Introduction
Chondrosarcoma is the second most common bone
cancer, after osteosarcoma (1).
Characteristically, chondrosarcomas contain chondroid cells and a
matrix. Surgical excision is the mainstay of treatment, with
radiotherapy as an alternative if surgery is contraindicated or if
a metastasis requires treatment. Although radiotherapy eliminates
tumor cells, normal cells are also harmed. As the side-effects of
radiotherapy are therefore serious, more effective
anti-chondrosarcoma drugs with minimal toxicity are urgently
required. Resveratrol (Res), a dietary phytochemical found in
almost 70 plant species, has attracted widespread attention due to
the anti-tumor activities it exerts on several types of cancer
cell, including hepatocellular carcinoma and gastric cancer cells.
However, its effect on chondrosarcoma cells remains unknown.
Sirtuin 1 (Sirt1), a histone deacetylase, is widely expressed in
various tumor types, including gastric cancer, osteosarcoma and
colon cancer. It is well known that Res is an effective agonist of
Sirt1 (2).
Signal transduction and activator of transcription 3
(STAT3), a protein constitutively expressed in numerous tissues and
cell types, regulates the proliferation, differentiation and
apoptosis of normal cells, and maintains normal physiological
processes. STAT3 is upregulated in several tumor cells, and it has
become increasingly accepted that such upregulation is closely
associated with tumorigenesis. Furthermore, previous studies have
shown that Res inhibits phosphorylation within the STAT3 signaling
pathway in numerous types of tumor cells. However, it remains
unknown whether Res exerts similar actions in chondrosarcoma cells
and, if so, whether Res activates Sirt1 in such cells. The present
study assessed the inhibitory effects of Res on chondrosarcoma
cells and the underlying mechanisms. In the present study, it was
shown that Res induced apoptosis, inhibited cell proliferation and
suppressed phosphorylation within the STAT3 signaling pathway by
activating Sirt1 in chondrosarcoma cells.
Materials and methods
Cell culture and reagents
Chondrosarcoma SW1353 cells (Chinese Academy of Life
Sciences; Shanghai, China) were cultured in Dulbecco's modified
Eagle's medium (DMEM)/F12 medium, supplemented with 10% (v/v) fetal
bovine serum (FBS), penicillin and streptomycin, at 37°C in a
humidified atmosphere containing 5% (v/v) CO2. Res and
the Hoechst 33258 reagent were purchased from Sigma-Aldrich (St.
Louis, MO, USA). Rabbit antibodies against B-cell lymphoma (BCL)-2
(cat. no. 4223), BCL-2 associated X protein (Bax; cat. no. 5023),
caspase 3 (cat. no. 9662), STAT3 (cat. no. 4904), and
phosphorylated (p-)STAT3 (cat. no. 9145) were purchased from Cell
Signaling Technology, Inc., (Danvers, MA, USA). The cell counting
kit (CCK)-8 reagent and Crystal Violet staining solution were
purchased from the Beyotime Institute of Biotechnology (Shanghai,
China). The Endofectin™-Plus transfection reagent was purchased
from Genecopoeia (Guangzhou, China). A specific Sirt1-small
interfering (si) RNA was purchased from GenePharma (Shanghai,
China).
CCK-8 assay
SW1353 cells were seeded into 96-well plates at a
density of 1×104 cells/well and divided into three
groups: Blank, Control and Res-treated (2, 5, 10, 25, 50 or 100
µmol/l) groups. After 24 h treatment, 10 µl CCK-8
solution was added to each well and the plate was incubated at 37°C
for 1 h. Cell viability was determined by measuring the absorbance
(A) at 450 nm using a microplate reader (Thermo Fisher Scientific,
Inc.). The percentage of proliferative cells were calculated as
follows: Relative viability (%) = (A450treated −
A450blank) / (A450control −
A450blank) × 100.
Colony formation assay
The cells were seeded into 12-well plates at a
density of 1,000 cells/well (1 ml/well) and divided into a control
and a Res-treated group (25 or 50 µmol/l). After 24 h
treatment, the plate was incubated in DMEM/F12 medium for 10 days.
Following incubation, the cells were fixed in 4% (v/v)
paraformaldehyde for 10 min, washed three times with
phosphate-buffered saline (PBS) and stained with crystal violet for
10 min at 25°C. The number of visible colonies were then counted
and images were captured.
Hoechst 33258 staining
The cells were seeded into 6-well plates at density
of 1×105 cells/ml (1 ml//well) and were divided into a
control and a Res-treated group (25 or 50 µmol/l). The cells
were incubated with 5% (v/v) CO2 at 37°C for 24 h,
washed three times with PBS, stained with 20 µM Hoechst
33258 solution for 20–30 min and were subsequently washed again
three times with PBS. Cell morphology was assessed under a
fluorescence microscope (Nikon Corporation, Tokyo, Japan).
Sirt1-siRNA transfection
The sequence of chemically modified siRNA was
5′-CGGGAAUCCAAAGGAUAAUTT-3′. The cells were grown overnight and
were subsequently transfected with siRNA using Endofectin™-Plus,
according to the manufacturer's protocol. Following incubation for
24 h, the cells were treated with 50 µmol/l Res for 24 h and
cell protein levels were measured by western blotting.
Western blotting
The total protein in was extracted using
radioimmunoprecipitation lysis buffer (Beyotime Insititute of
Biotechnology). The protein concentrations were determined using a
bicinchoninic acid protein assay kit (Sigma-Aldrich, Shanghai,
China). A total of 2 µg/µl protein was resolved by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were
electroblotted onto nitrocellulose membranes (Beyotime Insititute
of Biotechnology). The membranes were subsequently blocked for 2 h
in Tris-buffered saline with 0.5% Tween-20 (TBST), containing 5%
(w/v) non-fat milk. Following blocking, the membranes were
incubated with monoclonal antibodies directed against caspase-3,
BCL-2, Bax, STAT3 and p-STAT3 (all 1:1,000) overnight at 4°C.
Following three washes in TBST, the proteins were detected by
incubation with horseradish peroxidase-conjugated secondary goat
anti-rabbit immunoglobulin G (cat. no. BS13271; 1:5,000) for 2 h.
The bands were visualized using enhanced chemiluminescence. A
rabbit β-actin antibody (cat. no. AP0060; 1:3,000; Bioworld
Technology, Inc., Nanjing, China) served as a loading control and
band densities were quantified using Image Lab version 3.0
software.
Statistical analysis
The data are expressed as the mean ± standard
deviation. All statistical analyses were performed using SPSS
version 19.0 (IBM SPSS, Chicago, IL, USA). A one-way analysis of
variance and Tukey's post-hoc test were performed. P<0.05 was
considered to indicate a statistically significant difference.
Results
Res suppresses the proliferation of
SW1353 cells in a dose-dependent manner
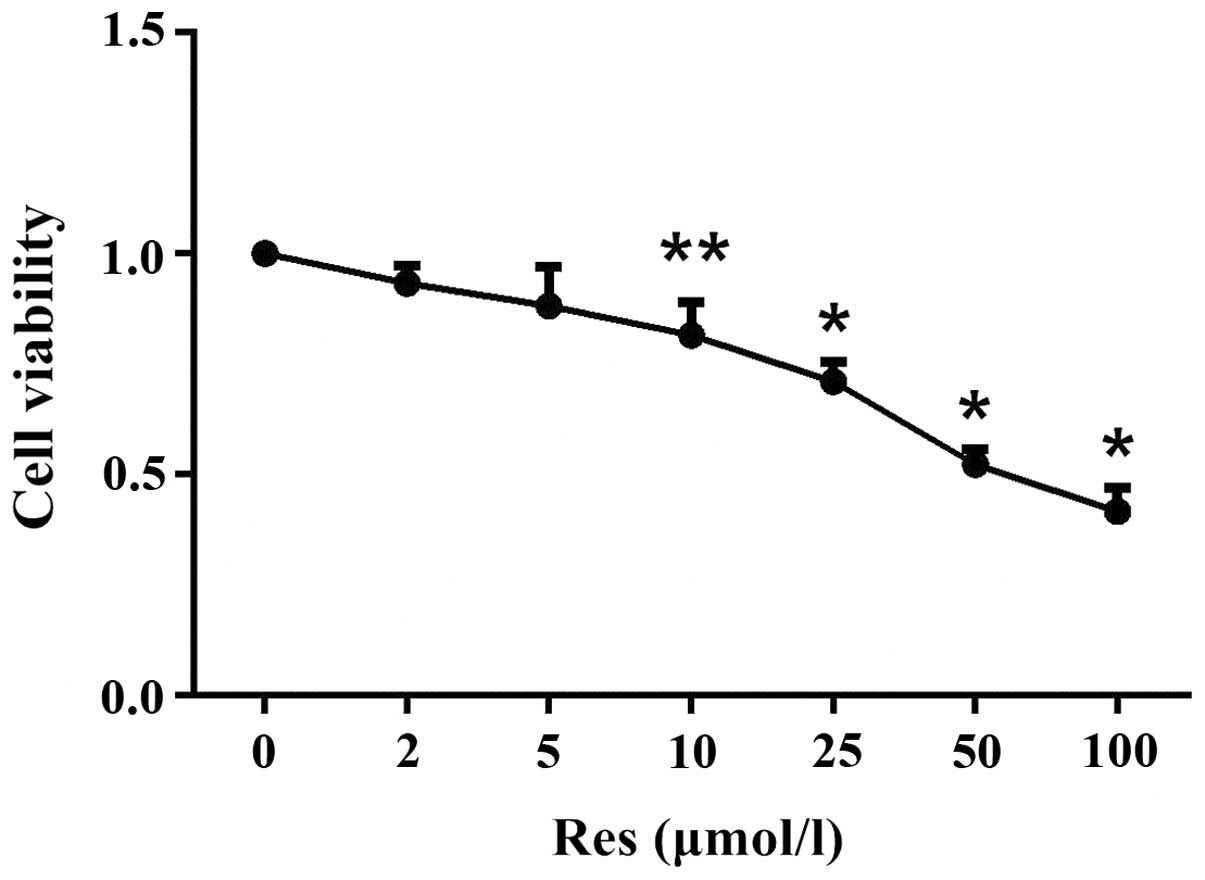
Cell viability can affect proliferation. Fig. 1 revealed that treatment with Res
(10, 25, 50, or 100 µmol/l for 24 h) affected the viability
of SW1353 cells in a dose-dependent manner. The relative
viabilities were 0.8161±0.0754 (P=0.015), 0.7102±0.0444 (P=0.0001),
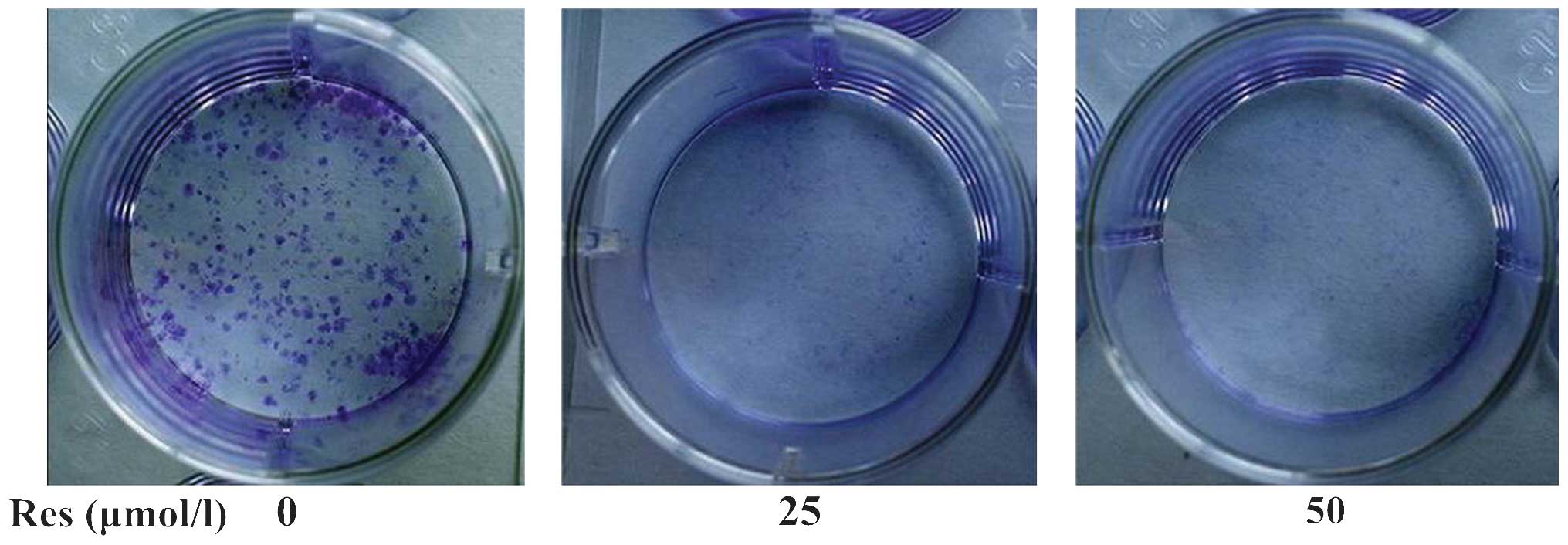
0.5226±0.0361 (P=0.0001) and 0.4166±0.0542 (P=0.0001). Furthermore,
a colony formation assay demonstrated that Res (25 or 50
µmol/l) significantly reduced cell proliferation compared
with the control (Fig. 2).
Res induces the apoptosis of SW1353
cells
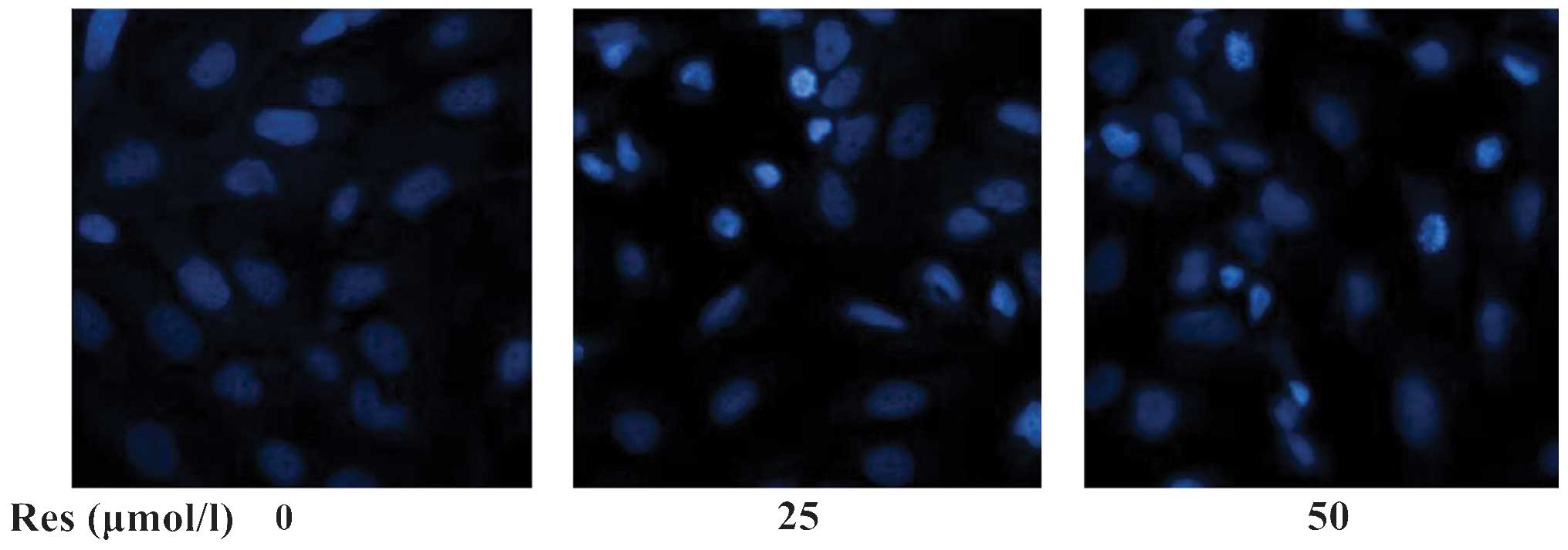
The Hoechst 33258 fluorochrome is concentrated in
the nucleus of apoptotic cells. As shown in Fig. 3, the rate of apoptosis of
Res-treated cells was significantly higher compared with that of
controls.
Res increases the expression levels of
Bax and cleaved caspase 3, and reduces the expression levels of
Bcl-2 and the Bcl-2/Bax ratio
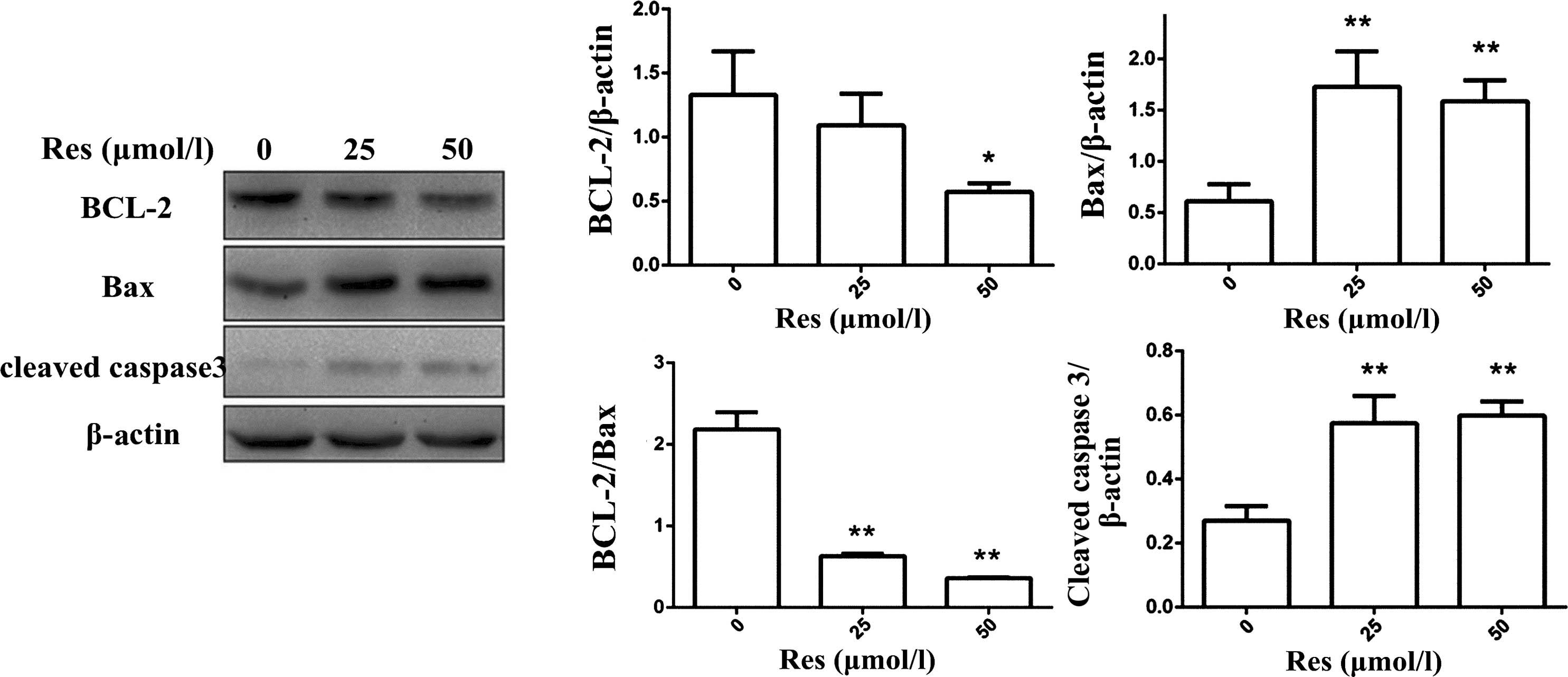
Bcl-2, Bax, and cleaved caspase-3 serve important
roles in the mitochondrial pathway of apoptosis. Res (25 or 50
µmol/l) significantly upregulated the expression levels of
Bax and cleaved caspase-3 (P<0.01; Fig. 4). In addition, Res (50
µmol/l) reduced the expression of Bcl-2 (P<0.01), but
only at 50 µmol/l. Res (25 or 50 µmol/l) reduced the
Bcl-2/Bax ratio (Fig. 4E).
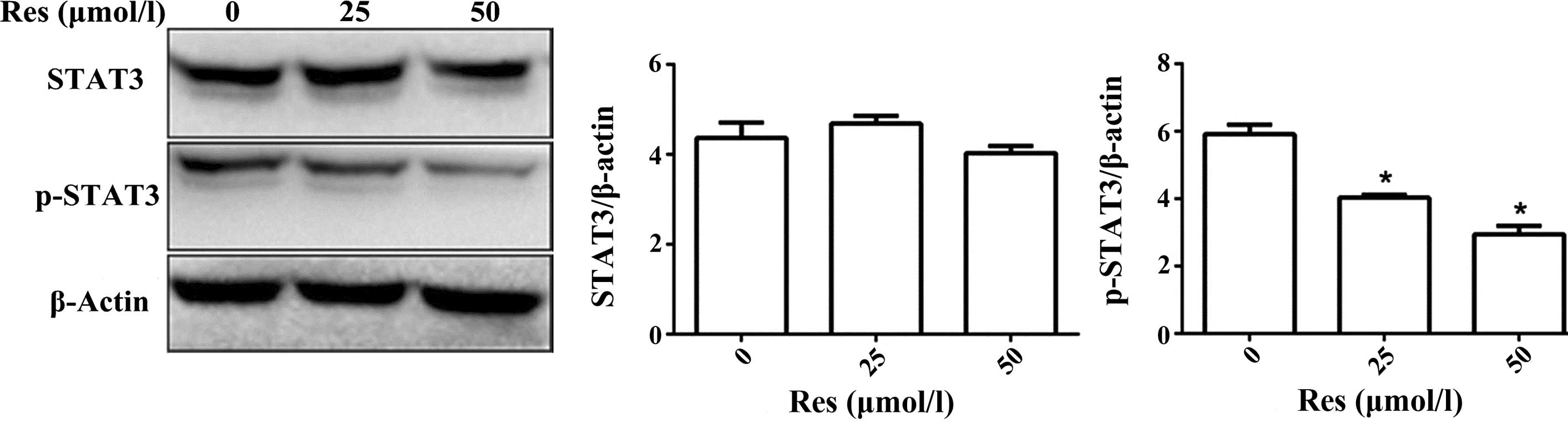
Res reduces the expression of p-STAT3 in
SW1353 cells
Western blotting was performed to measure the
expression levels of p-STAT3 and STAT3 in SW1353 cells treated with
Res (0, 25 or 50 mmol/l). p-STAT3 was downregulated in a
Res-dependent manner (P<0.01), however, the total STAT3 level
did not significantly change (Fig.
5).
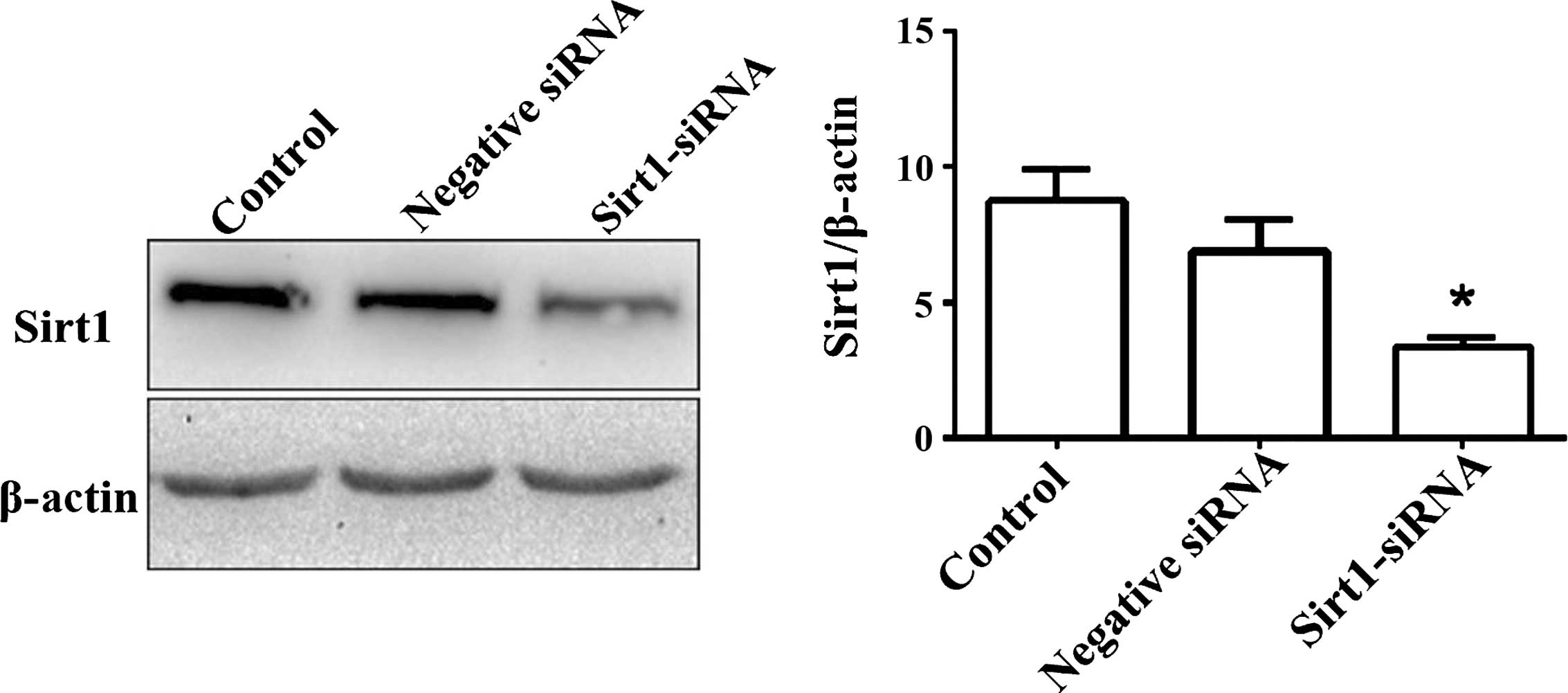
Effect of Sirt1 downregulation on the
STAT3 signaling pathway
Sirt1-siRNA significantly suppressed Sirt1 protein
expression compared with its expression in the control or
negative-siRNA-transfected group (P<0.01; Fig. 6). The expression levels of Sirt1,
p-STAT3, total STAT3 and β-actin were assessed in SW1353 cells
(control, Res-treated, siRNA and Res + siRNA). Res (50
µmol/l) activated the expression of Sirt1 (P<0.05), but
not after siRNA transfection. Treatment with Res (50 µmol/l)
suppressed the expression of p-STAT3 and caused no significant
effect on the total STAT3 levels. p-STAT3 expression was not
inhibited in the siRNA or Res+siRNA group (P<0.01), no
significant difference was identified between the total STAT3
levels in the siRNA and Res + siRNA group (P=0.14; Fig. 7).
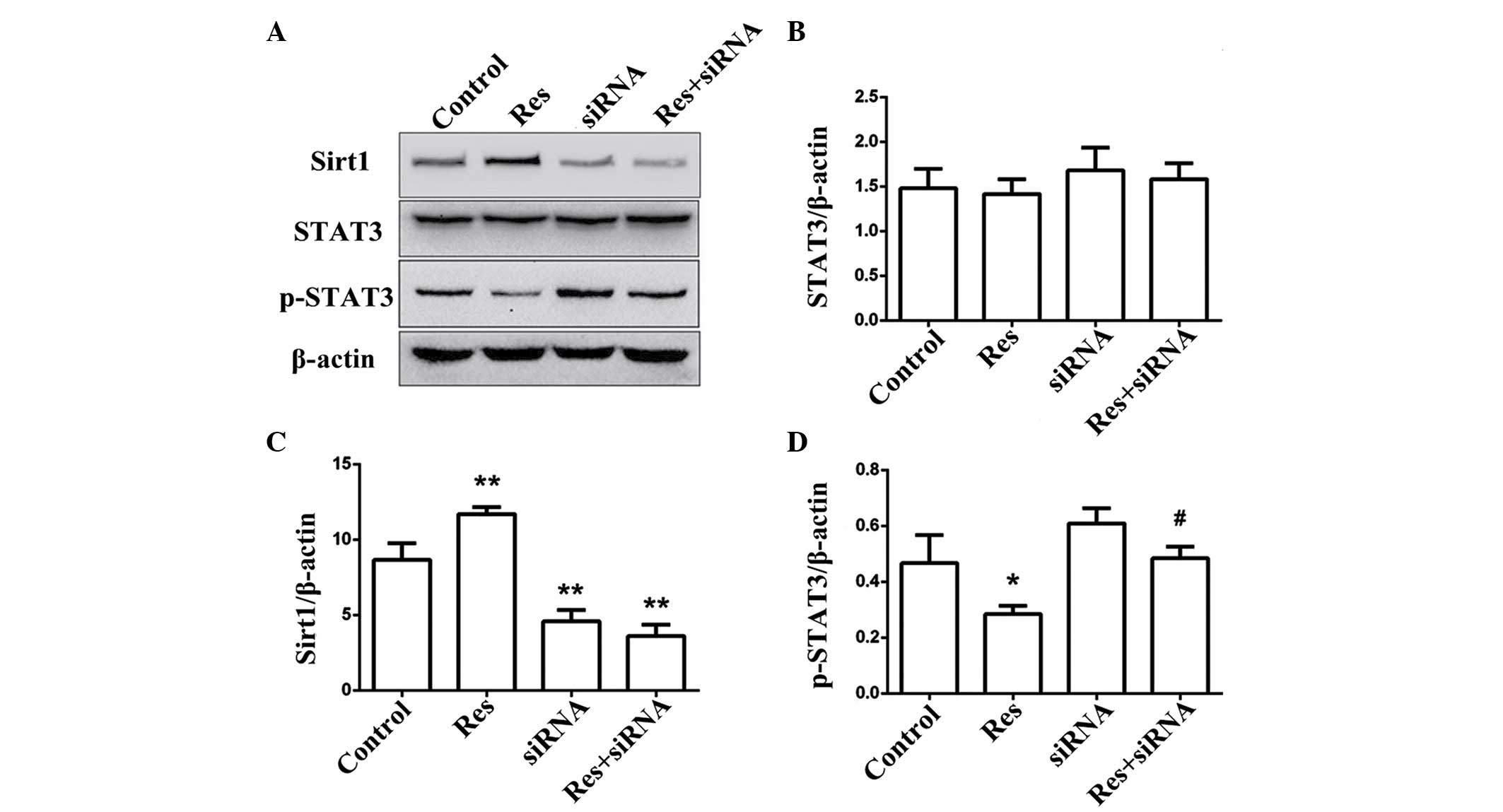 | Figure 7Res activates Sirt1 in SW1353 cells
and its suppressive action on the protein expression of p-STAT3 via
the activation of Sirt1 was weakened in Sirt1-siRNA cells,. (A) The
expression levels of Sirt1, total STAT3, and p-STAT3 in control,
Res-treated, Sirt1-siRNA-transfected and Res-treated +
Sirt1-siRNA-transfected groups were determined via Western
blotting. (B–D) The relative expression levels are shown in
histograms. The data are expressed as the mean ± standard deviation
(*P<0.05, **P<0.01 compared with the control group;
#P<0.05 compared with the Res group).. Sirt1, sirtuin
1; siRNA, small interfering RNA; p-, phosphorylated; Res,
resveratrol; STAT, signal transduction and activator of
transcription 3. |
Discussion
The effects of res, which was first discovered in
1940, on different types of tumor cells have been studied to
varying degrees. In hepatocellular carcinoma, gastric cancer and
breast cancer cells, Res inhibits cell proliferation, induces
apoptosis and inhibits the cell cycle (3–5). In
HepG2 hepatocellular carcinoma cells, Res induces apoptosis by
activating p53 and upregulating the expression levels of Bax and
p21 (6). In addition, in cutaneous
carcinoma cells, Res activates mitochondrial proteins, including
caspases-3, -8 and -9, and poly (ADP-ribose) polymerase, triggers
the release of cytochrome c, thus activating Bax, and
suppresses the expression of Bcl-2. Apoptosis follows this process
(7).
The present study found that Res reduced the
proliferation and induced the apoptosis of SW1353 cells. Res
increased the expression levels of Bax and cleaved caspase-3, and
downregulated the expression of Bcl-2. Res also significantly
reduced the Bcl-2/Bax ratio, indicating that Res can inhibit cell
proliferation and induce apoptosis via the mitochondrial
pathway.
STAT3, expressed by numerous cells and tissues, is
an important member of the STAT protein family. Continuous STAT3
activation triggers the ingravescence of tumor cells and tissues,
indicating that the STAT3 signaling pathway is intimately involved
in tumor cell proliferation and apoptosis (5). STAT3 phosphorylation upregulates the
expression of apoptosis-inhibitory proteins, including Bcl-2,
Bcl-xL, Mcl-1, and Survivin, and downregulates the expression of
Bax. STAT3 also activates the expression of cyclin D1, cell
division cycle 2, c-myc, cyclinB1, c-jun and c-fos, which may
trigger malignant proliferation (8–11).
The present study found that Res suppressed phosphorylation of
STAT3, showing that the inhibitory effects of Res on chondrosarcoma
proliferation were partly attributable to the phosphorylation of
STAT3.
Sirt1, a member of the class III nicotinamide
adenine (+)-dependent histone deacetylase Sirt family, is involved
in various physiological processes, including differentiation,
apoptosis and metabolism (12).
Res is an effective Sirt1 agonist and the covalent binding of Res
to Sirt1 alters the conformation of Sirt1, increasing the affinity
of the protein for its substrate (13). Numerous previous reports have shown
that Sirt1 gene knockdown induces the expression of STAT3 in
fibroblast cells and that Sirt1 upregulation inhibits acetylation
within the STAT3 signaling pathway of keratinocytes (14–16).
These data indicated that the STAT3 signaling pathway is regulated
by Sirt1. The present study found that Res induced Sirt1 expression
and suppressed phosphorylation within the STAT3 signaling pathway.
Additionally, STAT3 phosphorylation was significantly inhibited by
Res, however, this was negated by Sirt1-siRNA. Taken together, the
data revealed that Res suppresses phosphorylation within the STAT3
signaling pathway by activating Sirt1 in chondrosarcoma cells.
In conclusion, res, a natural anti-tumor material,
exerts diverse anti-tumor effects, including induction of
apoptosis, inhibition of cell proliferation and suppression of
phosphorylation within the STAT3 signaling pathway by activating
Sirt1 in chondrosarcoma cells. However, the mechanism by which
Sirt1 affects phosphorylation within the STAT3 pathway remains to
be elucidated.
Acknowledgments
The authors would like to thank Mr. L.Y. Cai, Mr. N.
Majid and Mr. L. Chen (Wenzhou Medical University, Wenzhou, China)
for their comments and advice. The present study was supported by
the Zhejiang Provincial Medical Science and Technology Project (no.
2014RCA017).
Abbreviations:
|
Res
|
resveratrol
|
|
STAT3
|
signal transduction and activator of
transcription 3
|
|
Sirt1
|
sirtuin 1
|
|
p-
|
phosphorylated
|
|
FBS
|
fetal bovine serum
|
|
BCL-2
|
B-cell lymphoma-2
|
|
Bax
|
BCL-2 associated X protein
|
|
CCK-8
|
Cell Counting Kit-8
|
|
PBS
|
phosphate-buffered saline
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
siRNA
|
small interfering RNA
|
|
TBST
|
Tris-buffered saline with Tween-20
|
References
|
1
|
Liang W, Li X, Li Y, Li C, Gao B, Gan H,
Li S, Shen J, Kang J, Ding S, et al: Gallic acid induces apoptosis
and inhibits cell migration by upregulating miR-518b in SW1353
human chondrosarcoma cells. Int J Oncol. 44:91–98. 2014.
|
|
2
|
Villalba JM and Alcaín FJ: Sirtuin
activators and inhibitors. Biofactors. 38:349–359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang Q, Wang B, Zang W, Wang X, Liu Z, Li
W and Jia J: Resveratrol inhibits the growth of gastric cancer by
inducing G1 phase arrest and senescence in a Sirt1-dependent
manner. PloS One. 8:e706272013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mezzanotte L, An N, Mol IM, Löwik CW and
Kaijzel EL: A new multicolor bioluminescence imaging platform to
investigate NF-κB Qactivity and apoptosis in human breast cancer
cells. PloS One. 9:e855502014. View Article : Google Scholar
|
|
5
|
Carter LG, D'Orazio JA and Pearson KJ:
Resveratrol and cancer: Focus on in vivo evidence. Endocr Relat
Cancer. 21:R209–R225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuo PL, Chiang LC and Lin CC:
Resveratrol-induced apoptosis is mediated by p53-dependent pathway
in Hep G2 cells. Life Sci. 72:23–34. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kalra N, Roy P, Prasad S and Shukla Y:
Resveratrol induces apoptosis involving mitochondrial pathways in
mouse skin tumorigenesis. Life Sci. 82:348–358. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu H, Pardoll D and Jove R: STATs in
cancer inflammation and immunity: A leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Germain D and Frank DA: Targeting the
cytoplasmic and nuclear functions of signal transducers and
activators of transcription 3 for cancer therapy. Clin Cancer Res.
13:5665–5669. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tebbutt NC, Giraud AS, Inglese M, Jenkins
B, Waring P, Clay FJ, Malki S, Alderman BM, Grail D, Hollande F, et
al: Reciprocal regulation of gastrointestinal homeostasis by SHP2
and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat
Med. 8:1089–1097. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ranger JJ, Levy DE, Shahalizadeh S,
Hallett M and Muller WJ: Identification of a Stat3-dependent
transcription regulatory network involved in metastatic
progression. Cancer Res. 69:6823–6830. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng CX: SIRT1, is it a tumor promoter or
tumor suppressor? Int J Biol Sci. 5:147–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Borra MT, Smith BC and Denu JM: Mechanism
of human SIRT1 activation by resveratrol. J Biol Chem.
280:17187–17195. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bernier M, Paul RK, Martin-Montalvo A,
Scheibye-Knudsen M, Song S, He HJ, Armour SM, Hubbard BP, Bohr VA,
Wang L, et al: Negative regulation of STAT3 protein-mediated
cellular respiration by SIRT1 protein. J Biol Chem.
286:19270–19279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sestito R, Madonna S, Scarponi C,
Cianfarani F, Failla CM, Cavani A, Girolomoni G and Albanesi C:
STAT3-dependent effects of IL-22 in human keratinocytes are
counterregulated by sirtuin 1 through a direct inhibition of STAT3
acetylation. FASEB J. 25:916–927. 2011. View Article : Google Scholar
|
|
16
|
Li Y, Zhu W, Li J, Liu M and Wei M:
Resveratrol suppresses the STAT3 signaling pathway and inhibits
proliferation of high glucose-exposed HepG2 cells partly through
SIRT1. Oncol Rep. 30:2820–2828. 2013.PubMed/NCBI
|