Introduction
Inhalational anesthetics have been used in clinical
practice for >170 years; they have been extensively applied due
to their numerous advantages, including anesthetic efficacy and
safety, and the ease at which they can be used to regulate
anesthetic depth (1). In the
United States, the annual number of cases where general anesthesia
is used is ~40,000,000, and anesthesia has an important role in the
safety and success of surgery. In recent years, inhalational
anesthetics have been reported to protect against ischemic injury
in the cardiovascular system, brain, kidney and other important
organs (2); and the potential
clinical application of these drugs may be wider than at present.
However, further studies are required on how inhalational
anesthetics induce unconsciousness, immobilization and analgesia,
and other general anesthetic effects. In addition, it is necessary
to determine how inhaled anesthesia induces intraoperative
awareness and postoperative agitation, nausea and vomiting, and
postoperative cognitive decline (POCD), and how it impacts the
intelligence and central nervous system development of young
children (3). Such issues have
attracted extensive clinical attention. Clarification of the
aforementioned pathogenetic mechanisms not only has far-reaching
significance on revealing the underlying mechanism of general
anesthesia, but may also be used to guide clinical medication,
improve the safety of clinical anesthesia, and for the development
of novel general anesthetics and antagonists (4). Understanding the underlying
mechanisms of anesthesia is conducive to understanding the
neuroscience of consciousness, memory, perception, movement and
awakening (5).
The pathogenetic mechanism of POCD is complex, and
research regarding the mechanism is diverse. It has previously been
indicated that halothane can induce cognitive impairment (6). In addition, it has been reported that
increased hippocampal inflammatory cytokine expression may induce
transitory cognitive impairment (7). In mice with an interleukin (IL)-1
receptor knockout, peripheral operation-induced hippocampal
neuronal inflammation and IL-1β-induced autoimmune response are
associated with memory impairment. In addition, continuous
excessive expression of hippocampal IL-1β may lead to contextual
memory and spatial memory impairment of mice (8).
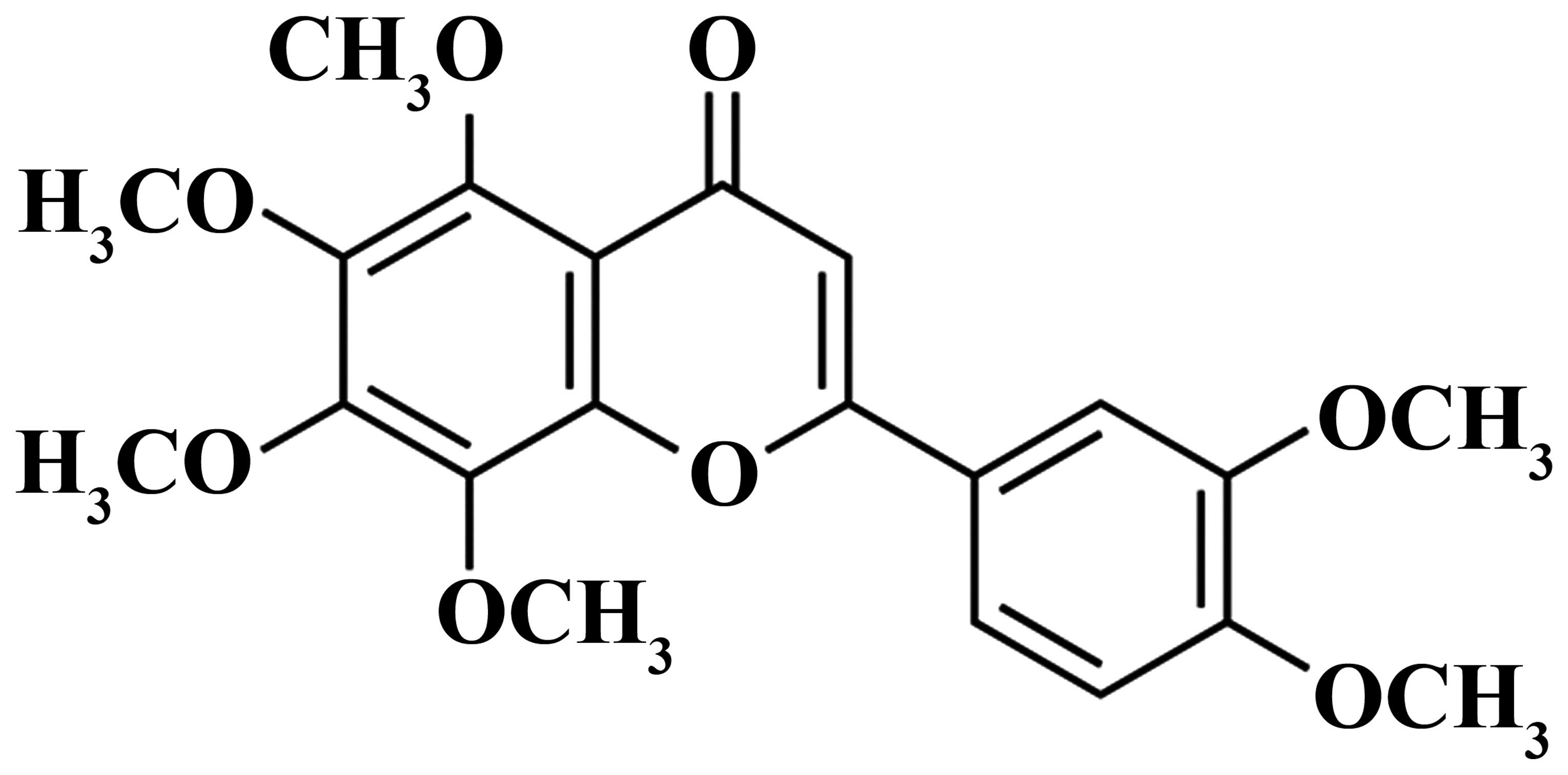
Nobiletin (Fig. 1)
is a type of polymethoxyflavonoid, which is predominantly found in
Pericarpium Citri Reticulatae, a traditional Chinese medicine
(9). Nobiletin exists in the
pericarp of citrus fruits, and exerts extensive physiological
effects that are beneficial to health. Therefore, nobiletin has
attracted clinical attention (10). As well as its anti-inflammatory,
antioxidant and anticancer activity, nobiletin has roles in
anti-atherosclerosis, reducing blood glucose levels, liver
protection and nerve nutrition (11). Previous studies have demonstrated
that rat hippocampal neurons cultivated in nobiletin may enhance
the signal channel of protein kinase A/extracellular
signal-regulated kinase/cAMP response element binding protein
(CREB) to alleviate memory deterioration caused by P-amyloid
protein in mice with Alzheimer's disease model and amyloid
precursor protein genetically modified, and to improve learning and
memory disorder caused by cerebral ischemia (12,13).
The aim of the present study was to determine the neuroprotective
effects of nobiletin, and to evaluate whether it could ameliorate
cognitive impairment via antioxidant, anti-inflammatory and
anti-apoptotic effects in isoflurane-treated aging rats.
Materials and methods
Ethical approval and experimental
animals
The animal protocol was approved by the Standing
Committee on Animals at Shandong University (Jinan, China). Male
Sprague-Dawley rats (age, 18 months) were acquired from the
Institute of Experimental Animals, Shandong University. All rats
were given ad libitum access to food and water, and were
maintained under controlled laboratory conditions: 12/12 h
light/dark cycle, 55±5% humidity and 23±2°C.
Rat model of isoflurane-induced
cognitive impairment
All aging rats were allowed to acclimate to the
environment for 1 week, and were then randomly separated into four
groups: i) Isoflurane group (n=20); ii) nobiletin (10) group (n=20); iii) nobiletin
(25) group (n=20); and iv) sham
group (n=10). Rats in the isoflurane and nobiletin (10) and (25) groups were administered 1.4%
isoflurane in a 100% oxygen environment for 2 h in an
anesthetization chamber. Rats were maintained at 37.5±0.5°C using a
heating pad. In the nobiletin (10) and (25) groups, the rats were
intraperitoneally injected with nobiletin (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) at 10 or 25 mg/kg/day for 3 days,
respectively (1).
Behavioral testing
A circular pool (150×50 cm; depth, 31 cm; 22±1°C)
was used to conduct the Morris water maze test. The pool area was
divided into SW, NW, SE and NE quadrants. A transparent platform
(diameter, 9 cm) 2 cm below water level was placed in the center of
the SW quadrant. All rats were trained to find the escape platform
in order to test reference memory. The rats underwent eight trials
for 30 min every day. The maximum swimming time was set at 120 sec
and ended with the animal finding the platform. Each rat was gently
guided to the platform, which remained for 30 sec, if the time
limit was exceeded. Escape latency, path length (length taken to
reach the platform) and swimming speed were all analyzed. After the
last reference memory test, the spatial probe test was performed.
Briefly, the rats were placed into the water and were allowed to
swim for 120 sec. Path length and time spent in the target quadrant
were recorded. The number of platform crossings were analyzed using
Actimetrics motion detection software for the Morris Water Maze
(Actimetrics Software, Evanston, IL, USA).
Enzyme-linked immunosorbent assay
(ELISA)
Whole blood samples were immediately collected by
centrifugation at 4,000 × g for 10 mins at 4°C to measure
serum nuclear factor (NF)-κB, tumor necrosis factor (TNF)-α, IL-1β
and IL-6 concentration using ELISA kits obtained from Nanjing
Jiancheng Bioengineering Institute (Nanjing, China). Glutathione
(GSH; Nanjing Jiancheng Bioengineering Institute), GSH peroxidase
(GSH-PX; Nanjing Jiancheng Bioengineering Institute), superoxide
dismutase (SOD; Elabscience Biotechnology Co. Ltd., Wuhan, China)
and malondialdehyde (MDA; Elabscience Biotechnology Co. Ltd.)
concentrations were also determined using ELISA kits, according to
the manufacturers' protocols.
Western blot analysis
The rats were anesthetized with 30 mg/kg
pentobarbital sodium and then sacrificed by decollation. Hippocampi
were then immediately removed and maintained in liquid nitrogen.
Frozen hippocampi were subsequently weighed, and ~50 mg tissue was
homogenized in RIPA buffer with protease inhibitors (Beyotime
Insititute of Biotechnology, Haimen, China). The homogenates were
centrifuged at 12,000 × g for 10 min at 4°C, and the
supernatant was collected in order to measure protein content using
bicinchoninic acid assay kit according to the manufacturer's
protocol (Beyotime Insititute of Biotechnology). Equal amounts of
protein samples (50 µg) were separated by 10–12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and were transferred to
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). After blocking with 5% skimmed milk in Tris-buffered saline
1% Tween-20 for 1 h, the membranes were probed with the following
primary antibodies: Anti-B-cell lymphoma 2-associated X protein
(Bax; cat. no. sc-6236; 1:500; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), anti-phosphorylated (p)-Akt (cat. no. sc-135650;
1:500; Santa Cruz Biotechnology, Inc.), anti-p-CREB (cat. no.
sc-81486; 1:500; Santa Cruz Biotechnology, Inc.),
anti-brain-derived neurotrophic factor (BDNF; cat. no. sc-20981;
1:500; Santa Cruz Biotechnology, Inc.) and anti-β-actin (cat. no.
sc-130656; 1:500; Santa Cruz Biotechnology, Inc.) overnight at 4°C.
Proteins were detected using horseradish peroxidase-conjugated
anti-rabbit secondary antibodies (cat. no. sc-2054; 1:1,000; Santa
Cruz Biotechnology, Inc.) at room temperature for 1 h. Blots were
visualized using an enhanced chemiluminescence kit (Santa Cruz
Biotechnology, Inc.). The membranes were analyzed using Image-Pro
Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD,
USA).
Statistical analysis
Results are presented as the mean ± standard
deviation and were analyzed using one-way analysis of variance,
followed by Tukey post-hoc multiple comparisons test using GraphPad
Prism version 4.0 (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Memory testing
To examine whether nobiletin exerted neuroprotective
effects on isoflurane-induced cognitive impairment, memory testing
was conducted. As shown in Fig. 2A and
B, treatment with isoflurane induced an increase in escape
latency and path length compared with in the sham group. Treatment
with nobiletin effectively decreased the escape latency and path
length in a dose-dependent manner. In addition, there was a
significant reduction in time spent in the target quadrant and the
number of times the rat crossed the platform in the isoflurane
model group compared with in the sham group (Fig. 2C and D). However, treatment with
nobiletin significantly increased time spent in the target quadrant
and the number of times the rat crossed the platform (Fig. 2C and D).
Antioxidant effects
To examine whether nobiletin exerted neuroprotective
effects on isoflurane-induced oxidative damage, GSH-PX, GSH, SOD
and MDA concentrations were analyzed using ELISA kits. As shown in
Fig. 3A-D, isoflurane inhibited
GSH-PX, GSH and SOD concentrations, whereas MDA concentration was
increased in the isoflurane group compared with in the sham group.
Conversely, treatment with nobiletin significantly increased
GSH-PX, GSH and SOD concentrations, and reduced MDA concentration
in isoflurane-treated rats (Fig.
3A-D).
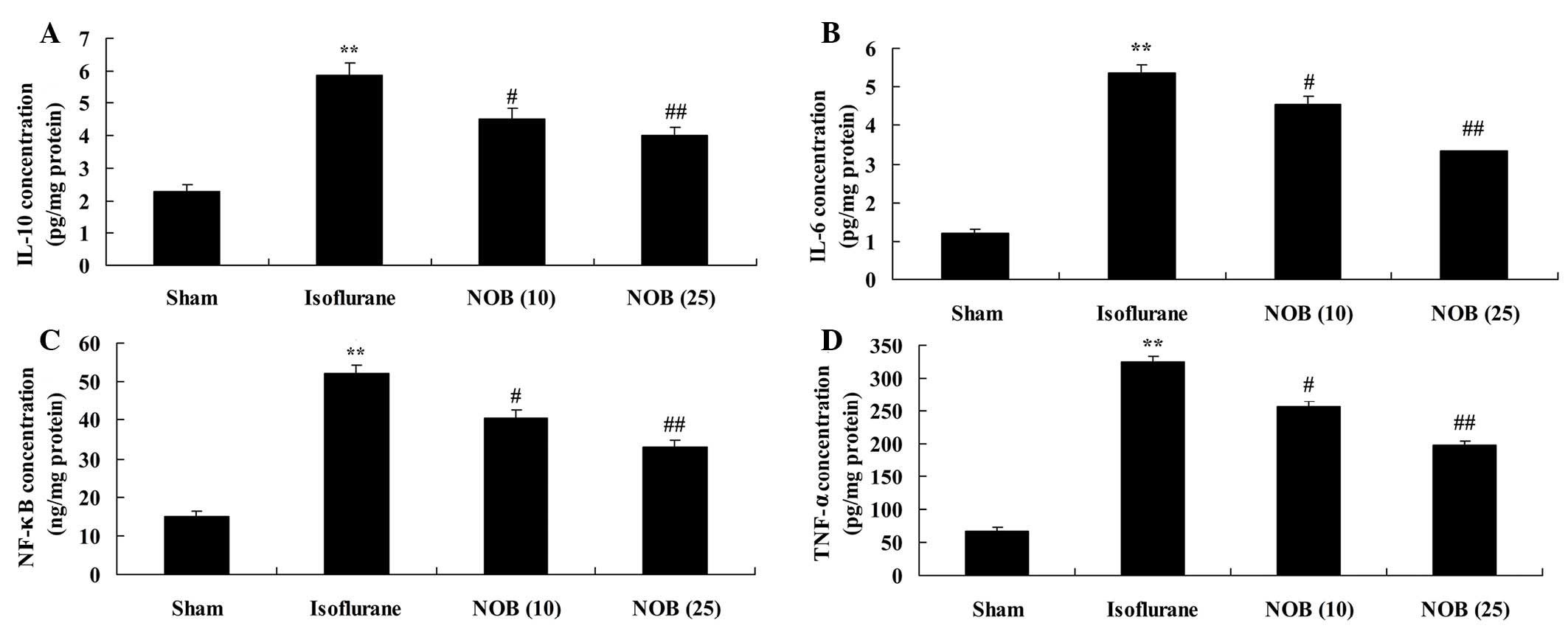
Anti-inflammatory effects
To examine whether nobiletin exerted neuroprotective
effects on isoflurane-induced inflammation, NF-κB, TNF-α, IL-1β and
IL-6 concentrations were analyzed using ELISA kits. As shown in
Fig. 4A-D, isoflurane enhanced
NF-κB, TNF-α, IL-1β and IL-6 concentrations compared with in the
sham group. However, treatment with nobiletin significantly reduced
NF-κB, TNF-α, IL-1β and IL-6 concentrations compared with in the
isoflurane-treated rats (Fig.
4A-D).
Protein expression of Bax
The present study assessed the effects of nobiletin
on the protein expression levels of Bax by western blot analysis.
Bax protein expression was activated by isoflurane compared with in
the sham group (Fig. 5).
Conversely, treatment with nobiletin significantly suppressed Bax
protein expression in isoflurane-treated rats (Fig. 5).
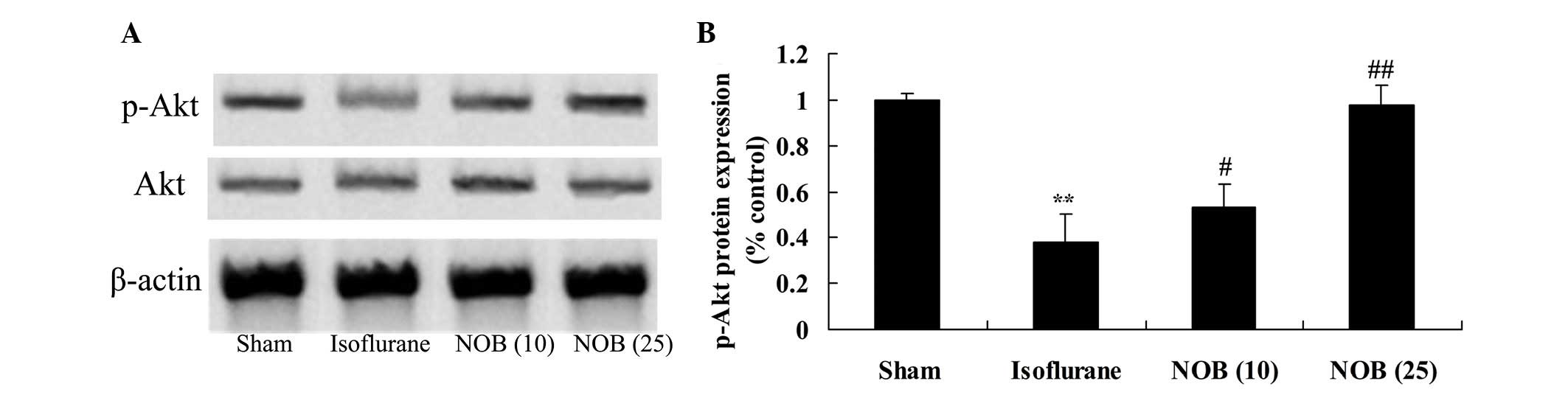
Protein expression of p-Akt
The protein expression levels of p-Akt were detected
in every group; p-Akt protein expression was decreased following
treatment with isoflurane compared with in the sham group (Fig. 6). However, treatment with nobiletin
markedly increased p-Akt protein expression compared with in the
isoflurane-treated group (Fig.
6).
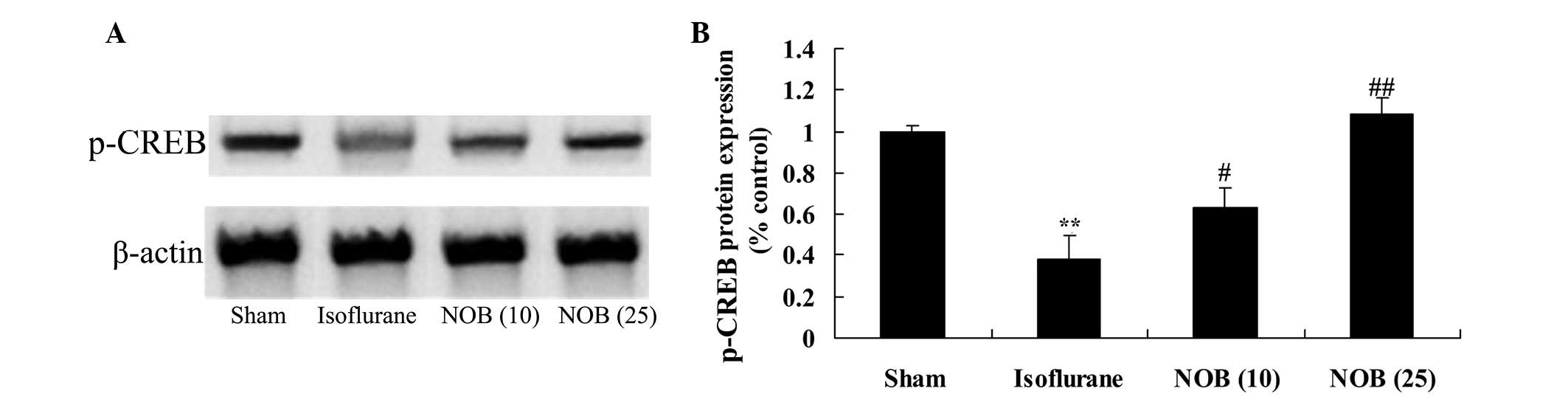
Protein expression of p-CREB
As shown in Fig. 7,
exposure to isoflurane markedly decreased the protein expression
levels of p-CREB compared with in the sham group. Treatment with
nobiletin increased p-CREB protein expression compared with in the
isoflurane-treated group (Fig.
7).
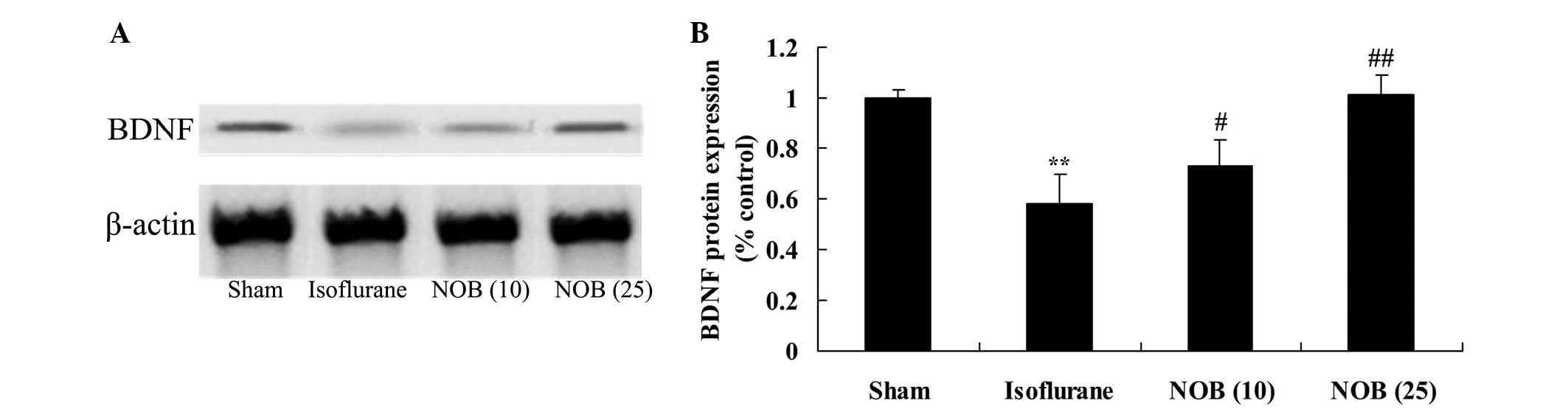
Protein expression of BDNF
To investigate the mechanism underlying the effects
of nobiletin on isoflurane-induced cognitive impairment, the
protein expression levels of BDNF were detected using western blot
analysis. Compared with the sham group, BDNF protein expression was
decreased following exposure to isoflurane anesthesia (Fig. 8). Conversely, the protein
expression levels of BDNF were increased following treatment with
nobiletin (Fig. 8).
Discussion
The primary cause of POCD is the use of anesthesia,
including frequently-used isoflurane, sevoflurane and other
inhalational anesthetics, as well as intravenous anesthetics, such
as ketamine and propofol (4).
Surgical and anesthetic complications can accelerate POCD. POCD
induced by isoflurane and other inhalational anesthetics is a
complex issue, which has attracted attention from surgeons and
anesthesiologists (14). At
present, several studies have focused on cognitive impairment
induced by isoflurane; however, whether the concentration of
frequently-used isoflurane (1–3%) is able to induce cognitive
impairment in patients undergoing surgery, as well as neuronal
apoptosis associated with cognitive function, requires further
study (15–17). The present study demonstrated that
the neuroprotective effects of nobiletin ameliorated
isoflurane-induced cognitive impairment in rats.
Proinflammatory and anti-inflammatory cytokines
exist in the body; the balance between them is regulated by the
neuroendocrine and immune systems. An imbalance between
proinflammatory and anti-inflammatory cytokines is a significant
cause leading to inflammatory injury (18). Among the types of inflammatory
damage that affect visceral organs, IL-1β is one of the earliest
proinflammatory cytokines. The synergistic effects of IL-1β and
TNF-α activate the inflammatory reaction-associated transcription
factor, NF-κB, in immune cells and non-immune cells, which induces
the inflammatory cascade reaction, promotes aggregation of
granulocytes, and lead to tissue damage (19). The present study demonstrated that
nobiletin significantly reduced isoflurane-induced NF-κB, TNF-α,
IL-1β and IL-6 concentrations in rats. Guan et al (20) reported that nobiletin attenuated
reactive oxygen species (ROS) production and the expression of
nuclear NF-κB p65 in a rat model of carotid artery injury (20). Jang et al (12) revealed that nobiletin ameliorated
scratching behavior via inhibiting the activation of NF-κB,
activator protein-1 and p38 in mice.
Dysfunctional energy metabolism and oxidative damage
have a role in cognitive impairment, since brain tissue is
particularly sensitive to tissue and free radical damage (21). In addition, the brain tissue exerts
strict demands on oxygen, and accounts for 20% of human body oxygen
consumption. However, GSH has obviously decreased in brain tissues
of isoflurane-induced aged rats (22). Sufficient evidence has revealed the
relationship between oxidative stress and cognitive function.
Antioxidants are able to reverse the memory impairment of aged rats
(23,24). Through lipid metabolite detection
and behavioral testing, it has been demonstrated that oxidative
damage can aggravate transitory cognitive impairment (25). The learning capacity of mice is
higher compared to that of aged rats. SOD is an essential
antioxidant in vivo; however, a large amount of SOD may
enhance oxidative stress (25).
Therefore, decreased SOD levels may 5reflect increasing of
oxidative stress levels. MDA is a lipid peroxidation product
associated with increased ROS levels. MDA levels can reflect the
degree of body lipid peroxidation, or indirectly reflect the degree
of tissue and cell damage (22).
The present study demonstrated that nobiletin significantly
increased GSH-PX, GSH and SOD concentrations, and reduced MDA
concentration in isoflurane-treated rats. Lo et al (11) reported that nobiletin inhibits
low-density lipoprotein oxidation in THP-1 cells.
The Akt signal transduction pathway is associated
with growth, proliferation and regulation of differentiation
(26). The Akt pathway promotes
survival, and its activation has an important role in nerve cell
protection, particularly in hypoxic ischemic neuronal injury. It
has recently attracted extensive attention (27). The activation of Akt can promote
endothelial cell survival, decrease nerve damage, reduce
inflammatory cell death and obstruct damage to thermoregulatory
neurons (27). The present study
demonstrated that nobiletin markedly increased isoflurane-induced
p-Akt protein expression. Zhang et al (28) reported that nobiletin was able to
activate p-Akt, p-CREB, BDNF and B-cell lymphoma 2 (Bcl-2) pathways
in order to protect against cerebral ischemia in rats.
Apoptosis is a complex type of programmed cell
death, which is associated with the regulation of several genes.
The Bcl-2 gene family has an important role during the regulatory
process. The Bcl-2 family can be divided into two categories,
according to effects on the apoptotic process: Proapoptotic genes,
including Bax, Bcl-2 antagonist/killer 1 and Bcl-2 associated
agonist of cell death; and anti-apoptotic genes, including Bcl-2,
Bcl-extra large and Bcl-w. Interactions between Bcl-2 family
proteins may affect cell survival and apoptosis. The interaction
between Bax and Bcl-2 is of particular importance. Increased levels
of Bax promote cell apoptosis; however, increased levels of Bcl-2
inhibit cell apoptosis. In the present study, nobiletin
significantly suppressed Bax protein expression in
isoflurane-treated rats. Malik et al (29) suggested that nobiletin may
ameliorate cisplatin-induced acute kidney injury by increasing the
expression of Bax, and exerting antioxidant, anti-inflammatory and
anti-apoptotic effects.
Previous studies regarding learning memory behaviors
have indicated that CREB, as ‘the third messenger’, is important in
the long-term memory process (30–32).
It is believed, as the optimal nuclear transcription factor, to
adapt to external stimulus as well as long term memory. Both the
cortical neuronal at plasticity forming process and hippocampal
neuron under long range increase stimulation and memory training
task, CREB phosphorylation and CRE reporter gene expression can be
detected. However, in mice with decreased spatial memory ability,
the phosphorylation level of CREB in the hippocampus has been
reported to be markedly decreased (33). In the present study, the protein
expression levels of CREB were enhanced following treatment with
nobiletin, as compared with in the isoflurane-induced model group.
Zhang et al (28)
demonstrated that nobiletin activated p-Akt, CREB, BDNF and Bcl-2
pathways for protection against cerebral ischemia in rats.
BDNF is an important member of the glial cell
line-derived neurotrophic factor family, which is predominantly
distributed throughout the central nervous system, particularly the
hippocampal area. BDNF exerts differentiative, proliferative and
nutritive effects on several types of neuron (34). In addition, it exerts promoting
effects on the synthesis of neurotransmitter and neurotrophic
factors, and is closely associated with learning, memory and
cognitive processes (35). At
present, it is believed that the occurrence of POCD is caused by
neuronal necrosis and apoptosis via multiple pathways. Previous
animal experiments have indicated that at the onset of POCD, the
expression of BDNF is markedly enhanced, which may significantly
alleviate ischemic brain injury, inhibit neuronal death, and exert
neuroprotective effects (35,36).
In the present study, the protein expression levels of BDNF were
increased following treatment of isoflurane-treated rats with
nobiletin. Zhang et al (28) reported that nobiletin activated
p-Akt, p-CREB, BDNF and Bcl-2 pathways in order to protect against
cerebral ischemia in rats. In addition, Li et al (37) suggested that nobiletin may
ameliorate hippocampal deficits via BDNF.
In conclusion, the present study demonstrated that
nobiletin exerts neuroprotective effects and ameliorates
isoflurane-induced cognitive impairment in aging rats. Nobiletin
exerted antioxidant, anti-inflammatory and anti-apoptotic effects
via the p-Akt, p-CREB, BDNF and Bcl-2 signaling pathways.
References
|
1
|
Zhang Y, Dong Y, Xu Z and Xie Z: Propofol
and magnesium attenuate isoflurane-induced caspase-3 activation via
inhibiting mitochondrial permeability transition pore. Med Gas Res.
2:202012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wiese AJ, Brosnan RJ and Barter LS:
Effects of acetylcholinesterase inhibition on quality of recovery
from isoflurane-induced anesthesia in horses. Am J Vet Res.
75:223–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng B, Zhang Y, Wang A, Dong Y and Xie
Z: Vitamin C attenuates Isoflurane-Induced Caspase-3 activation and
cognitive impairment. Mol Neurobiol. 52:1580–1589. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ni C, Li Z, Qian M, Zhou Y, Wang J and Guo
X: Isoflurane induced cognitive impairment in aged rats through
hippocampal calcineurin/NFAT signaling. Biochem Biophys Res Commun.
460:889–895. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sanders RD, Xu J, Shu Y, Januszewski A,
Halder S, Fidalgo A, Sun P, Hossain M, Ma D and Maze M:
Dexmedetomidine attenuates isoflurane-induced neurocognitive
impairment in neonatal rats. Anesthesiology. 110:1077–1085. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scuteri A and Wang H: Pulse wave velocity
as a marker of cognitive impairment in the elderly. J Alzheimers
Dis. 42:(Suppl 4). S401–S410. 2014.PubMed/NCBI
|
|
7
|
Shu Y, Zhou Z, Wan Y, Sanders RD, Li M,
Pac-Soo CK, Maze M and Ma D: Nociceptive stimuli enhance
anesthetic-induced neuroapoptosis in the rat developing brain.
Neurobiol Dis. 45:743–750. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao L, Li L, Lin D and Zuo Z: Isoflurane
induces learning impairment that is mediated by interleukin 1β in
rodents. PLoS One. 7:e514312012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakajima A, Ohizumi Y and Yamada K:
Anti-dementia activity of Nobiletin, a Citrus Flavonoid: A review
of animal studies. Clin Psychopharmacol Neurosci. 12:75–82. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamakuni T, Nakajima A and Ohizumi Y:
Pharmacological action of nobiletin, a component of AURANTII
NOBILIS PERICARPIUM with anti-dementia activity and its application
for development of functional foods. Nihon Yakurigaku Zasshi.
132:155–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lo YH, Pan MH, Li S, Yen JH, Kou MC, Ho CT
and Wu MJ: Nobiletin metabolite,
3′,4′-dihydroxy-5,6,7,8-tetramethoxyflavone, inhibits LDL oxidation
and down-regulates scavenger receptor expression and activity in
THP-1 cells. Biochim Biophys Acta. 1801:114–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jang SE, Ryu KR, Park SH, Chung S, Teruya
Y, Han MJ, Woo JT and Kim DH: Nobiletin and tangeretin ameliorate
scratching behavior in mice by inhibiting the action of histamine
and the activation of NF-κB, AP-1 and p38. Int Immunopharmacol.
17:502–507. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Al Rahim M, Nakajima A, Saigusa D, Tetsu
N, Maruyama Y, Shibuya M, Yamakoshi H, Tomioka Y, Iwabuchi Y,
Ohizumi Y and Yamakuni T: 4′-Demethylnobiletin, a bioactive
metabolite of nobiletin enhancing PKA/ERK/CREB signaling, rescues
learning impairment associated with NMDA receptor antagonism via
stimulation of the ERK cascade. Biochemistry. 48:7713–7721. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kupershmidt L, Amit T, Bar-Am O, Weinreb O
and Youdim MB: Multi-target, neuroprotective and neurorestorative
M30 improves cognitive impairment and reduces Alzheimer's-like
neuropathology and age-related alterations in mice. Mol Neurobiol.
46:217–220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang G, Ward C, Peng J, Zhao Y, Huang B
and Wei H: Isoflurane causes greater neurodegeneration than an
equivalent exposure of sevoflurane in the developing brain of
neonatal mice. Anesthesiology. 112:1325–1334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang N, Liang Y, Yang P, Wang W, Zhang X
and Wang J: TNF-alpha receptor antagonist attenuates
isoflurane-induced cognitive impairment in aged rats. Exp Ther Med.
12:463–468. 2016.PubMed/NCBI
|
|
17
|
Wang X, Zhao B and Li X: Dexmedetomidine
attenuates isoflurane-induced cognitive impairment through
antioxidant, anti-inflammatory and anti-apoptosis in aging rat. Int
J Clin Exp Med. 8:17281–17288. 2015.PubMed/NCBI
|
|
18
|
Kim YK, Na KS, Myint AM and Leonard BE:
The role of pro-inflammatory cytokines in neuroinflammation,
neurogenesis and the neuroendocrine system in major depression.
Prog Neuropsychopharmacol Biol Psychiatry. 64:277–284. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Castanon N, Lasselin J and Capuron L:
Neuropsychiatric comorbidity in obesity: Role of inflammatory
processes. Front Endocrinol (Lausanne). 5:742014.PubMed/NCBI
|
|
20
|
Guan S, Tang Q, Liu W, Zhu R and Li B:
Nobiletin inhibits PDGF-BB-induced vascular smooth muscle cell
proliferation and migration and attenuates neointimal hyperplasia
in a rat carotid artery injury model. Drug Dev Res. 75:489–496.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Golechha M, Sarangal V, Bhatia J, Chaudhry
U, Saluja D and Arya DS: Naringin ameliorates
pentylenetetrazol-induced seizures and associated oxidative stress,
inflammation and cognitive impairment in rats: Possible mechanisms
of neuroprotection. Epilepsy Behav. 41:98–102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu GS, Zhang ZS, Yang B and He W:
Resveratrol attenuates oxidative damage and ameliorates cognitive
impairment in the brain of senescence-accelerated mice. Life Sci.
91:872–877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leite MR, Wilhelm EA, Jesse CR, Brandao R
and Nogueira CW: Protective effect of caffeine and a selective A2A
receptor antagonist on impairment of memory and oxidative stress of
aged rats. Exp Gerontol. 46:309–315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haider S, Saleem S, Perveen T, et al:
Age-related learning and memory deficits in rats: role of altered
brain neurotransmitters, acetylcholinesterase activity and changes
in antioxidant defense system. Age (Dordr). 36:96532014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang C, He L, Yan M, Zheng GY and Liu XY:
Effects of polyprenols from pine needles of Pinus massoniana on
ameliorating cognitive impairment in a D-galactose-induced mouse
model. Age (Dordr). 36:96762014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li T and Wang G: Computer-aided targeting
of the PI3K/Akt/mTOR pathway: Toxicity reduction and therapeutic
opportunities. Int J Mol Sci. 15:18856–18891. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu W, Xu Z, Zhang M and Zuo Y: MiR-19a
promotes epithelial-mesenchymal transition through PI3K/AKT pathway
in gastric cancer. Int J Clin Exp Pathol. 7:7286–7296.
2014.PubMed/NCBI
|
|
28
|
Zhang L, Zhao H, Zhang X, Chen L, Zhao X,
Bai X and Zhang J: Nobiletin protects against cerebral ischemia via
activating the p-Akt, p-CREB, BDNF and Bcl-2 pathway and
ameliorating BBB permeability in rat. Brain Res Bull. 96:45–53.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Malik S, Bhatia J, Suchal K, Gamad N,
Dinda AK, Gupta YK and Arya DS: Nobiletin ameliorates
cisplatin-induced acute kidney injury due to its anti-oxidant,
anti-inflammatory and anti-apoptotic effects. Exp Toxicol Pathol.
67:427–433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alijanpour S, Rezayof A, Sepehri H and
Delphi L: Alterations in the hippocampal phosphorylated CREB
expression in drug state-dependent learning. Behav Brain Res.
292:109–115. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ortega-Martinez S: A new perspective on
the role of the CREB family of transcription factors in memory
consolidation via adult hippocampal neurogenesis. Front Mol
Neurosci. 8:462015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu L, Zhao QS, Li TW, et al: Yifei Xuanfei
Jiangzhuo formula, a Chinese herbal decoction, improves memory
impairment through inhibiting apoptosis and enhancing PKA/CREB
signal transduction in rats with cerebral ischemia/reperfusion. Mol
Med Rep. 12:4273–4283. 2015.PubMed/NCBI
|
|
33
|
Aguiar AS Jr, Castro AA, Moreira EL,
Glaser V, Santos AR, Tasca CI, Latini A and Prediger RD: Short
bouts of mild-intensity physical exercise improve spatial learning
and memory in aging rats: Involvement of hippocampal plasticity via
AKT, CREB and BDNF signaling. Mech Ageing Dev. 132:560–567. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Y, Lan R, Wang J, Li XY, Zhu DN, Ma
YZ, Wu JT and Liu ZH: Acupuncture reduced apoptosis and
up-regulated BDNF and GDNF expression in hippocampus following
hypoxia-ischemia in neonatal rats. J Ethnopharmacol. 172:124–132.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang F, Zhu ZQ, Liu DX, Zhang C, Gong QH
and Zhu YH: Emulsified isoflurane anesthesia decreases
brain-derived neurotrophic factor expression and induces cognitive
dysfunction in adult rats. Exp Ther Med. 8:471–477. 2014.PubMed/NCBI
|
|
36
|
Rak K, Völker J, Frenz S, Scherzad A,
Schendzielorz P, Radeloff A, Jablonka S, Hagen R and Mlynski R:
Effects of the neurotrophic factors BDNF, NT-3, and FGF2 on
dissociated neurons of the cochlear nucleus. Neuroreport.
25:960–964. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li J, Zhou Y, Liu BB, Liu Q, Geng D, Weng
LJ and Yi LT: Nobiletin ameliorates the deficits in Hippocampal
BDNF, TrkB and Synapsin I induced by chronic unpredictable mild
stress. Evid Based Complement Alternat Med. 2013:3596822013.
View Article : Google Scholar : PubMed/NCBI
|