Introduction
Asthma is a complex respiratory disease that
involves the remodeling of airway tissues and chronic inflammation
(1). Eosinophils are considered to
be key inflammatory effector cells that are implicated in the
pathogenesis of various diseases, including asthma (2). Previous studies have indicated that
eosinophils exert numerous proinflammatory effects in allergic
disorders through the release of various proinflammatory cytokines,
chemokines and cytotoxic molecules, which may ultimately lead to
the manifestation of allergic asthma (3,4).
Besides their recruitment and activation, an increase in the
survival and a reduction in the apoptosis rate of eosinophils has
been proposed as a putative mechanism that accounts for the
accumulation of inflammatory cells in allergic asthma (5). Currently, inhaled corticosteroids are
the mainstay pharmacotherapy for asthma. The broad
anti-inflammatory effects of these agents are divided into delayed
actions through alterations in protein expression, and rapid
actions probably through the membrane-bound glucocorticoid receptor
and direct interaction with the airway vasculature. However, a
small number of asthmatic patients do not respond well to inhaled
corticosteroids. In addition, side-effects of corticosteroids
include glaucoma, osteoporosis, growth retardation in children,
wound healing and metabolic effects. Therefore, in order to improve
the therapeutic strategies for patients with allergic inflammation,
including asthma, the development of novel agents that inhibit
eosinophil accumulation and survival is required.
Resveratrol (3,5,4′-trans-trihydroxystilbene) is a
natural polyphenol and phytoestrogen found in peanuts, mulberries
and grapes (Fig. 1A) (6,7). An
increasing number of previous studies have indicated that
resveratrol possesses a variety of pharmacological properties,
including antioxidant (8),
antiplatelet (9), anticancer
(10) and anti-inflammatory
activities (11). A previous study
indicated that resveratrol downregulates the production of monocyte
chemoattractant protein-1 by endothelial cells, which is a key
mediator that stimulates the infiltration of inflammatory cells
into the lungs, through the inhibition of p38 mitogen-activated
protein kinase activation in a rat model of acute pulmonary
thromboembolism (12). Previously,
it was reported that resveratrol notably inhibited the
proliferation of fibroblasts from human hypertrophic scars, and
decreased collagen expression in these cells (13). However, the effect of resveratrol
on the proliferation and survival of human eosinophils isolated
from asthmatic patients remains unclear. Based on the various
biological activities of resveratrol, particularly its
anti-inflammatory properties, the present study hypothesized that
resveratrol may regulate the survival and apoptosis of human
eosinophils. The results of the present study demonstrated an
inhibitory effect of resveratrol on eosinophils from asthmatic
patients through the prevention of cell cycle progression and the
induction of apoptosis.
Materials and methods
Chemicals and reagents
Resveratrol was obtained from Sigma-Aldrich (EMD
Millipore, Billerica, MA, USA) and was dissolved in
dimethylsulfoxide (DMSO). Resveratrol was subsequently diluted with
RPMI-1640 medium (Clonetics Corporation, San Diego, CA, USA) when
used to treat cells in culture. The final concentration of DMSO was
maintained at <0.1% in all cell culture experiments. At this
concentration, no obvious effects on cell growth were observed.
Ethical approval
The present study was approved by the Human Research
and Ethics Committee of The First Affiliated Hospital of Xinjiang
Medical University (Xinjiang, China). Written informed consent was
obtained from a total of 10 asthma patients (6 male, aged 25–30
years; 4 female, aged 20–30 years) that were recruited to the
study.
Eosinophil purification and
culture
Human eosinophils were purified and cultured as
described previously (14).
Briefly, eosinophils were purified from peripheral blood obtained
from the asthmatic patients using Percoll media (Sigma-Aldrich; EMD
Millipore) and density-gradient centrifugation was used to separate
the mononuclear cells from granulocytes. The lysis buffer (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China)
containing erythrocytes was subsequently removed, and purification
of eosinophils by negative selection was performed with CD16
immunomagnetic microbeads (catalog no. 130-045-701, Miltenyi Biotec
GmbH, Bergisch Gladbach, Germany) was performed. Purified
eosinophils were cultured in RPMI-1640 medium supplemented with 10%
fetal calf serum at 37°C in 5% (v/v) CO2. The purity of
eosinophils was assessed by staining blood smears with Hemacolor
Rapid Staining reagent (Ocon Chemicals Ltd., Cork, Ireland), and
was >98%. The viability of esosinophils was evaluated by trypan
blue staining and was >98%.
Cell proliferation assay
The effect of resveratrol on the proliferation of
eosinophils was determined using the Cell Counting Kit-8 (CCK8;
Sigma-Aldrich; EMD Millipore). Briefly, eosinophils at passage 4
were seeded into 96-well plates at a density of 1×105
cells/well and incubated at 37°C overnight. The cells were
subsequently treated with 0, 20, 40, 80, 160 or 320 µM resveratrol
for 24, 48 and 72 h before 10 µl CCK8 solution was added to each
well. Following incubation for 4 h at 37°C, the plates were read at
570 nm using an automated spectrophotometric plate reader
(PerkinElmer, Inc., Waltham, MA, USA). Experiments were performed
three times.
Cell cycle analysis
Purified eosinophils were seeded into 6-well plates
at a density of 2×105 cells/well. After 12 h, the cells
were treated with 0, 80 or 160 µM resveratrol for 24 and 48 h
before they were collected by centrifugation at 1,000 × g
for 5 min, at 4°C and washed with phosphate-buffered saline. The
cells were subsequently fixed in 75% ethanol for 2 h, resuspended
in 1 ml staining buffer (Beyotime Institute of Biotechnology,
Shanghai, China) containing 10 µg/ml RNase A for 30 min and were
subsequently stained with 15 µg/ml propidium iodide (PI) for 30
min. The cells were analyzed using a flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA) to determine cell cycle
progression.
Detection of apoptosis by flow
cytometry
Eosinophils were seeded into 6-well plates at a
density of 2×105 cells/well. After 12 h, the cells were
treated with 0, 80 or 160 µM resveratrol for 48 h, and were
collected and washed as before. The cells were subsequently labeled
with annexin V-fluorescein isothiocyanate (FITC)/PI and detected
using a flow cytometer with CellQuest Pro software version 5.1 (BD
Biosciences). The number of apoptotic cells, including those
undergoing early apoptosis (annexin
V-FITC+/PI−), late apoptosis (annexin
V-FITC+/PI+) and necrosis (annexin
V-FITC+/PI+), were expressed as a percentage
of the total number of cells.
Western blot analysis
After eosinophils were treated with various doses of
resveratrol (0, 80 and 160 µM), the cells were lysed, and the total
protein was extracted and collected, as described previously
(7). The protein concentration was
determined using a bicinchoninic acid assay. A total of 20 µg
protein from each sample was separated using a 10% SDS-PAGE gel.
The proteins were subsequently transferred onto a polyvinylidene
difluoride membrane. The membrane was then blocked in Tris-buffered
saline-Tween-20 (TBST; pH 7.4; 1.5 M NaCl; 20 mM Tris-HCl; 0.05%
Tween-20) containing 5% (w/v) non-fat milk for 2 h at room
temperature. The membrane was subsequently incubated overnight at
4°C with the following rabbit primary antibodies:
Anti-cyclin-dependent kinase 2 (CDK2; 1:1,000; catalog no. ab32147)
and anti-cyclin A (1:1,000; catalog no. ab181591), purchased from
Abcam (Shanghai, China); and anti-p53 (1:1,000; catalog no. 2527),
anti-p21 (1:1,000; catalog no. 2947), anti-cyclin E (1:1,000;
catalog no. 20,808), anti-Bim (1:800; catalog no. 2933), anti-Bax
(1:800; catalog no. 5023), anti-Bcl-2 (1:800; catalog no. 4223) and
anti-GAPDH (1:500; catalog no. 5174), purchased from Cell Signaling
Technology, Danvers, USA. After washing three times with TBST, the
membranes were incubated with an anti-rabbit peroxidase-conjugated
secondary antibody (1:800; catalog no. 611-7302; Rockland
Immunochemicals, Inc., Pottstown, PA, USA) at room temperature for
1 h. Protein bands were visualized with an enhanced
chemiluminescence reagent (Wuhan Boster Biological Technology,
Ltd., Wuhan, China) and detected using a LAS-3000 Luminescent
Imaging System (Fujifilm, Tokyo, Japan).
Statistical analysis
The data are expressed as the mean ± standard
deviation, and statistical analysis was performed using SPSS
software (version 13.0; SPSS, Inc., Chicago, IL, USA). Differences
between two groups were analyzed using the Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Resveratrol inhibits the proliferation
of eosinophils
In order to investigate the effects of resveratrol
on the eosinophil cell growth, purified eosinophils were treated
with various concentrations of resveratrol (0, 20, 40, 80, 160 or
320 µM) for 24, 48 and 72 h. The results of CCK8 assay demonstrated
that resveratrol effectively inhibited the proliferation of
eosinophils in a dose- and time-dependent manner (Fig. 1B). The greatest reduction in
eosinophil proliferation was observed using 320 µM resveratrol for
72 h.
Resveratrol induces cell cycle arrest
in eosinophils
To investigate the effects of resveratrol on cell
cycle progression, eosinophils were exposed to 0, 80 or 160 µM of
resveratrol for 24 and 48 h, and the cell cycle distribution
profiles were determined using a flow cytometer. Compared with
untreated controls, the percentage of eosinophils in G1
phase was increased, while the percentage of cells in S and
G2/M phases was decreased (Table I). These results suggested that
resveratrol treatment may arrest the cell cycle progression of
eosinophils in G1 phase.
 | Table I.Effect of resveratrol on the cell
cycle distribution of eosinophils. |
Table I.
Effect of resveratrol on the cell
cycle distribution of eosinophils.
|
| 24 h incubation | 48 h incubation |
|---|
|
|
|
|
|---|
| Resveratrol (µM) | G1
(%) | S (%) | G2/M
(%) | G1
(%) | S (%) | G2/M
(%) |
|---|
|
0 | 43.25±1.41 | 35.97±1.28 | 20.18±1.72 | 49.27±1.53 | 33.29±1.81 | 17.46±1.73 |
| 80 |
52.86±1.65a |
30.51±1.69a | 18.34±1.53 |
59.63±2.75a |
25.52±2.08a | 14.25±2.07 |
| 160 |
59.18±1.49b |
25.94±1.38b |
15.76±1.25a |
66.71±1.29b |
20.27±1.54b |
13.27±1.09a |
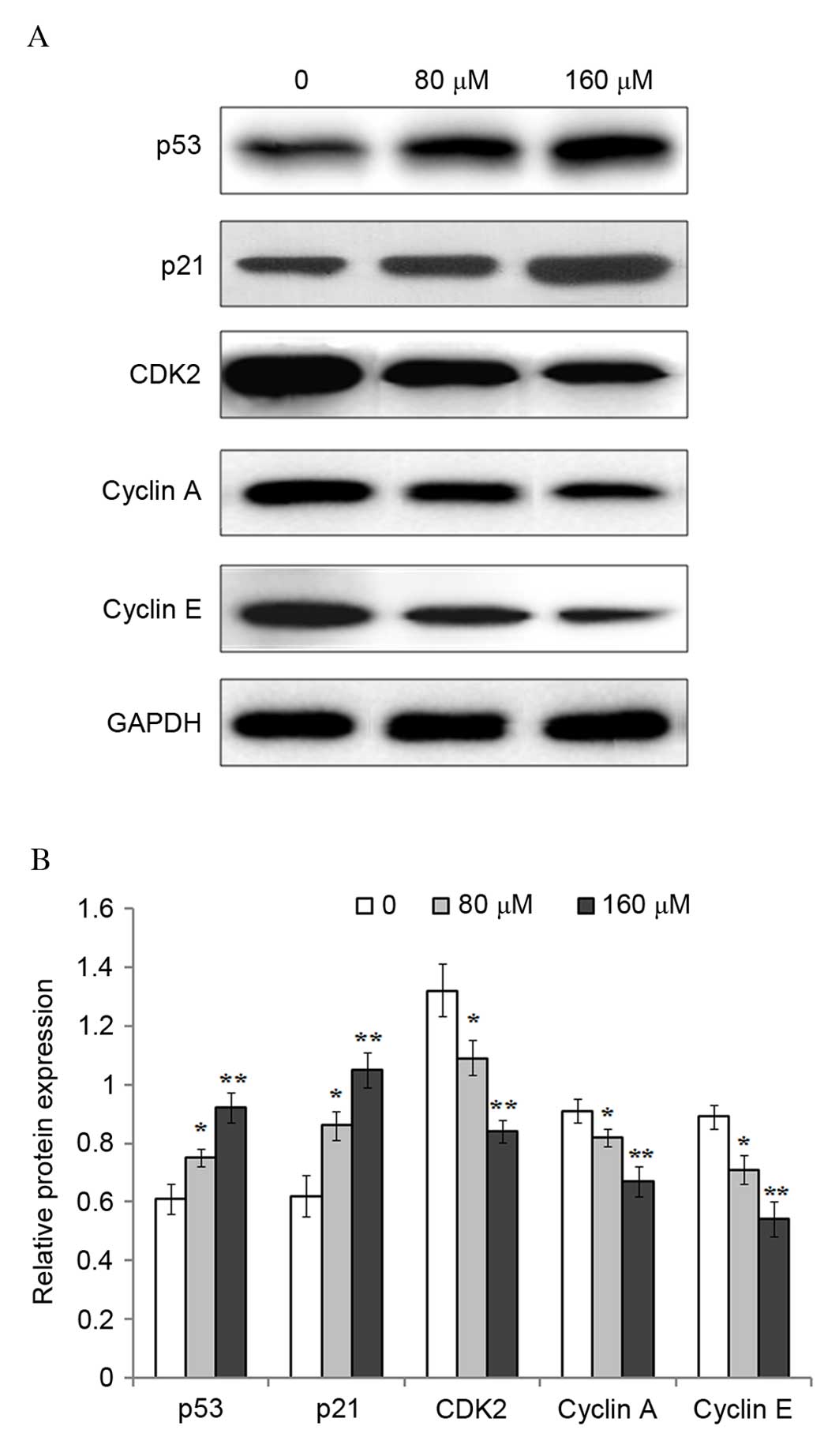
Previous studies have suggested that the p53 gene
serves an important role in the regulation of cell cycle
progression (15–17). Therefore, the protein expression
levels of p53 and its downstream target gene p21, as well as CDK2,
cyclin A and cyclin E cell cycle mediators were investigated
following exposure of eosinophils to 0, 80 or 160 µM resveratrol
for 48 h. Western blot analysis demonstrated that the protein
expression levels of p53 (P=0.0378; P=0.0085), and p21 (P=0.0327;
P=0.0054) were significantly increased following treatment with 80
and 160 µM resveratrol when compared with untreated controls
(Fig. 2). By contrast, the protein
expression levels of CDK2 (P=0.0342; P=0.0034), cyclin A (P=0.0476;
P=0.0085), and cyclin E (P=0.0401; P=0.0072) were significantly
reduced following exposure to 80 and 160 µM resveratrol compared
with the untreated controls.
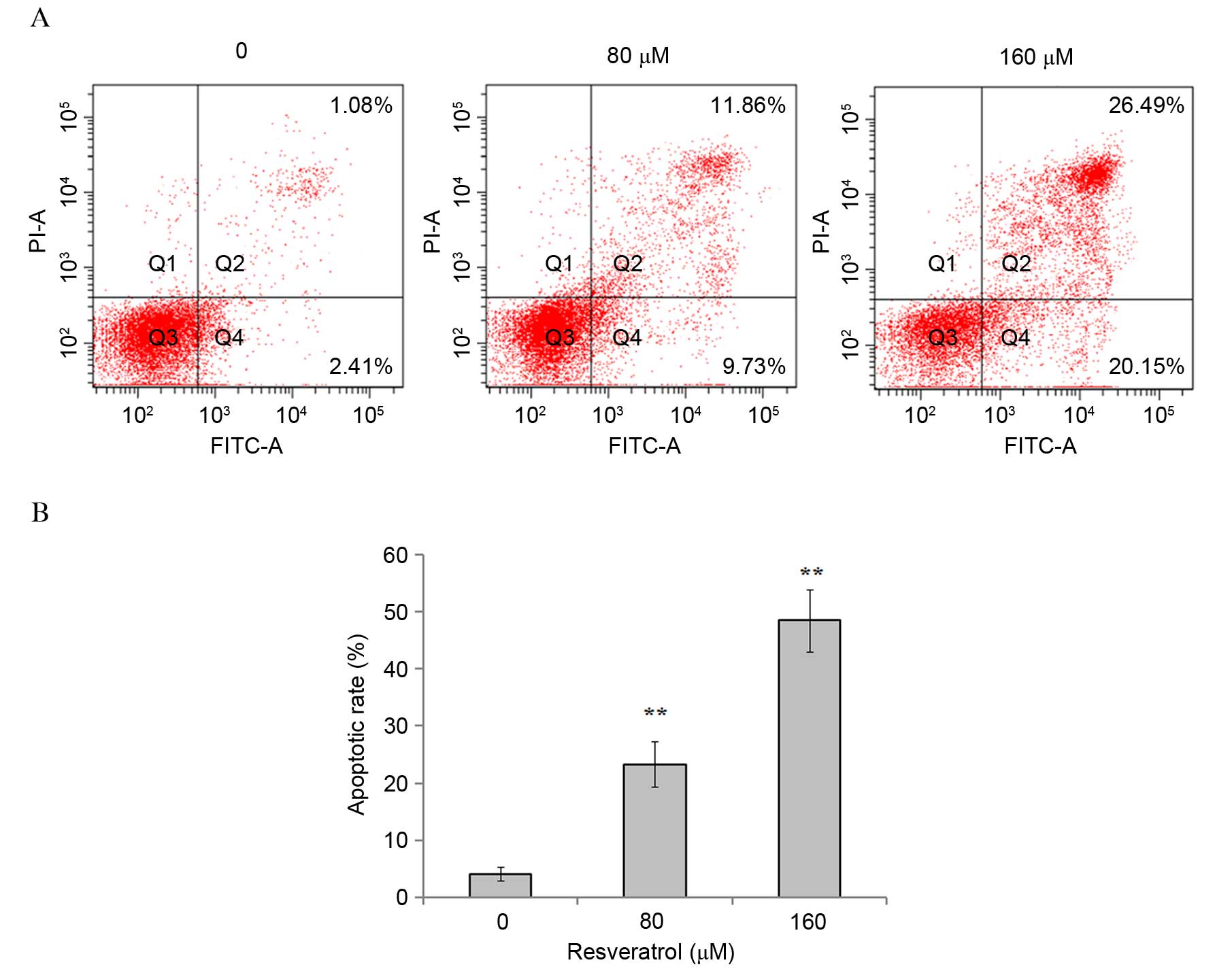
Resveratrol induces apoptosis in
eosinophils
In order to investigate whether the inhibitory
effect of resveratrol on eosinophil cell proliferation was
associated with induction of apoptosis, eosinophils were treated
with 0, 80 or 160 µM resveratrol for 48 h, and the percentage of
apoptotic cells was determined using a flow cytometer. The results
demonstrated that resveratrol significantly induced apoptosis in
eosinophils, in a dose-dependent manner [1.08±0.67 vs. 23.29±3.95
(P=0.0091) and 48.51±5.47% (P=0.0036) following exposure to 80 and
160 µM resveratrol, respectively (Fig.
3)].
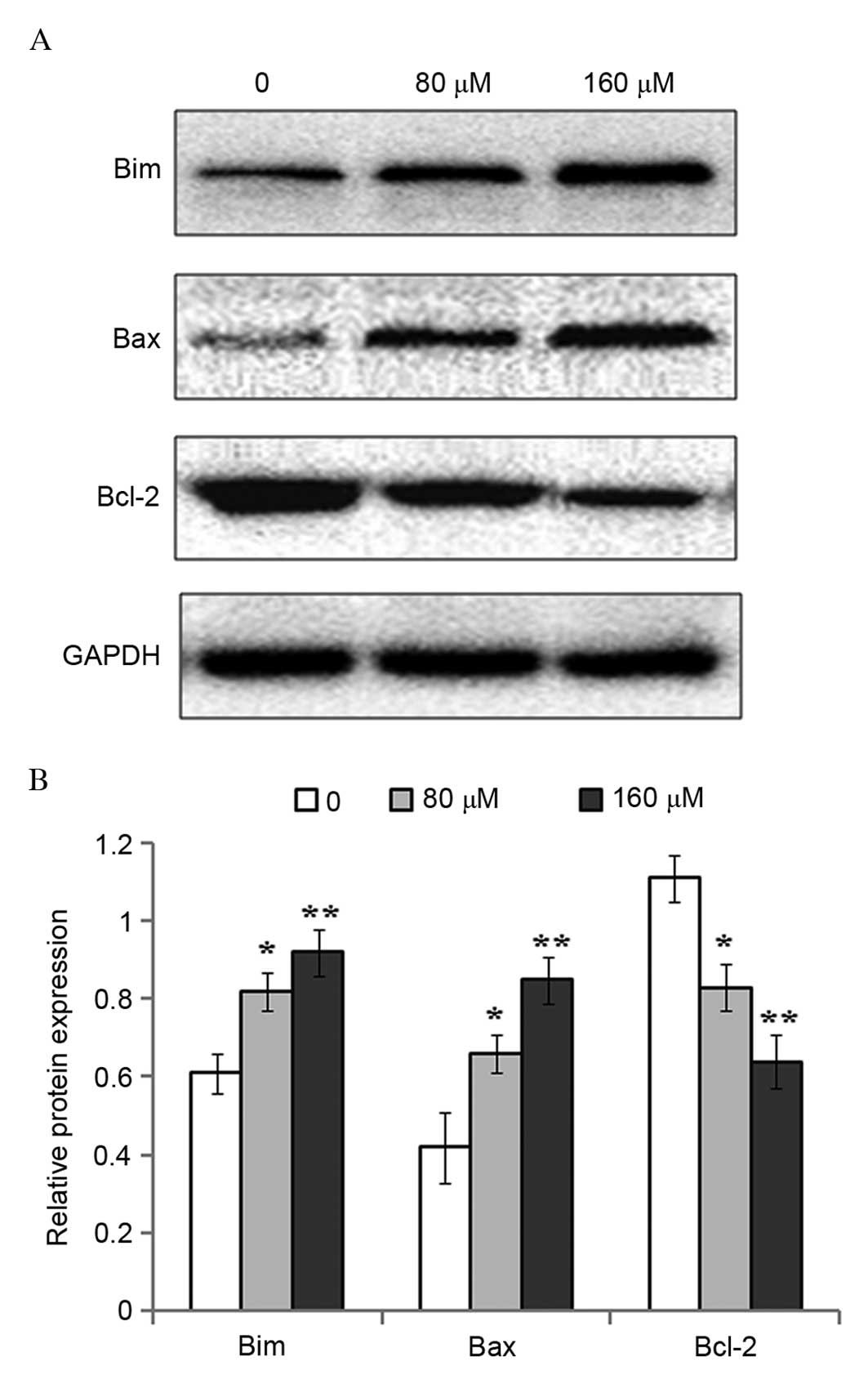
To investigate the potential molecular mechanisms
underlying resveratrol-induced apoptosis, the protein expression
levels of pro-apoptotic Bim and Bax, and anti-apoptotic Bcl-2, were
measured by western blot analysis. Compared with the untreated
controls, the protein expression levels of Bim (P=0.0355; P=0.0074)
and Bax (P=0.0406; P=0.0081) were increased significantly following
exposure of eosinophils to 80 and 160 µM resveratrol for 48 h,
whereas Bcl-2 protein expression levels were significantly
downregulated (P=0.0373; P=0.0056; Fig. 4).
Discussion
Asthma is a common chronic inflammatory disease,
which has increased in incidence in the United States over the past
two decades, and accounts for significant healthcare costs
(18). Eosinophils are considered
to serve a critical role in the pathogenesis of allergic asthma,
since they release a variety of factors that maintain and
exacerbate proinflammatory effects (4). The accumulation of eosinophils in the
lungs is commonly observed in 50% of patients with allergic asthma.
Eosinophil survival is considered to be prolonged in these patients
due to the production of proinflammatory factors, which may explain
the lack of eosinophil apoptosis in inflamed airways for several
days (19). Therefore, the
induction of eosinophil apoptosis may present a possible strategy
for the resolution of eosinophil-induced inflammation in the
treatment of allergic asthma. In the present study, resveratrol
effectively inhibited the proliferation of eosinophils isolated
from asthmatic patients in a dose and time-dependent manner. In
addition, resveratrol significantly induced eosinophil apoptosis,
likely through the upregulation of Bim and Bax protein expression
and the downregulation of Bcl-2 expression.
Resveratrol is a polyphenolic natural phytoalexin
found in various food products, and has antiproliferative,
anti-inflammatory and immunomodulatory properties. For these
reasons, resveratrol may be a potential agent for the treatment of
inflammatory disorders (20). It
has been reported that resveratrol attenuated oxidative stress and
suppressed the inflammatory response in the
diethylnitrosamine-initiated rat hepatocarcinogenesis (21). An additional study demonstrated
that resveratrol inhibited the proliferation of human aortic and
pulmonary aortic endothelial cells in a dose- and time-dependent
manner, as well as disrupted cell cycle progression (22). These findings are consistent with
the results of the present study demonstrating that resveratrol
decreased the proliferation of eosinophils in a dose- and
time-dependent manner. In addition, the results of the present
study suggested that the antiproliferative effect of resveratrol on
eosinophils may be associated with cell cycle inhibition. Following
exposure of eosinophils to 80 and 160 µM resveratrol, a greater
percentage of cells in G1 phase, and a reduced
percentage of cells in S and G2/M phases were observed
when compared with the untreated controls, which is similar to a
previous report involving human hypertrophic scar fibroblasts
(13). In order to investigate the
mechanism of resveratrol-mediated cell cycle arrest in eosinophils
further, the protein expression levels of p53, p21, CDK2, cyclin A
and cyclin E were determined by western blotting. The results
demonstrated that the protein expression levels of p53 and p21 were
significantly decreased following resveratrol treatment. Previous
studies have indicated that p53 serves an important role in
determining the response of cells to different types and levels of
stressful stimuli, and regulates downstream cell cycle, cell
metabolism, DNA repair and apoptotic signaling pathways (23–25).
In addition, p53 regulates the expression of downstream targets,
including p21, which contributes to the modulation of cell cycle
progression (26). CDK2 is a
catalytic subunit of the cyclin-dependent protein kinase complex,
and is essential for the G1/S phase transition of the
cell cycle (27). In the absence
of cyclin A and CDK2, apoptotic signaling pathways are induced. The
results of the present study demonstrated that resveratrol
treatment of eosinophils significantly downregulated the protein
expression levels of CDK2, cyclin A and cyclin E. The authors
hypothesized that the increase in p53 and p21, and the concurrent
reduction in CDK2, cyclin A and cyclin E protein expression levels
in eosinophils, may provide an explanation for the
resveratrol-mediated cell cycle arrest in G1/S
phase.
It is possible that a defect in apoptosis may
contribute to the chronic tissue eosinophilia associated with
asthma (28). Eosinophil apoptosis
is delayed in asthma, which may be partly explained by production
of granulocyte macrophage colony-stimulating factor (28). In a previous study, the apoptotic
index of peripheral blood eosinophils from asthmatic patients and
healthy volunteers was observed to be different. Eosinophils from
asthmatic patients not taking steroid medication exhibited a lower
apoptosis index (0.25) than those from healthy control subjects
(0.40) (28). In addition,
eosinophils isolated from asthmatic patients not undergoing
treatment with steroid medication survived for a longer period of
time compared with those from healthy control subjects. The
induction of eosinophil apoptosis is a current strategy used for
the treatment of allergic asthma, and forms the basis of a number
of available anti-asthmatic agents, including glucocorticoids,
theophylline and leukotriene modifiers (19). The results of the present study
demonstrated that the treatment of eosinophils with resveratrol
induced apoptosis in a dose-dependent manner. Further investigation
demonstrated that the expression levels of proapoptotic Bim and Bax
proteins were upregulated, while the expression of the
antiapoptotic protein Bcl-2 was reduced following exposure to
resveratrol. This may explain the observed increase in the
proportion of apoptotic eosinophils following exposure to
resveratrol.
In conclusion, the results of the present study
demonstrated that resveratrol treatment effectively suppressed the
proliferation of eosinophils from asthmatic patients. In addition,
resveratrol exposure increased the percentage of eosinophils in
G1 phase, and reduced the percentage of cells in S and
G2/M phases, potentially through the upregulation of p53
and p21 and the downregulation of CDK2, cyclin A and cyclin E
protein expression levels. Furthermore, resveratrol induced
apoptosis in eosinophils, likely through increasing Bim and Bax
protein expression and reducing Bcl-2 protein expression levels.
These findings suggested that resveratrol may be a potential
candidate for the treatment of asthma.
Acknowledgements
The authors would like to thank Dr Peter Rancourt
(Doküz Eylul University, Izmir, Turkey) for proofreading the
manuscript. The present study was partly supported by the National
Natural Science Foundation of China (grant nos. 81160004 and
81100026).
References
|
1
|
Haj-Salem I, Fakhfakh R, Bérubé JC,
Jacques E, Plante S, Simard MJ, Bossé Y and Chakir J: MicroRNA-19a
enhances proliferation of bronchial epithelial cells by targeting
TGFβR2 gene in severe asthma. Allergy. 70:212–219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hogan SP, Rosenberg HF, Moqbel R, Phipps
S, Foster PS, Lacy P, Kay AB and Rothenberg ME: Eosinophils:
Biological properties and role in health and disease. Clin Exp
Allergy. 38:709–750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lemière C, Ernst P, Olivenstein R,
Yamauchi Y, Govindaraju K, Ludwig MS, Martin JG and Hamid Q: Airway
inflammation assessed by invasive and noninvasive means in severe
asthma: Eosinophilic and noneosinophilic phenotypes. J Allergy Clin
Immunol. 118:1033–1039. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wong CK, Hu S, Leung KM, Dong J, He L, Chu
YJ, Chu IM, Qiu HN, Liu KY and Lam CW: NOD-like receptors mediated
activation of eosinophils interacting with bronchial epithelial
cells: A link between innate immunity and allergic asthma. Cell Mol
Immunol. 10:317–329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nutku E, Aizawa H, Hudson SA and Bochner
BS: Ligation of Siglec-8: A selective mechanism for induction of
human eosinophil apoptosis. Blood. 101:5014–5020. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alexander NS, Hatch N, Zhang S, Skinner D,
Fortenberry J, Sorscher EJ and Woodworth BA: Resveratrol has
salutary effects on mucociliary transport and inflammation in
sinonasal epithelium. Laryngoscope. 121:1313–1319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hiroto Y, Tadokoro K, Tsuda T, Nakazono E,
Ohnaka K, Takayanagi R, Hamasaki N and Tsuda H: Resveratrol, a
phytoestrogen found in red wine, down-regulates protein S
expression in HepG2 cells. Thromb Res. 127:e1–e7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yao J, Wang JY, Liu L, Li YX, Xun AY, Zeng
WS, Jia CH, Wei XX, Feng JL, Zhao L and Wang LS: Anti-oxidant
effects of resveratrol on mice with DSS-induced ulcerative colitis.
Arch Med Res. 41:288–294. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pervaiz S and Holme AL: Resveratrol: Its
biologic targets and functional activity. Antioxid Redox Signal.
11:2851–2867. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bishayee A, Politis T and Darvesh AS:
Resveratrol in the chemoprevention and treatment of hepatocellular
carcinoma. Cancer Treat Rev. 36:43–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Glehr M, Fritsch-Breisach M, Lohberger B,
Walzer SM, Moazedi-Fuerst F, Rinner B, Gruber G, Graninger W,
Leithner A and Windhager R: Influence of resveratrol on rheumatoid
fibroblast-like synoviocytes analysed with gene chip transcription.
Phytomedicine. 20:310–318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chun C, Yang W, Xueding C, Qi Z, Xiaoying
H, Honglei X, Fangyou Y, Chan C, Yuanyuan L, Weixi Z, et al:
Resveratrol downregulates acute pulmonary thromboembolism-induced
pulmonary artery hypertension via p38 mitogen-activated protein
kinase and monocyte chemoattractant protein-1 signaling in rats.
Life Sci. 90:721–727. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeng G, Zhong F, Li J, Luo S and Zhang P:
Resveratrol-mediated reduction of collagen by inhibiting
proliferation and producing apoptosis in human hypertrophic scar
fibroblasts. Biosci Biotechnol Biochem. 77:2389–2396. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Na HJ, Hudson SA and Bochner BS: IL-33
enhances Siglec-8 mediated apoptosis of human eosinophils.
Cytokine. 57:169–174. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kheirollahi M, Mehr-Azin M, Kamalian N and
Mehdipour P: Expression of cyclin D2, P53, Rb and ATM cell cycle
genes in brain tumors. Med Oncol. 28:7–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fischer M, Quaas M, Steiner L and Engeland
K: The p53-p21-DREAM-CDE/CHR pathway regulates G2/M cell cycle
genes. Nucleic Acids Res. 44:167–174. 2016. View Article : Google Scholar
|
|
17
|
Lukin DJ, Carvajal LA, Liu WJ,
Resnick-Silverman L and Manfredi JJ: P53 promotes cell survival due
to the reversibility of its cell-cycle checkpoints. Mol Cancer Res.
13:16–28. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang ZC, Yi MJ, Ran N, Wang C, Fu P, Feng
XY, Xu L and Qu ZH: Transforming growth factor-β1 induces bronchial
epithelial cells to mesenchymal transition by activating the Snail
pathway and promotes airway remodeling in asthma. Mol Med Rep.
8:1663–1668. 2013.PubMed/NCBI
|
|
19
|
Ilmarinen P and Kankaanranta H: Eosinophil
apoptosis as a therapeutic target in allergic asthma. Basic Clin
Pharmacol Toxicol. 114:109–117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian J, Chen JW, Gao JS, Li L and Xie X:
Resveratrol inhibits TNF-α-induced IL-1β, MMP-3 production in human
rheumatoid arthritis fibroblast-like synoviocytes via modulation of
PI3kinase/Akt pathway. Rheumatol Int. 33:1829–1835. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bishayee A, Barnes KF, Bhatia D, Darvesh
AS and Carroll RT: Resveratrol suppresses oxidative stress and
inflammatory response in diethylnitrosamine-initiated rat
hepatocarcinogenesis. Cancer Prev Res. 3:753–763. 2010. View Article : Google Scholar
|
|
22
|
Hsieh TC, Lu X, Guo J and Wu JM:
Differential regulation of proliferation, cell cycle control and
gene expression in cultured human aortic and pulmonary artery
endothelial cells by resveratrol. Int J Mol Med. 26:743–749.
2010.PubMed/NCBI
|
|
23
|
Kruse JP and Gu W: Modes of p53
regulation. Cell. 137:609–622. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang H, Xu Z, Zhong P, Ren Y, Liang G,
Schilling HA, Hu Z, Zhang Y, Wang X, Chen S, et al: Cell cycle and
p53 gate the direct conversion of human fibroblasts to dopaminergic
neurons. Nat Commun. 6:101002015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang X, Zhang S, Qi H, Wang Z, Chen HW,
Shao J and Shen J: JMJD5 interacts with p53 and negatively
regulates p53 function in control of cell cycle and proliferation.
Biochim Biophys Acta. 1853:2286–2295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Armstrong MJ, Stang MT, Liu Y, Gao J, Ren
B, Zuckerbraun BS, Mahidhara RS, Xing Q, Pizzoferrato E and Yim JH:
Interferon regulatory factor 1 (IRF-1) induces p21(WAF1/CIP1)
dependent cell cycle arrest and p21(WAF1/CIP1) independent
modulation of survivin in cancer cells. Cancer Lett. 319:56–65.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fei Q, Guo C, Xu X, Gao J, Zhang J, Chen T
and Cui D: Osteogenic growth peptide enhances the proliferation of
bone marrow mesenchymal stem cells from osteoprotegerin-deficient
mice by CDK2/cyclin A. Acta Biochim Biophys Sin (Shanghai).
42:801–806. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kankaanranta H, Lindsay MA, Giembycz MA,
Zhang X, Moilanen E and Barnes PJ: Delayed eosinophil apoptosis in
asthma. J Allergy Clin Immunol. 106:77–83. 2000. View Article : Google Scholar : PubMed/NCBI
|