Introduction
Gastrointestinal (GI) motility disorders comprise a
family of digestive problems, which are caused by poorly understood
neuromuscular dysfunction of the gut, and spastic or failed
propulsion (motility) of food through the digestive system. GI
motility patterns are highly integrated, require coordination
between smooth muscle cells, and utilize regulatory inputs from
neurons, and interstitial, endocrine and immune cells (1). The interstitial cells of Cajal (ICCs)
are pacemaker cells, which are responsible for the production of
gut movements alongside enteric neurons and smooth muscle cells
(2–4). Transient receptor potential (TRP)
melastatin 7 (TRPM7) is required for the pacemaking activity in
murine small intestine (4) and
Ca2+-activated Cl− channel [anoctamin 1
(ANO1)] is involved in slow wave current in ICCs (5). Therefore, TRPM7 and ANO1 are
potentially promising novel targets for the pharmacological
treatment of GI motility disorders. Several neurotransmitters and
hormones may affect ICC activity, thus modulating gut motility
(5). For example, Jatrorrhizine is
one of the major protoberberine alkaloids isolated from
manymedicinal plants, including Berberis aristata and
Coptis chinensis. Traditional oriental medicine uses the
extracts of these plants for the treatment of gastroenteritis and
diarrhea (6). Numerous traditional
oriental medicines have been used to treat GI motility disorders
(6).
Berberine is an isoquinoline alkaloid purified from
Berberis sp., which exerts various biochemical and
pharmacological effects (7,8). In
addition, it possesses significant antimicrobial activity towards
several organisms, including bacteria, fungi, protozoans and
helminths (9,10). Berberine is traditionally used as
an antidiarrheal agent (11,12),
and this effect is thought to be dependent on its antibacterial
activity (12,13). Furthermore, berberine has been
reported to inhibit acetylcholine- or Ba2+-induced
contraction of guinea pig ileum and colonic smooth muscle (12). However, to the best of our
knowledge, the effects of berberine on ICCs have not been
previously investigated. Therefore, the present study aimed to
investigate the effects of berberine on the pacemaker potentials
(PPs) of cultured ICC clusters from the murine small intestine.
Materials and methods
Ethics
Animal care and experiments were conducted in
accordance with the guidelines issued by the ethics committee of
Pusan National University (Busan, South Korea; approval no.
PNU-2015-1036) and the National Institutes of Health (NIH) Guide
for the Care and Use of Laboratory Animals (NIH publication no.
85-23; 1996 revision).
Preparation of cells and cell
cultures
BALB/c mice (age, 3–7 days weight, 1.9–2.2 g;
Samtako Bio Korea Inc., Osan-si, Korea) were anesthetized with 0.1%
ether and euthanized by cervical dislocation. They were maintained
under controlled conditions (temperature, 21±3°C; humidity 50±6%;
12 h light/dark cycles) and were allowed free access to food and
water. Small intestines were excised from 1 cm below the pyloric
ring to the cecum, and were opened along the mesenteric border.
Luminal contents were removed using Krebs Ringer bicarbonate
solution, tissues were pinned to the bases of Sylgard dishes, and
mucosae were removed by sharp dissection. Small tissue strips of
intestinal muscle, which consist of circular and longitudinal
muscles, were equilibrated for 30 min in Ca2+-free
Hank's solution containing 5.36 mM KCl, 125 mM NaCl, 0.34 mM NaOH,
0.44 mM Na2HCO3, 10 mM glucose, 2.9 mM
sucrose, and 11 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic
acid (HEPES); pH 7.4. Cells were then dispersed in an enzyme
solution containing collagenase (1.3 mg/ml; Worthington Biochemical
Corporation, Lakewood, NJ, USA), bovine serum albumin (2 mg/ml;
Sigma-Aldrich; Merck Millipore, Darmstadt, Germany), trypsin
inhibitor (2 mg/ml; Sigma-Aldrich; Merck Millipore) and ATP (0.27
mg/ml), and were plated onto sterile glass coverslips coated with
murine collagen (2.5 mg/ml; BD Biosciences, Franklin Lakes, NJ,
USA) in 35 mm culture dishes. Subsequently, the cells were cultured
at 37°C in an atmosphere containing 95% oxygen/5% carbon dioxide in
smooth muscle growth medium (Clonetics™; Lonza, Basel, Switzerland)
supplemented with 2% antibiotics/antimycotics (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and murine stem cell factor (5
ng/ml; Sigma-Aldrich; Merck Millipore). All experiments on ICC
clusters were performed following 12 h of culture. ICCs were
identified immunologically by incubating with a
phycoerythrin-conjugated rat anti-mouse c-Kit monoclonal antibody
(cat. no. 12-1172; eBioscience, Inc., San Diego, CA, USA), at a
dilution of 1:50 for 20 min at 37°C. Since the ICC morphology
differed from the other cell types in culture, identification was
possible under a phase contrast microscope following incubation
with the anti-c-Kit antibody.
Patch clamp experiments
Physiological salt solution [5 mM KCl, 135 mM NaCl,
2 mM CaCl2, 10 mM glucose, 1.2 mM MgCl2, and
10 mM HEPES (adjusted to pH 7.4 with NaOH)] was used to bathe
cultured ICC clusters (Na+-Tyrode). The pipette solution
used to examine pacemaker activity consisted of the following
reagents: 140 mM KCl, 5 mM MgCl2, 2.7 mM dipotassium
ATP, 0.1 mM sodium guanosine-5′-triphosphate, 2.5 mM creatine
phosphate disodium, 5 mM HEPES and 0.1 mM ethylene glycol
tetra-acetic acid (adjusted to pH 7.2 with KOH). Patch clamp
techniques were conducted in whole-cell configuration to record
potentials (i.e., current clamp mode) from cultured ICCs using
Axopatch I-D and Axopatch 200B amplifiers (Axon Instruments, Inc.,
Foster, CA, USA). Command pulses were applied using an
IBM-compatible personal computer (Compaq Computer Corporation,
Houston, TX, USA) and pClamp software (versions 6.1 and 10.0; Axon
Instruments, Inc.). Data were filtered at 5 kHz and displayed on an
oscilloscope, a computer monitor, and/or a pen recorder (Gould
2200; Gould Instruments, Inc., Valley View, OH, USA). Results were
analyzed using pClamp and Origin software (version 6.0; MicroCal,
Northampton, MA, USA). All experiments were performed at
30–33°C.
Cyclic guanosine monophosphate (cGMP)
assay
ICCs were preincubated with 100 mM
3-isobutyl-1-methylxanthine (Sigma-Aldrich; Merck Millipore) for 30
min at 37°C to inhibit cGMP degradation, and were then incubated
with berberine (50 μM; Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) for 10 min. Following homogenization in a
buffer containing 4 mM ethylenediaminetetraacetic acid to prevent
degradation of enzymatic cGMP, homogenates were heated for 5 min in
a boiling water bath to coagulate proteins, and were then
centrifuged at 3,950 × g for 5 min at 4°C. The super-natants
subsequently obtained were transferred to fresh tubes and were
stored at 4°C. Samples were assayed for cGMP using cGMP
enzyme-linked immunosorbent assay kits (Enzo Life Sciences, Inc.,
Farmingdale, NY, USA). These assays were conducted according to the
manufacturer's protocol.
Drugs
Opioid receptor antagonists were purchased from
Tocris Bioscience (Minneapolis, MN, USA); all other drugs were
obtained from Sigma-Aldrich (Merck Millipore). Stock solutions were
prepared and stored in accordance with the manufacturers'
protocols. Chemicals were dissolved in Na+-Tyrode
solution to obtain their final concentrations immediately prior to
use. Berberine was dissolved in methanol to produce a 50 mmol/l
stock solution, which was subsequently added to the bathing
solution at a final concentration of 50 μM on the day of the
experiment for 5 min. The final concentration of methanol in the
bathing solution was <0.1% and preliminary experiments confirmed
that this concentration of methanol did not affect results. In
addition, glibenclamide (Sigma-Aldrich; Merck Millipore) was
dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck
Millipore) to produce a 10 mmol/l stock solution, which was added
to the bathing solution at a final concentration of 10 μM on
the day of the experiment for 5 min. Next,
nor-Binaltorphimine dihydrochloride (Tocris Bioscience) was
dissolved in distilled water to produce a 25 mmol/l stock solution,
which was added to the bathing solution at a final concentration of
100 nM on the day of the experiment. ICI 174,864 (Tocris
Bioscience) and CTOP (Tocris Bioscience) was directly added to the
bathing solution at a concentration of 20 μM and 10
μM on the day of the experiment. Both SQ-22536
(Sigma-Aldrich; Merck Millipore) and ODQ (Sigma-Aldrich; Merck
Millipore) were dissolved in DMSO to produce a 100 mmol/l stock
solution, which was added to the bathing solution at a final
concentration of 100 μM on the day of the experiment for 5
min. Also, KT-5720 (Sigma-Aldrich; Merck Millipore) and KT-5823
Sigma-Aldrich; Merck Millipore) were dissolved in DMSO to produce 1
mmol/l stock solutions, which were added to the bath solution at a
final concentration of 1 μM on the day of the experiment for
5 min.
Statistical analysis
The data are presented as the mean ± standard error
of the mean. Student's t-test and one-way analysis of variance
followed by Bonferroni's post-hoc test were used to determine
significance. P<0.05 was considered to indicate a statistically
significant difference. For statistical analyses, Prism version 5.0
(GraphPad, Software Inc., La Jolla, CA, USA) and Origin version 8.0
(OriginLab Corporation, Northampton, MA, USA) were used. The n
values reported in the text refer to the number of cells used in
patch clamp experiments. Experiments were repeated 6–8 times.
Results
Effects of berberine on PPs in cultured
ICC clusters
The ICCs generated PPs under whole cell patch
current clamp mode (I=0) (Fig.
1) at a mean frequency of 24.1±2.1 cycles/min and a mean
amplitude of 23.8±1.5 mV. Treatment with berberine (10–50
μM) inhibited PPs and decreased their amplitudes in a
concentration-dependent manner (Fig.
1A–C). In the presence of berberine, mean PP frequencies were
23.1±3.2 cycles/min at 10 μM, 22.2±3.1 cycles/min at 30
μM, and 2.2±1.3 cycles/min at 50 μM (Fig. 1D, n=18), whereas mean amplitudes
were 23.5±1.4 mV at 10 μM, 12.3±1.5 mV at 30 μM, and
1.8±0.9 mV at 50 μM (Fig.
1E, n=18). These results suggest that berberine may
dose-dependently inhibit the PPs of ICCs.
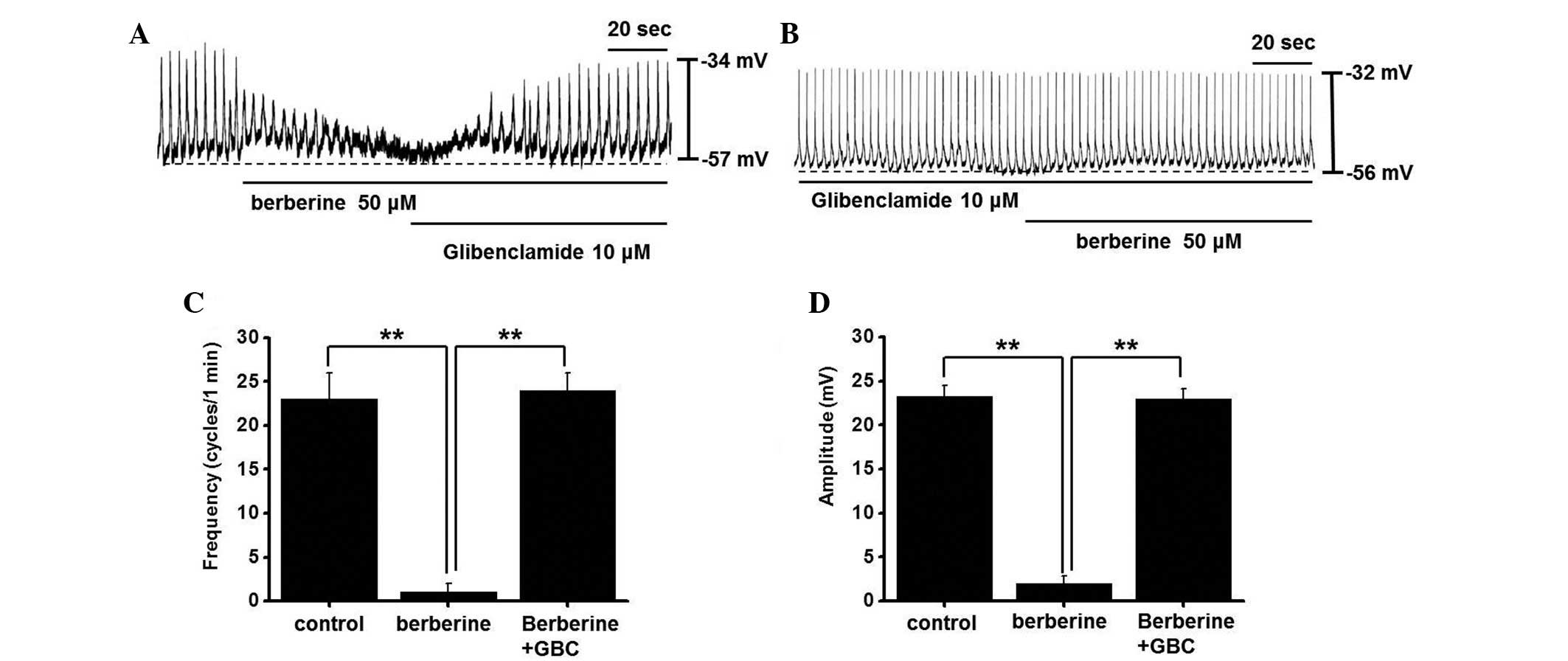
Berberine activates ATP-sensitive
K+ channels in cultured ICC clusters
In our previous study, pinacidil (an ATP-sensitive
K+ channel opener) decreased the frequency and amplitude
of PPs, and these pinacidil-induced effects were reversed by
treatment with glibenclamide (an ATP-sensitive K+
channel blocker) (14). In the
present study, berberine-induced ICC PP inhibition was suppressed
by glibenclamide (Fig. 2A), and
following glibenclamide pretreatment, berberine exerted no effects
on PPs (Fig. 2B). The effects of
berberine and glibenclamide on PPs are presented in Fig. 2C and D. These results suggest that
berberine may inhibit PPs via ATP-sensitive K+ channels
in ICCs.
Identification of berberine receptor
subtypes in cultured ICC clusters
To investigate the association between berberine and
its receptors in cultured ICCs, the opioid receptors were
investigated, since they are known to be involved in the regulation
of GI motility (15–17). There are three major classes of
opioid receptor (mu, delta and kappa) in the GI tract (15–17).
To identify the opioid receptor subtypes associated with the
effects of berberine, ICCs were pretreated with opioid receptor
antagonists, followed by treatment with berberine.
Nor-binaltorphimine dihydrochloride (a kappa opioid receptor
antagonist), ICI 174,864 (a delta opioid receptor antagonist) and
CTOP (a mu opioid receptor antagonist) were used to pretreat the
cells for 5 min, followed by berberine treatment (50 μM)
(Fig. 3). Treatment with these
opioid receptor antagonists alone had no effect on PPs; however,
pretreatment with ICI 174,864 or CTOP suppressed berberine-induced
PP inhibition (Fig. 3B and C). In
the presence of ICI 174,864 or CTOP, the mean amplitudes of
berberine-induced PPs were 14.6±1.1 and 24.1±1.2 mV, respectively
(n=6; Fig. 3E). Conversely,
pretreatment with nor-binaltorphimine dihydrochloride did
not suppress berberine-induced PP inhibition (Fig. 3A). In the presence of
nor-binaltorphimine dihydrochloride, the mean frequency and
amplitude of berberine-induced PPs were 3.1±1.4 cycles/min and
2.4±0.5 mV, respectively (n=6; Fig. 3D
and E). These results suggest that berberine may affect ICCs
via mu and delta opioid receptors.
Association of guanylate cyclase and
protein kinase G (PKG) with berberine-induced PP inhibition
To determine whether berberine-induced PP inhibition
is mediated by a cyclic nucleotide-dependent pathway, an adenylate
cyclase inhibitor (SQ-22536) and guanylate cyclase inhibitor
(1H-[1,2,4] oxadi-azolo [4,3-a] quinoxalin-1-one; ODQ) were used to
treat the ICC clusters. Preincubation with SQ-22536 (100 μM)
alone for 5 min had no effect on PPs, and in the presence of
SQ-22536, berberine (50 μM) still inhibited PPs (Fig. 4A). However, ODQ (100 μM)
suppressed berberine-induced PP inhibition (Fig. 4B). In the presence of SQ-22536, the
mean frequency and amplitude of berberine-induced PPs were 1.8±0.9
cycles/min and 1.6±0.9 mV, respectively (n=6; Fig. 4C and D); the ODQ corresponding
values were 23.7±1.0 cycles/min and 23.8±0.8 mV, respectively (n=6;
Fig. 4C and D). In addition, the
effects of a protein kinase A inhibitor (KT-5720) and a PKG
inhibitor (KT-5823) were detected. Preincubation of ICCs with
KT-5720 or KT-5823 alone had no effect on PPs. Furthermore, in the
presence of KT-5720 (1 μM), berberine (50 μM)
inhibited PPs (Fig. 5A); however,
preincubation with KT-5823 (1 μM) suppressed
berberine-induced PP inhibition (Fig.
5B). In addition, intracellular cGMP contents were measured
under basal and berberine-stimulated conditions, and berberine was
revealed to stimulate cGMP production [Fig. 6; control (12.1±0.6 pmol/mg protein)
vs. berberine (14.3±0.9 pmol/mg protein)]. These results suggest
that cGMP and PKG may have roles in berberine-induced PP
inhibition.
Discussion
The present study investigated the effects of
berberine on PPs in cultured ICC clusters from the mouse small
intestine, and sought to identify the receptors involved and the
underlying mechanisms of action. The results demonstrated that
berberine may inhibit the pacemaker activity of ICC clusters via
ATP-sensitive K+ channels and the cGMP-PKG-dependent
pathway by stimulating mu and delta opioid receptors. These
findings suggested that berberine offers a basis for the
development of novel treatments for GI motility dysfunction.
Berberine is an isoquinoline alkaloid, which is
present in numerous plant species, including Coptis sp. and
Berberis sp. Berberine is component in traditional Chinese
medicines, and is used to treat diarrhea and gastroenteritis.
Berberine possesses antimicrobial, antimotility and antisecretory
properties (18), and berberine
and its derivatives exert potent analgesic (19), anti-inflammatory (20) and anticancer (21) activities in the GI tract.
Furthermore, berberine reportedly exerts potential therapeutic
effects on diabetes (22),
hyperlipidemia (23),
cardiovascular diseases (24) and
central nervous system disorders (25). In addition, berberine affects GI
motility, since it is able to significantly reduce smooth muscle
contractility and intestinal motility, and may delay intestinal
transit times in rodents, as determined by intestinal myoelectric
activity experiments (16,26). The inhibitory effects of berberine
may be explained by the upregulation of somatostatin and
glucagon-like peptide-1 levels, and the downregulation of motilin
and gastrin levels (16,26). Berberine has also been reported to
block muscarinic receptors in guinea pig longitudinal muscle
isolated from the ileum, and therefore may reduce intestinal
motility (27). Furthermore,
berberine exerts dopamine D2 receptor antagonist and
5-HT1A receptor agonist properties, and exhibits
significant potential as a therapeutic agent for the treatment of
functional dyspepsia (28).
Berberine has also been reported to exert stimulatory effects in
low contractile states, and inhibitory effects in high contractile
states on rat jejunal segments (29). Berberine-induced GI motility
regulation could also be explained by the endogenous opioid system,
since opioid receptors are associated with the regulation of GI
motility, and inhibition of the opioid system suppresses the
inhibitory effects of berberine on intestinal activity (16,30).
ICCs act as gut pacemaker cells, and previous
studies have suggested that ICC networks coordinate peristaltic
movement (4,5). Due to the important roles of ICCs,
hormones and neurotransmitters that affect ICC activity are
considered to exert a significant influence on gut motility
(2).
The present study demonstrated that berberine may
decrease the frequency and amplitude of PPs in a dose-dependent
manner in ICCs (Fig. 1), and that
these effects may be mediated in a cGMP (Fig. 4) and PKG-dependent manner (Fig. 5) via ATP-sensitive K+
channels (Fig. 2). In addition, mu
and delta opioid receptors were revealed to be mechanistically
associated with the effects of berberine (Fig. 3). Opioids and opiates affect
various GI functions, including motility, secretion, and the
transport of electrolytes and fluids, by activating the three major
classes of opioid receptor (delta, kappa and mu) (15,31,32).
Mu and kappa opioid receptor immunoreactivities have been described
in the enteric neurons of rats and guinea pigs, and in the porcine
GI tract, whereas delta opioid receptor immunoreactivity has been
reported in porcine ileum. In addition, mu and kappa opioid
receptors are localized in ICCs of the myenteric plexus and deep
muscular plexus in rats (33). The
various effects of opioids and opiates are dependent on opioid
receptor activation. These receptors belong to the seven
transmembrane G-protein-coupled receptor super-family (15,31,32).
In the present study, mu and delta opioid receptors were shown to
be involved in berberine-induced PP inhibition (Fig. 3). Future studies should focus on
investigating the immunohistochemical expression level of opioid
receptors in murine GI tract.
The results of the present study indicated that
berberine inhibits ICC function. However, since GI motility
patterns are highly integrated, and require coordination between
smooth muscle cells and regulatory inputs from interstitial cells,
neurons, and endocrine and immune cells (1), further investigations regarding the
effects of berberine on smooth muscle cells, the enteric nervous
system, and endocrine and immune cells in vitro, and on GI
motility in vivo. are required.
In conclusion, the results of the present study
suggested that berberine reduces the amplitude and frequency of ICC
PPs in a cGMP-, and PKG-dependent manner via ATP-sensitive
K+ channels by stimulating mu and delta opioid
receptors. These findings indicated that berberine may be a
potential drug development candidate for the treatment of GI
motility disorders, including spasms, pain and transit
disturbances.
Acknowledgments
The present study was supported by a National
Research Foundation of Korea (NRF) grant funded by the Korean
government (MSIP) (grant no. 2014R1A5A2009936).
References
|
1
|
Sanders KM, Koh SD, Ro S and Ward SM:
Regulation of gastrointestinal motility - insights from smooth
muscle biology. Nat Rev Gastroenterol Hepatol. 9:633–645. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu HN, Ohya S, Nishizawa Y, Sawamura K,
Iino S, Syed MM, Goto K, Imaizumi Y and Nakayama S: Serotonin
augments gut pacemaker activity via 5-HT3 receptors. PLoS One.
6:e249282011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takaki M: Gut pacemaker cells: The
interstitial cells of Cajal (ICC). J Smooth Muscle Res. 39:137–161.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim BJ, Lim HH, Yang DK, Jun JY, Chang IY,
Park CS, So I, Stanfield PR and Kim KW: Melastatin-type transient
receptor potential channel 7 is required for intestinal pacemaking
activity. Gastroenterology. 129:1504–1517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nam JH, Kim WK and Kim BJ: Sphingosine and
FTY720 modulate pacemaking activity in interstitial cells of Cajal
from mouse small intestine. Mol Cells. 36:235–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang B, Cao A, Zhou J, Hu Z and Wu D:
Effect of jatrorrhizine on delayed gastrointestinal transit in rat
postoperative ileus. J Pharm Pharmacol. 64:413–419. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuo CL, Chi CW and Liu TY: The
anti-inflammatory potential of berberine in vitro and in vivo.
Cancer Lett. 203:127–137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jantova S, Cipak L and Letasiova S:
Berberine induces apoptosis through a mitochondrial/caspase pathway
in human promonocytic U937 cells. Toxicol In Vitro. 21:25–31. 2007.
View Article : Google Scholar
|
|
9
|
Basha SA, Mishra RK, Jha RN, Pandey VB and
Singh UP: Effect of berberine and (+/−)-bicuculline isolated from
Corydalis chaerophylla on spore germination of some fungi. Folia
Microbiol (Praha). 47:161–165. 2002. View Article : Google Scholar
|
|
10
|
Hwang BY, Roberts SK, Chadwick LR, Wu CD
and Kinghorn AD: Antimicrobial constituents from goldenseal (the
Rhizomes of Hydrastis canadensis) against selected oral pathogens.
Planta Med. 69:623–627. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okunade AL, Hufford CD, Richardson MD,
Peterson JR and Clark AM: Antimicrobial properties of alkaloids
from Xanthoriza simplicissima. J Pharm Sci. 83:404–406. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tai YH, Feser JF, Marnane WG and Desjeux
JF: Antisecretory effects of berberine in rat ileum. Am J Physiol.
241:G253–G258. 1981.PubMed/NCBI
|
|
13
|
Takase H, Yamamoto K, Ito K and Yumioka E:
Pharmacological studies on antidiarrheal effects of berberine and
geranii herba. Nihon Yakurigaku Zasshi. 102:101–112. 1993.In
Japanese. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee S, Gim H, Shim JH, Jung Kim H, Lee JR,
Kim SC, Kwon YK, Ha KT, So I and Kim BJ: The traditional herbal
medicine, Ge-Gen-Tang, inhibits pacemaker potentials by nitric
oxide/cGMP dependent ATP-sensitive K(+) channels in cultured
interstitial cells of Cajal from mouse small intestine. J
Ethnopharmacol. 170:201–209. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sternini C, Patierno S, Selmer IS and
Kirchgessner A: The opioid system in the gastrointestinal tract.
Neurogastroenterol Motil. 16(Suppl 2): 3–16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng Y, Li Y, Chen C, Lin X, Yang Y, Cai
H, Lv Z, Cao M, Li K, Xu J, et al: Inhibiting roles of berberine in
gut movement of rodents are related to activation of the endogenous
opioid system. Phytother Res. 27:1564–1571. 2013.PubMed/NCBI
|
|
17
|
Duraffourd C, Kumala E, Anselmi L, Brecha
NC and Sternini C: Opioid-induced mitogen-activated protein kinase
signaling in rat enteric neurons following chronic morphine
treatment. PLoS One. 9:e1102302014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen C, Yu Z, Li Y, Fichna J and Storr M:
Effects of berberine in the gastrointestinal tract - a review of
actions and therapeutic implications. Am J Chin Med. 42:1053–1070.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang QL, Lai ML, Zhong YF, Wang AM, Su JK
and Zhang MQ: Antinociceptive effect of berberine on visceral
hypersensitivity in rats. World J Gastroenterol. 19:4582–4589.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mo C, Wang L, Zhang J, Numazawa S, Tang H,
Tang X, Han X, Li J, Yang M, Wang Z, et al: The crosstalk between
Nrf2 and AMPK signal pathways is important for the
anti-inflammatory effect of berberine in LPS-stimulated macrophages
and endotoxin-shocked mice. Antioxid Redox Signal. 20:574–588.
2014. View Article : Google Scholar :
|
|
21
|
Tan W, Li Y, Chen M and Wang Y: Berberine
hydrochloride: Anticancer activity and nanoparticulate delivery
system. Int J Nanomedicine. 6:1773–1777. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsu YY, Tseng YT and Lo YC: Berberine, a
natural antidiabetes drug, attenuates glucose neurotoxicity and
promotes Nrf2-related neurite outgrowth. Toxicol Appl Pharmacol.
272:787–796. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong H, Zhao Y, Zhao L and Lu F: The
effects of berberine on blood lipids: A systemic review and
meta-analysis of randomized controlled trials. Planta Med.
79:437–446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Derosa G, Maffioli P and Cicero AF:
Berberine on metabolic and cardiovascular risk factors: An analysis
from preclinical evidences to clinical trials. Expert Opin Biol
Ther. 12:1113–1124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bhutada P, Mundhada Y, Bansod K, Tawari S,
Patil S, Dixit P, Umathe S and Mundhada D: Protection of
cholinergic and antioxidant system contributes to the effect of
berberine ameliorating memory dysfunction in rat model of
streptozotocin-induced diabetes. Behav Brain Res. 220:30–41. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gu L, Li N, Yu W, Gong J, Li Q, Zhu W and
Li J: Berberine reduces rat intestinal tight junction injury
induced by ischemia-reper-fusion associated with the suppression of
inducible nitric oxide synthesis. Am J Chin Med. 41:1297–1312.
2013. View Article : Google Scholar
|
|
27
|
Tsai CS and Ochillo RF: Pharmacological
effects of berberine on the longitudinal muscle of the guinea-pig
isolated ileum. Arch Int Pharmacodyn Ther. 310:116–131.
1991.PubMed/NCBI
|
|
28
|
Lee TH, Kim KH, Lee SO, Lee KR, Son M and
Jin M: Tetrahydroberberine, an isoquinoline alkaloid isolated from
Corydalis tuber, enhances gastrointestinal motor function. J
Pharmacol Exp Ther. 338:917–924. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen DP, Xiong YJ, Lv BC, Liu FF, Wang L,
Tang ZY and Lin Y: Effects of berberine on rat jejunal motility. J
Pharm Pharmacol. 65:734–744. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cosola C, Albrizio M, Guaricci AC, De
Salvia MA, Zarrilli A, Sciorsci RL and Minoia R: Opioid
agonist/antagonist effect of naloxone in modulating rabbit jejunum
contractility in vitro. J Physiol Pharmacol. 57:439–449.
2006.PubMed/NCBI
|
|
31
|
Reisine T and Bell GI: Molecular biology
of opioid receptors. Trends Neurosci. 16:506–510. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Minami M and Satoh M: Molecular biology of
the opioid receptors: Structures, functions and distributions.
Neurosci Res. 23:121–145. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bagnol D, Mansour A, Akil H and Watson SJ:
Cellular localization and distribution of the cloned mu and kappa
opioid receptors in rat gastrointestinal tract. Neuroscience.
81:579–591. 1997. View Article : Google Scholar : PubMed/NCBI
|