Introduction
Malignant melanoma is the most aggressive form of
cutaneous malignancy, and accounts for ~3% of all cases of
malignant tumor (1). The global
incidence of melanoma has been rising, including an annual increase
of between 3 and 7% for Caucasians in developed countries (2). Although melanoma accounts for only 4%
of all skin cancer cases, it is responsible for ~80% of all skin
cancer-related cases of mortality due to its extreme aggressiveness
and resistance to current therapeutic agents (3). To date, a number of promising
targeted agents, including the B-Raf inhibitors vemurafenib and
dabrafenib, are undergoing or have completed phase III clinical
trials; however, the majority of these have been demonstrated to be
ineffective and the 5-year survival rate of the vast majority of
patients diagnosed with metastatic melanoma is <5% (4). Therefore, novel treatment strategies
are required for patients with metastatic melanoma.
Malignant transformation is often characterized by
major changes in the organization of the cytoskeleton, decreased
cell-to-cell adhesion and increased cell motility. The loss of the
cellular adhesion molecule and single-span transmembrane
glycoprotein, E-cadherin, is positively associated with tumor
invasiveness, metastatic dissemination and poor patient prognosis
(5). E-cadherin is expressed in
epithelial cells, and intercellular homophilic interactions with
E-cadherin expressed by neighboring cells, leads to the formation
of cell-to-cell adherens junctions (AJs) and a tight polarized cell
layer (6). Loss of E-cadherin
expression triggers epithelial-to-mesenchymal transition (EMT),
which provides cancer cells with increased motility and
invasiveness (7). Clinical studies
have shown that the majority of patients with melanoma treated with
targeted B-Raf proto-oncogene, serine/threonine kinase inhibitors,
such as vemurafenib and dabrafenib, develop resistance to these
therapies after only a several months due to decreased E-cadherin
expression in the tumor tissues of patients (8). β-Catenin, the principal effector of
the Wnt signaling cascade, serves a crucial role in morphogenesis
and human cancer through its dual function in mediating
cell-to-cell AJs, and as a signaling molecule in numerous signaling
pathways (9). β-catenin forms an
AJ complex with E-cadherin through binding to the intracellular
domain of E-cadherin, which sequesters it at the plasma membrane
and hinders its entry into the nucleus (10). Numerous studies have demonstrated
that β-catenin signaling is implicated in melanoma progression and
metastasis (11,12). In recent years, a number of
plant-derived natural products have been described as potential
alternative therapies for metastatic melanoma, that function by
increasing E-cadherin expression and by disrupting the β-catenin
signaling pathway (13). For
instance, catechins, a class of flavonoids, exhibit anti-melanoma
activity through the restoration of E-cadherin and the suppression
of N-cadherin expression levels (14). In addition, the flavonoid complex
silymarin, blocks the invasion of melanoma cells by inhibiting
β-catenin translocation (15).
Therefore, inhibiting the proliferative, migratory and invasive
capacity of cancer cells with chemopreventative dietary factors,
may provide a potential strategy for melanoma prevention and/or
treatment.
Among the dietary factors, n-3 polyunsaturated fatty
acids (PUFAs) have gained attention as potential preventative
and/or adjuvant agents in the treatment of malignant melanoma
(16). A number of preclinical
in vitro and in vivo studies have attempted to
clarify the possible mechanisms underlying the proposed anticancer
effects of eicosapentaenoic acid (EPA) and docosahexaenoic acid
(DHA), which are the two principal n-3 PUFAs found in fish and
seafood (17,18). A number of published preclinical
and epidemiological studies provide evidence to suggest that
dietary or exogenously derived fatty acids may serve a vital role
in the development and progression of malignant melanoma (19,20);
however, conflicting results and discrepancies between studies
preclude definitive conclusions (21,22).
For instance, Kirkpatrick et al (23) reported no association between the
consumption of PUFAs and melanoma risk, whereas, Salem et al
(24) demonstrated that fish oil
supplementation of B16 melanoma-bearing mice enhanced melanoma cell
growth and metastases and decreased mouse survival rates. These
conflicting results reflect numerous confounding dietary elements.
Indeed, fish and corn oil, which are generally used to investigate
the effects of the ratio of n-3/n-6 PUFAs on disease development
and progression, consist not only of n-3 and n-6 PUFAs, but also
additional fatty acids and lipid soluble vitamins (25).
In the present study, the potential of high n-3
PUFAs supplementation in the prevention of melanoma cell growth was
investigated using omega-3 fatty acid desaturase (fat-1) transgenic
mouse xenografts, which carry the fat-1 gene from the
Caenorhabditis elegans roundworm (26). This gene, which is not present in
mammals, encodes an n-3 PUFA desaturase that catalyzes the
conversion of n-6 to n-3 PUFAs. Therefore, the fat-1 transgenic
mice were endogenously enriched with n-3 PUFAs and were
characterized by a lower ratio of n-6/n-3 PUFAs compared with their
wild-type (WT) littermates, by using a single diet high in n-6
PUFAs. The need for dietary n-3 PUFAs supplementation was
eliminated and corresponding confounding factors of diet were
removed. Therefore, the fat-1 transgenic mouse model is ideal for
addressing the effects of the tissue n-6/n-3 fatty acid ratio in
melanoma tumorigenesis.
Endogenous n-3 PUFAs have been previously reported
to exert their antitumor effects in melanoma through the
prostaglandin (PG) E3-mediated activation of the phosphatase and
tensin homologue deleted on chromosome 10 signaling pathway in
fat-1 transgenic mice injected subcutaneously with melanoma B16-F0
cells (27). However, whether n-3
PUFA-derived lipid mediators and the E-cadherin/β-catenin signaling
pathway are associated with n-3 PUFA-mediated antitumor effects in
malignant melanoma remains largely unknown. In the present study,
B16-F10 mouse melanoma cells, which have a higher metastatic
potential compared with B16-F0 melanoma cells, were injected into
fat-1 transgenic and WT mice in order to evaluate its
tumorigenicity. The results demonstrated that increased E-cadherin
expression, inhibition of the β-catenin signaling pathway and
biosynthesized n-3 PUFA-derived bioactive mediators, are involved
in the antitumor effects of n-3 PUFAs in melanoma.
Materials and methods
Cells and reagents
The murine B16-F10 melanoma cell line (American Type
Culture Collection, Manassas, VA, USA) was maintained in a
humidified incubator at 37°C and 5% CO2 in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), supplemented with 10% fetal bovine serum.
The primary antibodies, mouse anti-E-cadherin (catalog no. 14472),
mouse anti-N-cadherin (catalog no. 14215), rabbit anti-β-catenin
(catalog no. 9582), rabbit anti-zinc finger E-box 1 (ZEB1; catalog
no. 3396), rabbit anti-Snail (catalog no. 3879), rabbit anti-Slug
(catalog no. 9585), rabbit anti-c-Myc (catalog no. 13987), rabbit
anti-Akt (pan; catalog no. 4685), rabbit anti-phosphorylated
(p)-Akt (Thr308; catalog no. 13038), rabbit anti-nuclear factor κB
p65 (NF-κB p65; catalog no. 2765), rabbit anti-p-NF-κB p65 (Ser536;
catalog no. 3033), rabbit anti-glycogen synthase kinase-3β (GSK-3β;
catalog no. 9315), rabbit anti-p-GSK-3β (Ser9; cataolog no. 9322),
rabbit anti-signal transducer and activator of transcription 3
(STAT3; catalog no. 12640), rabbit anti-p-STAT3 (Tyr705; catalog
no. 9145), rabbit anti-epidermal growth factor receptor (EGFR;
catalog no. 4267), rabbit anti-GAPDH (catalog no. 5174) and the
horseradish peroxidase-linked secondary antibodies, anti-rabbit IgG
(catalog no. 7074) and anti-mouse IgG (catalog no. 7076) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
The primary mouse anti-twist (catalog no. ab175430) and rabbit
anti-smad interacing protein 1 (ZEB2; catalog no. ab138222)
antibodies were obtained from Abcam (Cambridge, MA, USA). Primary
and secondary antibodies were used at dilutions of 1:1,000 and
1:4,000, respectively. Vitamin E (Vit E) powder was purchased from
Tianjin Jianfeng Natural Product R&D Co., Ltd. (Tianjin,
China). Chloral hydrate was purchased from Shanghai Meilian
Biotechnology Co., Ltd. (Shanghai, China).
Animals and diet
The fat-1 transgenic mice were donated by Dr Jing X.
Kang at Massachusetts General Hospital and Harvard Medical School
(Boston, MA, USA). A total of 13 male heterozygous fat-1 mice were
bred with 26 WT C57BL/6 female mice (26). Each mouse was genotyped and
phenotyped by polymerase chain reaction and gas chromatography
using isolated DNA and total lipids from mice tails, respectively.
Specific pathogen-free transgenic and WT animals were fed a 10%
safflower oil diet, and housed in standard cages in temperature
under humidity-controlled conditions with a 12-h light/dark cycle.
At 10–12 weeks of age, heterozygous fat-1 male mice and
nontransgenic littermate controls were used for the purposes of the
present study. Their diets (per 100 g) consisted of 4.5 g sucrose,
18.6 g casein, 8.6 g cellulose, 50 g wheat starch, 0.3 g
DL-methionine, 7 g mineral mix, 1 g vitamin mix and 10 g safflower
oil. A total of 10 fat-1 and 10 WT mice were assigned to Vit E
groups and another 10 fat-1 and 10 WT mice were assigned to
negative control groups. The Vit E-treatment group was administered
with 100 IU/kg Vit E (dissolved in sterile water) via oral gavage
for 3 weeks. Sterile water was administered in the same manner to
the control group. All animal experiments were conducted in
accordance with the guidelines for the use and care of laboratory
animals, and approved by the Ethical Committee of Ningbo University
(Ningbo, China).
Cell injections and tumor
measurement
Cultured B16-F10 melanoma cells were collected by
trypsin digestion (0.05% trypsin-EDTA) for 2 min and washed twice
with DMEM before the cell number was ascertained. Each male mouse
was injected subcutaneously with 2.5×106 cells suspended
in 100 μl DMEM into the area overlying the abdomen. The day
of the injection of B16-F10 cells was designated day 0. Tumor
volume was assessed by conducting caliper measurements every 2
days, and calculated according to the following formula: Tumor
volume = Shortest diameter2 × the largest diameter ×
0.5. Following 15 days of cell injection, the mice were
anesthetized with an intraperitoneal injection of 350 mg/kg chloral
hydrate and sacrificed by exsanguination. The tumors were removed
and stored at −80°C for downstream analyses.
Analysis of fatty acids and lipid
mediators
Fatty acid composition was determined using gas
chromatography (GC) as described previously (28). Briefly, tissues were ground to a
powder form using liquid nitrogen, and the methyl esters of the
fatty acids from the lipid extract were transesterified with
H2SO4 in methanol (5%, v/v), together with
toluene, in sealed tubes at 70°C for 2 h. Fatty acid methyl esters
were analyzed using the Shimadzu GC-14C gas chromatograph (Shimadzu
Co., Kyoto, Japan) equipped with a flame-ionization detector and a
60 m × 0.25 mm (internal diameter) ×0.25 μm (film thickness)
fused silica bonded phase column (DB-23; Agilent Technologies,
Inc., Santa Clara, CA, USA). The fatty acid methyl esters were
identified by comparing sample mixtures with standard mixtures of
fatty acid methyl esters (Nu-Chek Prep, Inc., Elysian, MN, USA). A
1 μl aliquot of each sample was injected onto the column.
Nitrogen was the carrier gas and the flow rate was 4 ml/min. The
injector and detector temperature was 270°C. The column temperature
was 180°C and held for 10 min; the temperature was then increased
to 200°C at a rate of 20°C/min and held for 10.5 min; the
temperature was further increased to 215°C at a rate of 20°C/min
and held for 5 min; finally, it was increased to 215°C at a rate of
20°C/min and held for 4.75 min. Quantification of sample fatty acid
compositions was achieved by comparing peak areas with the
nonadecanoic acid internal standard (Sigma-Aldrich, St. Louis, MO,
USA), which was added to the samples (1 mg internal standard/500 mg
sample) prior to extraction. The composition of fatty acids was
expressed as the relative percentage of the total fatty acids
according to their peak areas.
Lipid mediators from n-3 and n-6 fatty acids were
identified using liquid chromatography (LC)-tandem mass
spectrometry (MS) methods as described previously (29). In brief, each tumor sample was
homogenized in 2 ml 15% v/v ice-cold methanol. The internal
standards PGB2-d4, PGD2-d4,
15-HETE-d8 and resolvin (RV) E1-d4 (20 ng per
sample each) were added to each sample and n-3 and n-6 fatty acid
mediators were extracted using Strata-X reversed-phase SPE columns
(8B-S100-UBJ; Phenomenex, Torrance, CA, USA). An Acquity ultra
performance (UP) LC system (Waters Corporation, Milford, MA, USA)
was used. Reversed-phase separation was performed on an Acquity
UPLC BEH shield RP18 column [2.1×100 mm (internal diameter); 1.7
μm (film thickness); Waters Corporation]. The mobile phase
consisted of (A) acetonitrile/water/acetic acid (60/40/0.02% v/v)
and (B) acetonitrile/isopropenyl acetate (50/50% v/v). Gradient
elution was carried out for 5 min at a flow rate of 0.5 ml/min.
Gradient conditions were as follows: 0–4.0 min, 0.1–55% B; 4.0–4.5
min, 55–99% B; 4.5–5.0 min, 99% B. A 10 μl aliquot of each
sample was injected onto the column. The column temperature was
maintained at 40°C. Mass spectrometry was performed on an ABI/Sciex
6500 QTRAP hybrid, triple quadrupole, linear ion trap mass
spectrometer (AB Sciex, Framingham, MA, USA) equipped with a Turbo
V ion source. Lipid mediators were detected in negative
electrospray ion mode. Curtain gas, nebulizer gas and turbo-gas
were set at 10, 30 and 30 psi, respectively. The electrospray
voltage was −4.5 kV, and the turbo ion spray source temperature was
525°C, and a methanol:water:acetate (60:40:0.02%) mobile phase was
used with a 0.5 ml/min flow rate. The following multiple reaction
monitoring transitions were used: PGE2 m/z
351>271, PGE3 m/z 349>269,
12-hydroxyeicosapentaenoic acid (HEPE) m/z
317>179, 15-HEPE m/z 317>219, RVD2
m/z 375>175, RVE1 m/z 349>195 and
Maresin1 m/z 359>177.
Western blot analysis
Tumor tissues (30 mg) were homogenized in Triton-X
protein lysis buffer (20 mM Tris-HCl, 1 mM EDTA, 140 mM NaCl, 1%
NonidetP-40, 1% aprotinin, 1 mM phenylmethylsulfonyfluoride and 1
mM sodium vanadate; pH 7.6). The sample protein concentration was
determined using a bicinchoninic acid protein assay. Aliquots of
protein (50 μg) were fractionated by 10% SDS-PAGE and
transferred onto nitrocellulose membranes. After nonspecific
binding sites were blocked with 5% nonfat milk in Tris-buffered
saline, blots were incubated overnight at 4°C with primary
antibodies, and then incubated for 1 h at room temperature with
horseradish peroxidase-conjugated secondary antibodies. Reactive
protein bands were analyzed using enhanced chemiluminescence.
Lipid peroxidation analysis
Lipid peroxidation in urine samples was determined
using an malondialdehyde (MDA) assay kit (Beyotime Institute of
Biotechnology, Haimen, China) according to the manufacturer's
instructions. Thiobarbituric acid (TBA; 200 μl) reagent was
added to 100 μl urine sample. The reaction mixture was
subsequently incubated in a water bath at 100°C for 15 min. After
cooling to room temperature, the mixture was centrifuged at 1,000 ×
g for 10 min at room temperature and the supernatant was separated,
before the absorbance was read at 532 nm. The concentration of MDA
was determined using a standard curve.
Statistical analysis
For each treatment group, data were presented as the
arithmetical mean ± standard error. Statistical significance
between groups was determined using the Student's t-test.
P<0.05 and P<0.01 was considered to indicate a statistically
significant difference.
Results
n-3 PUFAs inhibited the tumorigenicity of
B16-F10 melanoma cells in fat-1 transgenic mice
In order to test the hypothesis that a balanced
n-6/n-3 fatty acid ratio in fat-1 transgenic mice is associated
with a decreased risk of melanoma, B16-F10 murine melanoma cells
were injected into transgenic fat-1 and WT mice, and the
tumorigenicity of inoculated tumor cells was examined. As shown in
Fig. 1, a marked difference in the
tumor growth rate was observed between fat-1 transgenic (n=10) and
WT mice (n=10). Over an observation period of 15 days, all mice
initially developed a palpable tumor by day 7. The tumors in fat-1
transgenic mice exhibited a markedly slower growth rate when
compared to those in WT mice.
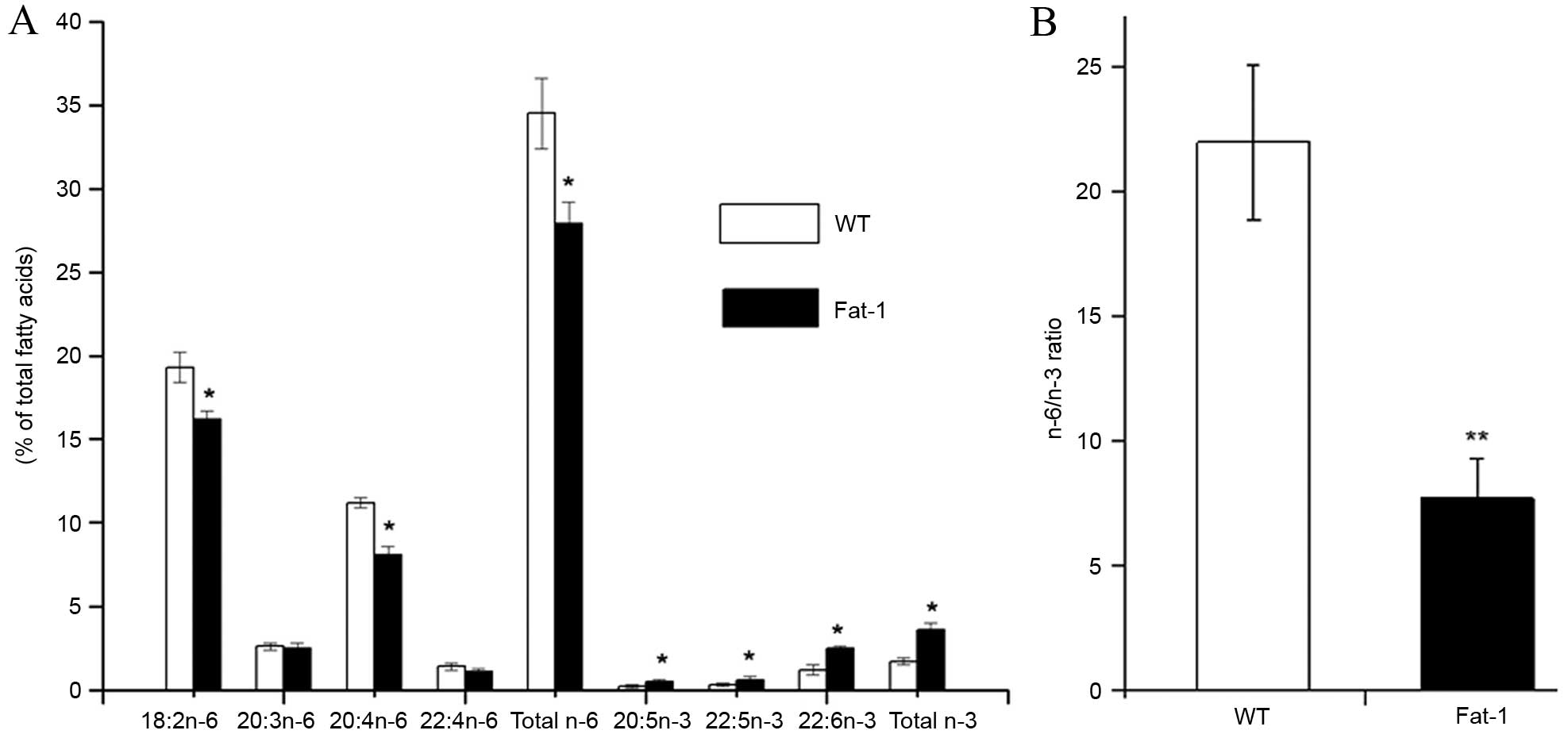
Fatty acid profiles in the tumor tissues
of fat-1 and WT mice
Analysis of the total lipids extracted from the
tumor tissues of fat-1 and WT mice revealed distinct lipid profiles
between the two groups (Fig. 2).
Compared with WT mice, fat-1 transgenic mice displayed
significantly increased levels of n-3 fatty acids, including
20:5n-3 (EPA; P=0.0441), 22:5n-3 [docosapentaenoic acid (DPA;
P=0.0482)] and 22:6n-3 (DHA; P=0.0151), and exhibited decreased
concentrations of n-6 fatty acids, including 18:2n-6 (linoleic
acid), 20:3n-6 [dihomo-gamma-linolenic acid (DGLA)], 20:4n-6
[arachidonic acid (AA)] and 22:4n-6 (adrenic acid) in the tumor
tissues (Fig. 2A). In particular,
the levels of linoleic acid (P=0.0356) and AA (P=0.0122) n-6 fatty
acids were significantly reduced in tumors from fat-1 mice compared
with WT animals. As shown in Fig.
2B the n-6/n-3 fatty acid ratio in tumors from fat-1 mice
(7.7±1.6) was significantly lower than that of WT mice (22.0±3.1;
P=0.0097), despite the animals being fed the same diet. These
results indicate the fat-1 transgene is functionally active in
vivo, and endogenously catalyzes the conversion of n-6 to n-3
PUFAs.
Lipid mediators derived from AA (prostanoids,
leukotrienes, lipoxins and epoxyeicosatrienoic acids), EPA
(E-series resolvins) and DHA (D-series resolvins, protectins and
maresins) exert their potent actions through the promotion or
resolution of inflammation (30).
Therefore, the levels of n-6 and n-3 PUFA-derived lipid mediators
in the melanoma tumor tissues of WT and fat-1 transgenic mice were
determined using UPLC-UV-tandem MS analysis, in order to determine
whether the observed difference in tumor growth between these two
groups was associated with differences in the levels of lipid
mediators. As shown in Fig. 3, a
significant increase in the levels of fatty acid metabolites
derived from EPA, [12-HEPE (P=0.0187), 15-HEPE (P=0.0224), PGE3
(P=0.0320) and RVE1 (P=0.0098)] and DHA (RVD2 (P=0.0092) and
Maresin1 (P=0.042)) were observed in tumors from fat-1 mice
compared with WT controls. In contrast, no significant difference
in the levels of proinflammatory PGE2 was observed between fat-1
and WT mice.
Differential protein expression levels of
E-cadherin and its master regulators in fat-1 transgenic and WT
mice
In order to determine whether fat-1 expression, and
the associated increase in n-3 PUFA levels, affects E-cadherin and
N-cadherin expression levels, melanoma tumor tissues from
transgenic fat-1 mice and WT controls were analyzed for the
expression of E-cadherin and N-cadherin by western blotting. As
shown in Fig. 4, the protein
expression levels of E-cadherin and N-cadherin were markedly
upregulated and downregulated in the tumor tissues of fat-1 mice
compared with those of the WT controls, respectively. This implies
that E-cadherin and N-cadherin expression may be modulated by n-3
PUFAs. Several transcription factors, including Snail, Slug, ZEB1,
ZEB2 and Twist, function as transcriptional repressors of
E-cadherin (31). Therefore,
western blotting was used to investigate whether the observed
increase in E-cadherin expression levels in the melanoma tissues of
fat-1 mice may be mediated by alterations in the protein expression
levels of these transcriptional repressors. As shown in Fig. 4, the protein expression levels of
ZEB1 and Snail were markedly decreased in the tumors of fat-1 mice
compared with those of WT mice. In contrast, no significant
alterations in the expression of Slug, ZEB2 and Twist proteins were
observed (data not shown). The transcription factors NF-κB and
STAT3 are activated in a range of human cancers and are thought to
promote tumorigenesis, in part, through their ability to regulate
the expression levels of E-cadherin transcriptional repressors
(32). As shown in Fig. 4, fat-1 mouse tumor tissues
exhibited a slight decrease in NF-κB protein expression levels, but
a marked decrease in p-NF-κB (Ser536), compared to those from WT
mice. Similarly, despite observing no significant alterations in
the expression levels of STAT3, p-STAT3 (Tyr705) expression was
substantially decreased in the tumor tissues of fat-1 mice. These
results suggest that NF-κB and STAT3 may be involved in modulating
E-cadherin expression.
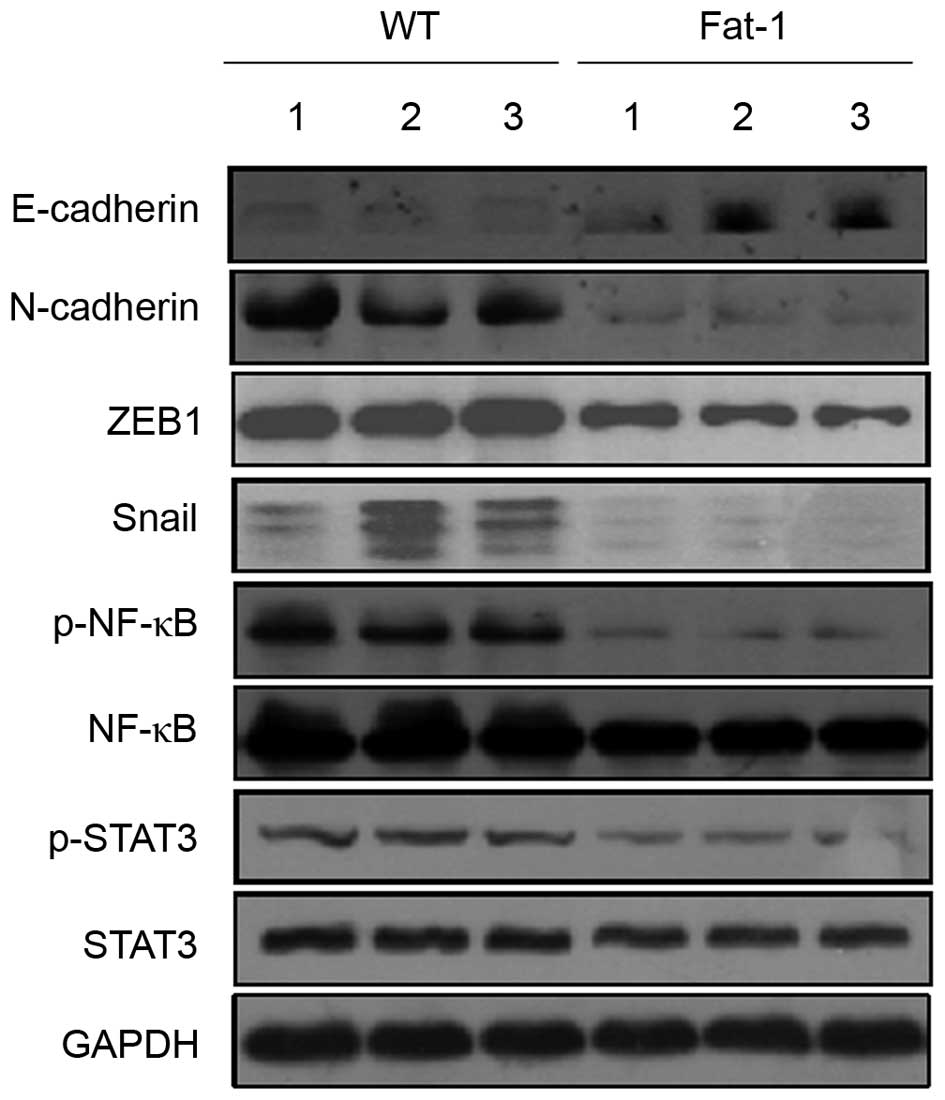 | Figure 4Endogenous n-3 PUFAs may upregulate
E-cadherin expression by inhibiting the expression of its master
regulators. Western blotting analysis of E-cadherin, N-cadherin,
Snail, ZEB1, NF-κB, p-NF-κB, STAT3 and p-STAT3 protein expression
levels in B16-F10 mouse melanoma tumor tissues from three WT (lanes
1–3) and three transgenic fat-1 mice (lanes 4–6). PUFAs,
polyunsaturated fatty acids; ZEB1, zinc finger E-box 1; NF-κB,
nuclear factor κB; STAT3, signal transducer and activator of
transcription 3; p-NF-κB, phosphorylated nuclear factor κB; WT,
wild-type; fat-1, omega-3 fatty acid desaturase. |
Inhibition of EGFR/Akt/β-catenin
signaling pathway by n-3 PUFAs in fat-1 transgenic mice
β-catenin functions as a transcriptional regulator
in the canonical and noncanonical Wnt signaling pathways, and
serves a critical role in regulating development, cell
proliferation, differentiation and neoplastic transformation
(10). To determine whether the
low n-6/n-3 PUFA ratio in transgenic fat-1 mice affects β-catenin
signaling pathways, western blotting was used to examine the
protein expression levels of β-catenin and c-Myc; a known
transcriptional target of β-catenin. As shown in Fig. 5, fat-1 tumor tissues exhibited a
marked decrease in the expression levels of β-catenin and c-Myc
compared with those from WT mice. β-catenin is activated, not only
by Wnt ligands, but also by additional signaling pathways, such as
the EGFR/Akt/GSK-3β pathway (33).
Cytoplasmic β-catenin is controlled by the GSK-3β-containing
destruction complex. When GSK-3β is phosphorylated and inactivated,
cytoplasmic β-catenin is stabilized and translocated to the nucleus
(34). To determine whether
β-catenin degradation may occur through inhibition of the
phosphorylation of GSK-3β and upstream kinases, EGFR and Akt, the
protein expression levels of GSK-3β, p-GSK-3β, EGFR, Akt and p-Akt
were examined. As shown in Fig. 5
no significant differences in GSK-3β and Akt protein expression
levels were observed in the melanoma tumor tissues of fat-1 and WT
mice, whereas the expression levels of p-GSK-3β, p-Akt and EGFR
were markedly decreased in fat-1 tissues compared with those of WT
mice. This indicates that the EGFR/Akt/GSK-3β signaling pathway may
be involved in controlling endogenous n-3 PUFA-induced β-catenin
degradation.
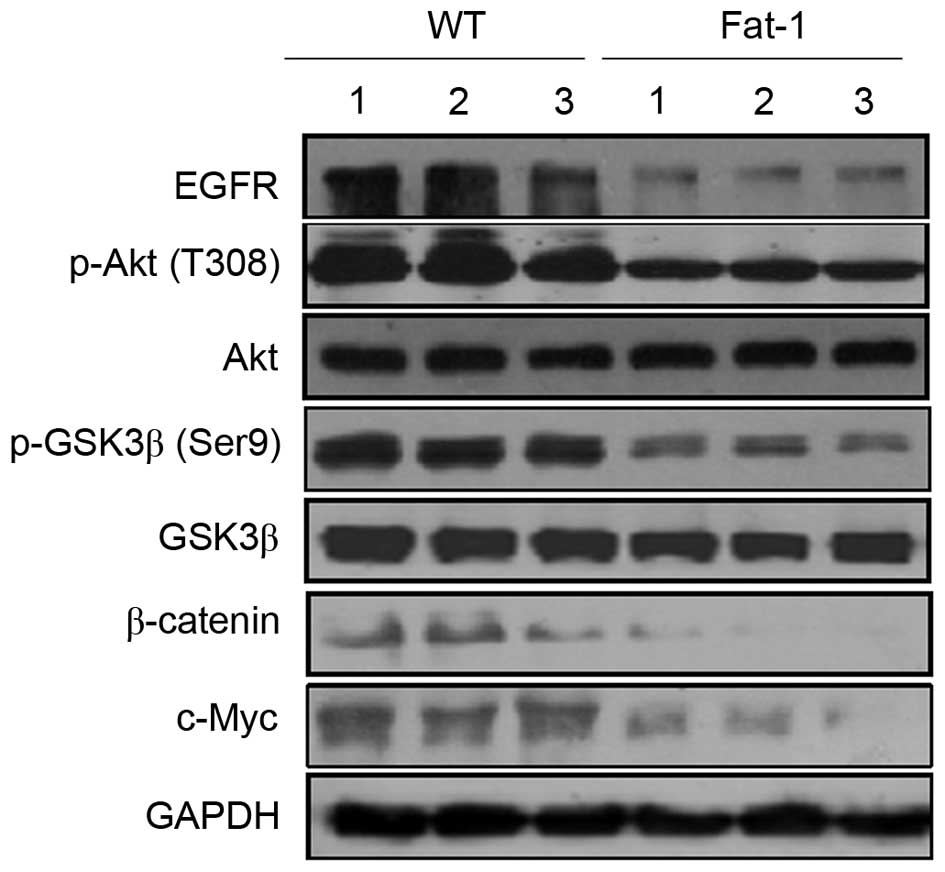 | Figure 5Endogenous n-3 PUFAs modulate
β-catenin signaling. Western blotting analysis of β-catenin, c-Myc,
EGFR and Akt, p-Akt, GSK-3β and p-GSK-3β protein expression levels
in B16-F10 mouse melanoma tumor tissues from three WT (lanes 1–3)
and three fat-1 transgenic mice (lanes 4–6). PUFAs, polyunsaturated
fatty acids; EGFR, epidermal growth factor receptor; p-Akt,
phosphorylated Akt; GSK-3β, glycogen synthase kinase-3β; WT,
wild-type; fat-1, omega-3 fatty acid desaturase. |
Tumor growth inhibition was not
associated with n-3 PUFA-induced oxidative stress in fat-1
mice
A lipid peroxidation assay was performed to
determine lipid peroxidation levels in fat-1 and WT mice with or
without Vit E administration. As shown in Fig. 6A, the urine from fat-1 mice
demonstrated an increased level of MDA compared with that of WT
mice (20.3±2.1 vs. 11.7±2.7 μM; P=0.0293). Notably, no
significant difference in the levels of MDA was observed between
fat-1 and WT mice (12.9±3.2 vs. 8.3±1.8 μM) following the
administration of Vit E. In addition, tumor growth was suppressed
in fat-1 and WT mice, and fat-1 mice exhibited decreased tumor
growth compared with WT-mice following Vit E supplementation
(Fig. 6B).
Discussion
Malignant melanoma is one of the most common
life-threatening cancers among young western populations due to its
rapid progression and high metastatic potential (35). The development of new therapies for
malignant melanoma, including immunotherapy and targeted-molecular
therapy, faces challenges due to the occurrence of drug resistance
and harmful side effects (8).
Notably, there is increasing evidence that natural products, such
as dietary lipids, are considered to be of crucial importance in
regulating melanoma cell growth (36). However, the molecular mechanisms
underlying the role of n-3 PUFAs in melanoma development,
progression and prevention are not fully understood. In the present
study, to evaluate the antitumor effects of n-3 PUFAs on melanoma
in vivo, B16-F10 mouse melanoma cells, which metastasize to
the lungs, were injected into fat-1 and WT mice. The fat-1
transgenic mouse is a suitable experimental model as the mice can
maintain a balanced n-6/n-3 fatty acid ratio in all organs and
tissues, due to the ubiquitous expression of the Caenorhabditis
elegans desaturase gene (26).
The genetic approach presented in this study enabled the production
of two different fatty acid profiles in tumor tissues whilst using
identical diets rich in linoleic acid (18:2, n-6) but lacking in
n-3 fatty acids. Although the formation of tumors was not
significantly inhibited in fat-1 transgenic mice compared with
those of WT mice, endogenous production of n-3 PUFAs in fat-1 mice
markedly decreased the growth rate of the xenografts. Moreover,
melanoma tumor growth inhibition in fat-1 mice was correlated with
the production of n-3 PUFA-antitumor derivatives, the modulation of
E-cadherin expression and inhibition of β-catenin signaling.
E-cadherin is an important adhesion molecule in
epithelial cells that functions to mediate cell-to-cell adhesion
and is a potent invasion/tumor suppressor (5). In the present study, B16-F10 melanoma
tumors from fat-1 mice displayed a significant upregulation in the
protein expression levels of the E-cadherin epithelial biomarker
and a marked decrease in N-cadherin expression (a marker of
mesenchymal stage), compared with those of WT mice. This suggests
that the reverse EMT process, known as mesenchymal-epithelial
transition, may have occurred in fat-1 tumor tissues. Consistent
with these results, recent studies have demonstrated that breast
and colorectal cancer tissues from fat-1 mice display increased
levels of E-cadherin expression, which indicates that its
expression may be modulated by endogenous n-3 PUFAs (37,38).
However, in the present study, the formation of metastases in the
lungs or other organ sites in fat-1 and WT mice was not observed
(data not shown). One possible reason for this is that the
subcutaneous tumor model may not favor lung metastases of B16-F10
cells, compared with the intravenous injection tumor model. In
addition, it is virtually impossible for cancer cells to
disseminate from a primary tumor to a more distant tissue site
within a period of 15 days (39).
Therefore, further studies to investigate the anti-metastatic
activities of n-3 PUFAs using the intravenous injection tumor model
are required. In the present study, the protein expression levels
of the transcriptional repressors of E-cadherin, ZEB1 and Snail,
were markedly decreased, suggesting that ZEB1 and Snail may have
been involved in regulating E-cadherin expression in the fat-1
tumor tissues. Consistent with these results, Galindo-Hernandez
et al (40) reported that
the expression of Snail and Twist was correlated with linoleic acid
(n-6 PUFAs) -mediated downregulation of E-cadherin expression in
breast cancer cells. NF-κB and STAT3 transcription factors are
principle regulators of inflammation, and have been implicated in
the pathogenesis of immune disorders and cancer (41). The results of the present study
demonstrated that the mouse melanoma tumor tissues from fat-1 mice
exhibited lower levels of p-NF-κB and p-STAT3 protein expression
compared with those of WT mice. NF-κB and STAT3 have been
identified as important regulators of EMT in several different
types of cancer (42). Therefore,
it is possible that NF-κB and STAT3 modulated E-cadherin expression
in fat-1 mice, through regulating the transcriptional repressors of
E-cadherin.
A notable finding of the present study was the
observed inhibition of the β-catenin signaling pathway in fat-1
mice. β-catenin, a key component of the Wnt signaling pathway and
the cadherin/catenin-based adhesion process, serves an important
role in the development of melanoma and its evasion of the immune
system (43,44). Therefore, we hypothesize that the
observed disruption to the β-catenin signaling pathway may be
attributed to the effective chemopreventative and antitumorigenic
effects of n-3 PUFAs. Decreased tumor growth was associated with
fat-1-mediated downregulation of β-catenin, p-GSK-3β and c-Myc
protein expression levels. These results are consistent with those
of other studies demonstrating that endogenous n-3 PUFAs were
associated with a reduction in p-GSK-3β levels and a subsequent
downregulation of β-catenin in prostate and pancreatic cancer
tissues, which supports our hypothesis that the antitumor effects
of n-3 PUFAs in melanoma may involve GSK-3β-mediated β-catenin
degradation (45,46). In contrast, Castellone et al
(47) demonstrated that PGE2, an
AA (n-6 PUFA) -derived prostanoid, enhances the stability of
β-catenin through promoting GSK-3β phosphorylation. n-3 PUFAs are
readily incorporated into cell membranes and lipid rafts, and their
incorporation may lead to changes in the levels of
membrane-associated signaling proteins, including Ras, Akt, EGFR
and human epidermal growth factor receptor 2 (48). In the present study and the study
by Pai et al (49), the
observed reductions in the expression levels of EGFR and p-Akt
protein in fat-1 tumor tissues suggest that the EGFR/Akt signaling
pathway may be involved in n-3 PUFA-mediated β-catenin degradation.
In addition, increased E-cadherin expression may also account for
the decreased protein expression levels of c-Myc through
sequestering β-catenin in the plasma membrane, thereby blocking its
translocation to the nucleus. Ultimately, the results of the
present study suggest that β-catenin signaling inhibition
contributes to the preventive effect of n-3 PUFAs against
melanoma.
It is generally accepted that n-3 PUFAs exert their
potentially beneficial anti-inflammatory effects by decreasing the
levels of AA-derived mediators, such as the predominant
proinflammatory prostanoids PGE2 and PGI2 (50). In the present study, no notable
alterations in the levels of PGE2 between fat-1 and WT mice were
observed. In contrast, marked differences in the levels of EPA
metabolites (PGE3, 12-HEPE, 15-HEPE and RVE1) and DHA-derived
mediators (RVD2 and Maresin1) were observed in the tumors of fat-1
mice compared with those of WT mice. Preclinical and clinical
trials have provided evidence to show that EPA and DHA-derived
lipid mediators exert their potential anti-inflammatory effects in
a wide range of inflammatory diseases, including arthritis, lung
inflammation and colorectal cancer (51). The decreased expression levels of
p-NF-κB and p-STAT3 in the melanoma tumors of fat-1 mice in the
present study, confirmed the anti-inflammatory effects of n-3
PUFA-derived mediators. Taking these findings into account, it is
possible that the endogenously biosynthesized lipid mediators
derived from n-3 PUFAs underlie the antitumor effects observed in
the fat-1 transgenic mice, instead of the decrease in AA-derived
mediators, such as 2-series prostaglandins (PGE2).
Oxidative stress has been reported to serve an
important role in tumor initiation and progression (52). The excessive intracellular
accumulation of reactive oxygen species leads to disruption of the
mitochondrial membrane potential, the release of cytochrome c and
ultimate cell apoptosis (53). DHA
and EPA unsaturated fatty acids are susceptible to lipid
peroxidation, which can lead to high levels of oxidative stress,
cell growth inhibition and cell death (54). Indeed, in the present study, a
significant increase in MDA levels was observed in the urine of
fat-1 mice compared with that of WT mice. However, no significant
alterations in the MDA levels between fat-1 and WT mice were
observed following administration of the Vit E antioxidant, which
suggests that the lipid peroxidation induced by endogenous PUFAs
may be counteracted by Vit E. Notably, tumor growth was suppressed
in fat-1 and WT mice following Vit E administration, and tumor
growth inhibition was greater in fat-1 mice supplemented with Vit E
compared with WT controls. Ultimately, these data suggest that the
protective role of n-3 PUFAs against melanoma progression, may not
be mediated by n-3 PUFA-induced lipid peroxidation, and
demonstrates that Vit E supplementation may further enhance the
antitumor activity of n-3 PUFAs.
In conclusion, using an in vivo model
involving fat-1 transgenic mice, the present study provides
encouraging preclinical evidence of the molecular mechanisms by
which n-3 PUFAs may regulate the malignant features of melanoma.
The results presented support a protective role of n-3 PUFAs in the
prevention of melanoma progression, and in the potential
development of clinical interventions that combine n-3 PUFAs with
conventional or innovative therapies for the treatment of patients
with melanoma.
Acknowledgments
This study was partly sponsored by the Fang Runhua
Fund of Hong Kong, the K.C. Wong Magna Fund of Ningbo University,
the National Science Foundation of China (grant nos. 81172660,
81450048, 281370165 and 31201284), the Scientific Innovation Team
Project of Ningbo (grant no. 2014B82002), the Zhejiang Provincial
Natural Science Foundation of China (grant nos. LY14H260001 and
LY15C200008), the projects of the Medical and Health Technology
Development Program of Zhejiang (grant no. 2014kya198), the Natural
Science Foundation of Ningbo (grant nos. 2015A610222 and
2013A610209), the Science Foundation of Ningbo University (grant
no. XKL14D2101) and the project of the Application of Public
Welfare Technology (Experimental Animals) of Zhejiang (grant no.
2016C37120). We are also grateful to Mr An-Shi Wang (Ningbo
University, Ningbo, China) for performing cell culture and Mr Fei
Zhou (Ningbo University) for providing helpful advice.
References
|
1
|
American Cancer Society: Cancer Facts and
Figures 2014. Atlanta, GA: American Cancer Society; 2014
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuphal S and Bosserhoff A: Recent progress
in understanding the pathology of malignant melanoma. J Pathol.
219:400–409. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Flaherty KT, Lee SJ, Zhao F, Schuchter LM,
Flaherty L, Kefford R, Atkins MB, Leming P and Kirkwood JM: Phase
III trial of carboplatin and paclitaxel with or without sorafenib
in metastatic melanoma. J Clin Oncol. 31:373–379. 2013. View Article : Google Scholar
|
|
5
|
van Roy F: Beyond E-cadherin: Roles of
other cadherin superfamily members in cancer. Nat Rev Cancer.
14:121–134. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schmalhofer O, Brabletz S and Brabletz T:
E-cadherin, beta-catenin, and ZEB1 in malignant progression of
cancer. Cancer Metastasis Rev. 28:151–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heerboth S, Housman G, Leary M, Longacre
M, Byler S, Lapinska K, Willbanks A and Sarkar S: EMT and tumor
metastasis. Clin Transl Med. 4:62015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sullivan RJ and Flaherty KT: Resistance to
BRAF-targeted therapy in melanoma. Eur J Cancer. 49:1297–1304.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nelson WJ and Nusse R: Convergence of Wnt,
beta-catenin, and cadherin pathways. Science. 303:1483–1487. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sinnberg T, Menzel M, Kaesler S,
Biedermann T, Sauer B, Nahnsen S, Schwarz M, Garbe C and Schittek
B: Suppression of casein kinase 1alpha in melanoma cells induces a
switch in beta-catenin signaling to promote metastasis. Cancer Res.
70:6999–7009. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lucero OM, Dawson DW, Moon RT and Chien
AJ: A re-evaluation of the 'oncogenic' nature of Wnt/β-catenin
signaling in melanoma and other cancers. Curr Oncol Rep.
12:314–318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jones V and Katiyar SK: Emerging
phytochemicals for prevention of melanoma invasion. Cancer Lett.
335:251–258. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Y, Lin Y, Liu H and Li J: Inhibition of
invasion and up-regulation of E-cadherin expression in human
malignant melanoma cell line A375 by
(−)-epigallocatechin-3-gallate. J Huazhong Univ Sci Technol Med
Sci. 28:356–359. 2008. View Article : Google Scholar
|
|
15
|
Vaid M, Prasad R, Sun Q and Katiyar SK:
Silymarin targets β-catenin signaling in blocking
migration/invasion of human melanoma cells. PLoS One. 6:e230002011.
View Article : Google Scholar
|
|
16
|
Serini S, Fasano E, Celleno L, Cittadini A
and Calviello G: Potential of long-chain n-3 polyunsaturated fatty
acids in melanoma prevention. Nutr Rev. 72:255–266. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Algamas-Dimantov A, Yehuda-Shnaidman E,
Hertz R, Peri I, Bar-Tana J and Schwartz B: Prevention of
diabetes-promoted colorectal cancer by (n-3) polyunsaturated fatty
acids and (n-3) PUFA mimetic. Oncotarget. 5:9851–9863. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Denkins Y, Kempf D, Ferniz M, Nileshwar S
and Marchetti D: Role of omega-3 polyunsaturated fatty acids on
cyclooxygenase-2 metabolism in brain-metastatic melanoma. J Lipid
Res. 46:1278–1284. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miller AB and Gaudette LA: Cancers of
skin, bone, connective tissues, brain, eye, thyroid and other
specified and unspecified sites in Inuit. Acta Oncol. 35:607–616.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bain C, Green A, Siskind V, Alexander J
and Harvey P: Diet and melanoma. An exploratory case-control study.
Ann Epidemiol. 3:235–238. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gallagher RP, Elwood JM and Hill GB: Risk
factors for cutaneous malignant melanoma: The Western Canada
Melanoma Study. Cancer Res. 102:38–55. 1986.
|
|
22
|
Osterlind A, Tucker MA, Stone BJ and
Jensen OM: The Danish case-control study of cutaneous malignant
melanoma. IV. No association with nutritional factors, alcohol,
smoking or hair dyes. Int J Cancer. 42:825–828. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kirkpatrick CS, White E and Lee JA:
Case-control study of malignant melanoma in Washington State. II.
Diet, alcohol, and obesity. Am J Epidemiol. 139:869–880.
1994.PubMed/NCBI
|
|
24
|
Salem ML, Kishihara K, Abe K, Matsuzaki G
and Nomoto K: N-3 polyunsaturated fatty acids accentuate B16
melanoma growth and metastasis through suppression of tumoricidal
function of T cells and macrophages. Anticancer Res. 20:3195–3203.
2000.PubMed/NCBI
|
|
25
|
Demchenko DV, Pozharitskaya ON, Shikov AN
and Makarov VG: Validated HPTLC Method for Quantification of
Vitamin D-3 in Fish Oil. Journal of Planar Chromatography.
24:487–490. 2011. View Article : Google Scholar
|
|
26
|
Kang JX, Wang J, Wu L and Kang ZB:
Transgenic mice: Fat-1 mice convert n-6 to n-3 fatty acids. Nature.
427:5042004. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xia S, Lu Y, Wang J, He C, Hong S, Serhan
CN and Kang JX: Melanoma growth is reduced in fat-1 transgenic
mice: Impact of omega-6/omega-3 essential fatty acids. Proc Natl
Acad Sci USA. 103:12499–12504. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bellenger J, Bellenger S, Clément L,
Mandard S, Diot C, Poisson JP and Narce M: A new hypotensive
polyunsaturated fatty acid dietary combination regulates oleic acid
accumulation by suppression of stearoyl CoA desaturase 1 gene
expression in the SHR model of genetic hypertension. FASEB J.
18:773–775. 2004.PubMed/NCBI
|
|
29
|
Wang Y, Armando AM, Quehenberger O, Yan C
and Dennis EA: Comprehensive ultra-performance liquid
chromatographic separation and mass spectrometric analysis of
eicosanoid metabolites in human samples. J Chromatogr A.
1359:60–69. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Serhan CN, Chiang N, Dalli J and Levy BD:
Lipid mediators in the resolution of inflammation. Cold Spring Harb
Perspect Biol. 7:a0163112014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial- mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin WL, Lai DY, Lee YJ, Chen NF and Tseng
TH: Antitumor progression potential of morusin suppressing STAT3
and NFκB in human hepatoma SK-Hep1 cells. Toxico Lett. 232:490–498.
2015. View Article : Google Scholar
|
|
33
|
Ge C, Yu M and Zhang C: G protein-coupled
receptor 30 mediates estrogen-induced proliferation of primordial
germ cells via EGFR/Akt/β-catenin signaling pathway. Endocrinology.
153:3504–3516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Novak A and Dedhar S: Signaling through
beta-catenin and Lef/Tcf. Cell Mol Life Sci. 56:523–537. 1999.
View Article : Google Scholar
|
|
35
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fortes C, Mastroeni S, Melchi F, Pilla MA,
Antonelli G, Camaioni D, Alotto M and Pasquini P: A protective
effect of the Mediterranean diet for cutaneous melanoma. Int J
Epidemiol. 37:1018–1029. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Algamas-Dimantov A, Yehuda-Shnaidman E,
Hertz R, Peri I, Bar-Tana J and Schwartz B: Prevention of
diabetes-promoted colorectal cancer by (n-3) polyunsaturated fatty
acids and (n-3) PUFA mimetic. Oncotarget. 5:9851–9863. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Algamas-Dimantov A, Davidovsky D, Ben-Ari
J, Kang JX, Peri I, Hertz R, Bar-Tana J and Schwartz B:
Amelioration of diabesity-induced colorectal ontogenesis by omega-3
fatty acids in mice. J Lipid Res. 53:1056–1070. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Simchuk EJ and Low DE: Direct esophageal
metastasis from a distant primary tumor is a submucosal process: A
review of six cases. Dis Esophagus. 14:247–250. 2001. View Article : Google Scholar
|
|
40
|
Galindo-Hernandez O, Serna-Marquez N,
Castillo-Sanchez R and Salazar EP: Extracellular vesicles from
MDA-MB-231 breast cancer cells stimulated with linoleic acid
promote an EMT-like process in MCF10A cells. Prostaglandins Leukot
Essent Fatty Acids. 91:299–310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bollrath J and Greten FR: IKK/NF-kappaB
and STAT3 pathways: Central signalling hubs in
inflammation-mediated tumour promotion and metastasis. EMBO Rep.
10:1314–1319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Min C, Eddy SF, Sherr DH and Sonenshein
GE: NF-kappaB and epithelial to mesenchymal transition of cancer. J
Cell Biochem. 104:733–744. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Damsky WE, Curley DP, Santhanakrishnan M,
Rosenbaum LE, Platt JT, Gould Rothberg BE, Taketo MM, Dankort D,
Rimm DL, McMahon M and Bosenberg M: β-catenin signaling controls
metastasis in Braf-activated Pten-deficient melanomas. Cancer Cell.
20:741–754. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Spranger S, Bao R and Gajewski TF:
Melanoma-intrinsic β-catenin signalling prevents anti-tumor
immunity. Nature. 523:231–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lu Y, Nie D, Witt WT, Chen Q, Shen M, Xie
H, Lai L, Dai Y and Zhang J: Expression of the fat-1 gene
diminishes prostate cancer growth in vivo through enhancing
apoptosis and inhibiting GSK-3 beta phosphorylation. Mol Cancer
Ther. 7:3203–3211. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Song KS, Jing K, Kim JS, Yun EJ, Shin S,
Seo KS, Park JH, Heo JY, Kang JX, Suh KS, et al:
Omega-3-polyunsaturated fatty acids suppress pancreatic cancer cell
growth in vitro and in vivo via downregulation of Wnt/Beta-catenin
signaling. Pancreatology. 11:574–584. 2011. View Article : Google Scholar
|
|
47
|
Castellone MD, Teramoto H, Williams BO,
Druey KM and Gutkind JS: Prostaglandin E2 promotes colon cancer
cell growth through a Gs-axin-beta-catenin signaling axis. Science.
310:15042005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Biondo PD, Brindley DN, Sawyer MB and
Field CJ: The potential for treatment with dietary long-chain
polyunsaturated n-3 fatty acids during chemotherapy. J Nutr
Biochem. 12:787–796. 2008. View Article : Google Scholar
|
|
49
|
Pai R, Nakamura T, Moon WS and Tarnawski
AS: Prostaglandins promote colon cancer cell invasion; signaling by
cross-talk between two distinct growth factor receptors. FASEB J.
17:1640–1647. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Calder PC: n-3 polyunsaturated fatty
acids, inflammation, and inflammatory diseases. Am J Clin Nutr.
83(6 Suppl): 1505S–1519S. 2006.PubMed/NCBI
|
|
51
|
Serhan CN: Pro-resolving lipid mediators
are leads for resolution physiology. Nature. 510:92–101. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lone AA, Ganai SA, Ahanger RA, Bhat HA,
Bhat TA and Wani IA: Free radicals and antioxidants: Myths, facts
and mysteries. African Journal of Pure and Applied Chemistry.
7:91–113. 2013. View Article : Google Scholar
|
|
53
|
Kim KY, Cho HJ, Yu SN, Kim SH, Yu HS, Park
YM, Mirkheshti N, Kim SY, Song CS, Chatterjee B and Ahn SC:
Interplay of reactive oxygen species, intracellular Ca2+ and
mitochondrial homeostasis in the apoptosis of prostate cancer cells
by deoxypodophyllotoxin. J Cell Biochem. 114:1124–1134. 2013.
View Article : Google Scholar
|
|
54
|
Azad MB, Chen Y and Gibson SB: Regulation
of autophagy by reactive oxygen species (ROS): Implications for
cancer progression and treatment. Antioxid Redox Signal.
11:777–790. 2009. View Article : Google Scholar
|