Introduction
Head and neck squamous cell carcinoma (HNSCC) is a
highly aggressive cancer type, with >400,000 new cases and
200,000 mortalities worldwide per year (1,2).
Current treatment of HNSCC includes surgery, chemotherapy,
radiation therapy, targeted therapy or a combination of treatments;
however, patients often suffer recurrences or distant metastases,
and the 5 year overall survival rate of HNSCC remains very poor,
particularly for the subgroup with an advanced stage at diagnosis
(3–5). The poor clinical outcome of HNSCC is
largely due to recurrence and metastasis at adjacent or distant
regions (3,6). Although recent advances in molecular
biology, cellular biology and genomics have provided insight into
the molecular pathogenesis of HNSCC, the fundamental molecular
mechanisms remain to be fully understood.
Lycopene (LP), a pivotal biological compound in
tomatoes, has received tremendous attention as potential candidate
for cancer therapy. Numerous epidemiological studies have indicated
an inverse association between dietary supplementation of LP and
the risk of human cancer (7,8). LP
has been reported to possess a broad spectrum of tumor suppressive
activities in multiple human carcinomas, including leukemia,
prostate, breast, colon and lung cancer (9–13).
The mechanisms by which LP exerts its anticancer effects are
comprehensive and diverse, targeting multiple cell signaling
pathways in the processes of cellular growth, invasion, metastasis
and angiogenesis. In addition, clinical intervention studies have
shown that LP supplementation in men with prostate cancer decreased
serum prostate-specific antigen concentrations and inhibited tumor
growth. However, little attention has been given to the role of LP
in the functions of HNSCC in the literature to date.
The present study aimed to determine the anticancer
effect of LP on HNSCC cells in vitro. Furthermore, the
present study investigated the molecular mechanisms of LP on HNSCC
cells were investigated.
Materials and methods
Cell culture and treatment
The human HNSCC cell lines, FaDu and Cal27, were
obtained from the Shanghai Type Culture Collection (Shanghai,
China). The cells were grown in Eagle's Minimum Essential Medium
(EMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
supplemented with 10% (v/v) fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin sodium and 100
µg/ml streptomycin sulfate (HyClone, Logan, UT, USA). The
cells were maintained in a humidified incubator under standard
conditions (5% CO2 and 95% air at 37°C).
LP (Sigma-Aldrich, St. Louis, MO, USA) was dissolved
in tetrahydrofurancontaining (THF) to produce a 4 mM stock solution
with minimal exposure to air and light. The cells were treated with
the indicated concentrations of LP or matching volumes of THF for
the controls.
Cell proliferation and viability
assays
Cell proliferation was determined using a Cell
Counting kit-8 (Dojindo Laboratories, Tokyo, Japan), according to
the manufacturer's protocol and incubation at 37°C for 2 h. The
cells were seeded into flat-bottomed 96-well plates at a density of
2,500 cells/well and were treated with various concentrations of LP
(5, 10, 25 and 50 µM) or THF (vehicle) for 48 h. The optical
density was measured at 450 nm using the Infinite 200 Pro NanoQuant
(Tecan Group, Ltd., Maennedorf, Switzerland). The data are shown as
the relative values in which the luminescence at a given drug
concentration was compared with that of the control THF cells. All
cell survival assays were performed at least three times with
triplicate samples in 96-well plates.
Colony formation assays
Colony formation assays were performed by seeding
5,000 cells/well into 6-well plates. The cells were treated with 25
µM LP or THF for 48 h. The treatment was removed and fresh
medium was added. The cells were incubated for 14 days and colonies
were subsequently stained with 0.5% crystal violet for 15 min at
room temperature. Colonies comprising >50 cells were defined as
one colony and the number of colonies in 10 randomly selected
fields were quantified under a microscope (DVM6; Leica
Microsystems, Inc., Buffalo Grove, IL, USA). The experiment was
performed in triplicate.
Cell transwell invasion assays
For the Matrigel invasion assay, cells were
harvested and resuspended in EMEM, containing 0.1% (w/v) bovine
serum albumin (Sangon Biotech Co., Ltd., Shanghai, China). These
cells (6×105 cells) were added to the inserts containing
8 µm pores (Corning Co, Ltd., Corning, NY, USA) coated with
24 mg/ml Matrigel (BD Biosciences, Oxford, UK). The bottom chambers
contained EMEM with 10% fetal bovine serum. LP was added into the
top chambers at a final concentration of 25 µM. Following
incubation for 36 h, the cells on the upper surface of the membrane
were removed using a cotton swab. The cells that had invaded
through the membrane were fixed with 4% paraformaldehyde for 30 min
at 25°C and were subsequently stained with 0.5% crystal violet for
15 min at 25°C. Following staining, the cells were counted in six
randomly distinct fields under a light microscope (DVM6; Leica
Microsystems, Inc.) at a magnification of ×100. Experiments for
each cell line were performed in triplicate.
Flow cytometry
The apoptosis rate of cells was measured using an
Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit
(BD Biosciences), according to the manufacturer's protocol.
Briefly, the cells were treated with 25 µM LP or THF for 48
h, washed with cold phosphate-buffered saline and were subsequently
stained with Annexin V-FITC and propidium iodide at room
temperature for 15 min. Apoptotic cells were analyzed immediately
using a flow cytometer (FACS Calibur 95; BD Biosciences) and the
data were analyzed using CellQuest 3.0 software (BD
Biosciences).
Immunoblotting analysis
Immunoblotting analyses were performed with standard
methods. Briefly, the total protein was extracted from cells using
radioimmunoprecipitation lysis buffer (Sigma-Aldrich), containing a
proteinase inhibitor cocktail (Roche Diagnostics, Basel,
Switzerland). Equal quantities of protein were separated by 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis and the
proteins were transferred onto polyvinylidene difluoride membrane
(EMD Millipore, Billerica, MA, USA). Following blocking with 5%
non-fat milk in Tris-buffered saline containing 0.1% Tween-20 at
room temperature, the blots were incubated with the following
antibodies overnight at 4°C: Rabbit anti-protein kinase B antibody
(AKT; 1:1,000; cat. no. 9272S), rabbit anti-phosphorylated (p-)AKT
antibody (Ser473; 1:1,000; cat. no. 4060S), rabbit
anti-extracellular signal-regulated kinase antibody (ERK)1/2
(1:1,000; cat. no. 4695S), p-ERK1/2 antibody (T202/Y204; 1:1,000;
cat. no. 4370S), rabbit anti-cyclin D1 antibody (1:1,000; cat. no.
2978S), rabbit anti-caspase-3 antibody (1:1,000; cat. no. 9665) and
rabbit anti-cleaved caspase-9 antibody (1:1,000; cat. no. 9501),
all from Cell Signaling Technology, Inc. (Danvers, MA, USA), and
rabbit anti-B-cell lymphoma (Bcl)-2 antibody (1:1,000; cat. no.
10783-1-AP) and rabbit anti Bcl-2-associated X protein antibody
(Bax; 1:1,000; cat. no. 50599-2-Ig), both from Proteintech Group,
Inc, Chicago, IL, USA). The protein bands were visualized using an
enhanced chemiluminescence detection kit (EMD Millipore) following
incubation with goat anti-rabbit horseradish peroxidase-conjugated
secondary antibody (1:5,000; Cwbiotech, Beijing, China; cat. no.
CW0103S). The intensity of the bands was analyzed using Image-Pro
Plus software version 6.0 (Media Cybernetics, Inc., Rockville, MD,
USA).
Statistical analysis
All data is presented as the mean ± standard
deviation from at least three independent experiments. Comparisons
between two groups were performed using Student's t-test.
Statistical analyses were performed with SPSS 15.0 statistical
software package (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
LP suppresses HNSCC proliferation and
colony formation
To determine the effect of LP on HNSCC cell growth,
various concentrations of LP were used to treat FaDu and Cal27
cells for 48 h. As shown in Fig.
1A, LP inhibited the viability of FaDu and Cal27 cells, with
significant effects detected at a concentration of 25 µM LP.
From this, it was decided that 25 µM LP would be used for
further experiments. To further characterize the time-dependent
toxicities of LP, FaDu and Cal27 cells were incubated with 25
µM LP. In response to THF, cell proliferation analysis
demonstrated that treatment with LP caused a significant cell
inhibitory effect onwards for 24, 48 and 72 h (Fig. 1B). In addition, a colony forming
assay was used to determine whether LP suppressed the proliferation
ability of FaDu and Cal27 cells. This assay revealed that colony
formation was significantly inhibited by treatment with LP. These
results revealed that exposure to LP causes a significant
inhibition of HNSCC cell proliferation.
LP induces caspase activation in HNSCC
cells
Previous studies have shown that anticancer drugs
can exert antiproliferative effects via the induction of apoptosis
of tumor cells (14,15). To examine the mechanisms by which
LP inhibited the proliferation of HNSCC cells, the present study
examined the effect of LP on the levels of apoptosis. LP
significantly induced apoptosis in both FaDu and Cal27 cells
(Fig. 2A). Since LP induced
apoptosis, it was next determined whether the apoptosis signaling
pathway was also affected by LP. As observed by western blotting,
both in FaDu and Cal27 cells, LP significantly downregulated the
expression of Bcl-2 (Fig. 2B), an
antiapoptotic regulator. By contrast, the protein expression of
Bax, caspase-3 and cleaved-caspase-9, the crucial apoptotic
executioner, was increased with following treatment with LP in both
FaDu and Cal27 cells (Fig. 2B).
The Bax:Bcl-2 ratio highlighted the susceptibility of cells to cell
death, and it was upregulated upon treatment with LP. These results
suggested that LP can induce apoptosis by inhibiting the levels of
Bcl-2 and by simultaneously increasing the expression levels of
Bax, caspase-3 and cleaved-caspase-9.
 | Figure 2Lycopene induces cell apoptosis and
activates the apoptosis signaling pathway in head and neck squamous
cell carcinoma cells. (A) Flow cytometric analysis was performed to
detect apoptotic cells. Quadrant 1 indicates dead cells, quadrant 2
indicates early apoptotic cells, quadrant 3 indicates normal cell,
and quadrant 4 indicates late apoptotic cells. The data are
presented as the mean±standard deviation (n=3;
**P<0.01 compared with the THF treated cells). (B)
Western blot analysis of the protein expression levels of Bcl-2,
Bax, caspase-3 and cleaved caspase-9 in the cell lines, in response
to THF or 25 µM lycopene. Bcl, B-cell lymphoma; Bax,
Bcl-2-associated X protein; THF, tretrahydrofurancontaining; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase. |
LP suppresses the invasion of human HNSCC
cells
Tumor cell migration and invasion are important in
cancer progression and metastasis (16). The present study aimed to examine
whether LP had a direct functional role in facilitating tumor cell
invasion in HNSCC. As shown in Fig.
3, treatment with LP led to a significant decrease in cell
invasion, in both FaDu and Cal27 cells, compared with THF control
cells.
Cell growth and survival signaling
networks are affected by LP
To elucidate the underlying mechanism of the
sensitivity of HNSCC cells to LP, certain key signaling pathways
were detected. Although numerous signaling pathways have been
involved in tumorigenesis, the PI3K/AKT and MAPK signaling pathways
are the most commonly assessed. Aberrant activation of PI3K/AKT and
MAPK signaling pathway has been implicated in tumorigenesis and the
development of a variety of tumor types, including breast, lung,
ovarian, pancreatic, prostatic and gastrointestinal cancers, and
HNSCC (17–21). The present study next investigated
whether treatment with LP decreased the phosphorylation of AKT and
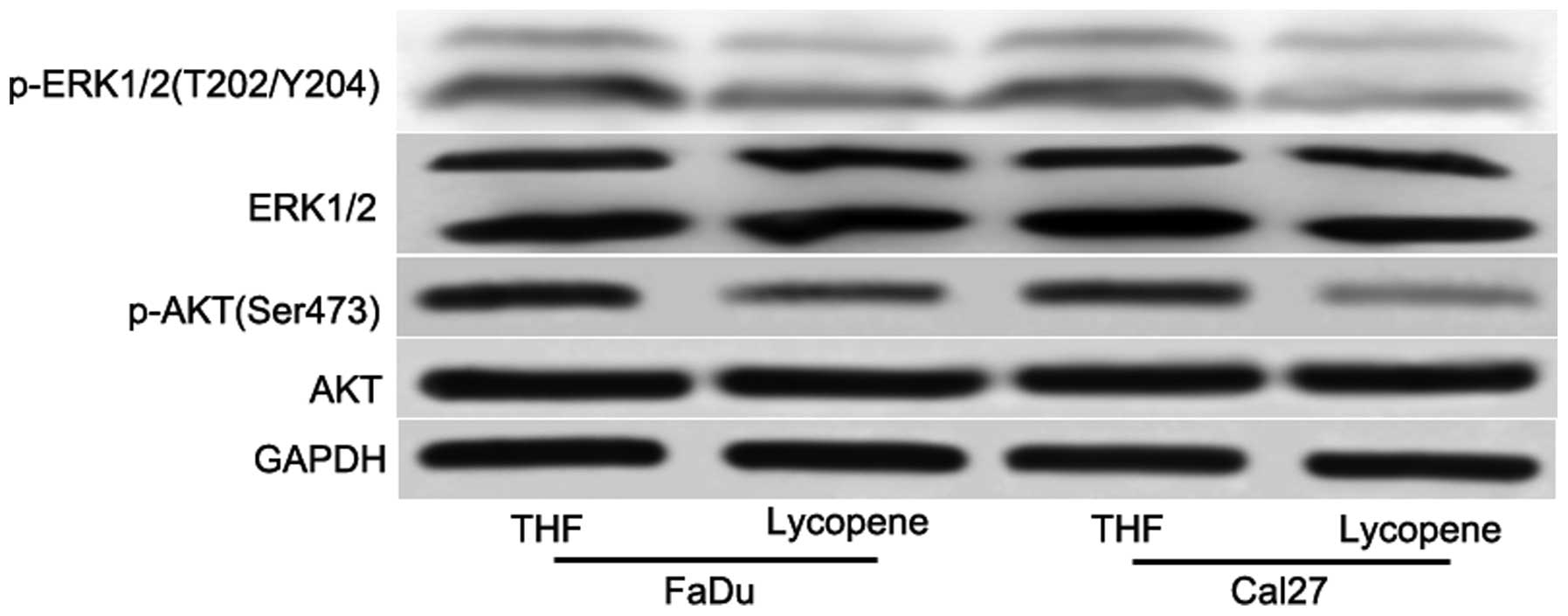
ERK. After 48 h treatment with 25 µM LP, it was revealed
that LP reduced the phosphorylation of AKT and ERK (Fig. 4), however, not the total AKT and
ERK levels (Fig. 4). Taken
together, these data indicated that the collective inhibition of
the PI3K/AKT and MAPK signaling pathways is an important feature of
LP-mediated antiproliferation in HNSCC.
Discussion
Natural products, including LP from tomatoes, that
exhibit the biological activity to suppress activation of cell
survival pathways while selectively inducing cell death of
malignant cell populations are of great importance. Numerous
previous studies have investigated the effects of LP on cultures of
human tumor cell lines and determined that LP exerts marked
antiproliferative activity and triggers apoptosis (7,11,13,14).
In addition, it has been previously reported that ingestion of LP
had preventive roles in prostate cancer (22,23).
Furthermore, tomato-paste extract, containing LP, induced arrest in
both the G0/G1 phase and the G2/M phase of the cell cycle in
prostate cancer cells via the upregulation of the expression levels
of p53, p21 and p27. In the present study, in a high density
culture, LP alone significantly inhibited proliferation, colony
formation, induced apoptosis and decreased the invasion ability in
HNSCC cells. Furthermore, it was observed that the change of
apoptosis, cell growth and survival signaling stemming from LP
exposure were responsible for the inhibition of the cell cycle and
cell apoptosis response triggered by LP.
It was previously reported that LP was associated
with cancer cell proliferation, mobility, migration and invasion in
numerous cancer types (11,13,24).
This was most likely by inducing antiproliferative and apoptosis
activities. The present study investigated and revealed that cell
proliferative was suppressed, and cell apoptosis was enhanced
following treatment with LP. Repression of cell growth may be due
to the induction of apoptosis, as demonstrated in the present
study. It was also demonstrated that Bcl-2, an antiapoptotic member
of the Bcl family, was downregulation upon treatment with LP. By
contrast, Bax, a pro-apoptotic protein, was induced by treatment
with LP. Additionally, other pro-apoptotic signaling, including
caspase 3 and caspase 9 were also significantly increased.
Previous studies have shown that treatment with LP
is associated with the migration and invasion capability of cancer
cells (25–27). The present study confirmed that
treatment with LP inhibited HNSCC cell invasion in vitro, in
association with decreased expression levels of p-AKT and p-ERK.
These findings provided additional mechanistic insight into the
ways in which LP inhibits the aggressive behavior of HNSCC.
In conclusion, this is the first report, to the best
of our knowledge, to investigate the effects of LP on HNSCC cell
lines. It was demonstrated that LP is a potential anticancer drug
from natural products for patients with HNSCC. In vitro
investigation revealed that treatment with LP inhibited cell
growth, induced apoptosis and decreased the invasion activity of
HNSCC cells by inducing apoptosis, and the PI3K/AKT and MAPK
signaling pathways. Further studies are underway to assess the
molecular mechanisms through which LP is likely to influence the
anti-tumor properties of more cancer cells in our laboratory.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leemans CR, Braakhuis BJ and Brakenhoff
RH: The molecular biology of head and neck cancer. Nat Rev Cancer.
11:9–22. 2011. View
Article : Google Scholar
|
|
4
|
Argiris A, Karamouzis MV, Raben D and
Ferris RL: Head and neck cancer. Lancet. 371:1695–1709. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tamagawa S, Beder LB, Hotomi M, Gunduz M,
Yata K, Grenman R and Yamanaka N: Role of miR-200c/miR-141 in the
regulation of epithelial-mesenchymal transition and migration in
head and neck squamous cell carcinoma. Int J Mol Med. 33:879–886.
2014.PubMed/NCBI
|
|
6
|
Wang T, Chen T, Niu H, Li C, Xu C, Li Y,
Huang R, Zhao J and Wu S: MicroRNA-218 inhibits the proliferation
and metastasis of esophageal squamous cell carcinoma cells by
targeting BMI1. Int J Mol Med. 36:93–102. 2015.PubMed/NCBI
|
|
7
|
Zhang YY, Lu L, Abliz G and Mijit F: Serum
carotenoid, retinol and tocopherol concentrations and risk of
cervical cancer among chinese women. Asian Pac J Cancer Prev.
16:2981–2986. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ansari MS and Sgupta NP: A comparison of
lycopene and orchidectomy vs orchidectomy alone in the management
of advanced prostate cancer. BJU Int. 95:4532005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gharib A and Faezizadeh Z: In vitro
anti-telomerase activity of novel lycopene-loaded nanospheres in
the human leukemia cell line K562. Pharmacogn Mag. 10(Suppl 1):
S157–S163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ford NA, Elsen AC, Zuniga K, Lindshield BL
and Erdman JW Jr: Lycopene and apo-12′-lycopenal reduce cell
proliferation and alter cell cycle progression in human prostate
cancer cells. Nutr Cancer. 63:256–263. 2011. View Article : Google Scholar
|
|
11
|
Takeshima M, Ono M, Higuchi T, Chen C,
Hara T and Nakano S: Anti-proliferative and apoptosis-inducing
activity of lycopene against three subtypes of human breast cancer
cell lines. Cancer Sci. 105:252–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang FY, Pai MH and Wang XD: Consumption
of lycopene inhibits the growth and progression of colon cancer in
a mouse xenograft model. J Agric Food Chem. 59:9011–9021. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Palozza P, Simone RE, Catalano A and Mele
MC: Tomato lycopene and lung cancer prevention: From experimental
to human studies. Cancers (Basel). 3:2333–2357. 2011. View Article : Google Scholar
|
|
14
|
Holzapfel NP, Holzapfel BM, Champ S,
Feldthusen J, Clements J and Hutmacher DW: The potential role of
lycopene for the prevention and therapy of prostate cancer: From
molecular mechanisms to clinical evidence. Int J Mol Sci.
14:14620–14646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goldar S, Khaniani MS, Derakhshan SM and
Baradaran B: Molecular mechanisms of apoptosis and roles in cancer
development and treatment. Asian Pac J Cancer Prev. 16:2129–2144.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wheeler SE, Suzuki S, Thomas SM, Sen M,
Leeman-Neill RJ, Chiosea SI, Kuan CT, Bigner DD, Gooding WE, Lai SY
and Grandis JR: Epidermal growth factor receptor variant III
mediates head and neck cancer cell invasion via STAT3 activation.
Oncogene. 29:5135–5145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zuo T, Liu TM, Lan X, Weng YI, Shen R, Gu
F, Huang YW, Liyanarachchi S, Deatherage DE, Hsu PY, et al:
Epigenetic silencing mediated through activated PI3K/AKT signaling
in breast cancer. Cancer Res. 71:1752–1762. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
da Silva HB, Amaral EP, Nolasco EL, de
Victo NC, Atique R, Jank CC, Anschau V, Zerbini LF and Correa RG:
Dissecting major signaling pathways throughout the development of
prostate cancer. Prostate Cancer. 2013:9206122013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi L, Wang L, Wang B, Cretoiu SM, Wang Q,
Wang X and Chen C: Regulatory mechanisms of betacellulin in CXCL8
production from lung cancer cells. J Transl Med. 12:702014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Giovannucci E, Ascherio A, Rimm EB,
Stampfer MJ, Colditz GA and Willett WC: Intake of carotenoids and
retinol in relation to risk of prostate cancer. J Natl Cancer Inst.
87:1767–1776. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wertz K: Lycopene effects contributing to
prostate health. Nutr Cancer. 61:775–783. 2009. View Article : Google Scholar
|
|
24
|
Aras A, Khokhar AR, Qureshi MZ, Silva MF,
Sobczak-Kupiec A, Pineda EA, Hechenleitner AA and Farooqi AA:
Targeting cancer with nano-bullets: Curcumin, EGCG, resveratrol and
quercetin on flying carpets. Asian Pac J Cancer Prev. 15:3865–3871.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chan CM, Fang JY, Lin HH, Yang CY and Hung
CF: Lycopene inhibits PDGF-BB-induced retinal pigment epithelial
cell migration by suppression of PI3K/Akt and MAPK pathways.
Biochem Biophys Res Commun. 388:172–176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Elgass S, Cooper A and Chopra M: Lycopene
treatment of prostate cancer cell lines inhibits adhesion and
migration properties of the cells. Int J Med Sci. 11:948–954. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang CS, Shih MK, Chuang CH and Hu ML:
Lycopene inhibits cell migration and invasion and upregulates
Nm23-H1 in a highly invasive hepatocarcinoma, SK-Hep-1 cells. J
Nutr. 135:2119–2123. 2005.PubMed/NCBI
|