal BDNF infusion in adult rats.
Exp Neurol. 192:348–356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Serafini G, Hayley S, Pompili M, Dwivedi
Y, Brahmachari G, Girardi P and Amore M: Hippocampal neurogenesis,
neurotrophic factors and depression: Possible therapeutic targets?
CNS Neurol Disord Drug Targets. 13:1708–1721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Son Y, Yang M, Kang S, Lee S, Kim J, Kim
J, Park S, Kim JS, Jo SK, Jung U, et al: Cranial irradiation
regulates CREB-BDNF signaling and variant BDNF transcript levels in
the mouse hippo-campus. Neurobiol Learn Mem. 121:12–19. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gage FH, Kempermann G, Palmer TD, Peterson
DA and Ray J: Multipotent progenitor cells in the adult dentate
gyrus. J Neurobiol. 36:249–266. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van Praag H, Kempermann G and Gage FH:
Neural consequences of environmental enrichment. Nat Rev Neurosci.
1:191–198. 2000. View
Article : Google Scholar
|
|
7
|
Murphy M, Greferath U, Nag N,
Nithianantharajah J and Wilson YM: Tracing functional circuits
using c-Fos regulated expression of marker genes targeted to
neuronal projections. Front Biosci. 9:40–47. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nithianantharajah J and Hannan AJ:
Enriched environments, experience-dependent plasticity and
disorders of the nervous system. Nat Rev Neurosci. 7:697–709. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nithianantharajah J, Levis H and Murphy M:
Environmental enrichment results in cortical and subcortical
changes in levels of synaptophysin and PSD-95 proteins. Neurobiol
Learn Mem. 81:200–210. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pang TY, Stam NC, Nithianantharajah J,
Howard ML and Hannan AJ: Differential effects of voluntary physical
exercise on behavioral and brain-derived neurotrophic factor
expression deficits in Huntington's disease transgenic mice.
Neuroscience. 141:569–584. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leger M, Paizanis E, Dzahini K,
Quiedeville A, Bouet V, Cassel JC, Freret T, Schumann-Bard P and
Boulouard M: Environmental enrichment duration differentially
affects behavior and neuroplasticity in adult mice. Cereb Cortex.
25:4048–4061. 2015. View Article : Google Scholar
|
|
12
|
Monteiro BM, Moreira FA, Massensini AR,
Moraes MF and Pereira GS: Enriched environment increases
neurogenesis and improves social memory persistence in socially
isolated adult mice. Hippocampus. 24:239–248. 2014. View Article : Google Scholar
|
|
13
|
Fan D, Li J, Zheng B, Hua L and Zuo Z:
Enriched environment attenuates surgery-induced impairment of
learning, memory and neurogenesis possibly by preserving BDNF
expression. Mol Neurobiol. 53:344–354. 2016. View Article : Google Scholar
|
|
14
|
Yang JL, Lin YT, Chuang PC, Bohr VA and
Mattson MP: BDNF and exercise enhance neuronal DNA repair by
stimulating CREB-mediated production of apurinic/apyrimidinic
endonuclease 1. Neuromolecular Med. 16:161–174. 2014. View Article : Google Scholar :
|
|
15
|
Kuzumaki N, Ikegami D, Tamura R, Hareyama
N, Imai S, Narita M, Torigoe K, Niikura K, Takeshima H, Ando T, et
al: Hippocampal epigenetic modification at the brain-derived
neurotrophic factor gene induced by an enriched environment.
Hippocampus. 21:127–132. 2011. View Article : Google Scholar
|
|
16
|
Williams BM, Luo Y, Ward C, Redd K, Gibson
R, Kuczaj SA and McCoy JG: Environmental enrichment: Effects on
spatial memory and hippocampal CREB immunoreactivity. Physiol
Behav. 73:649–658. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong L, Yan CH, Lu CQ, Xu J, Huang H and
Shen XM: Calmodulin activation is required for the enhancement of
hippocampal neurogenesis following environmental enrichment. Neurol
Res. 31:707–713. 2009. View Article : Google Scholar
|
|
18
|
Marquez-Curtis LA and Janowska-Wieczorek
A: Enhancing the migration ability of mesenchymal stromal cells by
targeting the SDF-1/CXCR4 axis. Biomed Res Int. 2013:5610982013.
View Article : Google Scholar
|
|
19
|
Cheng M and Qin G: Progenitor cell
mobilization and recruitment: SDF-1, CXCR4, α4-integrin and c-kit.
Prog Mol Biol Transl Sci. 111:243–264. 2012. View Article : Google Scholar
|
|
20
|
Cui L, Qu H, Xiao T, Zhao M, Jolkkonen J
and Zhao C: Stromal cell-derived factor-1 and its receptor CXCR4 in
adult neurogenesis after cerebral ischemia. Restor Neurol Neurosci.
31:239–251. 2013.PubMed/NCBI
|
|
21
|
Dwinell MB, Ogawa H, Barrett KE and
Kagnoff MF: SDF-1/CXCL12 regulates cAMP production and ion
transport in intestinal epithelial cells via CXCR4. Am J Physiol
Gastrointest Liver Physiol. 286:G844–G850. 2004. View Article : Google Scholar
|
|
22
|
Odemis V, Moepps B, Gierschik P and Engele
J: Interleukin-6 and cAMP induce stromal cell-derived factor-1
chemotaxis in astroglia by up-regulating CXCR4 cell surface
expression. Implications for brain inflammation. J Biol Chem.
277:39801–39808. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang X, Liu T, Zhou Z, Mu X, Song C, Xiao
T, Zhao M and Zhao C: Enriched environment altered aberrant
hippocampal neurogenesis and improved long-term consequences after
temporal lobe epilepsy in adult rats. J Mol Neurosci. 56:409–421.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qu HL, Zhao M, Zhao SS, Xiao T, Song CG,
Cao YP, Jolkkonen J and Zhao CS: Forced limb-use enhanced
neurogenesis and behavioral recovery after stroke in the aged rats.
Neuroscience. 286:316–324. 2015. View Article : Google Scholar
|
|
25
|
Dhanushkodi A and Shetty AK: Is exposure
to enriched environment beneficial for functional post-lesional
recovery in temporal lobe epilepsy? Neurosci Biobehav Rev.
32:657–674. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fares RP, Belmeguenai A, Sanchez PE,
Kouchi HY, Bodennec J, Morales A, Georges B, Bonnet C, Bouvard S,
Sloviter RS and Bezin L: Standardized environmental enrichment
supports enhanced brain plasticity in healthy rats and prevents
cognitive impairment in epileptic rats. PloS One. 8:e538882013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie H, Wu Y, Jia J, Liu G, Zhang Q, Yu K,
Guo Z, Shen L and Hu R: Enrichment-induced exercise to quantify the
effect of different housing conditions: A tool to standardize
enriched environment protocols. Behav Brain Res. 249:81–89. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Walker MD and Mason G: Female C57BL/6 mice
show consistent individual differences in spontaneous interaction
with environmental enrichment that are predicted by neophobia.
Behav Brain Res. 224:207–212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ambrogini P, Lattanzi D, Ciuffoli S, Betti
M, Fanelli M and Cuppini R: Physical exercise and environment
exploration affect synaptogenesis in adult-generated neurons in the
rat dentate gyrus: Possible role of BDNF. Brain Res. 1534:1–12.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Madroñal N, López-Aracil C, Rangel A, del
Río JA, Delgado-García JM and Gruart A: Effects of enriched
physical and social environments on motor performance, associative
learning and hippocampal neurogenesis in mice. PloS One.
5:e111302010. View Article : Google Scholar
|
|
31
|
Silva CF, Duarte FS, Lima TC and de
Oliveira CL: Effects of social isolation and enriched environment
on behavior of adult Swiss mice do not require hippocampal
neurogenesis. Behav Brain Res. 225:85–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alvarez PS, Simão F, Hemb M, Xavier LL and
Nunes ML: Effects of undernourishment, recurrent seizures and
enriched environment during early life in hippocampal morphology.
Int J Dev Neurosci. 33:81–87. 2014. View Article : Google Scholar
|
|
33
|
Shors TJ, Townsend DA, Zhao M,
Kozorovitskiy Y and Gould E: Neurogenesis may relate to some but
not all types of hippocampal-dependent learning. Hippocampus.
12:578–584. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rizzi S, Bianchi P, Guidi S, Ciani E and
Bartesaghi R: Impact of environmental enrichment on neurogenesis in
the dentate gyrus during the early postnatal period. Brain Res.
1415:23–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rochefort C, Gheusi G, Vincent JD and
Lledo PM: Enriched odor exposure increases the number of newborn
neurons in the adult olfactory bulb and improves odor memory. J
Neurosci. 22:2679–2689. 2002.PubMed/NCBI
|
|
36
|
Bonzano S, Bovetti S, Fasolo A, Peretto P
and De Marchis S: Odour enrichment increases adult-born
dopaminergic neurons in the mouse olfactory bulb. Eur J Neurosci.
40:3450–3457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martončiková M, Lievajová K, Orendáčová J,
Blaško J and Račeková E: Odor enrichment influences neurogenesis in
the rostral migratory stream of young rats. Acta Histochem.
113:326–332. 2011. View Article : Google Scholar
|
|
38
|
Petreanu L and Alvarez-Buylla A:
Maturation and death of adult-born olfactory bulb granule neurons:
Role of olfaction. J Neurosci. 22:6106–6113. 2002.PubMed/NCBI
|
|
39
|
Carlezon WA Jr, Duman RS and Nestler EJ:
The many faces of CREB. Trends Neurosci. 28:436–445. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li QQ, Shi GX, Yang JW, Li ZX, Zhang ZH,
He T, Wang J, Liu LY and Liu CZ: Hippocampal cAMP/PKA/CREB is
required for neuroprotective effect of acupuncture. Physiol Behav.
139:482–490. 2015. View Article : Google Scholar
|
|
41
|
Duffy SN, Craddock KJ, Abel T and Nguyen
PV: Environmental enrichment modifies the PKA-dependence of
hippocampal LTP and improves hippocampus-dependent memory. Learn
Mem. 8:26–34. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xie T, Wang WP, Jia LJ, Mao ZF, Qu ZZ,
Luan SQ and Kan MC: Environmental enrichment restores cognitive
deficits induced by prenatal maternal seizure. Brain Res.
1470:80–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chalasani SH, Baribaud F, Coughlan CM,
Sunshine MJ, Lee VM, Doms RW, Littman DR and Raper JA: The
chemokine stromal cell-derived factor-1 promotes the survival of
embryonic retinal ganglion cells. J Neurosci. 23:4601–4612.
2003.PubMed/NCBI
|
|
44
|
Opatz J, Küry P, Schiwy N, Järve A,
Estrada V, Brazda N, Bosse F and Müller HW: SDF-1 stimulates
neurite growth on inhibitory CNS myelin. Mol Cell Neurosci.
40:293–300. 2009. View Article : Google Scholar
|
|
45
|
Birch AM, McGarry NB and Kelly AM:
Short-term environmental enrichment, in the absence of exercise,
improves memory, and increases NGF concentration, early neuronal
survival, and synaptogenesis in the dentate gyrus in a
time-dependent manner. Hippocampus. 23:437–450. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Young D, Lawlor PA, Leone P, Dragunow M
and During MJ: Environmental enrichment inhibits spontaneous
apoptosis, prevents seizures and is neuroprotective. Nat Med.
5:448–453. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang L, Zhang J, Sun H, Liu H, Yang Y and
Yao Z: Exposure to enriched environment restores the mRNA
expression of mineralocorticoid and glucocorticoid receptors in the
hippocampus and ameliorates depressive-like symptoms in chronically
stressed rats. Curr Neurovasc Res. 8:286–293. 2011. View Article : Google Scholar : PubMed/NCBI
|