Introduction
Volatile anesthetics are frequently used during
pediatric surgery (1). Sevoflurane
[2,2,2-trifluoro-1-(trifluoromethyl) ethyl fluoro methyl ether] is
widely administered as a general anesthetic in pediatric patients,
due to its fast induction and recovery times, and it causes less
irritation to the airways compared with other inhaled anesthetics
(2). Accumulating evidence
indicates that volatile anesthetics induce neuronal apoptosis
(3–6) and affect neurogenesis in vitro
and in vivo (7,8). Furthermore, long-term neurocognitive
function was observed to be altered in 7 day-old rats (9).
Children aged <4 years that were exposed to
general anesthesia more than once have an increased risk of
developing learning disabilities (10,11).
Although sevoflurane is not as cytotoxic as isoflurane and
desflurane, sevoflurane exposure increases the risk of
neurodevelopmental impairments and cognitive dysfunction in
neonatal animal models (12–14).
The various mechanisms that underlie
anesthetic-mediated neuronal apoptosis in the evolving brain are
yet to be fully determined and numerous potential mechanisms have
been proposed, including: i) Disruption of intracellular calcium
homeostasis (15–17); ii) regulation of the cell cycle
(18); iii) inhibition of
N-methyl-D-aspartate receptors and activation of gamma-aminobutyric
acid receptors; and iv) associated impairment of synaptogenesis
(19–22).
Mitogen-activated protein kinases (MAPKs) are a
group of serine-threonine protein kinases that are important during
neurogenesis (23),
neurodegeneration (24) and brain
inflammation (25). The major
members of the MAPK family are c-Jun N-terminal kinase (JNK),
extracellular signal-regulated kinase (ERK) and p38 MAPK. Previous
studies have demonstrated an association between MAPK signaling
pathways and neurotoxicity induced by anesthetics. Wang et
al (26) reported that
N-stearoyl-l-tyrosine protects the developing brain against
sevoflurane-induced neurotoxicity by regulating the ERK1/2
signaling pathway. Furthermore, dexmedetomidine was demonstrated to
regulate the phosphorylation levels of ERK1/2 in the neonatal rat
brain (27) and provided
neuroprotection against isoflurane-induced neurodegeneration in the
hippocampus of neonatal rats by modulating the phosphoinositide
3-kinase (PI3K)/Akt signaling pathway (28). Zhao et al (29) reported that isoflurane causes
neurodegeneration via apoptosis through excessive activation of
inositol 1,4,5-trisphosphate receptors (InsP3Rs).
Strategies to potentially reduce the
anesthetic-induced neurotoxicity require further research. Previous
investigations focused on using plant-derived compounds for the
therapy of various medical conditions. Resveratrol, a phenolic
antioxidant present in grapes and berries, was demonstrated to
protect neuronal cells from isoflurane-induced cytotoxicity by
regulating the Akt signaling cascade (30). Caffeic acid phenethyl ester (CAPE)
is a phenolic chemical compound present in numerous plants and is
extracted from honeybee hive propolis (31). It is a strong antioxidant (32), and also exhibits anti-proliferative
(33) and anti-inflammatory
effects (32,34). Furthermore, the neuroprotective
effects of CAPE in in vivo and in vitro experimental
models have been demonstrated (35–37).
Thus, considering the biological effects of CAPE,
the present study aimed to investigate whether CAPE protects
against sevoflurane-induced neurotoxicity in a neonatal rat
model.
Materials and methods
Study animals
The present study was approved by the animal care
and ethical committee of Linyi People's Hospital (Linyi, China) and
was performed in accordance with the National Institutes of Health
Guide for the Use of Laboratory Animals. A total of 30 pregnant
female Sprague-Dawley rats from Guangdong Medical Laboratory Animal
Center (Foshan, China), were used in the present study. The animals
were housed in individual cages at ~22±1°C, and had access to water
and food ad libitum. The rats were observed closely for the
day of birth [postnatal day 0 (P0)]. The rat pups had access to
water ad libitum and were maintained under a 12-h light/dark
cycle at ~22±1°C. The treatment group rat pups received CAPE (10,
20 or 40 mg/kg body weight) orally each day along with standard
diet from P1 to P15.
Chemicals and antibodies
Sevoflurane and CAPE were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Fluoro-Jade C (0.001%) was
obtained from EMD Millipore (Billerica, MA, USA). Primary
antibodies against activated caspase-3 (cat. no. sc-7149), -8 (cat.
no. sc-56070), -9 (cat. no. sc-7885), B cell CCL/lymphoma 2 (Bcl-2;
cat. no. 509), Bcl-2-associated agonist of cell death (Bad; cat.
no. sc-8044), Bcl-2-like 1 (Bcl-xL; cat. no. sc-7195),
Bcl-2-associated X protein (Bax; cat. no. sc-493), phosphorylated
(p)-Bad (cat. no. sc-101640), β-actin (cat. no. sc-69879; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), Akt (cat. no. 2920),
p-Akt (cat. no. 4060), glycogen synthase kinase 3β (GSK3β; cat. no.
9315), p-GSK3β (cat. no. 9323), phosphatase and tensin homolog
(PTEN; cat. no. 9556), JNK (cat. no. 9252), p-JNK (cat. no. 9255),
p-c-Jun (cat. no. 2361), ERK1/2 (cat. no. 4615), p-ERK1/2 (cat. no.
4377), p38 (cat. no. 9228) and p-p38 (cat. no. 9215; Cell Signaling
Technology, Inc., Danvers, MA, USA) were used in the current study.
All chemicals used in this study were of analytical grade and
procured from Sigma-Aldrich unless specified.
Anesthesia exposure
At P7, groups of rat pups were exposed to
sevoflurane (2.9%). The pups were retained in a humid chamber with
total gas flow 2 l/min, using 25% O2 as the carrier.
Anesthetic agent fractions and O2 were measured using a
Capnomac Ultima gas analysis system (GE Health-care Life Sciences,
Chalfont, UK). During anesthetic exposure, the pups were placed on
a warm mat at 38±1°C. Neonatal rats were assigned to receive 2.9%
sevoflurane for 6 h in 30% O2 (38). On P7 the rats were administered
with CAPE (10, 20 or 40 mg/kg body weight) 1 h prior to sevoflurane
exposure. The control group received no anesthesia or CAPE. The
anesthetic control group received only anesthesia and were not
treated with CAPE. At the end of the study period, the animals (n=6
per group) were anesthetized with sodium thiopenthal (100 mg/kg;
Sigma-Aldrich) and were sacrificed after 25–30 min of thiopental
injection. Samples of hippocampal tissue were excised for analysis
of apoptosis by terminal deoxynucleotidyl transferase dUTP nick-end
labeling (TUNEL) assay, and protein expression by western
blotting.
Measurement of plasma S100 calcium
binding protein β (S100β) by enzyme-linked immunosorbent assay
(ELISA)
The S100 family of dimeric cytosolic calcium binding
proteins are expressed in astroglial and Schwann cells. The β
isomer of S100 is released into the extracellular space upon tissue
injury and enters the serum through the blood brain barrier
following mild brain injury, trauma, ischemia, hypoxia and exposure
to neurotoxins (39). The levels
of plasma S100β in neonatal rats were evaluated using a Sangtec 100
ELISA kit (DiaSorin S.P.A., Gerenzano, Italy) according to the
manufacturer's instructions. Briefly, 50 µl plasma from each
pup was added to a well of the 96-well plate and mixed with 150
µl tracer from the kit, and incubated for ~2 h. Following
incubation, 3,3′,5,5′tetramethylbenzidine substrate and stop
solution were added and the solution was mixed well. The optical
density was measured at 450 nm using a Bio-Rad iMark microplate
absorbance reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
(40).
Measurement of apoptosis by TUNEL
assay
TUNEL assay was performed to assess neuronal
apoptosis, as described previously by Li et al (6). Briefly, P7 rat pups exposed to
sevoflurane were sacrificed and the brain tissues were excised. The
sections were immersed in 10% buffered formalin for 15 min at room
temperature. Tissues were post-fixed for 48 h at 4°C, embedded in
paraffin and sections (5 µm) were used for the assay. A
TUNEL fluorescent assay was performed using the fluorometric TUNEL
system kit (Promega Corporation, Madision, WI, USA). The brain
tissue slides were protected from direct light and the nuclei were
stained using 2 µg/ml Hoechst for 10 min. TUNEL-positive
cells in the hippocampal dentate gyrus (DG), CA1 and CA3 regions
were analyzed in 10 fields using the NIS-Elements BR image
processing and analysis software (Nikon Corporation, Tokyo,
Japan).
Immunohistochemical analysis of cleaved
caspase-3
Apoptosis was analyzed by immunohistochemical
analysis of cleaved caspase-3 levels, as previously described by Li
et al (41). Briefly, the
brain tissue sections were incubated with anti-cleaved caspase-3
primary antibody at 4°C overnight, followed by biotin-conjugated
secondary antibody treatment (1:200; cat. no. sc-2040; Santa Cruz
Biotechnology, Inc.) for ~40 min at room temperature. The sections
were subsequently incubated with avidin-biotinylated peroxidase
complex (Vectastain ABC kit; Vector Laboratories, Inc., Burlingame,
CA, USA) for 40 min then stained with 3,3′-diaminobenzidine (Vector
Laboratories, Inc.). The sections were observed using an IX70
microscope (Olympus Corporation, Tokyo, Japan) with 6 randomly
chosen fields imaged per slide.
Immunoblotting
Hippocampi were isolated from the rat pups
immediately following exposure to sevoflurane then used for western
blotting as described previously (5,6). The
protein concentrations within the samples were determined using
bicinchoninic acid protein assay (Bio-Rad Laboratories, Inc.).
Protein samples (60 µg) were separated by SDS-PAGE and
electrotransferred to nitrocellulose membranes, then incubated with
primary antibodies (1:1,000). The positive reactive bands were
detected by Amersham ECL enhanced chemiluminescence western
blotting detection kit (GE Healthcare Life Sciences). The blots
were scanned using Image Master II scanner (GE Healthcare Life
Sciences) and densities analyzed using Image Quant TL software
(version 2003.03; GE Health-care Life Sciences). The band densities
were normalized to those of β-actin using anti-β-actin antibody.
Western blotting was repeated six times for quantification.
Statistical analysis
The experimental data are presented as the mean ±
standard deviation, obtained from three or six individual
experiments. The values were subjected to one-way analysis of
variance followed by post-hoc Duncan's multiple range test using
SPSS software (version 21.0; IBM SPSS, Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
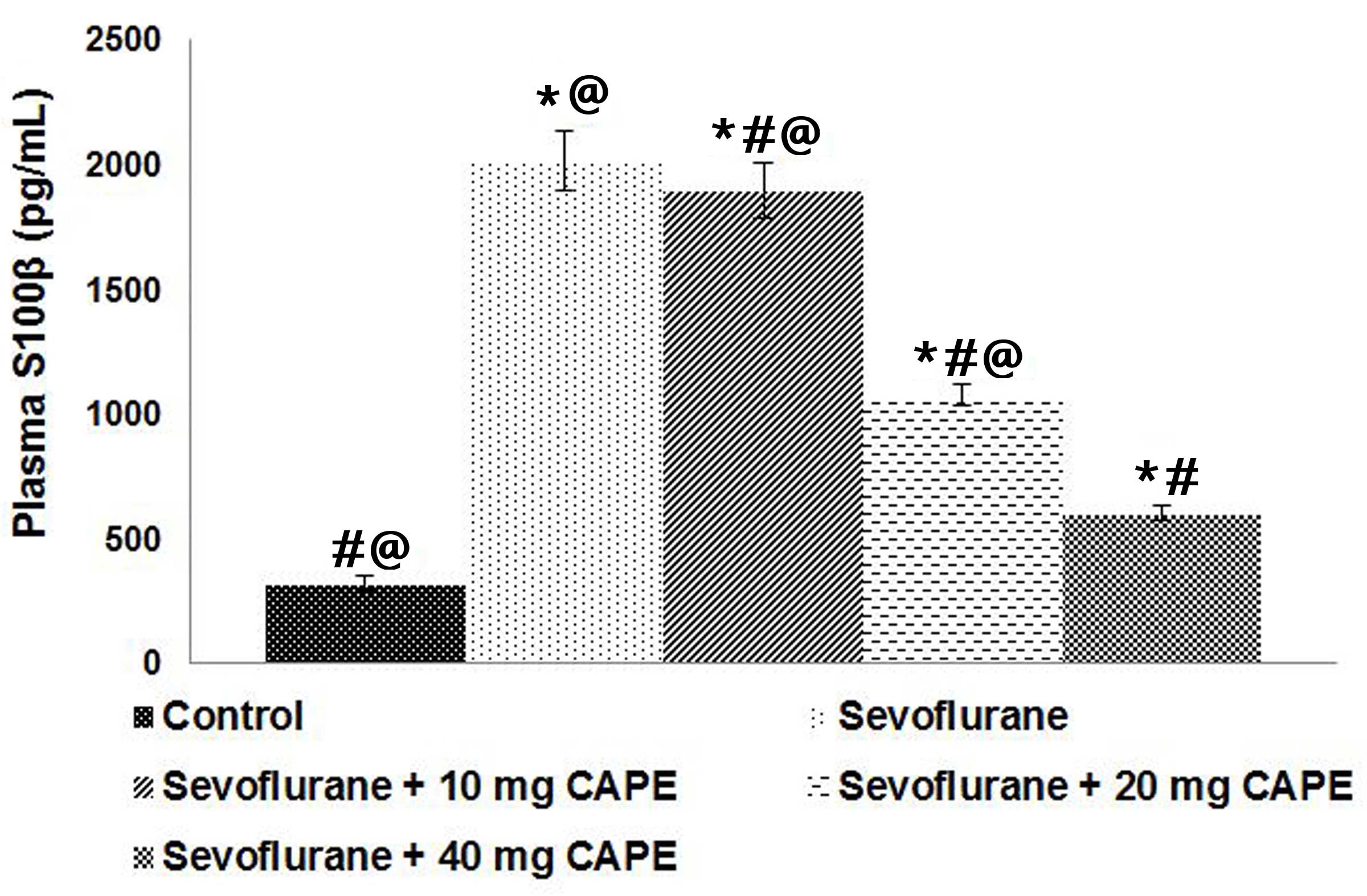
CAPE inhibits plasma S100β levels
Previous studies have demonstrated that
neuroapoptosis of the neonatal brain increases following exposure
to inhaled or intravenous anesthetic agents (7,42,43).
S100β, the β isomer of S100, has previously been identified as a
valuable biomarker for detecting anesthetic-induced
neurodegeneration (40,44). In the present study sevoflurane
(2.9%) caused ~4-fold increase in S100β levels, however
administration of CAPE caused a significant decrease in the plasma
levels of S100β (Fig. 1).
Supplementation with 40 mg CAPE exerted a more potent effect on the
plasma S100β levels than the lower doses (P<0.05).
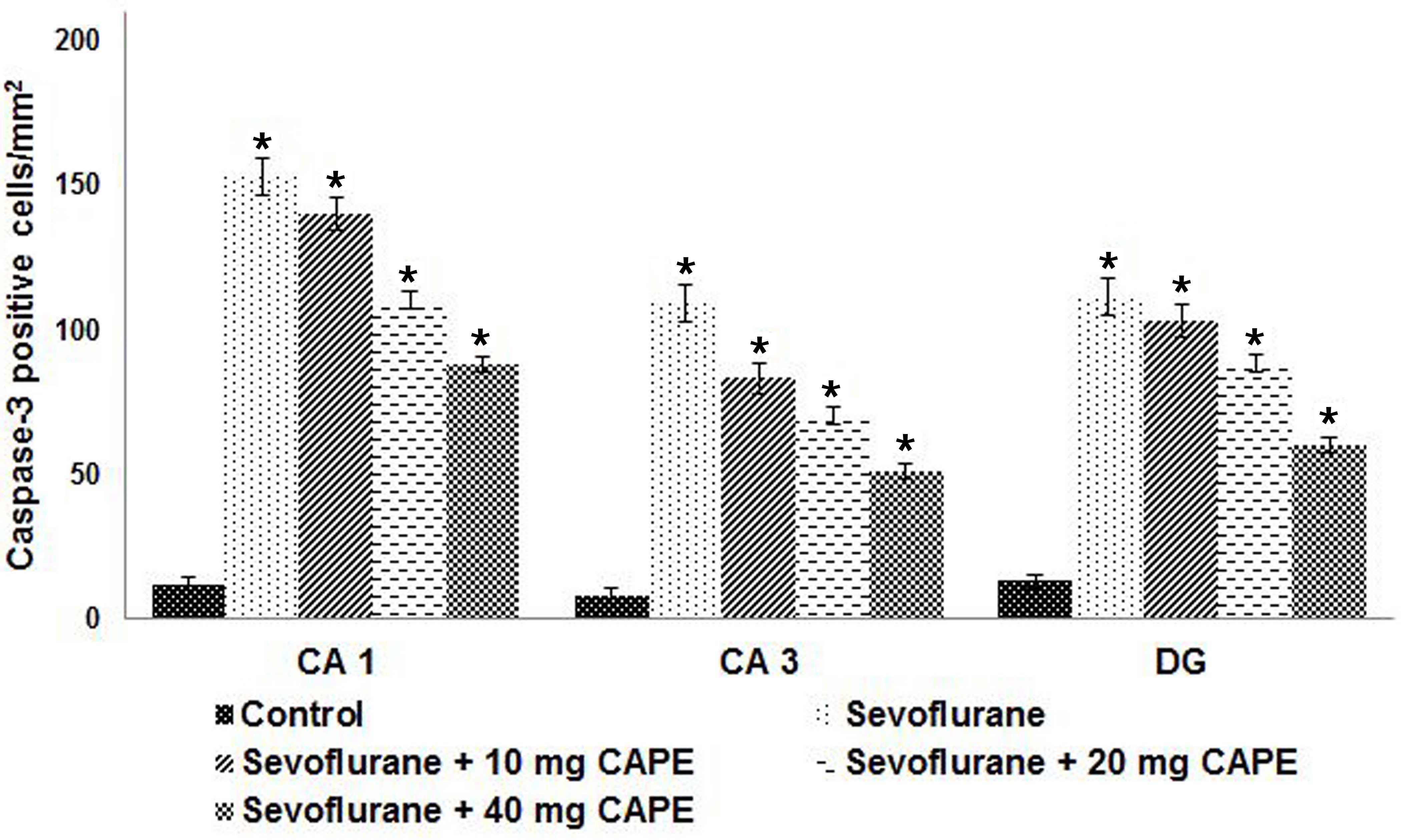
CAPE effectively inhibits
sevoflurane-induced neurodegeneration
Anesthetics have been shown to induce significant
neuronal apoptosis in the developing brain (4,7). In
the current study, a 6 h exposure to sevoflurane increased the
number of TUNEL-positive cells in the hippocampi of P7 rat pups;
the increase was greatest in CA1, followed by DG then CA3
(P<0.05; Fig. 2). Furthermore,
CAPE administration significantly decreased the number of
TUNEL-positive cells in the hippocampi of the rat pups (P<0.05).
The CA1 region of the hippocampus exhibited a higher number of
apoptotic cells when compared with the DG and CA3 regions
(P<0.05).
Activated caspase-3 is commonly used as a biomarker
for anesthesia-induced apoptosis (7,45).
Zheng et al (46)
demonstrated significant neural degeneration in the hippocampus
following exposure to 1% sevoflurane. Thus, the present study
examined the number of caspase-positive cells following sevoflurane
and CAPE exposure. Consistent with the S100β level results,
sevoflurane exposure significantly increased the number of
caspase-3-positive cells in the hippocampal regions of the neonatal
rats compared with the control. Administration of CAPE (10, 20 or
40 mg) resulted in a significant decrease in the number of
caspase-positive cells in a dose-dependent manner (P<0.05;
Fig. 3).
Furthermore, after 6 h exposure to inhaled
sevoflurane (2.9%), the expression levels of the pro-apoptotic
proteins, caspase-3, -8 and -9, were significantly upregulated
compared with control levels (P<0.05; Fig. 4), as demonstrated by western blot
analysis. Compared with those of the control, the expression levels
of Bad and Bax were significantly increased by sevoflurane
(P<0.05). Sevoflurane reduced the expression levels of
anti-apoptotic proteins, Bcl-2 and Bcl-xL, when compared with
control levels (P<0.05; Fig.
4). CAPE treatment significantly downregulated the expression
of caspases, and Bax and Bad compared with sevoflurane treatment
(P<0.05), whereas the expression levels of Bcl-xL and Bcl-2 were
increased (P<0.05). This indicated that CAPE may exert its
anti-apoptotic effects by modulating the expression of caspases and
apoptotic pathway proteins.
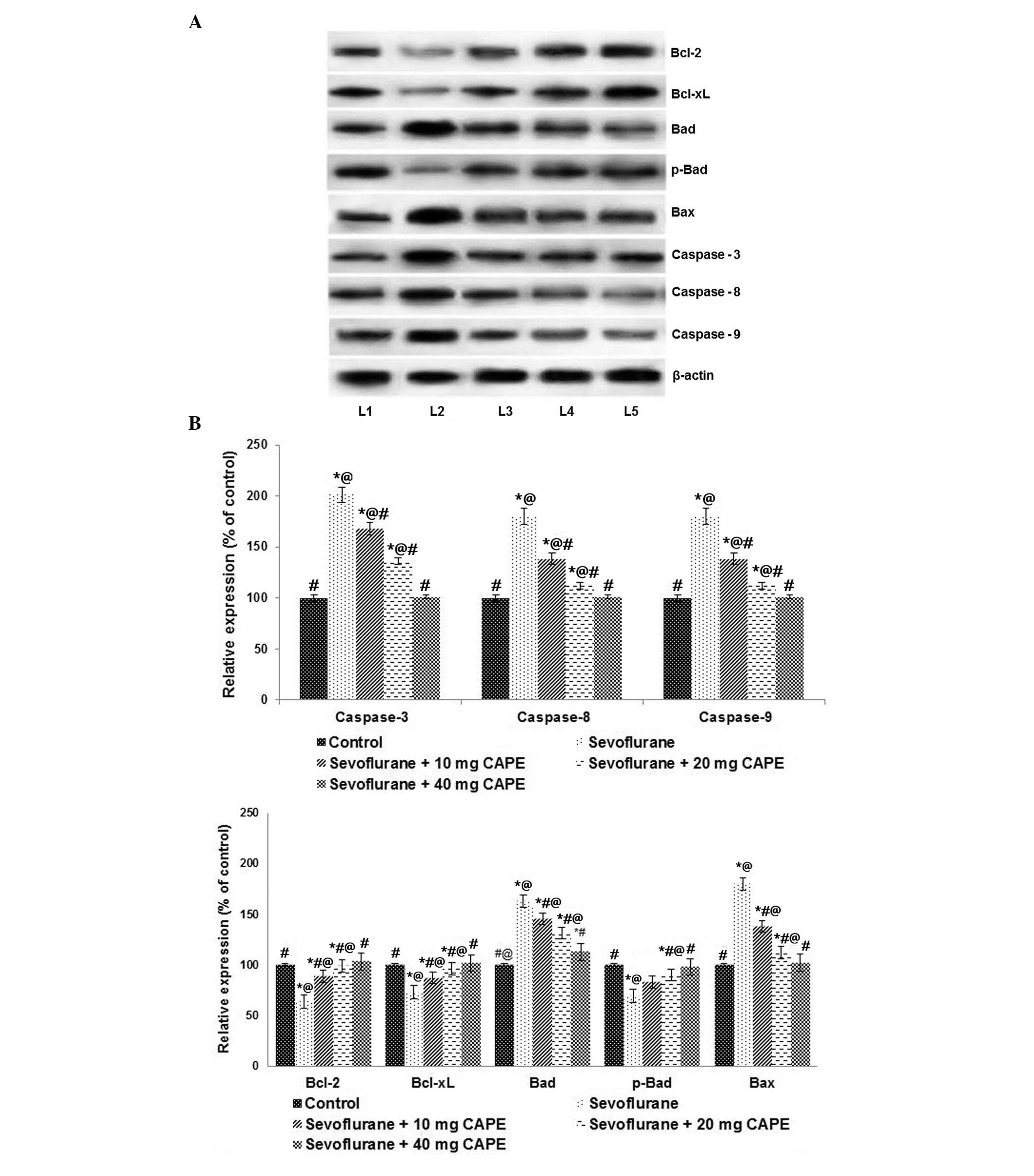 | Figure 4CAPE regulates the expression of
apoptotic pathway proteins following sevoflurane anesthesia. (A)
Sevoflurane significantly enhanced the expression levels of
caspases and the pro-apoptotic proteins, Bax and Bad. CAPE
supplementation caused marked protein expression regulation. Lane
1, control; lane 2, sevoflurane; lane 3, sevoflurane + 10 mg CAPE;
lane 4, sevoflurane + 20 mg CAPE; lane 5, sevoflurane + 40 mg CAPE.
(B) Relative expression levels of the proteins. Values are
presented as the mean ± standard deviation (n=3).
*P<0.05 vs. control; #P<0.05 vs.
sevoflurane; @P<0.05 vs. 40 mg CAPE; as determined by
one-way analysis of variance followed by Duncan's multiple range
test. Bcl-2, B cell CCL/lymphoma 2; Bcl-xL, Bcl-2-like 1; p-Bad,
phosphorylated Bcl-2-associated agonist of cell death; Bax,
Bcl-2-associated X protein; CAPE, caffeic acid phenethyl ester. |
Neuroprotection by CAPE involves the JNK,
ERK and p38 signaling pathways
To further investigate the molecular mechanisms
associated with neuroprotection by CAPE, the expression of MAPK
family proteins (JNK, ERK1/2 and p38 MAPK) were examined. Previous
studies have demonstrated that JNK, ERK1/2 and p38 are involved in
dexmedetomidine-induced neuroprotection (41,47,48).
The present study demonstrated that the levels of p-JNK and p-p38
kinases were significantly upregulated following sevoflurane
exposure compared with control (P<0.05; Fig. 5). However, the
sevoflurane-increased ERK1/2 levels were not as high as the JNK
levels. In addition to the enhanced expression of JNK, the levels
of p-c-Jun were increased compared with the control (P<0.05).
CAPE significantly downregulated the expression of p-JNK, p-ERK and
p-p38, and reduced the expression of p-c-Jun compared with the
sevoflurane group (P<0.05). Furthermore, CAPE significantly
downregulated the expression levels of total JNK, ERK1/2 and p38
when compared with sevoflurane treatment. However, comparatively,
CAPE exhibited a less potent effect on ERK1/2 and p-ERK1/2 levels
compared with JNK and p38. These results indicated that the MAPK
signaling pathway is involved in CAPE-mediated neuroprotection.
PI3K/Akt signaling pathway is involved in
neuroprotection of neonatal brain cells by CAPE
The mechanisms involved in inhalational
anesthetic-induced neuronal apoptosis in neonatal brains have been
widely investigated. The current study evaluated the affect of
sevoflurane on PI3K/Akt signaling pathway proteins. The
PI3K/Akt/mechanistic target of rapamycin signaling pathway is
important for regulating the cell cycle, and previous reports have
demonstrated that InsP3Rs and variations in
intracellular calcium homeostasis are involved in
anesthesia-induced neurodegeneration (29,49).
Sevoflurane exposure significantly reduced the levels of Akt and
p-Akt (P<0.05; Fig. 6).
Additionally, a significant decrease in the expression levels of
GSK3β and p-GSK3β levels were observed following 6 h of exposure to
sevoflurane compared with the control (P<0.05; Fig. 6). CAPE supplementation
downregulated the PI3K/Akt signaling pathway, as demonstrated by a
significant increase in Akt expression levels and enhanced GSK3β
expression (P<0.05). Additionally, PTEN expression levels were
observed to be enhanced by CAPE treatment compared with groups
exposed to sevoflurane only (P<0.05), suggesting that activation
of the PI3K/Akt pathway is involved in neuroprotection.
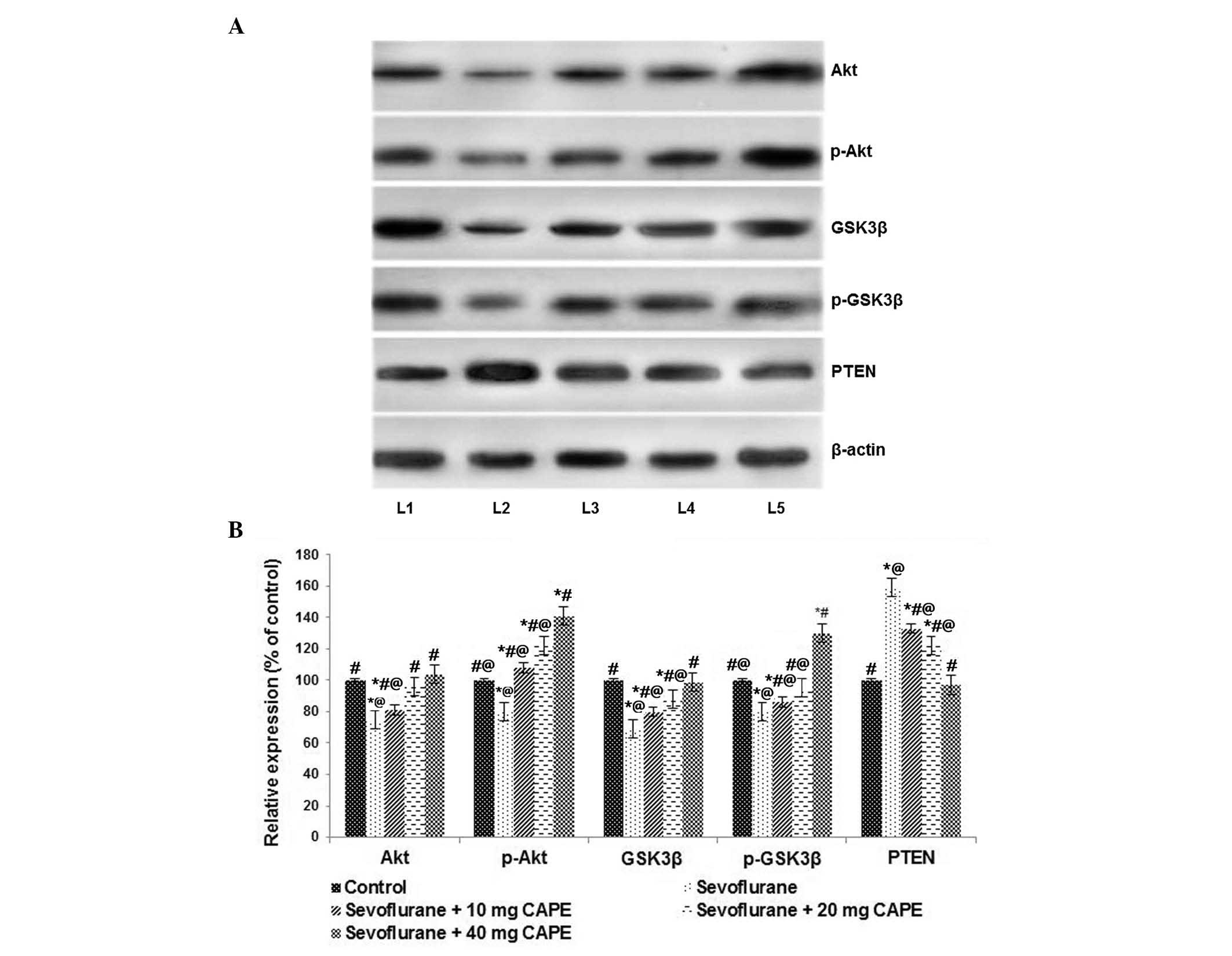 | Figure 6CAPE may activate the PI3K/Akt
signaling pathway following sevoflurane exposure. (A) Sevoflurane
at 2.9% significantly downregulated the PI3K/Akt signaling pathway.
CAPE treatment activated the PI3K/Akt signaling pathway. Lane 1,
control; lane 2, sevoflurane; lane 3, sevoflurane + 10 mg CAPE;
lane 4, sevoflurane + 20 mg CAPE; lane 5, sevoflurane + 40 mg CAPE.
(B) Values are presented as the mean ± standard deviation (n=6).
*P<0.05 vs. control; #P<0.05 vs.
sevoflurane; @P<0.05 vs. 40 mg CAPE; as determined by
one-way analysis of variance followed by Duncan's multiple range
test. p, phosphorylated; GSK3β, glycogen synthase kinase 3β; PTEN,
phosphatase and tensin homolog; PI3K, phosphoinositide 3-kinase
CAPE, caffeic acid phenethyl ester. |
Discussion
Growing experimental data have reported that
widespread neuroapoptosis occurs in developing brain cells
following early exposure to commonly used general anesthetics
(10,11,50,51).
Volatile anesthetic, sevoflurane, has previously been demonstrated
to induce apoptotic neurodegeneration in the developing rat brain
and to cause persistent learning/memory deficits (12,52).
Cell death by apoptosis is a vital aspect of normal
brain maturation that leads to the elimination of 50–70% of
progenitor cells and neurons during development (53,54).
However, neuronal apoptosis exceeding this natural apoptotic rate
is triggered by various pathologic conditions, including hypoxia,
ischemia or prolonged anesthetic exposure (55,56).
Accordingly, the current study examined the level of neuronal
apoptosis in the hippocampi of P7 rats exposed to 6 h of
sevoflurane anesthesia.
The expression of cleaved caspase-3 expression, a
validated marker of cell death, was measured to detect apoptosis.
Caspase-3, an aspartate-specific cysteine protease, is an important
executioner protein of the apoptosis pathway (57). In the present study,
immunohistochemistry and western blot analysis demonstrated that
sevoflurane exposure leads to a increase in the protein expression
of cleaved caspase-3 in the hippocampus. Neuronal apoptosis was
more severe in the CA1 region than the CA3 and DG regions, as
detected by immunohistochemistry and TUNEL assay. These findings
were consistent with those of previous studies (12,13,38).
Furthermore, the expression levels of initiator caspases (caspase-8
and -9) were observed to be enhanced by sevoflurane exposure.
Previous studies have demonstrated an association between
anesthetic-induced apoptosis and elevated plasma S100β levels,
which could potentially be used as a neurodegenerative biomarker
for brain damage following various types of stress (40,44).
In accordance with previous reports, sevoflurane exposure caused a
significant increase in plasma S100β levels. Downregulation of the
apoptotic cell counts and the expression of caspases (caspase-3, -8
and -9) by CAPE, suggests that CAPE suppresses sevoflurane-induced
apoptosis.
The balance between the anti-apoptotic (Bcl-2 and
Bcl-xL) and pro-apoptotic (Bad and Bax) Bcl-2 family proteins
regulates cell survival and death (58). Bad is activated through
phosphorylation (59) by
proto-oncogene proteins c-Akt that subsequently leads to the
binding of Bad with 14-3-3, a cytosolic protein, and causes the
release of anti-apoptotic protein, Bcl-xL. Bcl-xL binds to Bax and
consequently inhibits apoptosis (60,61).
Thus, Bcl-xL and Bcl-2 block Bax translocation to the mitochondria
and maintain the mitochondrial membrane potential to prevent
subsequent apoptosis (61). The
enhanced expression of Bad and Bax following sevoflurane exposure
observed in the current study suggests that the apoptosis rate is
elevated by sevoflurane, which correlates with suppression of
Bcl-xl and Bcl-2. Bcl-xL, expressed extensively in the central
nervous system (CNS), enriches cell survival by maintaining
mitochondrial membrane integrity and reducing cytochrome complex
release (58). An anesthesia
combination containing nitrous oxide, isoflurane and midazolam has
previously been reported to downregulate Bcl-xL, leading to
neurotoxicity (62). In the
present study, CAPE, at 10-, 20- and 40-mg doses, increased
neuronal cell survival, which was demonstrated by the upregulation
of anti-apoptotic proteins and significant inhibition of Bax and
Bad expression levels.
The PI3K/Akt intracellular signaling pathway is
associated with cellular quiescence, proliferation, cell survival
and cancer. Activated PI3K phosphorylates and activates Akt,
localizing it to the plasma membrane (63). The PI3K/Akt signaling pathway is
crucial in the decision between cell proliferation and renewal, as
opposed to differentiation and quiescence. The pathway is
antagonized by various factors, including PTEN (64) GSK3β (63) and homeobox gene Hb9 (65). Upon activation, Akt inhibits
apoptosis via phosphorylation of Bad and GSK3β (66,67).
Previous reports suggest a potential link between Akt and JNK
signaling, and Akt signaling is reported to be involved in the
apoptotic effect of JNK (68).
Furthermore, a selective JNK inhibitor, SP600125 (67) was demonstrated to exhibit
neuroprotective effects (69,70).
In the present study, sevoflurane exposure inhibited
the activation of Akt and upregulated the expression levels of
PTEN. In addition, sevoflurane reduced the level of p-GSK3β and
p-Akt, which promote the apoptosis of neuronal cells. CAPE
potentially activates the PI3K/Akt signaling pathway by
significantly increasing the expression and phosphorylation of Akt
and GSK3β. Silencing the InsP3R was previously
demonstrated to inhibit isoflurane-induced neuroapoptosis (29), potentially contributing to the
inhibition of neuroapoptosis by this mechanism. However, this
hypothesis requires further validation.
Furthermore, PTEN levels were suppressed by CAPE,
contributing to the effect of CAPE on the PI3K/Akt signaling
cascade. PTEN inhibition has previously been reported to promote
neuroprotection following CNS injury (71). Thus, inhibition of PTEN expression,
which was demonstrated in the current study, may also contribute to
the neuroprotective effects of CAPE.
JNK signaling is associated with neuronal apoptosis
activated by various stimuli that cause brain injury, including
ischemia/reperfusion and ethanol (72–74).
Previous studies have demonstrated that the JNK signaling pathway
is activated in isoflurane-induced neuronal apoptosis (6). SP600125, a JNK inhibitor, prevented
the phosphorylation of c-Jun, a substrate of JNK, and
neuroapoptosis induced by isoflurane (6,75).
In the present study, sevoflurane increased the levels of p-JNK and
p-c-Jun in the hippocampi of P7 rats, suggesting that the JNK
signaling pathway is activated in sevoflurane-induced neuronal
apoptosis. Expression of ERK1/2 and its phosphorylated forms, was
also enhanced marginally by sevoflurane. CAPE downregulated the
expression levels of JNK and ERK1/2 in a dose-dependent manner,
indicating that the effects of CAPE may involve the JNK and ERK
signaling pathways.
Previous reports have demonstrated that the p38 MAPK
signaling pathway is involved in anesthetic-induced
neurodegeneration (76) and that
p38 is enhanced in isoflurane-induced neuronal apoptosis (75). In the current study, CAPE prevented
the sevoflurane-induced increase in p-p38 expression levels,
suggesting that the p38 signaling pathway is involved in the
neuroprotective effect of CAPE. Treatment with dexmedetomidine and
p38 MAPK inhibitor, SB203580 was previously demonstrated to
decrease the expression level of p-p38, suggesting that the p38
signaling pathway is also involved in the neuroprotective effects
of dexmedetomidine (75).
In conclusion, the observations of the current study
indicate that CAPE inhibits sevoflurane-induced neuronal apoptosis
in the neonatal rat brain via modulating the expression of caspases
and regulating the critical pathways involved in neuronal
apoptosis, including the JNK/ERK/p38 MAPK and PI3K/Akt signaling
pathways. Thus, CAPE may be a potential candidate for reducing
anesthetic-induced neurotoxicity. However, further investigations
using specific JNK/ERK and Akt, and studies on the apoptotic
pathway inhibitors are required to assess the neuroprotective
effects of CAPE.
References
|
1
|
Istaphanous GK and Loepke AW: General
anesthetics and the developing brain. Curr Opin Anesthesiol.
22:368–373. 2009. View Article : Google Scholar
|
|
2
|
Patel SS and Goa KL: Sevoflurane: A review
of its pharmacodynamic and pharmacokinetic properties and its
clinical use in general anaesthesia. Drugs. 51:658–700. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Johnson SA, Young C and Olney JW:
Isoflurane-induced neuroapoptosis in the developing brain of
nonhypoglycemic mice. J Neurosurg Anesthesiol. 20:21–28. 2008.
View Article : Google Scholar
|
|
4
|
Brambrink AM, Evers AS, Avidan MS, Farber
NB, Smith DJ, Zhang X, Dissen GA, Creeley CE and Olney JW:
Isoflurane-induced neuroapoptosis in theneonatal rhesus macaque
brain. Anesthesiology. 112:834–841. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Y, Liu C, Zhao Y, Hu K, Zhang J, Zeng
M, Luo T, Jiang W and Wang H: Sevoflurane induces short-term
changes in proteins in the cerebral cortices of developing rats.
Acta Anaesthesiol Scand. 57:380–390. 2013. View Article : Google Scholar
|
|
6
|
Li Y, Wang F, Liu C, Zeng M, Han X, Luo T,
Jiang W, Xu J and Wang H: JNK pathway may be involved in
isoflurane-induced apoptosis in the hippocampi of neonatal rats.
Neurosci Lett. 545:17–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jevtovic-Todorovic V, Hartman RE, Izumi Y,
Benshoff ND, Dikranian K, Zorum-ski CF, Olney JW and Wozniak DF:
Early exposure to common anesthetic agents causes widespread
neurodegeneration in the developing rat brain and persistent
learning deficits. J Neurosci. 23:876–882. 2003.PubMed/NCBI
|
|
8
|
Zhu C, Gao J, Karlsson N, Li Q, Zhang Y,
Huang Z, Li H, Kuhn HG and Blomgren K: Isoflurane anesthesia
induced persistent, progressive memory impairment, caused a loss of
neural stem cells and reduced neurogenesis in young, but not adult,
rodents. J Cereb Blood Flow Metab. 30:1017–1030. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stratmann G, Sall JW, May LD, Bell JS,
Magnusson KR, Rau V, Visrodia KH, Alvi RS, Ku B, Lee MT and Dai R:
Isoflurane differentially affects neurogenesis and long-term
neurocognitive function in 60 day-old and 7 day-old rats.
Anesthesiology. 110:834–848. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
DiMaggio C, Sun LS and Li G: Early
childhood exposure to anesthesia and risk of developmental and
behavioral disorders in a sibling birth cohort. Anesth Analg.
113:1143–1151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ing C, DiMaggio C, Whitehouse A, Hegarty
MK, Brady J, von Ungern-Sternberg BS, Davidson A, Wood AJ, Li G and
Sun LS: Long-term differences in language and cognitive function
after childhood exposure to anesthesia. Pediatrics. 130:e476–e485.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Satomoto M, Satoh Y, Terui K, Miyao H,
Takishima K, Ito M and Imaki J: Neonatal exposure to sevoflurane
induces abnormal social behaviors and deficits in fear conditioning
in mice. Anesthesiology. 110:628–637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kodama M, Satoh Y, Otsubo Y, Araki Y,
Yonamine R, Masui K and Kazama T: Neonatal desflurane exposure
induces more robust neuroapoptosis than do isoflurane and
sevoflurane and impairs working memory. Anesthesiology.
115:979–991. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shih J, May LD, Gonzalez HE, Lee EW, Alvi
RS, Sall JW, Rau V, Bickler PE, Lalchandani GR, Yusupova M, et al:
Delayed environmental enrichment reverses sevoflurane-induced
memory impairment in rats. Anesthesiology. 116:586–602. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei HF, Liang G, Yang H, Wang Q, Hawkins
B, Madesh M, Wang S and Eckenhoff RG: The common inhalational
anesthetic isoflurane induces apoptosis via activation of inositol
1,4,5-trisphosphate receptors. Anesthesiology. 108:251–260. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lunardi N, Ori C, Erisir A and
Jevtovic-Todorovic V: General anesthesia causes long-lasting
disturbances in the ultrastructural properties of developing
synapses in young rats. Neurotox Res. 17:179–188. 2010. View Article : Google Scholar
|
|
17
|
Zhao X, Yang Z, Liang G, Wu Z, Peng Y,
Joseph DJ, Inan S and Wei H: Dual effects of isoflurane on
proliferation, differentiation and survival in human
neuroprogenitor cells. Anesthesiology. 118:537–549. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Soriano SG, Liu Q, Li J, Liu JR, Han XH,
Kanter JL, Bajic D and Ibla JC: Ketamine activates cell cycle
signaling and apoptosis in the neonatal rat brain. Anesthesiology.
112:1155–1163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sinner B, Friedrich O, Zink W, Zausig Y
and Graf BM: The toxic effects of s(+)-ketamine on differentiating
neurons in vitro as a consequence of suppressed neuronal
Ca2+ oscillations. Anesth Analg. 113:1161–1169. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao YL, Xiang Q, Shi QY, Li SY, Tan L,
Wang JT, Jin XG and Luo AL: GABAergic excitotoxicity injury of the
immature hippocampal pyramidal neurons' exposure to isoflurane.
Anesth Analg. 113:1152–1160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brambrink AM, Evers AS, Avidan MS, Farber
NB, Smith DJ, Martin LD, Dissen GA, Creeley CE and Olney JW:
Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus
macaque brain. Anesthesiology. 116:372–384. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Istaphanous GK, Ward CG, Nan X, Hughes EA,
Mccann JC, McAuliffe JJ, Danzer SC and Loepke AW: Characterization
and quantification of isoflurane-induced developmental apoptotic
cell death in mouse cerebral cortex. Anesth Analg. 116:845–854.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mousa A and Bakhiet M: Role of cytokine
signaling during nervous system development. Int J Mol Sci.
14:13931–13957. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harper SJ and Wilkie N: MAPKs: New targets
for neurodegeneration. Expert Opin Ther Targets. 7:187–200. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaminska B, Gozdz A, Zawadzka M,
Ellert-Miklaszewska A and Lipko M: MAPK signal transduction
underlying brain inflammation and gliosis as therapeutic target.
Anat Rec (Hoboken). 292:1902–1913. 2009. View Article : Google Scholar
|
|
26
|
Wang WY, Yang R, Hu SF, Wang H, Ma ZW and
Lu Y: N-stearoyl-l-tyrosine ameliorates sevoflurane induced
neuroapoptosis via MEK/ERK1/2MAPK signaling pathway in the
developing brain. Neurosci Lett. 541:167–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sanders RD, Sun P, Patel S, Li M, Maze M
and Ma D: Dexmedetomidine provides cortical neuroprotection: Impact
on anaesthetic-induced neuroapoptosisin the rat developing brain.
Acta Anaesthesiol Scand. 54:710–716. 2010. View Article : Google Scholar
|
|
28
|
Li Y, Zeng M, Chen W, Liu C, Wang F, Han
X, Zuo Z and Peng S: Dexmedetomidine reduces isoflurane-induced
neuroapoptosis partly by pre-serving PI3K/Akt pathway in the
hippocampus of neonatal rats. PLoS One. 9:e936392014. View Article : Google Scholar
|
|
29
|
Zhao Y, Liang G, Chen Q, Joseph DJ, Meng
Q, Eckenhoff RG, Eckenhoff MF and Wei H: Anesthetic-induced
neurodegeneration mediated via inositol 1,4,5-trisphosphate
receptors. J Pharmacol Exp Ther. 333:14–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bai T, Dong DS and Pei L: Resveratrol
mitigates isoflurane-induced neuroapoptosis by inhibiting the
activation of the Akt-regulated mitochondrial apoptotic signaling
pathway. Int J Mol Med. 32:819–826. 2013.PubMed/NCBI
|
|
31
|
Demestre M, Messerli SM, Celli N,
Shahhossini M, Kluwe L, Mautner V and Maruta H: CAPE (caffeic acid
phenethyl ester)-based propolis extract (Bio 30) suppresses the
growth of human neurofibromatosis (NF) tumor xenografts in mice.
Phytother Res. 23:226–230. 2009. View Article : Google Scholar
|
|
32
|
Natarajan K, Singh S, Burke TR Jr,
Grunberger D and Aggarwal BB: Caffeic acid phenethyl ester is a
potent and specific inhibitor of activation of nuclear
transcription factor NF-kappaB. Proc Natl Acad Sci USA.
93:9090–9095. 1996. View Article : Google Scholar
|
|
33
|
Lin HP, Jiang SS and Chuu CP: Caffeic acid
phenethyl ester causes p21 induction, Akt signaling reduction and
growth inhibition in PC-3 human prostate cancer cells. PLoS One.
7:e312862012. View Article : Google Scholar
|
|
34
|
Orban Z, Mitsiades N, Burke TR, Tsokos M
and Chrousos GP: Caffeic acid phenethyl ester induces leukocyte
apoptosis, modulates nuclear factor-kappaB and suppresses acute
inflammation. Neuroimmunomodulation. 7:99–105. 2000. View Article : Google Scholar
|
|
35
|
Irmak MK, Fadillioglu E, Sogut S, Erdogan
H, Gulec M, Ozer M, Yagmurca M and Gozukara ME: Effects of caffeic
acid phenethyl ester and alpha-tocopherol on reperfusion injury in
rat brain. Cell Biochem Funct. 21:283–289. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Altug ME, Serarslan Y, Bal R, Kontas T,
Ekici F, Melek IM, Aslan H and Duman T: Caffeic acid phenethyl
ester protects rabbit brains against permanent focal ischemia by
antioxidant action: A biochemical and planimetric study. Brain Res.
1201:135–142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kurauchi Y, Hisatsune A, Isohama Y,
Mishima S and Katsuki H: Caffeic acid phenethyl ester protects
nigral dopaminergic neurons via dual mechanisms involving haem
oxygenase-1 and brain-derived neurotrophic factor. Br J Pharmacol.
166:1151–1168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Istaphanous GK, Howard J, Nan X, Hughes
EA, McCann JC, McAuliffe JJ, Danzer SC and Loepke AW: Comparison of
the neuroapoptotic properties of equipotent anesthetic
concentrations of desflurane, isoflurane, or sevoflurane in
neonatal mice. Anesthesiology. 114:578–587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bloomfield SM, McKinney J, Smith L and
Brisman J: Reliability of S100B in predicting severity of central
nervous system injury. Neurocritical Care. 6:121–138. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang S, Peretich K, Zhao Y, Liang G, Meng
Q and Wei H: Anesthesia induced neurodegeneration in fetal rat
brains. Pediatr Res. 66:435–440. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li B, Du T, Li H, Gu L, Zhang H, Huang J,
Hertz L and Peng L: Signaling pathways for transactivation by
dexmedetomidine of epidermal growth factor receptors in astrocytes
and its paracrine effect on neurons. Br J Pharmacol. 154:191–203.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pearn ML, Hu Y, Niesman IR, Patel HH,
Drummond JC, Roth DM, Akassoglou K, Patel PM and Head BP: Propofol
neurotoxicity is mediated by p75 neurotrophin receptor activation.
Anesthesiology. 116:352–361. 2012. View Article : Google Scholar :
|
|
43
|
Creeley C, Dikranian K, Dissen G, Martin
L, Olney J and Brambrink A: Propofol induced apoptosis of neurones
and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br
J Anaesth. 110:i29–i38. 2013. View Article : Google Scholar
|
|
44
|
Liang G, Ward C, Peng J, Zhao Y, Huang B
and Wei H: Isoflurane causes greater neurodegeneration than an
equivalent exposure of sevoflurane in the developing brain of
neonatal mice. Anesthesiology. 112:1325–1334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dong Y, Zhang G, Zhang B, Moir RD, Xia W,
Marcantonio ER, Culley DJ, Crosby G, Tanzi RE and Xie Z: The common
inhalational anesthetic sevoflurane induces apoptosis and increases
beta-amyloid protein levels. Arch Neurol. 66:620–631. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zheng SQ, An LX, Cheng1 X and Wang YJ:
Sevoflurane causes neuronal apoptosis and adaptability changes of
neonatal rats. Acta Anaesthesiol Scand. 57:1167–1174. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Du T, Li B, Liu S, Zang P, Prevot V, Hertz
L and Peng L: ERK phosphorylationin intact, adult brain by
alpha(2)-adrenergic trans-activation of EGF receptors. Neurochem
Int. 55:593–600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang X, Wang J, Qian W, Zhao J, Sun L,
Qian Y and Xiao H: Dexmedetomidine inhibits tumor necrosis
factor-alpha and interleukin 6 inlipopolysaccharide-stimulated
astrocytes by suppression of c-Jun N-terminalkinases. Inflammation.
37:942–949. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang H, Liang G, Hawkins BJ, Madesh M,
Pierwola A and Wei H: Inhalational anesthetics induce cell damage
by disruption of intracellular calcium homeostasis with different
potencies. Anesthesiology. 109:243–250. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Flick RP, Katusic SK, Colligan RC, Wilder
RT, Voigt RG, Olson MD, Sprung J, Weaver AL, Schroeder DR and
Warner DO: Cognitive and behavioral outcomes after early exposure
to anesthesia and surgery. Pediatrics. 128:e1053–e1061. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bong CL, Allen JC and Kim JT: The effects
of exposure to general anesthesia in infancy on academic
performance at age 12. Anesth Analg. 117:1419–1428. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fang F, Xue Z and Cang J: Sevoflurane
exposure in 7 day-old rats affects neurogenesis, neurodegeneration
and neurocognitive function. Neurosci Bull. 28:499–508. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Oppenheim RW: Cell death during
development of the nervous system. Annu Rev Neurosci. 14:453–501.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rakic S and Zecevic N: Programmed cell
death in the developing human telencephalon. Eur J Neurosci.
12:2721–2734. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Blomgren K, Leist M and Groc L:
Pathological apoptosis in the developing brain. Apoptosis.
12:993–1010. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Loepke AW and Soriano SG: An assessment of
the effects of general anesthetics on developing brain structure
and neurocognitive function. Anesth Analg. 106:1681–1707. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gown AM and Willingham MC: Improved
detection of apoptotic cells in archival paraffin sections:
Immunohistochemistry using antibodies to cleaved caspase 3. J
Histochem Cytochem. 50:449–454. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhao H, Yenari MA, Cheng D, Sapolsky RM
and Steinberg GK: Bcl-2 overexpression protects against neuron loss
within the ischemic margin following experimental stroke and
inhibits cytochrome c translocation and caspase-3 activity. J
Neurochem. 85:1026–1036. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chong ZZ, Li F and Maiese K: Oxidative
stress in the brain: Novel cellular targets that govern survival
during neurodegenerative disease. Prog Neurobiol. 75:207–246. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hou J, Wang S, Shang YC, Chong ZZ and
Maiese K: Erythropoietin employs cell longevity pathways of SIRT1
to foster endothelial vascular integrity during oxidant stress.
Curr Neurovasc Res. 8:220–235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Koh PO: Nicotinamide attenuates the
ischemic brain injury-induced decrease of Akt activation and Bad
phosphorylation. Neurosci Lett. 498:105–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yon JH, Daniel-Johnson J, Carter LB and
Jevtovic-Todorovic V: Anesthesia induces neuronal cell death in the
developing rat brain via the intrinsic and extrinsic apoptotic
pathways. Neuroscience. 135:815–827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Peltier J, O'Neill A and Schaffer DV:
PI3K/Akt and CREB regulate adult neural hippocampal progenitor
proliferation and differentiation. Dev Neurobiol. 67:1348–1361.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wyatt LA, Filbin MT and Keirstead HS: PTEN
inhibition enhances neurite outgrowth in human embryonic stem
cell-derived neuronal progenitor cells. J Comp Neurol.
522:2741–2755. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ojeda L, Gao J, Hooten KG, Wang E,
Thonhoff JR, Dunn TJ, Gao T and Wu P: Critical role of
PI3K/Akt/GSK3β in motoneuron specification from human neural stem
cells in response to FGF2 and EGF. PLoS One. 6:e234142011.
View Article : Google Scholar
|
|
66
|
Luo HR, Hattori H, Hossain MA, Hester L,
Huang Y, Lee-Kwon W, Donowitz M, Nagata E and Snyder SH: Akt as a
mediator of cell death. Proc Natl Acad Sci USA. 100:11712–11717.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yeste-Velasco M, Folch J, Casadesús G,
Smith MA, Pallàs M and Camins A: Neuroprotection by c-Jun
NH2-terminal kinase inhibitor SP600125 against potassium
deprivation-induced apoptosis involves the Akt pathway and
inhibition of cell cycle reentry. Neuroscience. 159:1135–1147.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang W, Shi L, Xie Y, Ma C, Li W, Su X,
Huang S, Chen R, Zhu Z, Mao Z, et al: SP600125, a new JNK
inhibitor, protects dopaminergic neurons in the MPTP model of
Parkinson's disease. Neurosci Res. 48:195–202. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kuan CY and Burke RE: Targeting the JNK
signaling pathway for stroke and Parkinson's diseases therapy. Curr
Drug Targets CNS Neurol Disord. 4:63–67. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Walker CL, Walker MJ, Liu NK, Risberg EC,
Gao X, Chen J and Xu XM: Systemic bisperoxovanadium activates
Akt/mTOR, reduces autophagy and enhances recovery following
cervical spinal cord injury. PLoS One. 7:e300122012. View Article : Google Scholar
|
|
72
|
Guan QH, Pei DS, Zhang QG, Hao ZB, Xu TL
and Zhang GY: The neuroprotective action of SP600125, a new
inhibitor of JNK, on transient brain ischemia/reperfusion-induced
neuronal death in rat hippocampal CA1 via nuclear and non-nuclear
pathways. Brain Res. 1035:51–59. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Han JY, Jeong EY, Kim YS, Roh GS, Kim HJ,
Kang SS, Cho GJ and Choi WS: C-jun N-terminal kinase regulates the
interaction between 14-3-3 and bad in ethanol-induced cell death. J
Neurosci Res. 86:3221–3229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Fan J, Xu G, Nagel DJ, Hua Z, Zhang N and
Yin G: A model of ischemia and reperfusion increases JNK activity,
inhibits the association of bad and 14-3-3 and induces apoptosis of
rabbit spinal neurocytes. Neurosci Lett. 473:196–201. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Liao Z, Cao D, Han X, Liu C, Peng J, Zuo
Z, Wang F and Li Y: Both JNK and P38 MAPK pathways participate in
the protection by dexmedetomidine against isoflurane-induced
neuroapoptosis in the hippocampus of neonatal rats. Brain Res Bull.
107:69–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zheng S and Zuo Z: Isoflurane
preconditioning induces neuroprotection against ischemia via
activation of P38 mitogen-activated protein kinases. Mol Pharmacol.
65:1172–1180. 2004. View Article : Google Scholar : PubMed/NCBI
|