Introduction
Hypoxic-ischemic encephalopathy (HIE) is a common
cause of brain damage in neonates, and is the leading cause of
severe neurological sequelae in children (1). Of the ~130 million births worldwide
each year, four million infants will suffer from birth asphyxia,
and of these, one million will result in mortality and a similar
number will develop serious and long-term sequelae, including
neurodevelopmental disorders (2).
In China, the incidence rate of neonatal asphyxia is 1.14–11.7%,
and the incidence of HIE in full-term live birth infants is
1–2/1000 affected newborns. Approximately 15–20% of affected
newborns will succumb to the condition within the neonatal period,
and an additional 25–30% will develop severe and permanent
neurological handicaps (3),
including cerebral palsy, seizures, visual defects, mental
retardation, cognitive impairment and epilepsy (4). There is currently no specific
treatment for HIE. Previous studies have demonstrated that
endogenous neural stem cells (NSCs) exist in certain areas of the
brain, and that brain damage may stimulate the proliferation,
differentiation and self-repair mechanisms of these NSCs (5–7).
However, endogenous stem cells are limited in number, and their
survival may be affected by neurite growth inhibitory factors and
deficiencies of neurotrophic factors. Thus, the potential for
spontaneous brain repair is limited. When brain damage occurs, the
mechanisms of NSC proliferation and differentiation may provide a
method to enhance the autogenous repair functions of the brain,
thus, providing novel insight and treatment strategies for
hypoxic-ischemic brain damage (HIBD). β-Catenin is a crucial
molecule in the Wnt signaling pathway. During ischemic brain
injuries, β-catenin is important for the regulation of NSC
proliferation and differentiation (8–10).
Neurogenin 1 (Ngn1) is a downstream target gene of β-catenin, and
previous studies have demonstrated that Ngn1 is important during
the differentiation of NSCs into neurons (11,12).
As a member of the bone morphogenetic protein (BMP) family, the
synergy between BMP4 and β-catenin is important in determining the
differentiation pathway of NSCs (13,14).
The regulatory role of the Wnt/β-catenin signaling system during
HIBD remains unclear. Therefore, referring to the literature
(15), the present study cultured
NSCs in brain tissue homogenate from normal and HIBD brains to
simulate the respective microenvironments. Additionally, NSCs were
transfected with β-catenin small interfering RNA (siRNA) to
investigate the effects of β-catenin on NSC differentiation, and
the gene expression levels of Ngn1 and BMP4. The current study
aimed to investigate the potential mechanisms of NSC
differentiation in HIBD rats at the in vitro cell level.
Materials and methods
Isolation, sampling and culture of NSCs
from cerebral cortex of neonatal Sprague Dawley (SD) rats
A mix of male and female SD rats (n=50; age, 1–3
days; weight, 10.5±1.1 g) were provided by The Experimental Animal
Center of The First Affiliated Hospital of Xinjiang Medical
University (Ürümqi, China). They were sacrificed by abdominal
injection of 100 g/l chloral hydrate (Sigma-Aldrich, St. Louis, MO,
USA), then disinfected by soaking in 750 ml/l ethanol for 5 min.
The cerebral cortex tissues were isolated and digested in trypsin
(Sigma-Aldrich) at 37°C for 15 min. Digested tissue was filtered
through a 200-mesh filter (Fuzhou Maixin Biotechnology Development
Co., Ltd., Fuzhou, China), then centrifuged at 157 × g for 5 min,
the supernatant was discarded and cells were resuspended in
Dulbecco's modified Eagle's medium (DMEM)/F12 (Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA), containing 20 ml/l B27,
10 mg/l basic fibroblast growth factor and 20 mg/l epidermal growth
factor, all purchased from Gibco (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Cells were seeded into culture flasks and
cultured at 37°C and in an atmosphere of 50 ml/l CO2.
Half the medium was changed every 3–4 days, and the cells were
passaged once every 7 days. The present study was approved by the
ethics committee of The First Affiliated Hospital of Xinjiang
Medical University.
Cell transfection
Cells were harvested and centrifuged at 4°C, 640 × g
for 5 min, then resuspended in DMEM/F12 at room temperature at a
density of 2.5×109–2.5×1010 cells/l.
Electroporation apparatus (Multiporator 4308 electroporation
system) was purchased from Eppendorf (Hamburg, Germany). The cell
suspension was transferred into electrotransformation cuvettes, and
20 plasmid (pGCPU6/GFP/Neo siRNA expression vector; Shanghai Ji Kai
Gene Chemical Technology Co., Ltd., Shanghai, China) was added,
while an equal volume of electrotransformation solution was added
to the blank control group. Electroporation was performed at 300 V
for 60 μsec. The cells were transferred into culture flasks
5–10 min later and cultured at 37°C and in a 5 ml/l CO2
atmosphere.
Preparation of HIBD model and brain
tissue homogenate
Healthy male and female, SD rats (n=50; age, 7 days;
weight, 13.2±1.4 g) obtained from the Experimental Animal Center of
The First Affiliated Hospital of Xinjiang Medical University were
randomly divided into control and HIBD groups. The control group
(n=50) received no treatment. In the HIBD group (n=100), the
Rice-Vannucci method (16) was
used to perform the HIBD model. The rats were anesthetized by ether
inhalation (1.5 ml; Sigma-Aldrich) and the skin was disinfected
with 750 ml/l alcohol. Incision to the neck separated the left
common carotid artery and was ligatured with 7.0 sterilized silk
wire (Shanghai Nation Medical Equipment Co., Ltd., Shanghai,
China), the vessel was cut at the middle of ligation. Following 2 h
rest, the rat was placed into a plexiglass hypoxic chamber
(30×40×50 cm) at normal atmospheric pressure. Nitrogen-oxygen
mixture entered the chamber through a 1×1-cm hole on one side. A
hole at the other side was connected to a CY-12C portable digital
oxygen analyzer (Meicheng Electrochemical Analytical Instruments,
Hangzhou, China). The bottom of the chamber was covered with soda
lime to absorb CO2 and moisture. The oxygen
concentration inside the cabin was controlled at ~8 ml/l, the
temperature at 36±1°C, and humidity was 70±5 ml/l. The rats were
under hypoxic conditions for a 2 h period. After 24 h, the HIBD
rats and the control SD rats were sacrificed with 100 g/l chloral
hydrate by abdominal injection, and the whole left brain tissues
were removed and suspended in DMEM/F12 (9X volume of brain tissue).
The tissues were homogenized on ice and centrifuged at 3,913 × g at
4°C for 15 min. The supernatant was collected, divided into 1.5 ml
centrifuge tubes and stored at −80°C.
Co-culture of brain tissue supernatant
and NSCs
NSCs were collected 24 h after transfection and
centrifuged at 157 × g for 5 min. The supernatant was discarded and
the precipitate was resuspended in DMEM/F12. The clusters of cells
were pipetted into single cells by syringe needle, then seeded into
6-well plates. The brain tissue homogenate supernatants of normal
and HIBD rats were added to the cells, according to the different
experimental groupings, at an equal ratio of homogenate to
medium.
Experimental grouping
The second and third generation NSCs were randomly
divided into 5 groups as follows: i) Blank control group without
plasmid transfection (CON group); ii) NSCs transfected with
negative control (NC) plasmid for 24 h, co-cultured with normal
brain tissue homogenate (NC+N group); iii) NSCs transfected with NC
plasmid for 24 h, co-cultured with HIBD brain tissue homogenate
(NC+HIBD group); iv) NSCs transfected with β-catenin siRNA
(Shanghai Ji Kai Gene Chemical Technology Co., Ltd.) for 24 h,
co-cultured with normal brain tissue homogenate (siNSCs+N group);
and v) NSCs transfected with β-catenin siRNA for 24 h, co-cultured
with HIBD brain tissue homogenate (siNSCs+HIBD group).
Detection of NSC differentiation by
immunofluorescence
The immunofluorescence staining was performed 48 h
after transfection according to the methods of a previous study
(17). NSCs were fixed in
ReadiUse™ 4% formaldehyde fixation solution (AAT Bioquest,
Sunnyvale, CA, USA) for 15–20 min at room temperature, and then
permeabilized with 1% (vol/vol) Triton X-100 (Invitrogen; Thermo
Fisher Scientific, Inc.) in phosphate-buffered saline (PBS;
Hyclone; GE Healthcare Life Sciences) for 30 min, and incubated in
blocking buffer which contained 10% goat serum (Biorbyt, Cambridge,
UK) for 10 min. The cells were incubated with primary antibodies
overnight at 4°C and secondary antibodies for 30 min at room
temperature. The primary antibodies used were as follows: Mouse
forkhead box O4 (O4) antibody (1:50; Abcam, Cambridge, MA, USA;
cat. no. ab128908), rabbit enolase 2 (NSE) antibody (1:50; Abcam;
cat. no. ab53025), and rabbit glial fibrillary acidic protein
(GFAP) antibody (1:50; Abcam; cat. no. ab7260). The secondary
antibodies were fluorescein isothiocyanate (FITC)-conjugated goat
anti-rabbit IgG antibody (1:100; Wuhan Boster Biological
Technology, Ltd., Wuhan, China; cat. no. SA1064) and CY3-conjugated
goat anti-rabbit IgG antibody (1:100; Wuhan Boster Biological
Technology, Ltd.; cat. no. SA1074). Following removal of the
secondary antibodies, 50 μ1 Hoechst 33258 (10 μg/ml;
Wuhan Boster Biological Technology, Ltd.) was added and incubated
in darkness at room temperature for 20 min. Coverslips were mounted
with 50 ml/l buffered glycerol (Fuzhou Maixin Biotechnology
Development Co., Ltd.), and cells were imaged under a BX61
fluorescence microscope (Olympus Corporation). The experiment was
performed 6 times and 6 non-overlapping fields of vision were
captured. The differentiation percentages of NSCs to neurons or
glial cells were then calculated according to the following
formulae: (NSE positively stained cells/Hoechst 33258-stained
cells) × 100; (GFAP positively stained cells/Hoechst 33258-stained
cells) × 100; and (O4 positively stained cells/Hoechst
33258-stained cells) × 100.
Nestin immunofluorescence assay
The neurospheres cloned from single cells were
inoculated into each well of 24-well plates with
pre-polylysine-coated coverslips, and 1 ml serum-free medium was
then added to each well. The 24-well plates were then cultured at
37°C in 5% CO2 for 2 h to allow cell adhesion prior to
the Nestin immunofluorescence assay. The culture medium was removed
and 1 ml of 0.01 mol/l PBS containing 4% paraformaldehyde (pH 7.2)
was added for 15 min at room temperature to fix cells. The cells
were washed three times with 0.01 mol/l PBS (pH 7.2), and then 200
μ1 of 0.01 mol/l PBS containing 10% goat serum (pH 7.2) was
added into each well, and the plates were gently shaken at room
temperature for 30 min. Following removal of the blocking solution,
200 μ1 rabbit anti-Nestin polyclonal antibody (dilution,
1:150; Abcam; cat. no. ab92391) was added to each well, and
incubated with gentle shaking at room temperature for 1 h, followed
by overnight incubation at 4°C. Following washing three times with
PBS, FITC-conjugated goat anti-rabbit secondary antibody (dilution,
1:100) was added to each well, and gently shaken for 2 h in
darkness at room temperature. Following further washing three times
with PBS, glycerol phosphate buffer was used to mount the slices.
The neurospheres with positive Nestin immunofluorescence were then
observed using a fluorescence microscope with the excitation
wavelength of 495 nm and an absorption wavelength of 520 nm.
mRNA expression of Ngnl and BMP4 in NSCs
assessed by semiquantitative reverse transcription-polymerase chain
reaction (RT-PCR)
NSCs from the 5 experimental groups were collected
48 h after transfection and total RNA was extracted with TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Reverse transcription was performed to
obtain cDNA for the PCR reaction using PrimeScript™ RT kits (Takara
Biotechnology Co., Ltd., Dalian, China) according to the
manufacturer's instructions at 37°C for 15 min, followed by 85°C
for 5 sec. The reaction system (20 μ1 for each sample) was
as follows: 4 μ1 5X PrimeScript Buffer; 1 μ1
PrimeScript RT Enzyme mix I; 1 μ1 50 μmol/l Oligo dT
Primer; 1 μ1 100 μmol/l random hexamers; and 13 total
RNA. The PCR system (15 μ1 for each sample) was as follows:
7.5 μ1 2X Premix Ex Taq; 0.25 μ1 forward primer (10
μmol/l); 0.25 μ1 reverse primer (10 μmol/L); 3
μ1 cDNA (5 ng/μL); and 4 μ1 distilled water.
GAPDH was used as the reference gene. The following primers were
used to amplify the indicated fragments: Ngn 1, F
5′-CGGCCAGCGATACAGAGTC-3′ and R 3′-TACGGGATGAAGCAGGGTG-5′,
amplified fragment size 190 bp; BMP4, F 5′-AGAGCCAACACTGTGAGGA-3′
and R 3′-TGTCCAGGCACCATTTCT-5′, amplified fragment size 245 bp; and
GAPDH, F 5′-ACCACAGTCCATGCCATCAC-3′ and R 5′-TCCACC
ACCCTGTTGCTGTA-3′, amplified fragment size 450 bp. The reaction
cycling parameters were as follows on a CFX96 Touch™ real-time PCR
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA): Initial
step 95°C for 5 min; 35 cycles 94°C for 30 sec, 8°C for 30 sec,
72°C for 30 sec, and final step 72°C for 5 min. The PCR products
were then electrophoretically separated on a 5% agarose gel. The
optical density ratios of Ngn1 and BMP4 were normalized to GAPDH in
each group to reflect the relative optical density. Ethidium
bromide (Sigma-Aldrich) was used to visualize the DNA ladder.
Detection of Ngnl and BMP4 protein
expression levels in NSCs by western blot
Total protein was extracted from NSCs of the 5
groups 48 h after transfection using radioimmunoprecipitation assay
lysis buffer (Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Protein concentration was determined using the Coomassie brilliant
blue method and protein concentration of the samples was adjusted
to 50 μg/μl. The protein samples were loaded onto 12%
SDS-polyacrylamide gels for electrophoresis. The voltage used
through the concentration gel was 60 V, and 100 V for the
separating gel. Following electrophoresis, the proteins were
transferred to a nitrocellulose membrane. After washing three times
for 15 min in PBS, the nitrocellulose membrane was then immersed
into blocking solution of 1% bovine serum albumin (Fuzhou Maixin
Biotechnology Development Co., Ltd.) at room temperature for 2 h.
Following blocking, the membranes were incubated with primary
antibody overnight at 4°C, followed by washing with PBS. The
horseradish peroxidase-labeled secondary antibody was added,
followed by incubation at room temperature for 2 h. Visualization
was conducted using the enhanced chemiluminescence (ECL Plus
Western Blotting Detection reagent; GE Healthcare Life Sciences,
Little Chalfont, UK). The intensity of bands was calculated with
ImageJ 1.46 analysis software (imagej.nih.gov/ij/). The primary antibodies used were
as follows: Polyclonal mouse Ngn1 (1:500; Abcam; cat. no. ab66498);
monoclonal mouse BMP4 (1:500; Abcam; cat. no. ab39973) and
monoclonal mouse β-actin (1:100; Abcam; cat. no. ab6276). The
secondary antibody was horseradish peroxidase-conjugated sheep
anti-mouse IgG (1:5,000; Abcam; cat. no. ab6808).
Statistical analysis
The experiments were repeated 5 times. The
experimental data are expressed as χ±s. SPSS statistical analysis
software version 16.0 (SPSS, Inc., Chicago, IL, USA) was used for
analysis of variance tests to compare the intergroup difference.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Culture and identification of NSCs
NSCs were observed using an inverted phase contrast
microscope (CKX41; Olympus Corporation, Tokyo, Japan). The cultured
cells appeared scattered with round-like shape, and small cell
bodies, with good refraction. Following 3 days in culture, the
cells grew gradually and formed cell balls composed of several
cells. Following passage, single cells remained present in the
medium, there were also small cell clumps, and some single cells
undergoing cell division. Gradually, larger balls composed of more
cells were formed. As observed by immunofluorescence staining, the
primary and subcultured single cell balls were Nestin-positive.
Fetal calf serum (10%) was added to the culture medium for 2 days,
subsequently, the NSC balls quickly adhered to the walls of the
culture flasks and differentiated. Several processes were observed
to protrude from the edge of cell balls. After 7 days, the cell
balls disappeared, and the cells exhibited larger nuclei. The
refraction was improved and the axons partially intertwined with
each other forming a network.
Differentiation of NSCs
Simultaneous staining for NSE (neuronal marker),
GFAP (astrocyte marker) and O4 (oligodendrocyte precursor marker)
was performed (Fig. 1), and the
percentages of NSCs that differentiated into NSE-positive cells,
GFAP-positive cells and O4-positive cells were counted using the
fluorescence microscope (Table I,
Fig. 2). Compared with the CON
group, the differentiation into neurons and oligodendrocytes was
increased in the NC+N and the NC+HIBD groups (neuron
differentiation, P=0.001 and P=0.001, respectively; oligodendrocyte
differentiation, P=0.001 and P=0.002, respectively), while the
differentiation into astrocytes was reduced (each P= 0.001).
Compared with the NC+N group, the NC+HIBD group exhibited increased
differentiation into neurons (P=0.001), however, there was no
statistically significant difference in the number of astrocytes or
oligodendrocytes between the 2 groups. Compared with the CON group,
the 2 siNSC groups transfected with β-catenin siRNA exhibited
reduced differentiation into neurons (siNSC+N vs. CON, P=0.001;
siNSC+HIBD vs. CON, P=0.009) and increased differentiation into
astrocytes (siNSC+N vs. CON, P=0.001; siNSC+HIBD vs. CON, P=0.001).
Additionally, these siNSC groups exhibited increased
differentiation into oligodendrocytes compared with the CON group
(P=0.001), however, this was reduced compared with the NC group.
Compared with the siNSCs+N group, the siNSCs+HIBD group exhibited
increased differentiation into neurons and oligodendrocytes
(neuron, P=0.006; oligodendrocyte, P=0.001), however, these 2
groups showed no significant difference in the levels of
differentiation into astrocytes (Figs.
1 and 2, Table I).
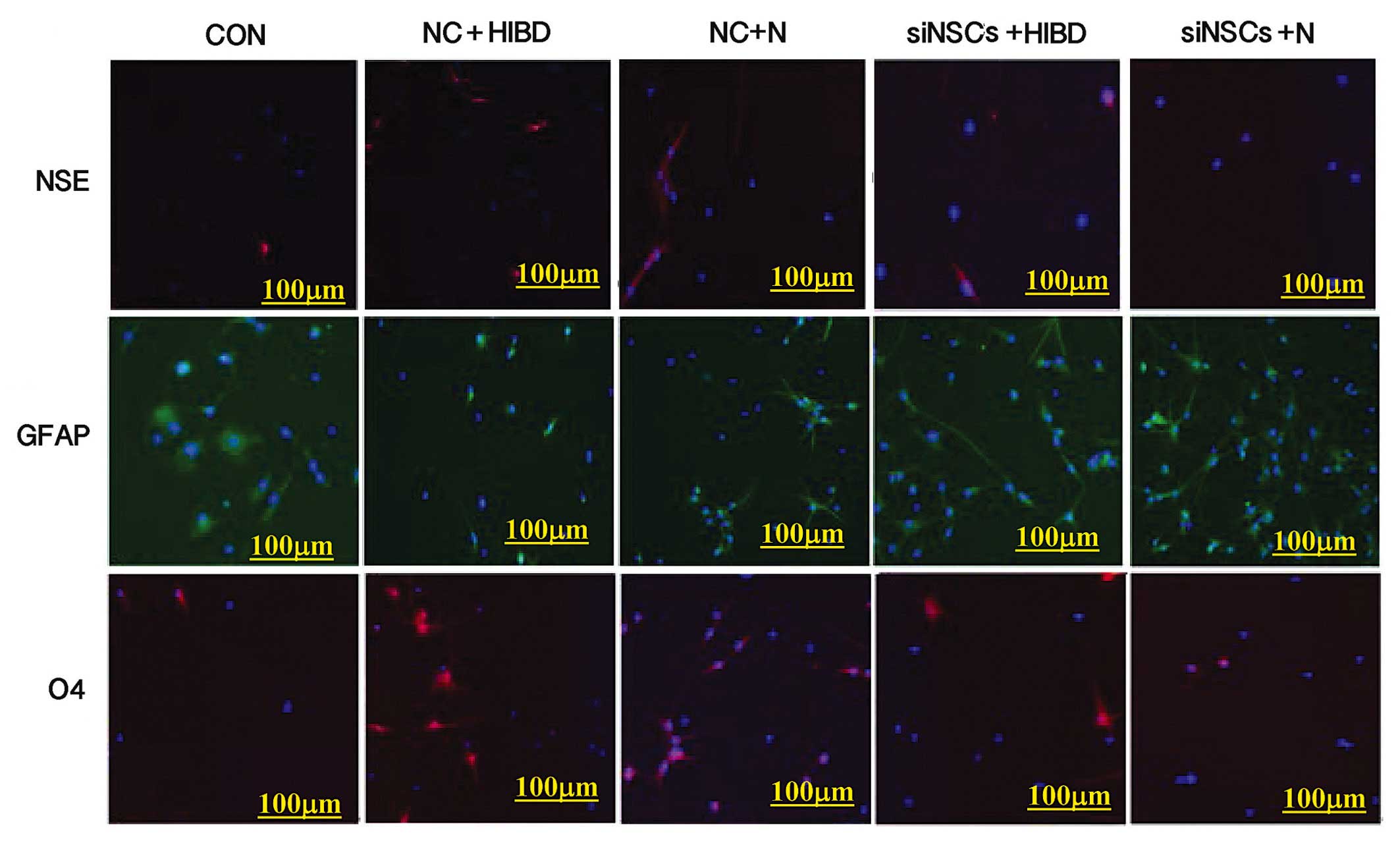 | Figure 1Expressions of NSE, O4 and GFAP in the
experimental groups indicating NSC differentiation by fluorescence
microscopy (×100). Immunofluorescence staining performed
simultaneously on each group, red (CY3) represents the expression
NSE or O4 in cytoplasm, green (FITC) represents the expression of
GFAP in cytoplasm, blue (Hoechst 33258) represents the nucleus.
NSC, neural stem cell; NSE, enolase 2; GFAP, glial fibrillary
acidic protein; O4, oligodendrocyte cell surface antigen O4; CON,
blank control group; NC, negative control plasmid-transfected
cells; N, normal brain tissue; HIBD, hypoxic-ischemic brain damage
tissue; siNSC, β-catenin small interfering RNA-transfected
cells. |
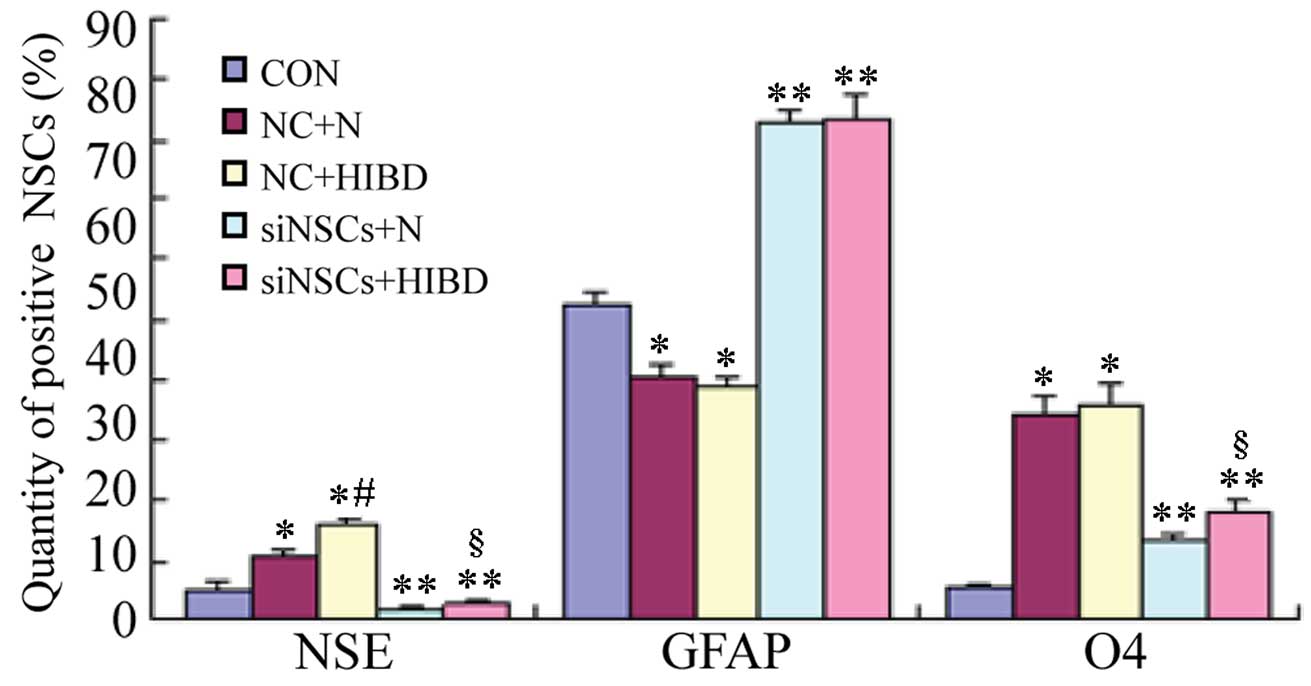 | Figure 2Positive ratios of NSCs-expressed
neural markers in the different groups. The immunofluorescence
staining was performed on the 5 groups simultaneously. The number
of positive cells was measured (%), and expressed as χ±s.
*P<0.05 and **P<0.01 vs. CON group;
#P<0.05 vs. NC+N group; §P<0.05 vs.
siNSCs+N group. NSC, neural stem cell; NSE, enolase 2; GFAP, glial
fibrillary acidic protein; O4, oligodendrocyte cell surface antigen
O4; CON, blank control group; NC, negative control
plasmid-transfected cells; N, normal brain tissue; HIBD,
hypoxic-ischemic brain damage tissue; siNSC, β-catenin small
interfering RNA-transfected cell. |
 | Table IComparison of the positive ratios of
NSC-expressed neural markers between the different groups. |
Table I
Comparison of the positive ratios of
NSC-expressed neural markers between the different groups.
| Group | NSE | GFAP | O4 |
|---|
| CON |
5.26±1.71 | 52.25±2.27 |
5.70±0.70 |
| NC+N | 10.81±0.90a | 40.48±1.97a | 34.42±2.77a |
| NC+HIBD | 15.88±1.05a,c | 38.83±1.63a | 35.62±3.91a |
| siNSCs+N |
1.48±0.53b | 82.77±2.43b | 13.25±1.08b |
| siNSCs+HIBD |
2.83±0.79b,d | 83.20±4.48b | 18.30±1.89b,d |
β-Catenin siRNA reduces Ngnl and
increases BMP4 mRNA expression levels in NSCs
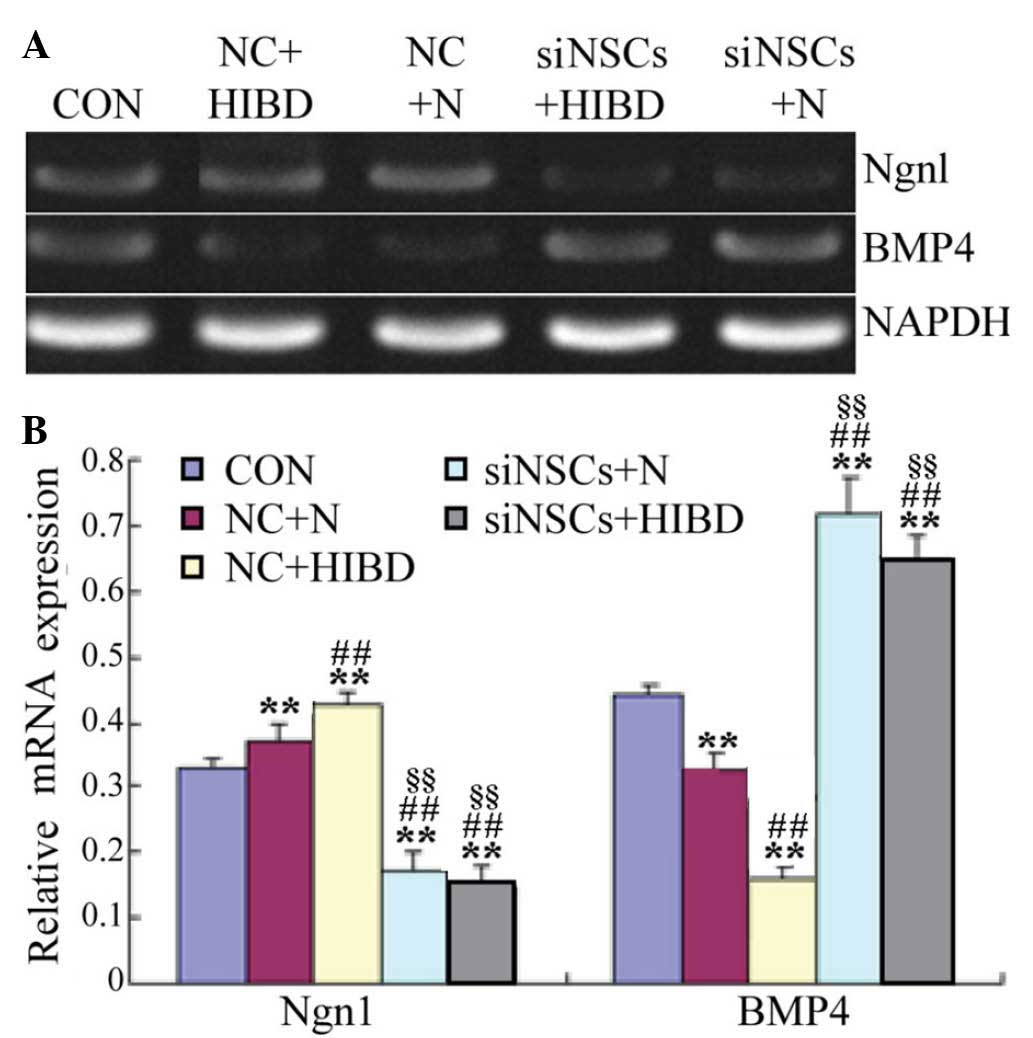
Semiquantitative RT-PCR results demonstrated that,
compared with the CON group, the expression levels of Ngn1 mRNA
were significantly increased in the NC+HIBD group (P=0.005;
Fig. 3), however, the BMP4 mRNA
expression levels were significantly reduced (P=0.001). Compared
with the NC+N group, the expression levels of Ngn1 mRNA were
significantly reduced in the NC+HIBD group (P=0.004), however, BMP4
mRNA levels were significantly increased (P=0.001). Compared with
the CON and NC groups, the Ngn1 mRNA levels were significantly
decreased in the siNSC groups (siNSC+N vs. CON, P=0.001; siNSC+N
vs. NC+N, P=0.001; siNSC+N vs. NC+HIBD, P=0.001; siNSC+HIBD vs.
CON, P=0.002; siNSC+HIBD vs. NC+N, P=0.001; siNSC+HIBD vs. NC+HIBD,
P=0.001), whereas, the BMP4 mRNA levels were significantly
increased (siNSC+N vs. CON, P=0.001; siNSC+N vs. NC+N, P=0.001;
siNSC+NC+HIBD, P=0.001; siNSC+HIBD vs. CON, P=0.003; siNSC+HIBD vs.
NC+N, P=0.002; siNSC+HIBD vs. NC+HIBD, P=0.001). No significant
difference was observed in the mRNA levels of Ngn1 and BMP4 mRNA
between the siNSCs+N and the siNSCs+HIBD groups (Fig. 3, Table II).
 | Table IIComparison of Ngn1 and BMP4 mRNA
expression levels the different NSC groups. |
Table II
Comparison of Ngn1 and BMP4 mRNA
expression levels the different NSC groups.
| Group | Ngn1 | BMP4 |
|---|
| CON | 0.33±0.02 | 0.44±0.01 |
| NC+N | 0.37±0.03a | 0.32±0.02a |
| NC+HIBD | 0.44±0.03a,b | 0.17±0.03a,b |
| siNSCs+N | 0.19±0.02a–c | 0.72±0.04a–c |
| siNSCs+HIBD | 0.17±0.03a–c | 0.65±0.06a–c |
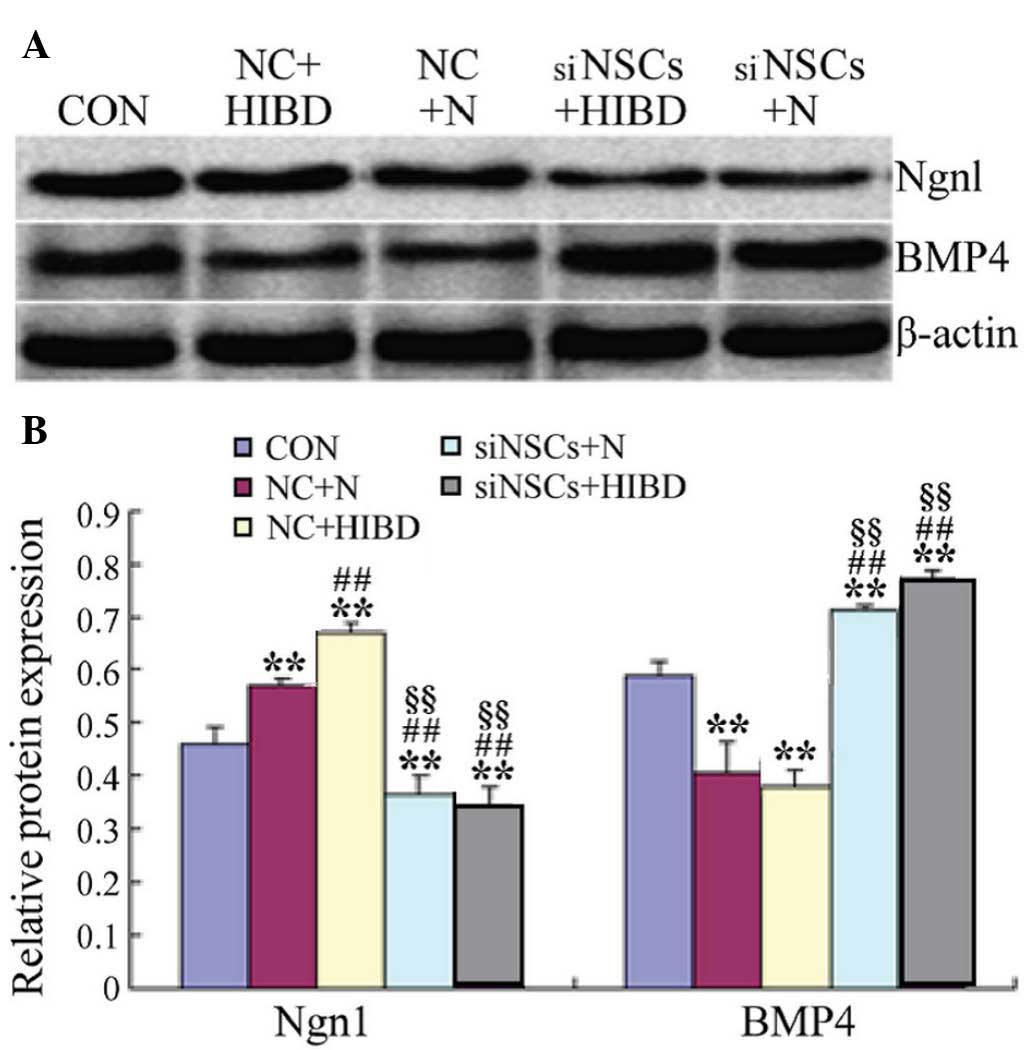
β-Catenin siRNA reduces Ngnl and
increases BMP4 protein levels in NSCs
As presented in Fig.
4 and Table III, western
blot analysis demonstrated that, compared with the CON group, the
levels of Ngn1 protein were significantly increased in the NC
groups (NC+N vs. CON, P=0.027; NC+HIBD vs. CON, P=0.001), however,
BMP4 protein levels were significantly reduced (NC+N vs. CON,
P=0.003; NC+HIBD vs. CON, P=0.008). Compared with the NC+N group,
the expression levels of Ngn1 protein were significantly increased
in the NC+HIBD group (P=0.002). The protein expression levels of
Ngn1 protein were decreased in the siNSC groups compared with the
CON and NC groups (siNSC+N vs. CON, P=0.048; siNSC+N vs. NC+N,
P=0.001; siNSC+N vs. NC+HIBD, P=0.001; siNSC+HIBD vs. CON, P=0.023;
siNSC+HIBD vs. NC+N, P=0.001; siNSC+HIBD vs. NC+HIBD, P=0.001;
Fig. 4), whilst the levels of BMP4
protein were significantly increased (siNSC+N vs. CON, P=0.023;
siNSC+N vs. NC+N, P=0.001; siNSC+N vs. NC+HIBD, P=0.002; siNSC+HIBD
vs. CON, P=0.001; siNSC+HIBD vs. NC+N, P=0.001; siNSC+HIBD vs.
NC+HIBD, P=0.001; Fig. 4). No
significant difference was observed between the levels of Ngn1 and
BMP4 protein in the siNSCs+N and the siNSCs+HIBD groups (Fig. 4, Table III).
 | Table IIIComparison of Ngn1 and BMP4 protein
expression levels in the different NSC groups. |
Table III
Comparison of Ngn1 and BMP4 protein
expression levels in the different NSC groups.
| Group | Ngn1 | BMP4 |
|---|
| CON | 0.46±0.03 | 0.59±0.03 |
| NC+N | 0.58±0.02a | 0.43±0.03a |
| NC+HIBD | 0.67±0.02a,b | 0.41±0.06a |
| siNSCs+N | 0.39±0.04a–c | 0.71±0.05a–c |
| siNSCs+HIBD | 0.37±0.03a–c | 0.77±0.01a–c |
Discussion
Previous experiments by Zhang et al (15) have successfully isolated and
cultured NSCs from the cortex tissues of newborn SD rats. They
confirmed that the cells were NSCs, and that they exhibited
continuous potential for proliferation and differentiation. RNA
interference is used to inhibit the expression of specific genes,
resulting in targeted gene silencing (18,19).
siRNAs are small non-coding RNA molecules of 21 to 25 nucleotides
in length. They are widely used to reduce the expression of a gene
to investigate its function. The siRNA vector used to target rat
β-catenin in the present study has been previously demonstrated to
specifically inhibit the expression of β-catenin (15,20).
In order to further explore the repair mechanisms
that occur following HIBD, the current study divided NSCs into 5
treatment groups. Immunocytochemical staining revealed that normal
and HIBD brain tissue homogenate promoted NSCs to differentiate
into neurons and oligodendrocytes, however, differentiation to
astrocytes was suppressed. Notably, the promotion and suppression
effects in HIBD brain tissue homogenates were greater than those in
normal brain tissue homogenates. β-catenin siRNA inhibited the
differentiation of NSCs to neurons, whereas, differentiation to
astrocytes was promoted. These results suggested that the local
microenvironment has an important impact on the differentiation of
NSCs, and that when damaged by hypoxia/ischemia, changes to the
brain microenvironments may stimulate proliferation and
differentiation of NSCs, thus, initiating autogenous healing
processes. Additionally, the results of the current study suggest
that β-catenin is important in facilitating the differentiation of
NSCs to neurons.
The differentiation of NSCs is dependent on the
microenvironment and the regulation of various genes (21–23).
Previous studies have demonstrated that the development of NSCs is
closely associated with the Wnt, BMP and sonic hedgehog signaling
systems. They have investigated how the Wnt/β-catenin signaling
pathway regulates the development and differentiation of NDCs
(24,25). Following activation, ß-catenin is
translocated into the nucleus, where the β-catenin/T-cell factor
complex directly regulates the transcription of Ngnl, thus,
regulating the differentiation of NSCs (26,27).
BMP4 is an important regulator of neural development. During the
development of the nervous system, there is 'crosstalk' between the
BMP and Wnt signaling pathways (28–30).
Therefore, the current study measured the expression levels of Ngn1
and BMP4 in NSCs exposed to HIBD brain tissue homogenate by
semiquantitative RT-PCR and western blotting. The results of the
present study suggested that the expression levels of Ngn1 protein
and mRNA were increased with the increased differentiation of NSCs
into neurons, and that the expression levels of BMP4 protein and
mRNA were reduced with the reduced differentiation of NSCs into
astrocytes. When ß-catenin expression was inhibited by siRNA, the
expression of Ngn1 protein and mRNA were reduced, and the
differentiation of NSCs into neurons was decreased. Additionally,
the expression level of BMP4 protein and mRNA was increased by
β-catenin siRNA, and differentiation of NSCs into astrocytes was
also increased. The results of the present study further
illustrated that β-catenin siRNA inhibited the differentiation of
NSCs to neurons and promoted the differentiation into astrocytes.
In HIBD, the damaged brain tissues repair themselves, which may be
associated with the fact that β-catenin promotes the
differentiation of NSCs to neurons, and this mechanism may be
mediated by β-catenin-induced upregulation of Ngn1. BMP4 and Ngn1
are important in the differentiation of NSCs in HIBD, and BMP4 may
inhibit the expression of Ngn1. The importance of Ngn1 in promoting
the differentiation of NSCs suggests that it may be an important
contributor for the repair of brain function following HIBD.
In conclusion, the damaged brain tissues in HIBD may
promote NSCs to differentiate into neurons for self-repair
processes. β-catenin, BMP4 and Ngn1 may be important in the
coordination of NSC proliferation and differentiation following
HIBD. The present study demonstrated that in HIBD, Ngn1 can promote
the differentiation of endogenous neural stem cells to neurons,
thus, repairing brain damage, and that this process is via the
Wnt/β-catenin signaling pathway. The current study also provides a
foundation for future studies using genetically modified neural
stem cells for the treatment of HIBD.
Acknowledgments
The current study was funded by the National Natural
Science Foundation of China (no. 81460195).
References
|
1
|
Nanavati T, Seemaladinne N, Regier M,
Yossuck P and Pergami P: Can we predict functional outcome in
neonates with hypoxic ischemic encephalopathy by the combination of
neuroimaging and electroencephalography? Pediatr Neonatol.
56:307–316. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buonocore G, Perrone S, Longini M,
Paffetti P, Vezzosi P, Gatti MG and Bracci R: Non protein bound
iron as early predictive marker of neonatal brain damage. Brain.
126:1224–1230. 2013. View Article : Google Scholar
|
|
3
|
Cai Q, Xue XD and Fu JH: Research status
and progress of neonatal hypoxic ischemic encephalopathy. Zhong Guo
Shi Yong Er Ke Za Zhi. 24:968–971. 2009.In Chinese.
|
|
4
|
Vanucci RC and Perlman JM: Interventions
for perinatal hypoxic-ischemic encephalopathy. Pediatrics.
100:1004–1014. 1997. View Article : Google Scholar
|
|
5
|
Sun D: Endogenous neurogenic cell response
in the mature mammalian brain following traumatic injury. Exp
Neurol. 275:405–410. 2016. View Article : Google Scholar
|
|
6
|
Sun D: The potential of endogenous
neurogenesis for brain repair and regeneration following traumatic
brain injury. Neural Regen Res. 9:688–692. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Edelmann K, Glashauser L, Sprungala S,
Hesl B, Fritschle M, Ninkovic J, Godinho L and Chapouton P:
Increased radial glia quiescence, decreased reactivation upon
injury and unaltered neuroblast behavior underlie decreased
neurogenesis in the aging zebrafish telencephalon. J Comp Neurol.
521:3099–3115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oliva CA and Inestrosa NC: A novel
function for Wnt signaling modulating neuronal firing activity and
the temporal structure of spontaneous oscillation in the
entorhinal-hippocampal circuit. Exp Neurol. 269:43–55. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oliva CA, Vargas JY and Inestrosa NC: Wnts
in adult brain: From synaptic plasticity to cognitive deficiencies.
Front Cell Neurosci. 7:2242013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Inestrosa NC and Varela-Nallar L: Wnt
signaling in the nervous system and in Alzheimer's disease. J Mol
Cell Biol. 6:64–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu W, Zhou H, Liu L, Zhao C, Deng Y, Chen
L, Wu L, Mandrycky N, McNabb CT, Peng Y, et al: Disruption of
neurogenesis and cortical development in transgenic mice
misex-pressing Olig2, a gene in the Down syndrome critical region.
Neurobiol Dis. 77:106–116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu F, Xuan A, Chen Y, Zhang J, Xu L, Yan
Q and Long D: Combined effect of nerve growth factor and brain
derived neurotrophic factor on neuronal differentiation of neural
stem cells and the potential molecular mechanisms. Mol Med Rep.
10:173917–173945. 2014.
|
|
13
|
Lei ZN, Liu F, Zhang LM, Huang YL and Sun
FY: Bcl-2 increases stroke-induced striatal neurogenesis in adult
brains by inhibiting BMP-4 function via activation of β-catenin
signaling. Neurochem Int. 61:34–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chuang CY, Lin KI, Hsiao M, Stone L, Chen
HF, Huang YH, Lin SP, Ho HN and Kuo HC: Meiotic competent human
germ cell-like cells derived from human embryonic stem cells
induced by BMP4/WNT3A signaling and OCT4/EpCAM (epithelial cell
adhesion molecule) selection. J Biol Chem. 287:14389–14401. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang XY, Yang YJ, Xu PR, Zheng XR, Wang
QH, Chen CF and Yao Y: The role of β-catenin signaling pathway on
proliferation of rats neural stem cells after hyperbaric oxygen
therapy in vitro. Cell Mol Neurobiol. 31:101–109. 2011. View Article : Google Scholar
|
|
16
|
Rice JE III, Vannucci RC and Brierley JB:
The influence of immaturity on hypoxic-ischemic brain damage in the
rat. Ann Neurol. 9:131–141. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen CF, Yang YJ, Wang QH, Yao Y and Li M:
Effect of hyperbaric oxygen administered at different pressures and
diffrernt exposure time on differentiation of neural stem cells in
vitro. Zhongguo Dang Dai Er Ke Za Zhi. 12:368–372. 2010.In Chinese.
PubMed/NCBI
|
|
18
|
Shyam R, Ren Y, Lee J, Braunstein KE, Mao
HQ and Wong PC: Intraventricular delivery of siRNA nanoparticles to
the central nervous system. Mol Ther Nucleic Acids. 4:e2422015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li TS, Yawata T and Honke K: Efficient
siRNA delivery and tumor accumulation mediated by ionically
cross-linked folic acid-poly(ethylene glycol)-chitosan
oligosaccharide lactate nanoparticles: For the potential targeted
ovarian cancer gene therapy. Eur J Pharm Sci. 52:48–61. 2014.
View Article : Google Scholar
|
|
20
|
Zhang XY, Yang YJ, Chen CF, Yao Y and Wang
QH: Construction and screening of eukaryotic expression plasmids
containing shRNA targeting β-catenin gene. J Med Mol Biol.
7:136–142. 2010.
|
|
21
|
Gigek CO, Chen ES, Ota VK, Maussion G,
Peng H, Vaillancourt K, Diallo AB, Lopez JP, Crapper L, Vasuta C,
et al: A molecular model for neurodevelopmental disorders. Transl
Psychiatry. 5:e5652015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen ES, Gigek CO, Rosenfeld JA, Diallo
AB, Maussion G, Chen GG, Vaillancourt K, Lopez JP, Crapper L,
Poujol R, et al: Molecular convergence of neurodevelopmental
disorders. Am J Hum Genet. 95:490–508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rak K, Völker J, Jürgens L, Völker C,
Frenz S, Scherzad A, Schendzielorz P, Jablonka S, Mlynski R,
Radeloff A and Hagen R: Cochlear nucleus whole mount explants
promote the differentiation of neuronal stem cells from the
cochlear nucleus in co-culture experiments. Brain Res. 1616:58–70.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schafer ST, Han J, Pena M, von Bohlen Und
Halbach O, Peters J and Gage FH: The Wnt adaptor protein ATP6AP2
regulates multiple stages of adult hippocampal neurogenesis. J
Neurosci. 35:4983–4998. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Liu Y, Li S, Long ZY and Wu YM:
Wnt signaling pathway participates in valproic acid-induced
neuronal differentiation of neural stem cells. Int J Clin Exp
Pathol. 8:578–585. 2015.PubMed/NCBI
|
|
26
|
Ma YX, Wu ZQ, Feng YJ, Xiao ZC, Qin XL and
Ma QH: G protein coupled receptor 50 promotes self-renewal and
neuronal differentiation of embryonic neural progenitor cells
through regulation of notch and wnt/β-catenin signalings. Biochem
Biophys Res Commun. 458:836–842. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yuan L and Hassan BA: Neurogenins in brain
development and disease: An overview. Arch Biochem Biophys.
558:10–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Imayoshi I and Kageyama R: bHLH factors in
self-renewal, multipotency, and fate choice of neural progenitor
cells. Neuron. 82:9–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Z, Shi Y, Zhao S, Li J, Li C and Mao
B: Xenopus Nkx6.3 is a neural plate border specifier required for
neural crest development. PLoS One. 9:e1151652014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
An SM, Ding Q, Zhang J, Xie J and Li L:
Targeting stem cell signaling pathways for drug discovery: Advances
in the Notch and Wnt pathways. Sci China Life Sci. 57:575–580.
2014. View Article : Google Scholar : PubMed/NCBI
|