Introduction
Betulinic acid (BA) is a lupane-type pentacyclic
triterpenoid saponin (3β-hydroxy-lup-20 (29)-en-28-oic acid; MW, 456.71; Fig. 1), which exists in the bark of a
variety of natural plants, principally in Betula. It has
been investigated extensively in previous decades due to its
beneficial properties, including anticancer, anti-inflammatory,
anti-angiogenic, and immunomodulatory effects, its anthelmintic
activity and its anti-human immunodeficiency virus effects. Its
antitumor effects are higher at a reduced pH (<6.8), a
characteristic of several types of tumor (1–3).
In previous decades, BA has been shown to have a
marked antitumor therapeutic effect in melanoma cells and several
types of solid tumor, including glioblastoma (4), lung carcinoma (5), breast carcinoma (6), colorectal carcinoma (7) and prostate carcinoma (8). In addition, the antitumor effects on
hematological malignancies have been investigated in our previous
studies and in those of others in previous years (1,9–11).
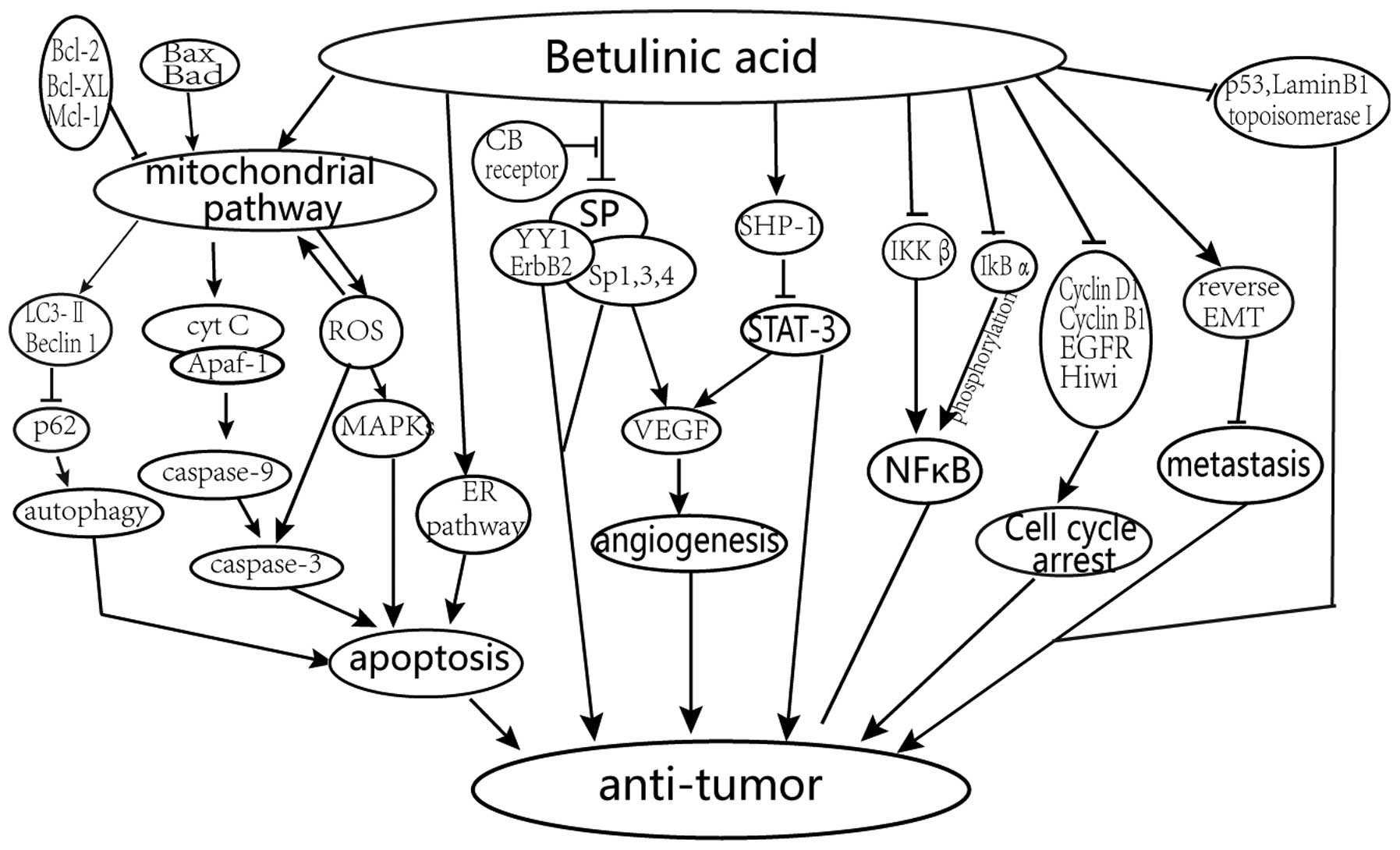
The reported primary mechanisms of the anticancer
effects of BA treatment are shown in Fig. 2 and described below.
Promotion of apoptosis by activation
of the mitochondrial pathway
BA improves the level of reactive oxygen species
(ROS) production and alters the mitochondrial membrane potential
gradient, followed by the release of cytochrome c (Cyt
c), which causes the mitochondrial-mediated apoptosis of
tumor cells via a caspase-dependent mechanism and apoptosis
inducing factor (1,12,13).
It has been demonstrated that there is a link between ROS and the
p38 and stress-activated protein (SAP) kinase/c-Jun N-terminal
kinase (JNK) in melanoma cells. This indicates that ROS act
upstream of the mitogen-activated protein kinases (MAPKs) in the
signaling pathway of BA (14). In
addition, autophagy has been shown to occur downstream of the
mitochondrial damage induced by BA (15).
Regulation of cell cycle and the
angiogenic pathway via specificity protein (Sp) transcription
factors, cyclin D1 and epidermal growth factor receptor (EGFR)
BA can inhibit cancer cell growth and proliferation
via cell cycle arrest. Drugs, including BA, can inhibit the protein
expression of Sp1, Sp2 and Sp4 through the microRNA
(miR)-27a-ZBTB10-Sp1 axis and slow down the aggressiveness of the
tumor (16–19).
Inhibition of the signal transducer
and activator of transcription 3 (STAT3) and nuclear factor (NF)-κB
signaling pathways
BA can downregulate the activation of STAT3 through
the upregulation of Src homology 2 domain-containing phosphatase 1
(SHP-1), and affect the STAT3/HIF-1/VEGF signal pathway (20–22).
The expression of NF-κB can be inhibited by reducing the activation
of inhibitor of NF-κB (IκBα) kinase (IKKβ) and phosphorylation of
IκBα with BA (23).
Prevention of the invasion and
metastasis
The invasion and metastasis of malignancies is
prevented via epithelial-mesenchymal transition (EMT) and
inhibition of topoisomerase I (24).
The aim of this review was to discuss the primary
pharmacological effects of BA in solid types of tumor and in
hematological malignancies, and to provide a valuable reference for
future investigations in the hematological system.
Sources of BA
BA is a type of pentacyclic triterpene acid, which
is found in the bark of several species of plant. As a natural
compound, it has a wide range of biological activities, and also
the characteristics of low toxicity and a high safety index. BA has
attracted increasing attention over previous years due to these
properties (25).
Previous studies have revealed three sources of BA.
Its direct extraction from plants is the earliest and most direct
source. The primary raw material used for BA extraction is
Betula bark. The bark of Platanus acerifolia,
Vochysia divergen, Euphorbiacea, Ficus
pandurata Hance, and the leaves of Vitex negundo and
Pterospermum heterophyllum Hance can also be used as raw
material to extract BA. However, the extraction rate (up to 3.3%)
is low due to the low content of BA in the bark of these plants. In
order to increase the extraction efficiency, the preparation of
semisynthetic BA has been introduced. This method provides a higher
rate of extraction from betulin, which is an associated natural
compound and important constituent of birch bark (22–30%), which
can be converted into BA in high yields through an oxidation
process (26,27). Another method used to extract BA is
microbial fermentation. Microbial transformation has several
advantages, including mild reaction conditions, low cost and
reduced pollution. Several types of microbes have been used, namely
Aspergillus oryzae AS 3.49, Aspergillus sp. WZ,
Aspergillus foetidus ZU-G1 and Trichoderma koningii
ZJ. However, further investigations are required for the
large-scale preparation of BA with the use of microbes (28).
Antitumor effects of BA in solid tumor
types
BA was initially confirmed as a selective inhibitor
of human melanoma cells (29). BA
has attracted attention due to its unique anticancer activities of
selective tumor growth inhibition or apoptosis, without damaging
normal cells, at a concentration >100 mg/kg body weight
(30). Several types of solid
cancer cell have been shown to be sensitive towards BA. The
following section focuses on the mechanisms underlying the effects
of BA in solid tumor treatment.
Malignant melanoma
Malignant melanoma, to which individuals of European
origin are vulnerable, accounted for 1.6% of new cancer cases in
2012 worldwide (31). As a
consequence, it is imperative to identify an effective treatment
approach. BA is a specific toxic reagent towards melanoma cells and
was first used for the treatment of melanoma. Tan et al
(14) demonstrated that treatment
of UISO-Mel-1 human melanoma cells with BA leads to the activation,
via phosphorylation, of pro-apoptotic MAPK proteins, P38 and
SAP/JNK, the formation of ROS and the upregulation of caspase
(14). Pisha et al
(29) initially reported that BA
induced the apoptosis of a number of melanoma cell lines, including
MEL-1, 2, 3 and 4, with half maximal effective dose values ranging
between 0.5 and 4.8 µg/ml. In addition, BA interferes with
EMT-associated changes, a mechanism to antagonize invasive melanoma
cells A375 at a concentration of 10 µM, whereas BA reduces A375
cell proliferation at a concentration of 50 µM (32).
Cervical cancer
BA activates the endoplasmic reticulum pathway and
the ROS-mediated mitochondrial pathway to induce apoptosis of HeLa
cells. Potze et al (33)
demonstrated that BA causes cell membrane rupture, apoptosis and
mitochondrial depolarization in HeLa cell lines, with an minimum
effective concentration of 7.5 µg/ml, reaching a plateau at 10
µg/ml.
BA increases the levels of microtubule-associated
protein 1 light chain 3 (LC3-II) more markedly in HeLa cell lines,
compared with DMSO-treated control groups, and the BA-treated HeLa
cell lines have a potent inducing effect on the expression of p62.
BA can also inhibit the autophagic flux by increasing the
degradation of long-lived proteins following 14 h medium
replacement (33).
The B cell lymphoma-2 (Bcl-2) family members
interact with each other to maintain mitochondrial integrity and
regulate cell apoptosis. The two predominant types of Bcl-2
proteins include anti-apoptotic proteins, including Bcl-2-A1,
Bcl-2, Bcl-extra large (Bcl-xL), Bcl-2-like protein 2, and myeloid
cell leukemia-1, and pro-apoptotic proteins, including
Bcl-2-associated death promoter, Bcl-2 homologous
antagonist/killer, Bcl-2-associated X protein (Bax),
BH3-interacting domain death agonist, Bcl-2-interacting killer,
Bcl-2-interacting mediator of cell death, activator of apoptosis
harakiri, phorbol-12-myristate-13-acetate-induced protein 1 and
p53-upregulated modulator of apoptosis. BA downregulates Bcl-2 and
upregulates the Bax gene in HeLa cell lines (34–36).
Breast cancer
Previous research demonstrated that Sp is
overexpressed in tumors (19).
Previous reports have shown that BA mediates antitumor activity by
downregulating the Sp1 transcription factor. Knocking down the
expression of Sp1 inhibits tumor growth and angiogenesis in
xenograft models (10,19). ZBTB10 is a transcriptional
repressor of Sp transcription factors, and drugs inhibit Sp
transcription factors through the microRNA (miR)-27a-ZBTB10-Sp1
axis. BA induces the apoptosis of MDA-MB-231
estrogen-receptor-negative breast cancer cell lines by
downregulating the mRNA and protein levels of Sp1, Sp3 and Sp4 at
concentrations of 2.5–10 µM, decreasing the expression of miR-27a
and increasing the levels of ZBTB10 in vitro and in
vivo (16,37,38).
Yin Yang 1 (YY1), an Sp-regulated gene, is a key
upstream regulator of ErbB2. BA inhibits the expression of YY1 in
BT474 and MDA-MB-453 cell lines. The activation of cannabinoid type
1 (CB1) and CB2 receptors, which modulate the miR-27a-ZBTB10-Sp1
axis, mediate the effects of Sp transcription factors and ErbB2 on
these two cell lines (39).
P53, a well-known tumor suppressor, mediates cell
cycle arrest and apoptosis (40).
BA induces the apoptosis of MCF-7 and T47D breast cancer cell lines
in an p53-independent apoptotic pathway with half maximal
inhibitory concentration (IC50) values of 12.3 and 9.8
µg/ml, respectively, following 72 h incubation (6,41).
Lung carcinoma, colorectal carcinoma and
gastric adenocarcinoma
Lung cancer and colorectal cancer are considered to
be major contributors to incidence and mortality rates (42,33).
In a previous study in nude mice, compared with control mice,
BA-treated transplanted tumors of A549 lung cancer or SW480 colon
cancer cell lines grew at a slower rate, with an IC50 of
4.3 µg/ml in the A549 cell lines, determined using MTT (44).
BA inhibits the proliferation of colon cancer cells
and xenograft tumor growth. It induces the proteasome-dependent and
-independent downregulation of Sp transcription factors, including
Sp1, Sp3 and Sp4, in SW480 and RKO cell lines, at concentrations of
5–10 µM. In addition, BA disrupts the expression of miR-27a and
ZBTB10 mRNA in RKO cell lines (45,46).
The expression levels of Sp-regulated genes, including cyclin D1,
p65, EGFR and Bcl-2 also decrease. In addition, BA can markedly
decrease the percentage of RKO cells in the G0/G1 and S phases, and
increase the percentage in the G2/M phase (47,48).
BA mediates G2/M cell cycle arrest and downregulates
the protein expression of Hiwi and cyclin B1 in the AGS human
gastric adenocarcinoma cell line, with an IC50 of 12.99
µg/ml (49).
Vascular endothelial cell growth factor (VEGF) is a
regulator of physiological and pathological angiogenesis. It is
expressed at high levels in several types of solid tumor, including
colon carcinoma and breast cancer. BA can decrease the expression
of VEGF via Sp proteins, thus having an antiangiogenic role
(50,51).
Pancreatic cancer and hepatocellular
carcinoma
The lamin B1 protein, an important member of the
lamin protein family (52),
regulates apoptosis, proliferation, invasion and metastasis
(53). The expression of lamin B1
is reduced in lung cancer, colon cancer, breast cancer, bronchial
carcinoma and gastric cancer (54,55),
whereas the expression of lamin B1 is increased in prostate cancer
and hepatocellular carcinoma (56,57).
The expression of lamin B1 is positively correlated
with the growth of cancer. Sp1, a lamin B1 downstream gene, may
regulate the expression of lamin B1. However, BA suppresses the
expression of lamin B1 in pancreatic cancer cells independent of
the Sp1 protein in vitro and in xenograft models (58–60).
The upregulation of lamin B1 in hepatocellular
carcinoma tumors correlates with tumor size, stage and nodule
number. Elevated levels of plasma lamin B1 can predict early stage
hepatocellular carcinoma with a sensitivity of 76% and a
specificity of 82% (61).
Prostate cancer, bladder cancer and
endometrial adenocarcinoma
The dysregulation of STAT3 is involved in tumor cell
survival, proliferation, apoptosis and metastasis. BA mediates
anticancer activity through inhibiting STAT3 in solid tumors. It
was reported that BA may be a potent anti-angiogenic drug in
prostate cancer, affecting the expression and transcription of
hypoxia-indicible factor (HIF)-1α, STAT3 and VEGF, and capillary
tube formation (20,22).
In endometrial adenocarcinoma cells, BA is vital in
cancer development and progression. It inhibits prolidase, which
catalyzes collagen degradation in the final step, and decreases the
expression of α1 and α2 integrin, HIF-1, VEGF, glucose
transporter-1, erythropoietin-1, carbonic anhydrase and
glyceraldehyde-3-phosphate dehydrogenase (62,63).
NF-κB, a key regulator of stress-induced
transcriptional activation, regulates cell survival, proliferation,
apoptosis, immune responses and adaptive responses to alterations
in cellular redox balance (23,64).
BA inhibits the expression of NF-κB, which leads to a decrease in
the activity of IKKβ and phosphorylation of IκBa in PC-3 human
prostate carcinoma cells. BA treatment for 24 h results in a
dose-dependent reduction in cell viability, which ranges between
2.9 and 91.2% in PC-3 cells at concentrations of 1–40 µM.
Furthermore, the protein expession of cyclin D1 is lowered in a
mouse model of prostate cancer treated with BA (10 mg/kg) (8).
The expression of EGFR is correlated with
vascularity. BA can significantly downregulate the expression of
the Sp-dependent gene, EGFR, through repression of the Sp1, Sp3 and
Sp4 proteins in 253JB-V and KU7 bladder cancer cells at a
concentration of 5 or 10 µM (19,65).
Head and neck carcinoma
The RET proto-oncogene, involved in recurrent
chromosomal rearrangements, is found in thyroid and lung cancer. Of
all papillary thyroid carcinoma (PTC) cases, ~20% are attributed to
RET/PTC rearrangements (66,67).
Topoisomerases, a class of ubiquitous enzymes
located in the cell nucleus, catalyze the fracture and combination
of DNA strands. BA secludes to topoisomerase I in the nucleoplasm.
Therefore, BA inhibits topoisomerase I DNA cleavage complex
formation. Coincidentally, fragile site breakage of the RET
proto-oncogene is affected by DNA topoisomerase I (68–70).
The BA derivative, compound 15, shows marked
inhibition of SW1736 anaplastic thyroid cancer cell lines in a
short duration with an IC50 of 3.54±0.66 µM (71). Compared with BA, B10, a
semi-synthetic glycosylated derivative of BA, shows a higher
cytotoxicity in glioma cell lines. B10 induces cell death by
inducing autophagy and lysosomal permeabilization in glioblastoma
cells. It induces autophagy and abrogates the autophagic flux on a
panel of glioblastoma cell lines. The release of lysosomal enzymes
contributes to B10-triggered cell death. B10 decreases the level of
poly ADP ribose polymerase, the apoptotic protein, and survivin
(72).
The phosphoinositide 3-kinase (PI3K)/Akt/mammalian
target of rapamycin (mTOR) pathway, integrating extra- and
intracellular survival signals, stimulates cell growth and inhibits
cell death (73,74). In addition, the PI3K/Akt signal can
regulate the activity and stability of lysosomes (75). The PI3K/Akt/mTOR signaling pathway
is inhibited in B10-treated (18 µM) U87MG cells by decreasing the
phosphorylation of Akt, a downstream target of PI3K, which is an
upstream target of mTOR. The activation of caspase-3, lysosomal
permeabilization and cell death are decreased significantly when
ATG7, ATG5 or BECN1 are downregulated by RNA interference (76).
Antitumor effects of BA in hematological
malignancies
Currently, the therapeutical effect of BA against
hematological malignancies predominantly focuses on multiple
myeloma, acute leukemia and lymphoma. This review elaborates on the
correlative functional mechanism of BA-treated cell lines.
Multiple myeloma
The U266 and MM.1S human multiple myeloma cell lines
have been investigated in order to determine whether BA can
modulate the STAT3 pathway. BA downregulates the activation of
STAT3 (22) through the
upregulation of SHP-1 (77). Thus,
BA inhibits the activation of STAT3, Src kinase, janus kinase
(JAK)1 and JAK2 (77). The ability
of BA to inhibit STAT3 activation is abolished and BA-induced cell
death is rescued when the SHP-1 gene is silenced. In multiple
myeloma, the expression levels of STAT3-regulated gene products,
including Bcl-extra large (Bcl-xL), Bcl-2, cyclin D1 and survivin,
are downregulated by BA (77).
In our previous study, it was demonstrated that BA
inhibits cell proliferation and autophagic flux, and induces
apoptosis in a time-dose-dependent manner in KM3 multiple myeloma
cells, which was bound up with the activation of caspase 3. These
experimental results indicated that the proliferation of the KM3
cells was suppressed when the cells were treated with BA (5–25
µg/ml). The IC50 values at 12, 24 and 36 h were 22.29,
17.36 and 13.06 µg/ml, respectively. However, the cells were
sensitized to BA-induced apoptosis when they were treated with
Z-DEVD-FMK, a specific inhibitor of caspase 3. The accumulation of
LC3-II and P62 in KM3 cells treated with dose-dependent BA
increased, which indicated the suppression of autophagic flux.
Furthermore, the expression of Beclin 1, an important inducer of
autophagy, was downregulated in the KM3 cells treated with BA
(78). Our previous study also
confirmed that BA can induce the apoptosis of RPMI-8226 multiple
myeloid cell lines via modulating the apoptosis-associated genes,
Bcl-xL and caspase 3 (79). This
efficiency showed a time- and dose-dependency. In the RPMI-8226
cell lines, BA also affected the cell cycle in the G1/S phase and
arrested cells in the G0/G1 phase. The IC50 values of BA
to RPMI-8226 cells at 24 and 48 h were 10.156±0.659 and 5.434±0.212
µg/ml, respectively (79).
Acute leukemia
Ehrhardt et al (80) found that BA induced marked
apoptosis in 65% of primary pediatric acute leukemia cells and in
all leukemia cell lines assessed through the mechanism of induction
of Cy c and second mitochondria-derived activator of
caspases. In all cell lines assessed, including the SKW6, HUT 78
and CEM T-cell leukemia cell lines, the BJAB, NALM6 and BOE B-cell
lines and the HL-60 myeloid cell line, the cells showed sensitivity
towards BA-induced apoptosis at a concentration of 10 µg/ml
(80). Kumar et al
(81) found that the methanolic
extract of Dillenia indica L. fruits showed significant
antileukemic activity in U937, HL60 and K562 human leukemic cell
lines. BA can induce the apoptosis of these leukemic cell lines
with IC50 values of 13.73±0.89, 12.84±1.23 and
15.27±1.16 µg/ml, respectively (81). Our previous study also showed that
BA inhibited the proliferation of K562 cells through the induction
of cell cycle arrest, and upregulation of the protein expression
levels of Bcl-2-associated X protein and caspase 3. BA was
cytotoxic towards K562 cells with an IC50 of 21.26 µg/ml
at 24 h (82). It was also found
that BA is important in T lymphocytic leukemia. BA is able to
inhibit the proliferation of Jurkat cells by regulating the cell
cycle, with arrest of cells at the G0/G1 phase and the induction of
apoptosis. The antitumor effects of BA were associated with the
downregulated expression levels of cyclin D3 and Bcl-xL. The
proliferation of Jurkat cells was decreased in the BA-treated group
with an IC50 value of 70 µmol/l at 24 h (83).
Lymphoma
Our previous study in the Raji Burkitt lymphoma cell
line showed that BA can induce cell cycle arrest and apoptosis via
suppressing the expression of the D-type cyclin, cyclin D3. The
IC50 values of BA at 24, 48 and 72 h were 39.44±0.65,
26.26±2.39 and 15.35±1.83 µg/ml, respectively. BA primarily caused
the arrest of Raji lymphoma cell lines in the G0/G1 phase at 24 h
(84).
Outlook
In conclusion, BA is a promising antitumor reagent.
It mediates selective cell death without cytotoxicity towards
normal cells and tissues. The antitumor activities described above
indicate BA as a veritable candidate for clinical cancer treatment.
In addition, previous studies have demonstrated that BA is involved
in the treatment of solid tumors, however, there are few reports on
BA-treated hematological malignancies, the elucidation of which may
be of potential value in such a novel field of research, and
indicates a direction for future investigations.
Acknowledgements
This study was supported by the grants from the
National Natural Science Foundation of China (grant nos. 81372541
and 81070429).
References
|
1
|
Gheorgheosu D, Duicu O, Dehelean C, Soica
C and Muntean D: Betulinic acid as a potent and complex antitumor
phytochemical: A minireview. Anticancer Agents Med Chem.
14:936–945. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dehelean CA, Soica C, Ledeti I, Aluas M,
Zupko I, Luşcan GA, Cinta-Pinzaru S and Munteanu M: Study of the
betulin enriched birch bark extracts effects on human carcinoma
cells and ear inflammation. Chem Cent J. 6:1372012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Csuk R: Betulinic acid and its
derivatives: A patent review (2008–2013). Expert Opin Ther Pat.
24:913–923. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jeremias I, Steiner HH, Benner A, Debatin
KM and Herold-Mende C: Cell death induction by betulinic acid,
ceramide and TRAIL in primary glioblastoma multiforme cells. Acta
Neurochir (Wien). 146:721–729. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsu TI, Wang MC, Chen SY, Huang ST, Yeh
YM, Su WC, Chang WC and Hung JJ: Betulinic acid decreases
specificity protein 1 (Sp1) level via increasing the sumoylation of
sp1 to inhibit lung cancer growth. Mol Pharmacol. 82:1115–1128.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tiwari R, Puthli A, Balakrishnan S, Sapra
BK and Mishra KP: Betulinic acid-induced cytotoxicity in human
breast tumor cell lines MCF-7 and T47D and its modification by
tocopherol. Cancer Inves. 32:402–408. 2014. View Article : Google Scholar
|
|
7
|
Jung GR, Kim KJ, Choi CH, Lee TB, Han SI,
Han HK and Lim SC: Effect of betulinic acid on anticancer
drug-resistant colon cancer cells. Basic Clin Pharmacol Toxicol.
101:277–285. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rabi T, Shukla S and Gupta S: Betulinic
acid suppresses constitutive and TNFalpha-induced NF-kappaB
activation and induces apoptosis in human prostate carcinoma PC-3
cells. Mole Carcinog. 47:964–973. 2008. View Article : Google Scholar
|
|
9
|
Gao Y, Jia Z, Kong X, Li Q, Chang DZ, Wei
D, Le X, Suyun H, Huang S, Wang L, et al: Combining betulinic acid
and mithramycin a effectively suppresses pancreatic cancer by
inhibiting proliferation, invasion and angiogenesis. Cancer Res.
71:5182–5193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soica C, Danciu C, Savoiu-Balint G, Borcan
F, Ambrus R, Zupko I, Bojin F, Coricovac D, Ciurlea S, Avram S, et
al: Betulinic acid in complex with a gamma-cyclodextrin derivative
decreases proliferation and in vivo tumor development of
non-metastatic and metastatic B164A5 cells. Int J Mol Sci.
15:8235–8255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mullauer FB, Kessler JH and Medema JP:
Betulinic acid, a natural compound with potent anticancer effects.
Anticancer Drugs. 21:215–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mullauer FB, Kessler JH and Medema JP:
Betulinic acid induces cytochrome c release and apoptosis in a
Bax/Bak-independent, permeability transition pore dependent
fashion. Apoptosis. 14:191–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gibellini L, Pinti M, Nasi M, De Biasi S,
Roat E, Bertoncelli L and Cossarizza A: Interfering with ROS
metabolism in cancer cells: The potential role of quercetin.
Cancers. 2:1288–1311. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan Y, Yu R and Pezzuto JM: Betulinic
acid-induced programmed cell death in human melanoma cells involves
mitogen-activated protein kinase activation. Clin Cancer Res.
9:2866–2875. 2003.PubMed/NCBI
|
|
15
|
Mizushima N: Methods for monitoring
autophagy using GFP-LC3 transgenic mice. Methods Enzymol.
452:13–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pathi SS, Jutooru I, Chadalapaka G,
Sreevalsan S, Anand S, Thatcher GR and Safe S: GT-094, a NO-NSAID,
inhibits colon cancer cell growth by activation of a reactive
oxygen species-microRNA-27a: ZBTB10-specificity protein pathway.
Mol Cancer Res. 9:195–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Safe SH, Prather PL, Brents LK,
Chadalapaka G and Jutooru I: Unifying mechanisms of action of the
anticancer activities of triterpenoids and synthetic analogs.
Anticancer Agents Med Chem. 12:1211–1220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Papineni S, Chintharlapalli S, Abdelrahim
M, Lee SO, Burghardt R, Abudayyeh A, Baker C, Herrera L and Safe S:
Tolfenamic acid inhibits esophageal cancer through repression of
specificity proteins and c-Met. Carcinogenesis. 30:1193–1201. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chadalapaka G, Jutooru I, Burghardt R and
Safe S: Drugs that target specificity proteins downregulate
epidermal growth factor receptor in bladder cancer cells. Mol
Cancer Res. 8:739–750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu Y, Zhao Q, Wang Z and Liu XY: Activated
STAT3 correlates with prognosis of non-small cell lung cancer and
indicates new anticancer strategies. Cancer Chemother Pharmacol.
75:917–922. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yadav VR, Prasad S, Sung B, Kannappan R
and Aggarwal BB: Targeting inflammatory pathways by triterpenoids
for prevention and treatment of cancer. Toxins (Basel).
2:2428–2466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shin J, Lee HJ, Jung DB, Jung JH, Lee HJ,
Lee EO, Lee SG, Shim BS, Choi SH, Ko SG, et al: Suppression of
STAT3 and HIF-1 alpha mediates anti-angiogenic activity of
betulinic acid in hypoxic PC-3 prostate cancer cells. PLoS One.
6:e214922011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wan Y, Wu YL, Lian LH, Xie WX, Li X,
Ouyang BQ, Bai T, Li Q, Yang N and Nan JX: The anti-fibrotic effect
of betulinic acid is mediated through the inhibition of NF-kappaB
nuclear protein translocation. Chem Biol Interact. 195:215–223.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chowdhury AR, Mandal S, Mittra B, Sharma
S, Mukhopadhyay S and Majumder HK: Betulinic acid, a potent
inhibitor of eukaryotic topoisomerase I: Identification of the
inhibitory step, the major functional group responsible and
development of more potent derivatives. Med Sci Monit.
8:BR254–BR265. 2002.PubMed/NCBI
|
|
25
|
Alakurtti S, Makela T, Koskimies S and
Yli-Kauhaluoma J: Pharmacological properties of the ubiquitous
natural product betulin. Eur J Pharm Sci. 29:1–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bruckner V, Kovacs J and Koczka I:
Occurrence of betulinic acid in the bark of the plane tree. J Chem
Soc. 1:948–951. 1948. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yogeeswari P and Sriram D: Betulinic acid
and its derivatives: A review on their biological properties. Curr
Med Chem. 12:657–666. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen QH, Liu J, Zhang HF, He GQ and Fu ML:
The betulinic acid production from betulin through
biotransformation by fungi. Enzyme Microb Tech. 45:175–180. 2009.
View Article : Google Scholar
|
|
29
|
Pisha E, Chai H, Lee IS, Chagwedera TE,
Farnsworth NR, Cordell GA, Beecher CW, Fong HH, Kinghorn AD, Brown
DM, et al: Discovery of betulinic acid as a selective inhibitor of
human melanoma that functions by induction of apoptosis. Nat Med.
1:1046–1051. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zuco V, Supino R, Righetti SC, Cleris L,
Marchesi E, Gambacorti-Passerini C and Formelli F: Selective
cytotoxicity of betulinic acid on tumor cell lines, but not on
normal cells. Cancer Lett. 175:17–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in globocan 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gheorgheosu D, Jung M, Ören B, Schmid T,
Dehelean C, Muntean D and Brüne B: Betulinic acid suppresses
NGAL-induced epithelial-to-mesenchymal transition in melanoma. Biol
Chem. 394:773–781. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Potze L, Mullauer FB, Colak S, Kessler JH
and Medema JP: Betulinic acid-induced mitochondria-dependent cell
death is counterbalanced by an autophagic salvage response. Cell
Death Dis. 5:e11692014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Williams MM and Cook RS: Bcl-2 family
proteins in breast development and cancer: Could Mcl-1 targeting
overcome therapeutic resistance? Oncotarget. 6:3519–3530. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng EH, Wei MC, Weiler S, Flavell RA,
Mak TW, Lindsten T and Korsmeyer SJ: BCL-2, BCL-X (L) sequester BH3
domain-only molecules preventing BAX- and BAK-mediated
mitochondrial apoptosis. Mol Cell. 8:705–711. 2011. View Article : Google Scholar
|
|
36
|
Willis SN, Chen L, Dewson G, Wei A, Naik
E, Fletcher JI, Adams JM and Huang DC: Proapoptotic Bak is
sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by
BH3-only proteins. Genes Dev. 19:1294–1305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li W, Liu M, Xu YF, Feng Y, Che JP, Wang
GC and Zheng JH: Combination of quercetin and hyperoside has
anticancer effects on renal cancer cells through inhibition of
oncogenic microRNA-27a. Oncol Rep. 31:117–124. 2014.PubMed/NCBI
|
|
38
|
Mertens-Talcott SU, Noratto GD, Li X,
Angel-Morales G, Bertoldi MC and Safe S: Betulinic acid decreases
ER-negative breast cancer cell growth in vitro and in vivo: Role of
Sp transcription factors and microRNA-27a: ZBTB10. Mol Carcinog.
52:591–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu X, Jutooru I, Lei P, Kim K, Lee SO,
Brents LK, Prather PL and Safe S: Betulinic acid targets YY1 and
ErbB2 through cannabinoid receptor-dependent disruption of
microRNA-27a: ZBTB10 in breast cancer. Mol Cancer Ther.
11:1421–1431. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Levine AJ: p53, the cellular gatekeeper
for growth and division. Cell. 88:323–331. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shi M, Liu D, Shen B and Guo N: Helpers of
the cellular gatekeeper-miRNAs dance in P53 network. Biochim
Biophys Acta. 1805:218–225. 2010.PubMed/NCBI
|
|
42
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2015 CA. Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar
|
|
43
|
GBD 2013 Mortality and Causes of Death
Collaborators, . Global, regional and national age-sex specific
all-cause and cause-specific mortality for 240 causes of death,
1990–2013: A systematic analysis for the global burden of disease
study 2013. Lancet. 385:117–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mullauer FB, van Bloois L, Daalhuisen JB,
Ten Brink MS, Storm G, Medema JP, Schiffelers RM and Kessler JH:
Betulinic acid delivered in liposomes reduces growth of human lung
and colon cancers in mice without causing systemic toxicity.
Anticancer Drugs. 22:223–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chintharlapalli S, Papineni S, Lei P,
Pathi S and Safe S: Betulinic acid inhibits colon cancer cell and
tumor growth and induces proteasome-dependent and-independent
downregulation of specificity proteins (Sp) transcription factors.
BMC Cancer. 11:3712011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lai Y, Zhang X, Zhang Z, Shu Y, Luo X,
Yang Y, Wang X, Yang G, Li L and Feng Y: The microRNA-27a:
ZBTB10-specificity protein pathway is involved in follicle
stimulating hormone-induced VEGF, Cox2 and survivin expression in
ovarian epithelial cancer cells. Int J Oncol. 42:776–784.
2013.PubMed/NCBI
|
|
47
|
Jutooru I, Chadalapaka G, Lei P and Safe
S: Inhibition of NFkappaB and pancreatic cancer cell and tumor
growth by curcumin is dependent on specificity protein
down-regulation. J Biol Chem. 285:25332–25344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jutooru I, Chadalapaka G, Abdelrahim M,
Basha MR, Samudio I, Konopleva M, Andreeff M and Safe S: Methyl
2-cyano-3,12-dioxooleana-1,9-dien-28-oate decreases specificity
protein transcription factors and inhibits pancreatic tumor growth:
Role of microRNA-27a. Mol Pharmacol. 78:226–236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang LJ, Chen Y, Ma Q, Fang J, He J, Cheng
YQ and Wu QL: Effect of betulinic acid on the regulation of Hiwi
and cyclin B1 in human gastric adenocarcinoma AGS cells. Acta
Pharmacol Sin. 31:66–72. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yuan P, Wang L, Wei D, Zhang J, Jia Z, Li
Q, Le X, Wang H, Yao J and Xie K: Therapeutic inhibition of Sp1
expression in growing tumors by mithramycin a correlates directly
with potent antiangiogenic effects on human pancreatic cancer.
Cancer. 110:2682–2690. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dvorak HF: Vascular permeability
factor/vascular endothelial growth factor: A critical cytokine in
tumor angiogenesis and a potential target for diagnosis and
therapy. J Clin Oncol. 20:4368–4380. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dittmer TA and Misteli T: The lamin
protein family. Genome Biol. 12:2222011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Broers JL, Ramaekers FC, Bonne G, Yaou RB
and Hutchison CJ: Nuclear lamins: Laminopathies and their role in
premature ageing. Physiol Rev. 86:967–1008. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Broers JL, Raymond Y, Rot MK, Kuijpers H,
Wagenaar SS and Ramaekers FC: Nuclear A-type lamins are
differentially expressed in human lung cancer subtypes. Am J
Pathol. 143:211–220. 1993.PubMed/NCBI
|
|
55
|
Moss SF, Krivosheyev V, de Souza A, Chin
K, Gaetz HP, Chaudhary N, Worman HJ and Holt PR: Decreased and
aberrant nuclear lamin expression in gastrointestinal tract
neoplasms. Gut. 45:723–729. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Coradeghini R, Barboro P, Rubagotti A,
Boccardo F, Parodi S, Carmignani G, D'Arrigo C, Patrone E and Balbi
C: Differential expression of nuclear lamins in normal and
cancerous prostate tissues. Oncol Rep. 15:609–613. 2006.PubMed/NCBI
|
|
57
|
Lim SO, Park SJ, Kim W, Park SG, Kim HJ,
Kim YI, Sohn TS, Noh JH and Jung G: Proteome analysis of
hepatocellular carcinoma. Biochem Biophys Res Commun.
291:1031–1037. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Maeno H, Sugimoto K and Nakajima N:
Genomic structure of the mouse gene (Lmnb1) encoding nuclear lamin
B1. Genomics. 30:342–346. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lin F and Worman HJ: Expression of nuclear
lamins in human tissues and cancer cell lines and transcription
from the promoters of the lamin A/C and B1 genes. Exp Cell Res.
236:378–384. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li L, Du Y, Kong X, Li Z, Jia Z, Cui J,
Gao J, Wang G and Xie K: Lamin B1 is a novel therapeutic target of
betulinic acid in pancreatic cancer. Clin Cancer Res. 19:4651–4661.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sun S, Xu MZ, Poon RT, Day PJ and Luk JM:
Circulating Lamin B1 (LMNB1) biomarker detects early stages of
liver cancer in patients. J Proteome Res. 9:70–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Karna E, Szoka L and Palka JA: Betulinic
acid inhibits the expression of hypoxia-inducible factor 1alpha and
vascular endothelial growth factor in human endometrial
adenocarcinoma cells. Mol Cell Biochem. 340:15–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Gleadle JM and Ratcliffe PJ: Induction of
hypoxia-inducible factor-1, erythropoietin, vascular endothelial
growth factor and glucose transporter-1 by hypoxia: Evidence
against a regulatory role for Src kinase. Blood. 89:503–509.
1997.PubMed/NCBI
|
|
64
|
Van Waes C: Nuclear factor-kappaB in
development, prevention and therapy of cancer. Clin Cancer Res.
13:1076–1082. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Patel HM, Rane R, Thapliyal N, Palkar M,
Shaikh M and Karpoormath R: Epidermal growth factor receptor (EGFR)
tyrosine kinase inhibitors from the natural origin: A recent
perspective. Anticancer Agents Med Chem. 15:988–1011. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Nikiforov YE: RET/PTC rearrangement in
thyroid tumors. Endocr Pathol. 13:3–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Nikiforov YE and Nikiforova MN: Molecular
genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol.
7:569–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wada S and Tanaka R: Betulinic acid and
its derivatives, potent DNA topoisomerase II in “hibitors, from the
bark of Bischofia javanica. Chem Biodivers. 2:689–694. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ganguly A, Das B, Roy A, Sen N, Dasgupta
SB, Mukhopadhayay S and Majumder HK: Betulinic acid, a catalytic
inhibitor of topoisomerase I, inhibits reactive oxygen
species-mediated apoptotic topoisomerase I-DNA cleavable complex
formation in prostate cancer cells but does not affect the process
of cell death. Cancer Res. 67:11848–11858. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Dillon LW, Pierce LC, Lehman CE, Nikiforov
YE and Wang YH: DNA topoisomerases participate in fragility of the
oncogene RET. PLoS One. 8:e757412013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kommera H, Kaluderovic GN, Kalbitz J and
Paschke R: Synthesis and anticancer activity of novel betulinic
acid and betulin derivatives. Arch Pharm (Weinheim). 343:449–457.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bache M, Bernhardt S, Passin S, Wichmann
H, Hein A, Zschornak M, Kappler M, Taubert H, Paschke R and
Vordermark D: Betulinic acid derivatives NVX-207 and B10 for
treatment of glioblastoma-an in vitro study of cytotoxicity and
radiosensitization. Int J Mol Sci. 15:19777–19790. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Shaw RJ and Cantley LC: Ras, PI (3)K and
mTOR signalling controls tumour cell growth. Nature. 441:424–430.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Madge LA, Li JH, Choi J and Pober JS:
Inhibition of phosphatidylinositol 3-kinase sensitizes vascular
endothelial cells to cytokine-initiated cathepsin-dependent
apoptosis. J Biol Chem. 278:21295–21306. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Gonzalez P, Mader I, Tchoghandjian A,
Enzenmuller S, Cristofanon S, Basit F, Debatin KM and Fulda S:
Impairment of lysosomal integrity by B10, a glycosylated derivative
of betulinic acid, leads to lysosomal cell death and converts
autophagy into a detrimental process. Cell Death Differ.
19:1337–1346. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Pandey MK, Sung B and Aggarwal BB:
Betulinic acid suppresses STAT3 activation pathway through
induction of protein tyrosine phosphatase SHP-1 in human multiple
myeloma cells. Int J Cancer. 127:282–292. 2010.PubMed/NCBI
|
|
78
|
Yang LJ, Chen Y, He J, Yi S, Wen L, Zhao
J, Zhang BP and Cui GH: Betulinic acid inhibits autophagic flux and
induces apoptosis in human multiple myeloma cells in vitro. Acta
Pharmacol Sin. 33:1542–1548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Cheng YQ, Chen Y, Wu QL, Fang J and Yang
LJ: Effect of betulinic acid on inducing apoptosis of human
multiple myeloma cell line RPMI-8226. Zhongguo Shi Yan Xue Ye Xue
Za Zhi. 17:1224–1229. 2009.(In Chinese). PubMed/NCBI
|
|
80
|
Ehrhardt H, Fulda S, Fuhrer M, Debatin KM
and Jeremias I: Betulinic acid-induced apoptosis in leukemia cells.
Leukemia. 18:1406–1412. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kumar D, Mallick S, Vedasiromoni JR and
Pal BC: Anti-leukemic activity of Dillenia indica L. fruit extract
and quantification of betulinic acid by HPLC. Phytomedicine.
17:431–435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wu Q, He J, Fang J and Hong M: Antitumor
effect of betulinic acid on human acute leukemia K562 cells in
vitro. J Huazhong Univ Technolog Med Sci. 30:453–457. 2010.
View Article : Google Scholar
|
|
83
|
Chen Z, Wu Q, Chen Y and He J: Effects of
betulinic acid on proliferation and apoptosis in Jurkat cells and
its in vitro mechanism. J Huazhong Univ Sci Technolog Med Sci.
28:634–638. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Chen Z, Wu QL, Chen Y and He J: Effect of
betulinic acid on proliferation, apoptosis, and cell cycle of human
lymphoma cell line Raji. Zhong Cao Yao Bian Ji Bu. 4:556–559.
2008.
|