Introduction
Hyperlipidemia comprises a heterogeneous group of
disorders, characterized by increased levels of one or more lipids
and/or lipoproteins in the circulation, including increased levels
of atherogenic free fatty acids, triglycerides (TG; known as
hypertriglyceridemia), low-density lipoprotein cholesterol (LDL-C;
known as hypercholesterolemia) and apolipoprotein B; in addition,
decreased levels of antiatherogenic high-density lipoprotein
cholesterol (HDL-C) are observed (1,2). The
increased prevalence of hyperlipidemia is a public and economic
problem worldwide. Hyperlipidemia can arise from the adoption of
unhealthy lifestyle choices, such as consuming an excess of
high-fat food (3) and alcohol
(4), or as a result of specific
diseases, including diabetes (5),
hypothyroidism (6), erythematosus
(7) and chronic renal diseases
(8). Hyperlipidemia is a risk
factor for cardiovascular disease (CVD), which is the most common
cause of mortality worldwide (9).
In addition to CVD, hyperlipidemia is closely associated with
diabetes, insulin resistance and obesity (10,11).
Considering the serious outcomes of this disease, strategies for
the prevention and treatment of hyperlipidemia are urgently
required and may have a significant clinical value.
Pharmacotherapy is the current primary method for
the treatment of hyperlipidemia. Specific agents, including statins
(12), fibrates (13), nicotinic acids (14) and bile acid sequestrants (15), have been demonstrated to possess
reliable lipid lowering effects and thus dominate the main drug
market. However, certain patients are intolerant to these drugs due
to their adverse effects, which include liver and kidney toxicity
(16), rhabdomyolysis (17) and hyperglycemia (18). This has led to concerns regarding
the safety of these drugs for clinical use. Therefore, the search
for alternative therapies has gained significant attention in
recent years. Traditional Chinese medicine (TCM) products are
frequently considered to be less toxic than synthetic agents, and
may be a preferred option for the treatment of hyperlipidemia
(19). Over the last few decades,
hundreds of TCM compounds (20),
extracts (21), single herbs
(22) or formulae (23) have been reported to be effective in
the prevention and treatment of hyperlipidemia, particularly in
cases caused by high-fat diet (HFD).
One particular TCM herb, Coptis chinensis (C.
chinensis), obtained from the dry rootstocks of C.
chinensis Franch. or Coptis teeta Wall., has been used
to treat gastrointestinal disorders and diarrhea for ~30 years
worldwide (24). Jatrorrhizine
hydrochloride (JH) is an active component of the C.
chinensis herb and has been demonstrated to have
anti-inflammatory (25),
antimicrobial (26) and
leukemia-prevention activities (27). Notably, a recent study revealed
that JH stimulated insulin secretion and decreased fasting blood
glucose levels in rats, indicating that this compound may have
beneficial effects on energy metabolism (28). Although the protective effects of
JH against hypercholesterolemia and diabetes have been reported by
independent studies (29,30), to the best of our knowledge, no
comprehensive investigation has been conducted to examine its
versatile functions in the regulation of glucose and lipid
metabolism. In the present study, an HFD-induced hyperlipidemic
mouse model was employed to evaluate the effects of JH in
vivo. JH was observed to prevent the development of
hyperlipidemia in these mice, possibly via the suppression of fatty
acid synthesis and by increasing the β-oxidation of lipids in the
liver.
Materials and methods
Animals
Animal procedures employed in the present study
conformed to the Guide for the Care and Use of Laboratory Animals
published by the US National Institutes of Health (NIH publication
no. 85-23; revised 1996) and the approved regulations established
by the Laboratory Animal Care Committee at the Huai'an First
People's Hospital Affiliated to Nanjing Medical University
(Huai'an, China; permit no. SYXK2016-0016, issued April 6, 2016). A
total of 28 7-week-old male C57BL/6 mice, weighing 18–22 g, were
purchased from the Model Animal Research Center of Nanjing
University (Nanjing, China) and maintained under a 12-h light/dark
cycle, in a temperature and humidity-controlled environment. Mice
were acclimated to lab conditions for 1 week and then divided into
four groups (n=7 mice per group), including the normal diet (ND),
the hyperlipidemia model (HFD), HFD and low-dose JH (HFD+JH-L), and
HFD and high-dose JH (HFD+JH-H) groups. In order to induce
hyperlipidemia in the mice, a HFD (60% fat content; Research Diets,
Inc., New Brunswick, NJ, USA) was given to the HFD, HFD+JH-L and
HFD+JH-H group mice, as described previously (3); by contrast, ND control group mice
were fed a normal rodent chow diet. Mice in the JH-L and JH-H
treatment groups received daily intragastric injections of 20 and
100 mg/kg body weight JH, respectively, while mice in the control
and hyperlipidemic groups received an equivalent volume of 0.9%
saline solution using the same procedure. The doses of JH
administered to mice were selected based on a previous study
demonstrating that 100 mg/kg JH decreases blood glucose levels in
diabetic mice (28). Mouse body
weights were measured every 3 days. Treatment was administered over
the course of 8 weeks. Upon completion of treatment, mice were
fasted for 6 h and sacrificed by cervical dislocation for the
collection of blood and liver samples. JH (purity, >98%;
Fig. 1) extracted from the
tuberous roots of Tinospora sagittata (Oliv.) Gagnep. was
purchased from Nanjing Zelang Medical Technology Co., Ltd.
(Nanjing, China).
Glucose and insulin tolerance tests
In order to conduct a glucose tolerance test, mice
were fasted for 16 h and then injected intraperitoneally with 1
g/kg body weight glucose. To conduct an insulin tolerance test,
mice were fasted for 6 h and then injected intraperitoneally with
0.75 U/kg body weight insulin. Blood glucose levels were measured
prior to injection and at 0, 15, 30, 60 and 90 min after injection
with glucose or insulin using a glucose monitor (Roche Diagnostics,
Basel, Switzerland) after injection.
Serological analysis
Blood samples were collected in non-heparinized
tubes and centrifuged at 1,000 × g for 10 min at 4°C. Kits
purchased from the Nanjing Jiancheng Bioengineering Institute
(Nanjing, China) were used to determine the levels of serum TG
(GPO-PAP assay; catalog no. A110-1), total cholesterol (TC; GPO-PAP
assay; catalog no. A111-1), LDL-C (catalog no. A113-1), HDL-C
(catalog no. A112-1), aspartate transaminase (AST; catalog no.
C010-2) and alanine aminotransferase (ALT; catalog no. C009-2), by
spectrophotometric analysis.
Liver histology
Mouse livers were isolated, fixed in a 4%
paraformaldehyde solution for 24 h in situ, paraffin
embedded and cut into 4-µm transverse sections for routine
hematoxylin-eosin (H&E) staining.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from mouse livers using the
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Total RNA (1 µg) was then
reverse-transcribed into cDNA using PrimeScript™ RT Master Mix
(Takara Bio, Inc., Otsu, Japan) under the following conditions:
37°C for 15 min and then 85°C for 5 sec. Target gene mRNA levels
were determined by qPCR analysis using the SYBR premix Ex Taq
(Takara Bio, Inc.) and the LightCycler Nano system (Roche
Diagnostics). The primer sequences were as follows: 36B4, sense,
5′-GAA ACT GCT GCC TCA CAT CCG-3′, and antisense, 5′-GCT GGC ACA
GTG ACC TCA CACG-3′; sterol regulatory element binding
transcription factor 1c (SREBP-1c) sense, 5′-GAT CAA AGA GGA GCC
AGT GC-3′, and antisense, 5′-TAG ATG GTG GCT GCT GAG TG-3′; fatty
acid synthase (FAS) sense, 5′-TCC TGG AAC GAG AAC ACG ATCT-3′, and
antisense, 5′-GAG ACG TGT CAC TCC TGG ACT TG-3′; peroxisome
proliferator activated receptor-α (PPARα) sense, 5′-ACG ATG CTG TCC
TCC TTG ATG-3′, and antisense, 5′-ACG ATG CTG TCC TCC TTG ATG-3′;
and carnitine palmitoyltransferase 1A (CPT1A) sense, 5′-CTC AGT GGG
AGC GAC TCT TCA-3′, and antisense, 5′-GGC CTC TGT GGT ACA CGA
CAA-3′. The qPCR reaction mixture (10 µl) consisted of 0.3
µl forward primer (10 µM), 0.3 µl reverse
primer (10 µM), 5 µl SYBR Premix Ex Taq (2X), 2
µl cDNA template and 2.4 µl double-distilled
H2O. Thermal cycling parameters were as follows: 95°C
for 10 sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 20
sec. The 36B4 gene was used as an internal control. In detail, a
quatification cycle (Cq value) was obtained for each amplification
curve, and a ΔCq value was first calculated by subtracting the Cq
value for 36B4 from the Cq value for each sample. Fold-changes
compared with the endogenous control were then determined by
calculating 2−ΔΔCq (31).
Western blotting
To determine the protein expression levels of FAS
and PPARα, mouse liver samples were first lysed in
radioimmunoprecipitation assay buffer, which contained 50 mM
Tris/HCl (pH 8.0), 150 mM NaCl, 1% Nonidet-P40, 1% sodium
deoxycholate, 0.1% SDS, 0.1 mM DTT, 0.05 mM PMSF, 0.002 mg/ml
aprotinin, 0.002 mg/ml leupeptin and 1 mM NaVO3. Protein
concentration was determined using a BCA Protein Quantitation assay
kit (catalog no. A045-3; Nanjing Jiancheng Bioengineering
Institute). Equal amounts of protein were loaded and separated by
10% SDS-PAGE prior to transfer onto polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). Membranes were
blocked in 5% bovine serum albumin blocking buffer (pH 7.5; Nanjing
Sunshine Biotechnology Co., Ltd., Nanjing, China) and incubated
with rabbit anti-FAS (1:500; catalog no. 10624-2-AP) and rabbit
anti-PPARα (1:500; catalog no 15540-1-AP) primary polyclonal
antibodies (Proteintech Group, Inc., Rosemont, IL, USA), and a
mouse anti-GAPDH monoclonal antibody (1:500; catalog no. KC-5G5;
KangChen Bio-tech, Inc., Shanghai, China) at 4°C overnight. Bound
antibodies were detected using the horseradish
peroxidase-conjugated secondary antibodies goat anti-rabbit IgG
(1:2,000; catalog no. sc-2004) and goat anti-mouse IgG (1:2,000;
catalog no. sc-2005) purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Membranes were washed with phosphate-buffered
saline containing 0.1% Tween-20 for 10 min between each step.
Proteins were visualized using an Enhanced Chemiluminescence kit
(catalog no. WBKLS0050; Merck Millipore, Darmstadt, Germany) and
quantified using ImageJ software (National Institutes of Health,
Bethesda, MA, USA).
Statistical analysis
Data were analyzed using one-way analysis of
variance followed by Fisher's least significant difference
post-hoc test. Calculations were performed using the Origin8
software (version 8.6; OriginLab, Northampton, MA, USA). Data are
presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of JH on body weight and insulin
resistance in HFD-fed mice
As expected, mice consuming an HFD gained
substantially more body weight when compared with ND control mice
over the course of 8 weeks (Fig.
2A). After 8 weeks of feeding, mice in the HFD group developed
severe obesity, as observed by the increase in body weight
(P<0.001, HFD vs. ND group; Fig. 2A
and B). By contrast, HFD-fed mice treated with JH exhibited a
dose-dependent reduction in body weight when compared with
untreated HFD-fed mice. Mice in the JH-H group exhibited a 27%
decrease in body weight relative to the HFD group after 8 weeks
(P<0.001, HFD+JH-H vs. HFD group). The food intake was reduced
in HFD-fed mice compared with ND mice; however, no significant
difference in food intake was observed among the HFD-fed groups
(P<0.001, HFD vs. ND group; Fig.
2C). Insulin is an important hormone for maintaining blood
glucose levels and mediating lipid homeostasis (32). Therefore, serum insulin
concentrations in each group were measured. As shown in Fig. 2D, serum insulin concentrations were
significantly increased in the HFD model group compared with the ND
group. Notably, JH treatment significantly suppressed the
HFD-induced increase in insulin concentrations. Greater effects
were observed in the HFD+JH-H group (P<0.001, HFD vs. ND group;
P=0.006, HFD+JH-L vs. HFD group; P<0.001, HFD+JH-H vs. HFD
group; Fig. 2D). Glucose
intolerance and insulin resistance are common syndromes observed in
obese individuals (33); since JH
treatment was found to decrease the body weight of obese mice, the
effects of JH treatment on these syndromes was investigated. As
shown in Fig. 2E and F, the
HFD-fed mice exhibited significantly impaired sensitivities to
glucose and insulin compared with the ND-fed mice. Notably, the
HFD+JH-H group demonstrated a significantly improved glucose
tolerance and insulin sensitivity.
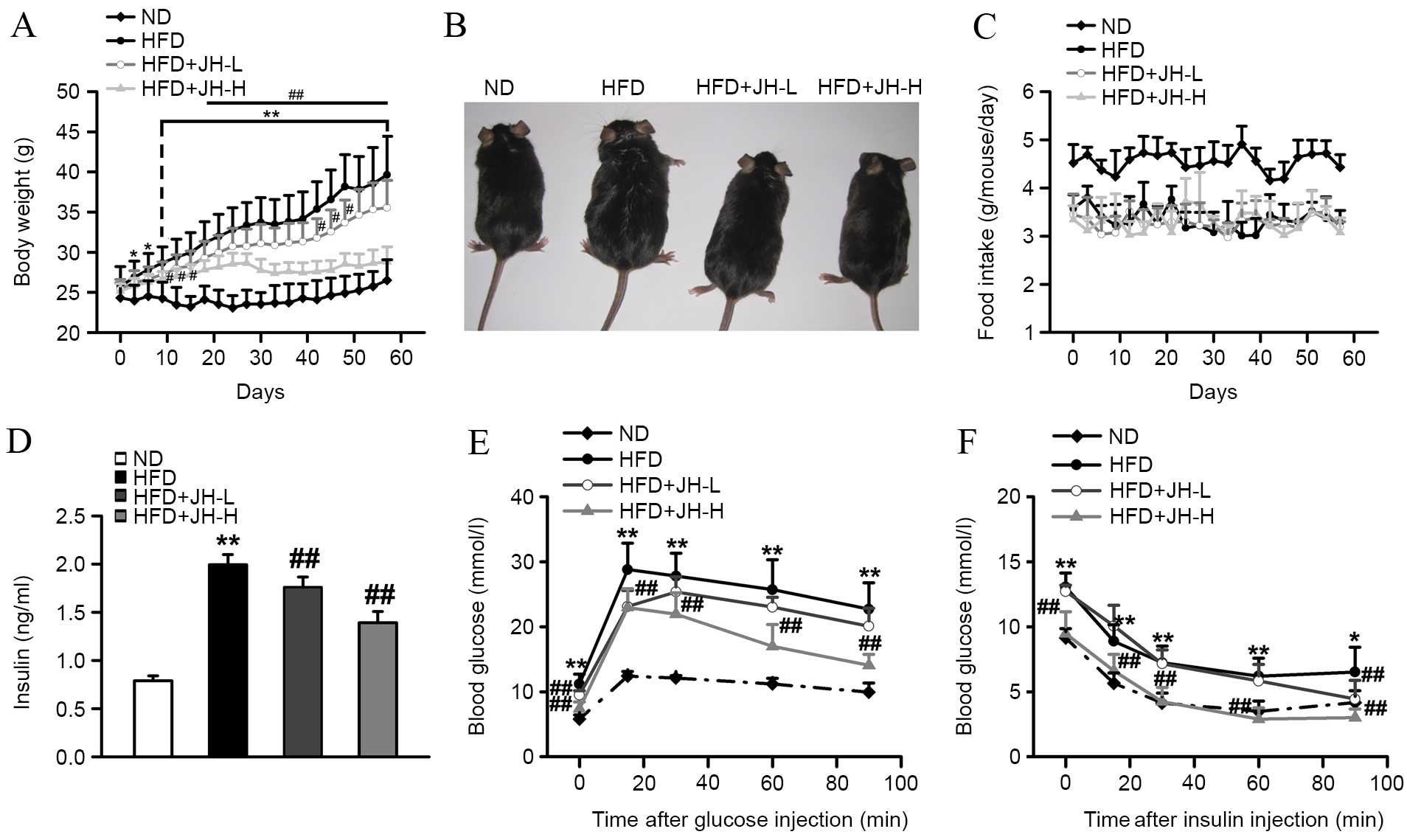 | Figure 2Effect of JH treatment on the body
weight, glucose and insulin sensitivity of HFD-fed mice. (A) Body
weight, (B) physiology, (C) food intake, (D) serum insulin levels,
(E) glucose tolerance test and (F) insulin tolerance test of the
ND, HFD, HFD+JH-L (20 mg/kg body weight) and HFD+JH-H (100 mg/kg
body weight) groups are shown (n=7 per group). Data are presented
as the mean ± standard deviation. *P<0.05 and
**P<0.01, HFD vs. ND group; #P<0.05 and
##P<0.01, HFD+JH-L or HFD+JH-H vs. HFD group. JH,
jatrorrhizine hydrochloride; HFD, high-fat diet; ND, normal
diet. |
Effect of JH treatment on the serum lipid
profile of HFD-fed mice
To determine the hypolipidemic effect of JH on
HFD-fed mice, the serum levels of TG, TC, LDL-C and HDL-C were
measured. As shown in Fig. 3A and
B, HFD-fed mice exhibited a significant increase in serum TG
and TC levels when compared with the ND group (1.9 and 1.7-fold
increase, P<0.001, HFD vs. ND group). However, JH treatment
significantly reduced the serum TG levels in a dose-dependent
manner when compared with the untreated HFD-fed mice, and TG levels
in the HFD+JH-H group were comparable to the baseline NC group
levels (P<0.001, HFD+JH-L vs. HFD group; P<0.001, HFD+JH-H
vs. HFD group; Fig. 3A).
Similarly, high-dose JH treatment of HFD-fed mice was associated
with a significant reduction in serum TC levels compared with the
untreated HFD-fed mice (P<0.001, HFD+JH-H vs. HFD group).
However, serum TC levels were reduced to a lesser extent in the
HFD+JH-H group (16% reduction in the HFD+JH-H vs. HFD group) than
the serum TG levels. Furthermore, maintaining a cholesterol balance
between potentially harmful LDL-C and beneficial HDL-C is essential
for body health (34). As shown in
Fig. 3C, JH treatment
significantly reduced the HFD-induced upregulation of serum LDL-C
levels. By contrast, serum HDL-C levels were significantly elevated
following JH treatment when compared with the untreated HFD-fed
mice, with a significant effect observed in the HFD+JH-H group
(Fig. 3D). These findings suggest
that JH may ameliorate HFD-induced hyperlipidemia and modify the
LDL-C/HDL-C balance by decreasing the ratio between LDL-C and
HDL-C.
 | Figure 3Effect of JH treatment on the serum
lipid profile of HFD-fed mice. Serum levels of (A) triglycerides,
(B) total cholesterol, (C) LDL-C and (D) HDL-C in the ND, HFD,
HFD+JH-L (20 mg/kg body weight) and HFD+JH-H (100 mg/kg body
weight) groups are shown (n=7 per group). **P<0.01,
HFD vs. ND group; ##P<0.01, HFD+JH-L or HFD+JH-H vs.
HFD group. Data are presented as the mean ± standard deviation. JH,
jatrorrhizine hydrochloride; HFD, high-fat diet; LDL-C, low-density
lipoprotein cholesterol; HDL-C, high-density lipoprotein
cholesterol; ND, normal diet. |
Effect of JH treatment on hepatic
steatosis in HFD-fed mice
As shown in Fig.
4A, morphological changes of the liver, such as enlargement and
a yellow color, were observed in the HFD group compared with the ND
group. Consistent with these observations, the liver-to-body weight
ratio was significantly increased in the HFD group compared with
the ND group due to lipid accumulation in the liver (P=0.001, HFD
vs. ND group; Fig. 4B). By
contrast, treatment with JH was shown to reverse these pathological
changes and significantly reduce the liver-to-body weight ratio
compared with that in untreated HFD-fed mice. Furthermore, H&E
staining was performed to further investigate the effect of JH
treatment on HFD-induced lipid accumulation in the liver. As
demonstrated in Fig. 4C, lipid
droplets were observed in the livers of HFD-fed mice, which was
accompanied by the swelling of hepatocytes. However, these
pathological alterations were attenuated following treatment with
JH. In order to evaluate liver function in all treatment groups,
the serum levels of AST and ALT were also determined. As shown in
Fig. 4D and E, AST and ALT levels
in HFD-fed mice were significantly increased when compared with
those in ND-fed mice, which suggests that the liver function was
impaired (P<0.001, HFD vs. ND group). However, treatment with JH
resulted in a significant decrease in serum AST and ALT levels when
compared to the HFD group (P<0.001, HFD+JH-H vs. HFD group;
Fig. 4D and E). Therefore, these
data preliminarily suggest that JH may counteract the development
of obesity and hepatic steatosis.
 | Figure 4Effect of JH treatment on hepatic
steatosis in HFD-fed mice. (A) Liver morphology, (B) liver-to-body
weight ratio, (C) liver pathology (as determined by
hematoxylineosin staining; magnification, x200), (D) serum AST and
(E) serum ALT levels in the ND, HFD, HFD+JH-L (20 mg/kg body
weight) and HFD+JH-H (100 mg/kg body weight) groups are shown (n=7
per group). **P<0.01, HFD vs. ND group;
#P<0.05 and ##P<0.01, HFD+JH-L or
HFD+JH-H vs. HFD group. Data are presented as the mean ± standard
deviation. JH, jatrorrhizine hydrochloride; HFD, high-fat diet;
AST, aspartate transaminase; ALT, alanine aminotransferase; ND,
normal diet. |
Effect of JH treatment on the hepatic
mRNA levels of lipid-associated genes in HFD-fed mice
Accelerated lipogenesis and decreased fatty acid
β-oxidation occur in the liver during the development of
hyperlipidemia (35). To elucidate
the molecular mechanisms underlying the hypolipidemic effect of JH,
RT-qPCR analysis was employed to quantify the expression levels of
hepatic genes involved in the regulation of lipid homeostasis. As
shown in Fig. 5A and B, the
expression levels of SREBP-1C and FAS, which are
lipogenesis-associated genes that promote the synthesis of de
novo monounsaturated fatty acids, were significantly increased
in the HFD group compared with the ND group (P<0.001, SREBP-1C;
P=0.026, FAS). By contrast, the HFD-induced increase in SREBP-1c
and FAS expression levels was inhibited by JH treatment, with a
significant effect observed in the HFD+JH-H group (P=0.005,
SREBP-1C; P=0.008, FAS; HFD+JH-H vs. HFD group). In addition, the
hepatic mRNA expression levels of PPAR-α and CPT1A, which are
involved in mediating fatty acid β-oxidation, were significantly
decreased in the HFD group compared with the ND group (Fig. 5C and D). However, JH
supplementation was associated with a significant increase in
PPAR-α and CPT1A mRNA expression levels when compared with the
untreated HFD group. Consistent with the mRNA expression levels,
the protein expression levels of FAS and PPAR-α were significantly
reduced and increased, respectively, following the administration
of JH in HFD-fed mice (Fig. 5E).
These results suggest that JH may improve HFD-induced
hyperlipidemia, in part, through the inhibition of fatty acid
synthesis and the activation of fatty acid β-oxidation in the
liver.
 | Figure 5Effect of JH treatment on the hepatic
mRNA levels of lipid-associated genes in HFD-fed mice. The mRNA
expression levels of (A) SREBP-1c, (B) FAS, (C) PPAR-α and (D)
CPT1A in the ND, HFD, HFD+JH-L (20 mg/kg body weight) and HFD+JH-H
(100 mg/kg body weight) groups are shown (n=7 per group). (E)
Western blot analysis and protein expression levels of FAS and
PPAR-α in the various groups are shown. *P<0.05 and
**P<0.01, HFD vs. ND group; #P<0.05 and
##P<0.01, HFD+JH-L or HFD+JH-H vs. HFD group. Data
are presented as the mean ± standard deviation. JH, jatrorrhizine
hydrochloride; HFD, high-fat diet; SREBP-1c, sterol regulatory
element binding transcription factor 1c; FAS, fatty acid synthase;
PPAR-α, peroxisome proliferator activated receptor-α; CPT1A,
carnitine palmitoyltransferase 1A; ND, normal diet. |
Discussion
The versatile pharmacological activities of JH have
attracted considerable attention in recent years. This compound is
a tetrahydroisoquinoline alkaloid and has a similar chemical
structure to berberine (36). The
reported beneficial effects of JH include bacteriostasis (37), tumor cell growth suppression
(38), reversal of multidrug
resistance (39) and the lowering
of blood glucose levels (28).
Nevertheless, to the best of our knowledge, no study has
investigated the effect of JH on hyperlipidemic animals or humans.
The results of the present study suggest that JH may prevent
metabolic disorders by ameliorating hyperlipidemia and insulin
resistance in HFD-fed obese mice. These beneficial effects may
occur via the suppression of lipogenesis and the promotion of fatty
acid β-oxidation.
In the present study, JH inhibited the gain in body
weight that was observed in HFD-fed mice in a dose-dependent
manner. This effect was not associated with food intake, as no
notable differences in diet consumption were observed among the
HFD-fed groups, suggesting that JH treatment may not affect
appetite. Obesity is known to be the most common cause of hepatic
steatosis, which is characterized by the pathological accumulation
of fat in the liver, and leads to liver damage in the form of
inflammation and fibrosis (40).
In the current study, hepatic steatosis was observed in HFD-fed
mice, which was evidenced by alterations in liver morphology
(enlargement and a yellow color) and histology (increased lipid
droplet accumulation). Notably, these pathological changes were
reversed by JH treatment. In addition, serum AST and ALT levels, a
hallmark of liver injury (41),
were significantly downregulated by JH treatment in HFD-fed mice.
Therefore, JH may be useful for the treatment of fatty liver
disease caused by hyperlipidemia. In addition, the severe glucose
intolerance, insulin resistance and hyperinsulinemia observed in
HFD-fed mice was significantly attenuated by JH treatment. Taken
together with its hypoglycemic effects, JH may be a potential
therapeutic agent for the prevention of diabetes. However, it
should be noted that the present study was designed for
investigating JH as a preventative agent for metabolic disorders.
JH was administered to mice when hyperlipidemia had not been fully
induced. Future studies should assess the protective roles of JH in
a therapeutic setting, with an extension of treatment time.
Lipid homeostasis is dependent on the balance
between lipogenesis (storage) and fatty acid β-oxidation
(consumption). A previous study demonstrated that the SREBP-1c and
PPARα transcription factors and the expression of their respective
target genes, FAS and CPT1A, serve crucial roles in the development
of hyperlipidemia (42). SREBP-1c
modulates the expression of a large number of genes involved in the
uptake of lipoproteins, and the synthesis of cholesterol, TG and
very-low-density lipoprotein cholesterol in the liver (43). In addition, PPARα regulates the
expression of genes involved in mitochondrial and liver fatty acid
β-oxidation (44,45). In the present study, HFD increased
the hepatic mRNA expression levels of SREBP1c and FAS, but
decreased the levels of hepatic PPAR-α and CPT1A. Notably,
treatment of HFD-fed mice with JH reversed these effects. These
data suggest that reducing the expression levels of genes that
inhibit lipogenesis (namely SREBP-1c and FAS) and upregulating the
expression levels of genes involved in promoting lipid metabolism
(namely PPARα and CPT1A) may underlie the beneficial effects of JH
on HFD-induced hyperlipidemia and liver damage in mice.
FAS and CPT1A are important enzymes involved in
hepatic lipogenesis and fatty acid β-oxidation. FAS catalyzes the
final step of the fatty acid biosynthesis process, while CPT1A
facilitates the transfer of long-chain fatty acids across the
mitochondrial membrane during β-oxidation (46). In the present study, the mRNA
expression levels of genes encoding these enzymes were analyzed. JH
treatment of HFD-fed mice suppressed FAS and promoted CPT1A mRNA
expression levels. It should be noted that these results provide a
preliminary insight into the mechanisms through which JH exerts its
pharmacological functions. Future studies should conduct additional
experiments in order to elucidate the beneficial functions of JH in
the prevention and/or treatment of metabolic disorders. For
instance, the protein expression and enzymatic activity levels of
these enzymes should be determined. In addition, gain and
loss-of-function strategies could be employed to artificially
manipulate FAS and CPT1A expression, in order to determine whether
these enzymes mediate the effects of JH on hyperlipidemia.
In conclusion, the results of the present study
suggest that JH may serve a role in ameliorating metabolic
disorders in HFD-fed obese mice. The underlying mechanisms of the
hypolipidemic effect of JH may involve the suppression of
lipogenesis and the promotion of fatty acid β-oxidation. These
results provide novel insights into the role of JH in regulating
liver energy metabolism, and suggest that treatment with JH may
present an effective and safe strategy for the prevention of
hyperlipidemia and other metabolic disorders. Further investigation
is required to elucidate the mechanisms by which this compound
protects against metabolic disorders and to determine whether
additional mechanisms are involved.
Acknowledgments
This study was supported by grants from the Applied
and Technologic Research Program of Huai'an (no. HAS2014009) and
the Research Fund for the Technology Development Project of Nanjing
Medical University (no. 2013NJMU226).
References
|
1
|
Betteridge DJ: Diabetic dyslipidaemia. Eur
J Clin Invest. 29(Suppl 2): S12–S16. 1999. View Article : Google Scholar
|
|
2
|
Goldberg IJ: Clinical review 124: Diabetic
dyslipidemia: Causes and consequences. J Clin Endocrinol Metab.
86:965–971. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin J, Yang R, Tarr PT, Wu PH, Handschin
C, Li S, Yang W, Pei L, Uldry M, Tontonoz P, et al: Hyperlipidemic
effects of dietary saturated fats mediated through PGC-1beta
coactivation of SREBP. Cell. 120:261–273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mendelson JH and Mello NK: Alcohol-induced
hyperlipidemia and beta lipoproteins. Science. 180:1372–1374. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dunn FL: Hyperlipidemia in diabetes
mellitus. Diabetes Metab Rev. 6:47–61. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karthick N, Dillara K, Poornima KN and
Subhasini AS: Dyslipidaemic changes in women with subclinical
hypothyroidism. J Clin Diagn Res. 7:2122–2125. 2013.PubMed/NCBI
|
|
7
|
Borba EF and Bonfá E: Dyslipoproteinemias
in systemic lupus erythematosus: Influence of disease, activity,
and anticardiolipin antibodies. Lupus. 6:533–539. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sentí M, Romero R, Pedro-Botet J, Pelegrí
A, Nogués X and Rubiés-Prat J: Lipoprotein abnormalities in
hyperlipidemic and normolipidemic men on hemodialysis with chronic
renal failure. Kidney Int. 41:1394–1399. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Keefe JH and Bell DS: Postprandial
hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a
cardiovascular risk factor. Am J Cardiol. 100:899–904. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haffner SM: Diabetes, hyperlipidemia, and
coronary artery disease. Am J Cardiol. 83:17F–21F. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O'Brien T, Nguyen TT and Zimmerman BR:
Hyperlipidemia and diabetes mellitus. Mayo Clin Proc. 73:969–976.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Farnier M and Davignon J: Current and
future treatment of hyperlipidemia: The role of statins. Am J
Cardiol. 82:3J–10J. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Staels B, Dallongeville J, Auwerx J,
Schoonjans K, Leitersdorf E and Fruchart JC: Mechanism of action of
fibrates on lipid and lipoprotein metabolism. Circulation.
98:2088–2093. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garg A and Grundy SM: Nicotinic acid as
therapy for dyslipidemia in non-insulin-dependent diabetes
mellitus. JAMA. 264:723–726. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ast M and Frishman WH: Bile acid
sequestrants. J Clin Pharmacol. 30:99–106. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Akoglu H, Yilmaz R, Kirkpantur A, Arici M,
Altun B and Turgan C: Combined organ failure with combination
antihyperlipidemic treatment: A case of hepatic injury and acute
renal failure. Ann Pharmacother. 41:143–147. 2007. View Article : Google Scholar
|
|
17
|
Thompson PD, Clarkson P and Karas RH:
Statin-associated myopathy. JAMA. 289:1681–1690. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miettinen TA, Taskinen MR, Pelkonen R and
Nikkilä EA: Glucose tolerance and plasma insulin in man during
acute and chronic administration of nicotinic acid. Acta Med Scand.
186:247–253. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ho YP, To KK, Au-Yeung SC, Wang X, Lin G
and Han X: Potential new antitumor agents from an innovative
combination of demethylcantharidin, a modified traditional Chinese
medicine, with a platinum moiety. J Med Chem. 44:2065–2068. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsu CL, Wu CH, Huang SL and Yen GC:
Phenolic compounds rutin and o-coumaric acid ameliorate obesity
induced by high-fat diet in rats. J Agric Food Chem. 57:425–431.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park SH, Ko SK, Choi JG and Chung SH:
Salicornia herbacea prevents high fat diet-induced hyperglycemia
and hyperlipidemia in ICR mice. Arch Pharm Res. 29:256–264. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang XX, Yan HM, Xu Q, Xia MF, Bian H,
Zhu TF and Gao X: The effects of berberine on hyperhomocysteinemia
and hyperlipidemia in rats fed with a long-term high-fat diet.
Lipids Health Dis. 11:862012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng Q, Gou XJ, Meng SX, Huang C, Zhang
YQ, Tang YJ, Wang WJ, Xu L, Peng JH and Hu YY: Qushi huayu
decoction inhibits hepatic lipid accumulation by activating
AMP-Activated protein kinase in vivo and in vitro. Evid Based
Complement Alternat Med. 2013:1843582013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khin-Maung-U, Myo-Khin, Nyunt-Nyunt-Wai,
Aye-Kyaw and Tin-U: Clinical trial of berberine in acute watery
diarrhoea. Br Med J (Clin Res Ed). 291:1601–1605. 1985. View Article : Google Scholar
|
|
25
|
Akhter MH, Sabir M and Bhide NK:
Anti-inflammatory effect of berberine in rats injected locally with
cholera toxin. Indian J Med Res. 65:133–141. 1977.PubMed/NCBI
|
|
26
|
Amin AH, Subbaiah TV and Abbasi KM:
Berberine sulfate: Antimicrobial activity, bioassay, and mode of
action. Can J Microbiol. 15:1067–1076. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin CC, Ng LT, Hsu FF, Shieh DE and Chiang
LC: Cytotoxic effects of Coptis chinensis and Epimedium sagittatum
extracts and their major constituents (berberine, coptisine and
icariin) on hepatoma and leukaemia cell growth. Clin Exp Pharmacol
Physiol. 31:65–69. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fu Y, Hu B, Tang Q, Fu Q, Zhang Q and
Xiang JZ: Effect of jatror-rhizine, berberine, Huanglian Decoction
and compound-mimic prescription on blood glucose in mice. Chung
Tsao Yao Tsa Chih Pien Chi Pu. 36:548–551. 2005.In Chinese.
|
|
29
|
Wu H, He K, Wang Y, Xue D, Ning N, Zou Z,
Ye X, Li X, Wang D and Pang J: The antihypercholesterolemic effect
of jatrorrhizine isolated from Rhizoma Coptidis. Phytomedicine.
21:1373–1381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fu Y, Hu B, Tang Q, Fu Q and Xiang J:
Hypoglycemic activity of jatrorrhizine. J Huazhong Univ Sci
Technolog Med Sci. 25:491–493. 2005. View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
32
|
Saltiel AR and Kahn CR: Insulin signalling
and the regulation of glucose and lipid metabolism. Nature.
414:799–806. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kopelman PG: Obesity as a medical problem.
Nature. 404:635–643. 2000.PubMed/NCBI
|
|
34
|
Toth PP: Cardiology patient page. The
'good cholesterol': High-density lipoprotein. Circulation.
111:e89–e91. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wiegman CH, Bandsma RH, Ouwens M, van der
Sluijs FH, Havinga R, Boer T, Reijngoud DJ, Romijn JA and Kuipers
F: Hepatic VLDL production in ob/ob mice is not stimulated by
massive de novo lipogenesis but is less sensitive to the
suppressive effects of insulin. Diabetes. 52:1081–1089. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu F, Li Z, Shi X and Zhong M:
Determination of berberine, palmatine and jatrorrhizine in rabbit
plasma by liquid chromatography-electrospray ionization-mass
spectrometry. J Pharm Biomed Anal. 56:1006–1015. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kong W, Zhao Y, Xiao X, Jin C, Liu Y and
Li Z: Comparison of anti-bacterial activity of four kinds of
alkaloids in Rhizoma Coptidis based on microcalorimetry.
Zhongguohuaxue (yingwenban). 27:1186–1190. 2009.In Chinese.
|
|
38
|
Liu R, Cao Z, Pan Y, Zhang G, Yang P, Guo
P and Zhou Q: Jatrorrhizine hydrochloride inhibits the
proliferation and neovascularization of C8161 metastatic melanoma
cells. Anticancer Drugs. 24:667–676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xiong C and Fang D: Effects of
jatrorrhizine on isolated guinea pig atria. Zhongguo Yaolixue Yu
Dulixue Zazhi. 4:255–259. 1989.In Chinese.
|
|
40
|
Reddy JK and Rao MS: Lipid metabolism and
liver inflammation. II. Fatty liver disease and fatty acid
oxidation. Am J Physiol Gastrointest Liver Physiol. 290:G852–G858.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Renner EL and Dällenbach A: Increased
liver enzymes: What should be done? Ther Umsch. 49:281–286. 1992.In
German. PubMed/NCBI
|
|
42
|
Nguyen P, Leray V, Diez M, Serisier S, Le
Bloc'h J, Siliart B and Dumon H: Liver lipid metabolism. J Anim
Physiol Anim Nutr (Berl). 92:272–283. 2008. View Article : Google Scholar
|
|
43
|
Eberlé D, Hegarty B, Bossard P, Ferré P
and Foufelle F: SREBP transcription factors: Master regulators of
lipid homeostasis. Biochimie. 86:839–848. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Janani C and Ranjitha Kumari BD: PPAR
gamma gene-A review. Diabetes Metab Syndr. 9:46–50. 2015.
View Article : Google Scholar
|
|
45
|
Pawlak M, Lefebvre P and Staels B:
Molecular mechanism of PPARα action and its impact on lipid
metabolism, inflammation and fibrosis in non-alcoholic fatty liver
disease. J Hepatol. 62:720–733. 2015. View Article : Google Scholar
|
|
46
|
Wakil SJ and Abu-Elheiga LA: Fatty acid
metabolism: Target for metabolic syndrome. J Lipid Res. 50(Suppl):
S138–S143. 2009. View Article : Google Scholar :
|



















