Introduction
Cognitive decline may occur following major surgery
and anesthesia, and has been reported by patients and their
families for decades. Elderly patients are particularly susceptible
to such an event, which is known as postoperative cognitive
dysfunction (POCD). POCD is self-limiting in the majority of
patients (1); however, in some
patients it may be long-term or permanent. Previous studies have
reported that ~25% of elderly patients exhibit POCD 1 week
following non-cardiac surgery, whereas ~10% of elderly patients
exhibit POCD 3 months after non-cardiac surgery (2,3);
however, the association with general anesthesia remains unclear.
Inhalational anesthetics, including isoflurane, sevoflurane and
desflurane, are generally considered to be safe in clinical
anesthesia; however, numerous studies have demonstrated that these
agents can induce cell damage, neurodegeneration or POCD (4–8).
These observations raise concerns regarding the possibly
deleterious effects of general anesthesia in elderly patients.
Isoflurane is a common inhalational anesthetic
agent, exposure to which may induce cytotoxicity in various
neuronal and non-neuronal tissues and cells. In addition,
isoflurane has been reported to trigger widespread neuronal
apoptosis in the developing rat brain, subsequently leading to
persistent learning deficits and cognitive dysfunction, which may
persist for several weeks following treatment in adults, and aged
rats and mice (2,3). Our previous study demonstrated that
exposure to isoflurane at 1 minimal alveolar concentration (MAC)
for 12 h, or at 2 MAC for 8 h, may decrease cell viability, and
these effects may be associated with the disruption of
intracellular calcium homeostasis (9). Intracellular calcium homeostasis is
primarily regulated by three protein receptors on the endoplasmic
reticulum (ER): Inositol 1,4,5-trisphosphate receptors (IP3R),
ryanodine receptor (RyR) and Ca2+-ATPases (2,3),
IP3R on the ER membrane is able to induce non-physiological calcium
release, thus leading to a depletion of ER calcium, and increased
cytosolic ([Ca2+]c) and mitochondrial
calcium; these effects may contribute to cell apoptosis (10). A presenilin-1 mutation associated
with familial Alzheimer's disease (AD) has been reported to render
neurons vulnerable to isoflurane toxicity, via the induction of
abnormal calcium release from the ER through IP3R activation
(8). Mutations in β-amyloid (Aβ)
precursor protein (APP) are also associated with AD; therefore, the
present study hypothesized that this mutation may increase cell
susceptibility to isoflurane-induced cytotoxicity.
The aim of the present study was to clarify whether
the APP mutation enhances susceptibility to isoflurane-mediated
apoptosis, and whether this effect was induced by Ca2+
dysregulation via IP3R overactivation.
Materials and methods
Cell culture
SH-SY5Y neuroblastoma cells can undergo neuronal
maturation and have been previously used as an in vitro cell
model for studying the mechanisms of neuronal differentiation and
neurotoxicity. The SH-SY5Y human neuroblastoma cell line was
obtained from the Shanghai Institute for Biological Sciences of the
Chinese Academy of Sciences (Shanghai, China) and were cultured in
Dulbecco's' modified Eagle's medium (DMEM; Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) supplemented with 10% fetal bovine
serum (Sigma-Aldrich; Merck Millipore), 200 µg/ml G418, 100 U/ml
penicillin and 100 µg/ml streptomycin. Monolayer cultures at a
density of 0.3×105 cells/cm2 were incubated
in plastic flasks in a humidified atmosphere containing 95% air and
5% CO2 at 37°C. The medium was changed every 2 days, and cells were
passaged once they had reached 70–80% confluence. When the SH-SY5Y
cells reached 70% confluence, the cells were transfected with
overexpression plasmid pcDNA3.1-APP695, containing a mutant APP695
gene (Wanleibio, Shenyang, China) using the Lipofectamine 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's instructions. Cells were
transfected with vector alone or mutated APP. Prior to isoflurane
exposure, medium was replaced with serum-free DMEM.
Anesthetic exposure
Cells grown in plastic flasks were exposed to
isoflurane (1 MAC; 8 h) in a gas-tight chamber inside a cell
culture incubator. Cells in the control group were exposed to
atmospheric gas (5%CO2/21%O2/balanced N2) for 8 h. Atmospheric gas
(5%CO2/21%O2/balanced N2) was pumped in via a calibrated
agent-specific vaporizer, as described previously (9). Gas phase concentrations in the
incubator were verified by infrared absorbance of the effluent gas,
and were constantly monitored and maintained at the appropriate
concentrations throughout the experiments using an infrared Ohmeda
5330 Agent Monitor (Datex-Ohmeda; GE Healthcare Life Sciences,
Pittsburgh, PA, USA). Since the experimental cell culture plates
were inside the sealed anesthetic exposure chamber, which was
continuously perfused with a constant concentration of anesthetic,
the anesthetic concentration in the cell culture medium remained
stable as previously reported (11). There was no evidence of anesthetic
degradation in the cell culture over an 8 h time period. A group of
cells were pretreated with xestospongin C (100 nM; Merck Millipore)
for 30 in at room temperature prior to isofluarne exposure.
Imaging analysis of Annexin V and
propidium iodide (PI)
One of the early indications of cell damage is the
translocation of the phospholipid phosphatidylserine from the inner
to the outer leaflet of the plasma membrane. Annexin V is a
phospholipid-binding protein with a high affinity for
phosphatidylserine, which binds to it once exposed to environmental
stress. PI is able to bind to nucleic acids following penetration
of a breached plasma membrane, which occurs in the later stages of
cell damage. Immediately after treatment, the cells were analyzed
using an Annexin V/PI apoptosis kit [cat. no. AP 101-30; Multi
Sciences (Lianke) Biotech Co., Ltd., Hangzhou, China]. A total of
1–5×105 cells were collected by centrifugation (5,000 ×
g, 4°C, 5 min) and were resuspended in 500 µl 1X binding
buffer, to which was added 5 µl Annexin V and 10 µl PI. The cells
were then incubated at room temperature for 5 min in the dark, and
the number of Annexin V- and/or PI-positive cells were determined
by flow cytometry.
Observation of the changes in cell
ultrastructure
Following experimental treatment, the cells were
fixed in 2.5% glutaraldehyde. Following dehydration, soaking and
embedding as described previously (12), the samples were sliced and stained
in order to prepare transmission electron microscopy (TEM)
specimens for the observation of cell ultrastructure alterations
using a Libra200 microscope (Zeiss GmbH, Jena, Germany).
Measurements of [Ca2+]c
[Ca2+]c was measured by flow cytometry.
Cells were washed and incubated for 24 h at 37°C in NaCl Ringers
solution containing 1 mM CaCl; or in Na-gluconate Ringers solution
[125 mM Na-D-gluconate, 5 mM K-D-gluconate, 1 mM MgSO4,
32 mM HEPES/NaOH (pH 7.4) and 5 mM glucose] containing 1 mM
Ca-D-gluconate2. Cells were then loaded with Fluo-3/AM in
CaCl2 (1 mM)-containing NaCl or Na-gluconate Ringers
solution with 2 µM Fluo-3/AM. Cells were incubated at 37°C for 15
min with agitation, and were then washed twice and resuspended in
CaCl2 (2 mM)-containing NaCl Ringers solution.
Ca2+-dependent Fluo-3/AM fluorescence intensity was then
measured in fluorescence channel FL-1, which represents changes in
[Ca2+]c.
Western blot analysis
Following treatment, the cells were harvested and
total proteins were obtained by centrifugation at 10,000 × g
for 30 min at 4°C. Protein concentration was determined using a
Bio-Rad Dc assay kit (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) and were samples were subjected to western blot analyses.
Proteins (1–5 µg/ml) were separated on by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis on 7.5% gels and
transferred onto polyvinylidene difluoride membranes. The membranes
were blocked at room temperature for 90 min in Tris-buffered
saline-Tween containing 5% skim milk. Membranes were incubated with
rabbit polyclonal anti-IP3R antibody (1:800; cat. no. 07-1213; EMD
Millipore, Billerica, MA, USA) for 1 h at room temperature to
detect the protein levels of IP3R (250 kDa). Anti-β-actin antibody
(1:10,000; cat. no. MABE89; Sigma-Aldrich; Merck Millipore) was
used to detect β-actin (42 kDa). Subsequently, the membrane was
incubated with horseradish peroxidase-conjugated secondary antibody
(cat. no. AP181P; Bio-Rad Laboratories, Inc.) for 1 h at room
temperature. The signal was visualized using a Kodak Image Station
2000R system (Kodak, Rochester, NY, USA) and RapidStep™ reagent
(Merck Millipore). Each band in presented western blots represents
an independent experiment. Results were averaged from between three
and 10 independent experiments. Briefly, signal intensity was
analyzed using the National Institutes of Health (NIH) image
program (NIH Image 1.62; NIH, Bethesda, MD, USA). Western blots
were semi-quantified according to two steps. Firstly, levels of
β-actin were used to normalize levels of IP3R to the control, in
order to account for any loading differences in total protein
amount. Secondly, changes in IP3R protein levels in treated cells
were presented as a percentage of those in control cells.
Statistical analysis
Data were analyzed by SPSS 13.0 statistical software
(SPSS, Inc., Chicago, IL, USA). All data met normality and
homogeneity of variance, and were presented as the mean ± standard
deviation. Results regarding the effects of isoflurane on mutated
APP-transfected cells and vector-transfected cells were analyzed
using unpaired two-tail t-test. All other data were analyzed by
one-way analysis of variance followed by Newman-Keuls multiple
comparison tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Assessment of control conditions
The present study initially assessed whether control
conditions (5% CO2/21% O2/balanced N2) were able to affect cell
apoptosis, [Ca2+]c and IP3R expression. There were no
significant differences in cell apoptosis, [Ca2+]c and
IP3R levels in the cells exposed to control conditions compared
with the cells exposed to standard cell incubator conditions (data
not shown).
Isoflurane induces apoptosis of
SH-SY5Y cells transfected with APP mutation
Our previous study demonstrated that exposure to
isoflurane at 1 MAC for 12 h, or at 2 MAC for 8 h (9), may decrease cell viability;
therefore, in SH-SY5Y cells, the effects of isoflurane exposure at
1 MAC were compared between cells transfected with an APP mutation
and those transfected with the vector. In order to confirm that
apoptosis observed in SH-SY5Y cells was induced by isoflurane, the
number of Annexin V-positive/PI-negative and Annexin
V-positive/PI-positive cells was counted following exposure to
isoflurane (1 MAC) for 8 h (Fig.
1). Isoflurane induced apoptosis in mutated APP-transfected
SH-SY5Y cells (Fig. 1A-C) and
vector-transfected SH-SY5Y cells (Fig.
1D-F). As shown in Fig. 1C,
treatment with 1.2% isoflurane (~1 MAC) for 8 h induced a
significant increase in the apoptotic rate of mutated
APP-transfected SH-SY5Y cells compared with the vector-transfected
SH-SY5Y cells (Fig. 1F).
Inhibition of IP3R activity with xestospongin C partly reduced
isoflurane-induced cell apoptosis (Fig. 1B and E).
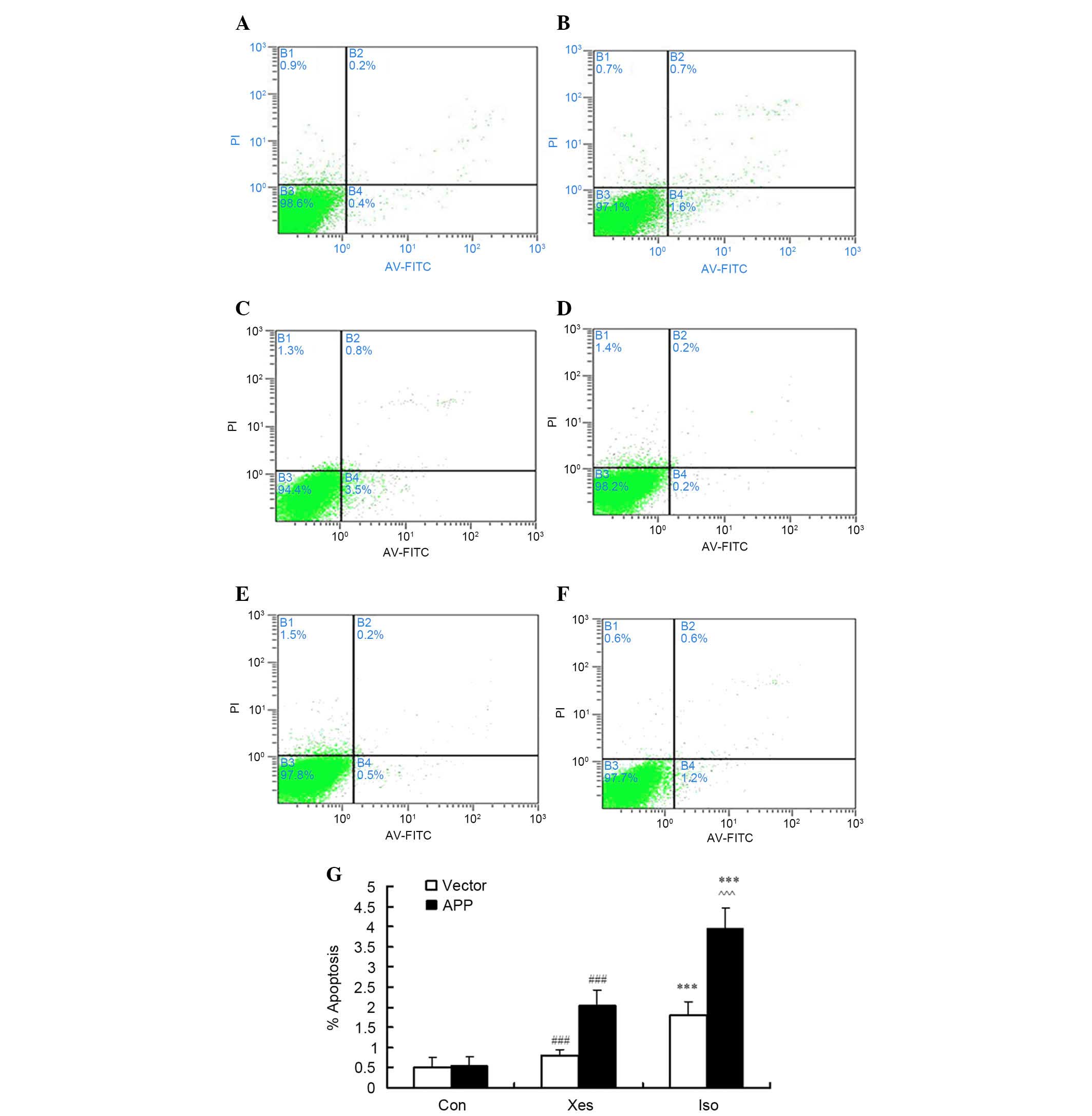 | Figure 1.After cells were treated with 1.2%
isoflurane for 8 h, apoptosis was determined by flow cytometry.
Briefly, the cells were harvested, stained with annexin
V-fluorescein isothiocyanate (FITC) and propidium iodide (PI), and
were then analyzed. (A-C) Representative results from one of six
independent experiments on mutated β-amyloid precursor protein
(APP)-transfected SH-SY5Y cells treated with (A) the control
condition (Con), (B) xestospongin C plus isoflurane (Xes) and (C)
isoflurane (Iso). (D-F) Representative results from
vector-transfected SH-SY5Y cells treated with (D) Con, (E) Xes and
(F) Iso. The quadrants of each plot exhibit the following: Viable
cells (Annexin−/PI−; lower left), early
apoptotic cells (Annexin+/PI−, lower right),
necrotic cells (Annexin+/PI+, upper right),
and late apoptotic cells (Annexin−/PI+, upper
left). (G) Data are presented as a sum of the percentage of
apoptotic cells in early apoptosis
(annexin+/PI−) and late apoptosis
(annexin+/PI+). Data are presented as the
mean ± standard deviation of at least three separate experiments.
***P<0.001 compared with Con and Xes; ###P<0.001
compared with Con; ^^^P<0.001 compared with vector
control group. |
Effects of isoflurane on the
ultrastructure of mutated APP-transfected SH-SY5Y cells
The results of a TEM analysis (Fig. 2) indicated that vector-transfected
SH-SY5Y cells were characterized by a smooth nuclear membrane, and
slight expansion and degranulation of the ER. Conversely, mutated
APP-transfected SH-SY5Y cells exhibited marked morphological
alterations; there were a large number of vacuoles in the
cytoplasm, organelle structure was incomplete, the ER exhibited a
moderate to high degree of swelling and marked degranulation,
mitochondrial cristae were disordered, the membrane structure was
damaged, and the number of microtubules was increased.
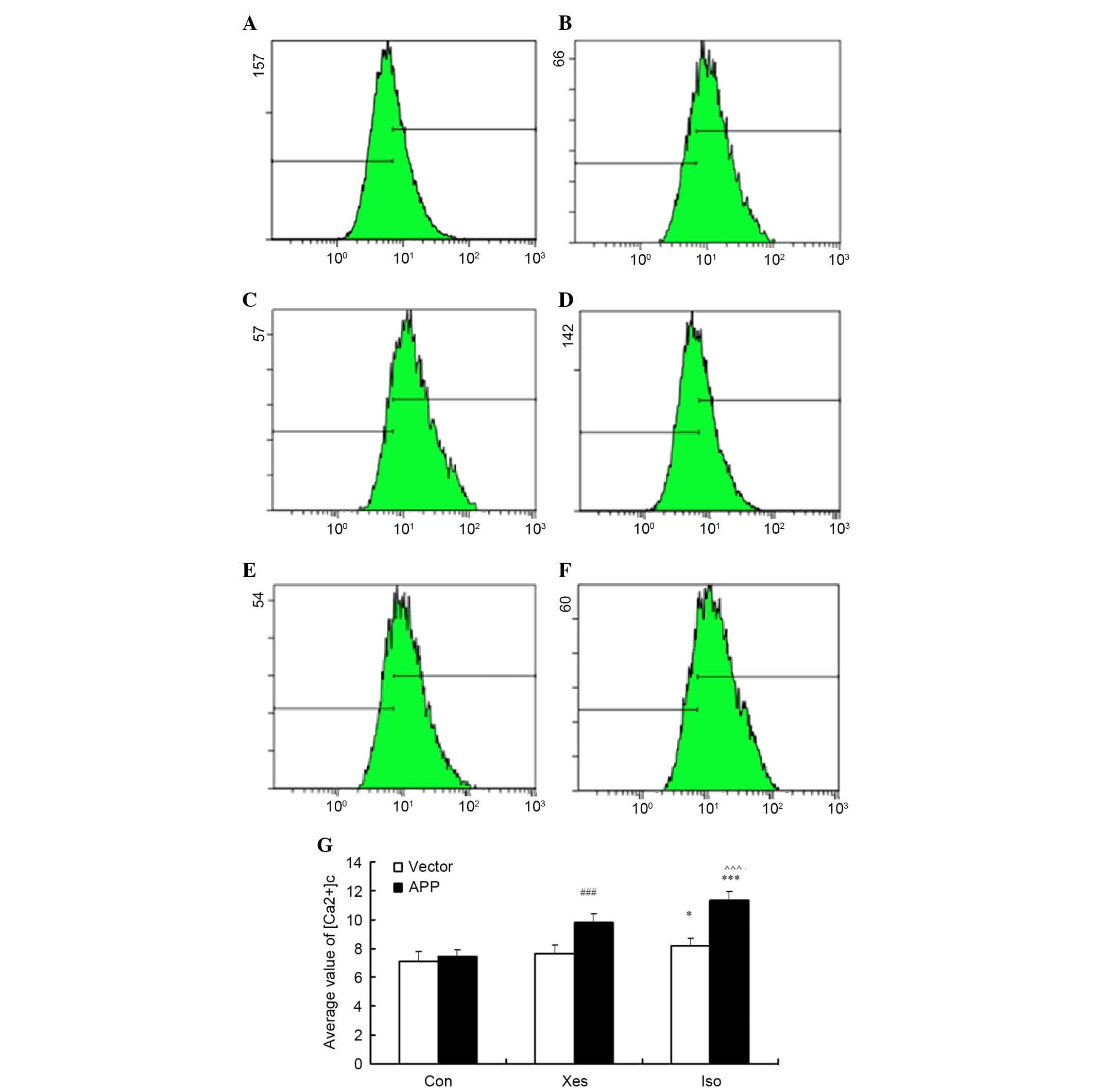
Effects of isoflurane on intracellular
calcium homeostasis
[Ca2+]c was determined by measuring the
average value of calcium fluorescence intensity by flow cytometry
in mutated APP-transfected SH-SY5Y cells (Fig. 3A-C) and vector-transfected SH-SY5Y
cells (Fig. 3D-F). Treatment with
isoflurane (1 MAC) induced a significant elevation in the
[Ca2+]c of mutated APP-transfected SH-SY5Y cells
(Fig. 3C) compared with in the
vector-transfected SH-SY5Y cells (Fig.
3F). In addition, it was determined whether calcium release
from the ER via IP3R contributed to isoflurane-induced elevation in
the [Ca2+]c of mutated APP-transfected SH-SY5Y cells.
Pretreatment with the potent IP3R antagonist, xestospongin C, for
30 min reduced isoflurane-induced calcium release from the ER
(Fig. 3B). These results suggest
that exposure to 1 MAC isoflurane for 8 h may significantly
increase intracellular calcium concentration via activation of IP3R
in mutated APP-transfected SH-SY5Y cells.
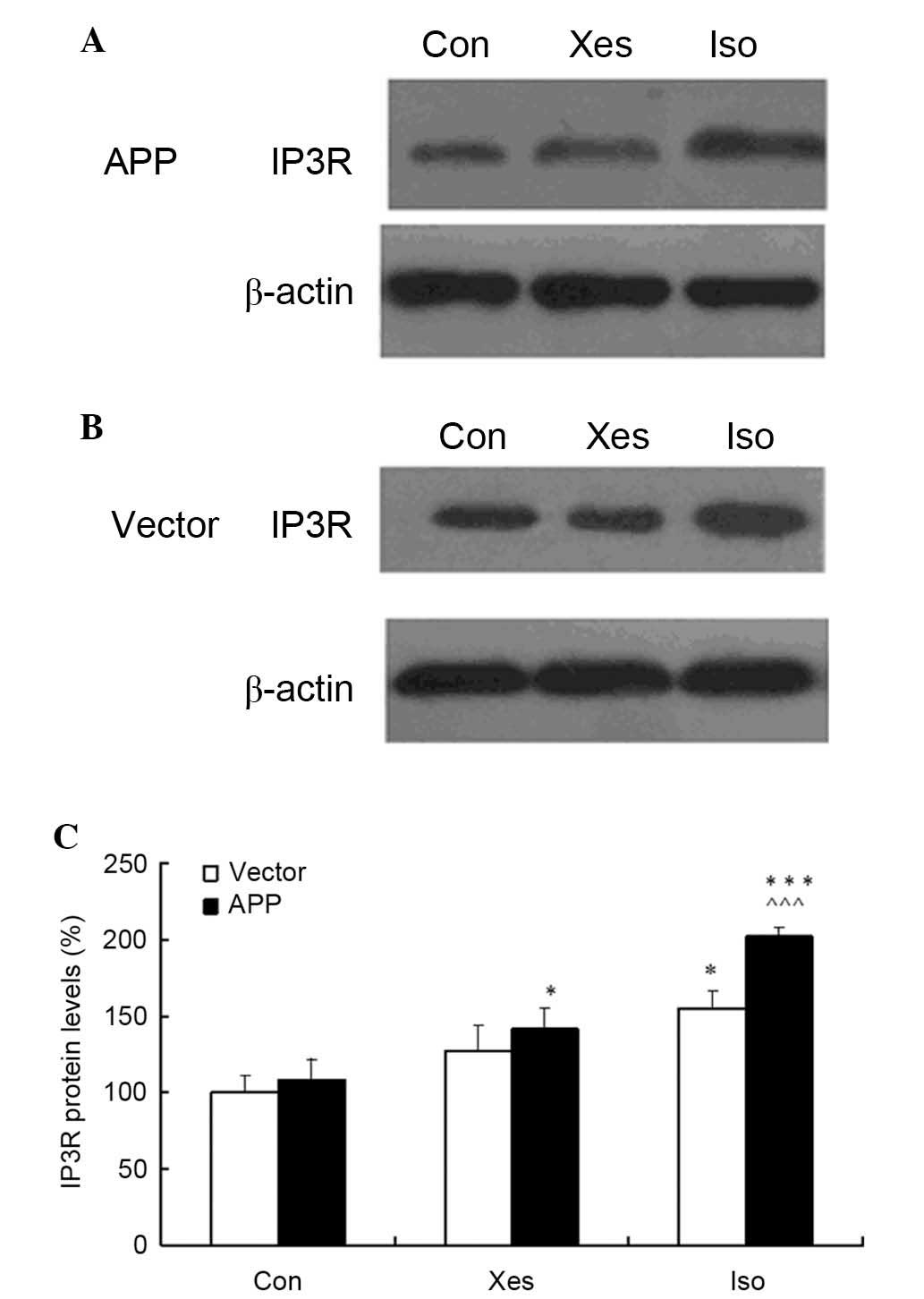
Isoflurane increases the protein
expression levels of IP3R
The present study assessed the effects of 1.2%
isoflurane on IP3R expression in SH-SY5Y cells. Treatment with 1.2%
isoflurane led to increases in the protein expression levels of
IP3R in mutated APP- and vector-transfected SH-SY5Y cells compared
with the cells in the control or xestospongin C groups (Fig. 4A and B). Compared with
vector-transfected SH-SY5Y cells, IP3R expression was increased in
mutated APP-transfected SH-SY5Y cells. These results indicate that
isoflurane exposure at 1 MAC for 8 h may increase IP3R protein
expression, and 100 nM xestospongin C could partly suppress
isoflurane-induced upregulation of IP3R protein expression.
Discussion
The present study demonstrated that treatment with
isoflurane at equipotent concentrations induced apoptosis of
SH-SY5Y cells by elevating [Ca2+]c levels,
whereas exposure to control conditions (5% CO2/21%
O2/N2) did not. Furthermore, isoflurane
induced a larger increase in apoptotic rate, and increased the
elevation of [Ca2+]c and IP3R protein levels
in mutated APP-transfected SH-SY5Y cells compared with in
vector-transfected control cells. These findings implicated IP3R as
the main source of calcium release from the ER.
Previous studies have proposed that inhalational
anesthetics induce apoptosis via dysregulated intracellular calcium
homeostasis (5,7,13).
Ca2+ regulation in neurons is complex.
[Ca2+]c in neurons is maintained at −100 nM,
a low level relative to the extracellular fluid (extracellular
[Ca2+]=1.2 mM). The dynamic balance of intracellular
Ca2+ homeostasis is maintained by Ca2+
transportation across cell membranes and regulation of
intracellular calcium stores. In response to stress, neurons will
instantly improve [Ca2+]c levels, in order to
trigger a series of physiological activities. The ER is the primary
source of releasable intracellular calcium in neurons (11) and has an important role in the
maintenance of intracellular calcium homeostasis, protein
synthesis, cell survival and apoptosis (14,15).
RyR and IP3R are calcium-activated calcium release channels that
are present on the ER membrane. Calcium release from the ER via RyR
activation can result in activation of IP3R and vice versa. In
neurons, isoflurane appears to induce calcium release from the ER;
however, it remains unclear whether this is due to direct or
indirect effects on IP3 or RyR. Our previously published and
current data suggested that overactivation of IP3R may contribute
to isoflurane-induced calcium elevation and cell apoptosis.
Excessive calcium release from the ER via IP3R may induce calcium
overload in the mitochondria and depletion of ER calcium (11), which may result in collapse of the
mitochondrial membrane potential and promotion of apoptosis.
To determine the importance of IP3R in
isoflurane-induced apoptosis, IP3R activity can be altered either
genetically or with the use of pharmacological agents. A previous
study in cultured chicken T lymphocytes with triple knock out of
IP3R indicated that the cells were resistant to inhalational
anesthetic-induced apoptosis, decreases in ER calcium
concentrations and increases in cytosolic and mitochondrial calcium
concentrations (5,7). Furthermore, rat pheochromocytoma
neurosecretory PC12 cells with elevated IP3R activity that were
transfected with presenilin-1 (L286V), or Q-111 rat striatal
neurons (a cell model of Huntington disease), were susceptible to
isoflurane-induced apoptosis and ER calcium release. However, these
effects were significantly attenuated following treatment with the
IP3R antagonist xestospongin C (5). These studies suggested that
activation of IP3R may have an important role in inhalational
anesthetic toxicity. In support of this viewpoint, the present data
clearly indicated that prolonged isoflurane exposure was able to
induce cell apoptosis via direct activation of IP3R, and treatment
with the IP3R antagonist xestospongin C reduced the rate of cell
apoptosis. Based on these findings, we aim to develop a therapeutic
approach that targets IP3R to protect patients undergoing
inhalational anesthesia from the potential deleterious side effects
of prolonged exposure.
The present study demonstrated that
isoflurane-induced enhancement in [Ca2+]c in
cells carrying an APP mutation is consistent with the higher degree
of neurotoxicity observed in these same cells after exposure to
isoflurane. These data indicated that a risk factor for early AD
may increase cell susceptibility to clinical concentrations of
isoflurane. A previous study reported that a clinically relevant
concentration of isoflurane was able to induce apoptosis, alter APP
processing, and increase Aβ levels in H4 human neuroglioma cells
stably transfected with an APP mutation, which is thought to be a
key feature in the pathogenesis of AD (16). Aβ has been demonstrated to augment
calcium release from the ER via RyR or IP3R; therefore,
anesthetic-induced increases in Aβ production may be considered
another indirect mechanism by which inhalational anesthetics
enhance ER calcium release (17).
Isoflurane induces apoptosis via calcium release from the ER, which
consequently increases the activity of beta-site APP-cleaving
enzyme and γ-secretase, which are associated with the generation of
Aβ proteins (18).
Isoflurane-mediated elevation and aggregation of Aβ proteins may
further induce apoptosis, resulting in the initiation of a vicious
cycle. However, due to the findings of xestospongin C experiments
in PC12 cells and rat cerebral cortical neurons it is likely that
isoflurane acts directly on IP3R to enhance calcium release.
In the present study, ultrastructural alterations to
cells following exposure to isoflurane were determined by TEM; the
results demonstrated that the most typical changes were swelling of
the mitochondria and the ER. Abnormal changes in ER structure can
induce calcium flux into the cytoplasm, resulting in a rapid
increase in intracellular calcium concentration and promotion of
cell apoptosis. Previous studies have proposed that ER-induced
apoptosis ultimately occurs via the mitochondrial pathway (19,20).
Abnormal alterations in the mitochondria may be caused by changes
to the ER. In addition, microtubule and microfilament content
increased following isoflurane exposure; microtubules are an
important component of the cytoskeleton, which are associated with
mitosis, intracellular translocation, overall cellular morphology,
cell markers, and various other functions. The structural integrity
of microtubules is the basis of nutrient transport between the
nerve cell body and axons. Several pathological clinical studies
demonstrated that neuritic plaques and hippocampal neurofibrillary
tangles are associated with dementia severity (21,22).
In the present study typical apoptotic bodies were not detected by
TEM, suggesting that apoptosis may occur at an early stage.
It has long been reported that anesthetics enhance
calcium release via the activation of RyR, which is the other major
calcium release channel complex on the ER (23). Similar to IP3R, RyR has an
important role in normal cell function and various
neurodegenerative diseases. Since IP3R and RyR interact, it remains
unclear as to whether one or both of these receptors are direct
targets of isoflurane; however, calcium influx from the
extracellular space also has a role in isoflurane cytotoxicity
(13). Memantine is a
noncompetitive partial antagonist of N-methyl-D-aspartate receptor
(NMDAR), which inhibits calcium influx and markedly suppresses
isoflurane-induced apoptosis and cell death (24). Further studies are required to
investigate how calcium release from the ER and calcium influx from
the extracellular space may contribute to anesthetic-associated
toxicity.
The present study only focused on an APP mutation;
however, there are other factors that contribute to
neurodegeneration in AD. These include tau, presenilin, various
secretases, apolipoprotein E, and perhaps heat shock proteins and
ferritins. Several of these factors may be modulated by calcium
(25–27).
A presenilin-1 mutation has been reported to render
neurons susceptible to isoflurane toxicity by inducing abnormal
calcium release from the ER via activation of IP3R (28). The measurements of intracellular
calcium conducted in the present study were limited to the
cytosolic compartment; therefore, secondary or primary alterations
in Ca2+ levels may be occurring in the ER and
mitochondria. Further studies are required to clarify the effects
of volatile anesthetics on calcium dynamics in these
organelles.
SH-SY5Y cells are not neurons; therefore, their
sensitivity to isoflurane exposure may differ, and the
concentration and duration of isoflurane exposure required to
induce cell apoptosis may be different. The results of the present
study were from cultured cell lines, more studies are required in
animals, such as rodents and primates, to investigate the effects
of isoflurane exposure on animal memory, cognition and
behavior.
In conclusion, the present study demonstrated that
an APP mutation associated with familial AD may render SH-SY5Y
cells more vulnerable to isoflurane-induced cytotoxicity. Calcium
release from IP3R on the ER may underlie the cytotoxic effects of
isoflurane. Notably, pharmacological inhibition of IP3R attenuated
isoflurane-induced cell apoptosis. Further investigation into the
cytotoxic effects of isoflurane is required in animal models and in
patients with risk factors for, or symptoms of AD. These findings
may improve the decision-making capabilities of anesthesiologists
with regards to the use of inhalational anesthetics in the elderly
population.
References
|
1
|
Bekker AY and Weeks EJ: Cognitive function
after anesthesia in the elderly. Best Pract Res Clin Anaesthesiol.
17:259–272. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moller JT, Cluitmans P, Rasmussen LS, Houx
P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD,
et al: Long-term postoperative cognitive dysfunction in the elderly
ISPOCD1 study. ISPOCD investigators. International Study of
Post-Operative Cognitive Dysfunction. Lancet. 351:857–861. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Newman MF, Kirchner JL, Phillips-Bute B,
Gaver V, Grocott H, Jones RH, Mark DB, Reves JG and Blumenthal JA:
Neurological Outcome Research Group and the Cardiothoracic
Anesthesiology Research Endeavors Investigators: Longitudinal
assessment of neurocognitive function after coronary-artery bypass
surgery. N Engl J Med. 344:395–402. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wei H and Xie Z: Anesthesia, calcium
homeostasis and Alzheimer's disease. Curr Alzheimer Res. 6:30–35.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jevtovic-Todorovic V, Hartman RE, Izumi Y,
Benshoff ND, Dikranian K, Zorumski CF, Olney JW and Wozniak DF:
Early exposure to common anesthetic agents causes widespread
neurodegeneration in the developing rat brain and persistent
learning deficits. J Neurosci. 23:876–882. 2003.PubMed/NCBI
|
|
6
|
Culley DJ, Baxter MG, Yukhananov R and
Crosby G: Long-term impairment of acquisition of a spatial memory
task following isoflurane-nitrous oxide anesthesia in rats.
Anesthesiology. 100:309–314. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Culley DJ, Baxter M, Yukhananov R and
Crosby G: The memory effects of general anesthesia persist for
weeks in young and aged rats. Anesth Analg. 96:1004–1009. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bianchi SL, Tran T, Liu C, Lin S, Li Y,
Keller JM, Eckenhoff RG and Eckenhoff MF: Brain and behavior
changes in 12-month-old Tg2576 and nontransgenic mice exposed to
anesthetics. Neurobiol Aging. 29:1002–1010. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang QJ, Wang XL, Zhao J, Zhao ZJ, Lv YX
and Zhu HX: Effects of different concentrations of isoflurane on
viability in rat primary cortical neurons. Chin J Anesthesiol.
30:673–675. 2010.
|
|
10
|
Luciani DS, Gwiazda KS, Yang TL, Kalynyak
TB, Bychkivska Y, Frey MH, Jeffrey KD, Sampaio AV, Underhill TM and
Johnson JD: Roles of IP3R and RyR Ca2+
channels in endoplasmic reticulum stress and beta-Cell death.
Diabetes. 58:422–432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Samtleben S, Wachter B and Blum R:
Store-operated calcium entry compensates fast ER calcium loss in
resting hippocampal neurons. Cell Calcium. 58:147–159. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Carvalho ND, Garcia CT, Ferreira AK,
Batista DR, Cassola AC, Maria D, Lebrun I, Carneiro SM, Afeche SC,
Marcourakis T and Sandoval MR: Neurotoxicity of coral snake
phospholipases A2 in cultured rat hippocampal neurons. Brain Res.
1552:1–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Geiger JE, Hickey CM and Magoski NS:
Ca2+ entry through a non-selective cation channel in
Aplysia bag cell neurons. Neuroscience. 162:1023–1038. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gallego-Sandín S, Alonso MT and
García-Sancho J: Calcium homoeostasis modulator 1 (CALHM1) reduces
the calcium content of the endoplasmic reticulum (ER) and triggers
ER stress. Biochem J. 437:469–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim HR, Kim MS, Kwon DY, Chae SW and Chae
HJ: Bosellia serrata-induced apoptosis is related with ER stress
and calcium release. Genes Nutr. 2:371–374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie Z, Dong Y, Maeda U, Alfille P, Culley
DJ, Crosby G and Tanzi RE: The common inhalation anesthetic
isoflurane induces apoptosis and increases amyloid beta protein
levels. Anesthesiology. 104:988–994. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ferreira IL, Ferreiro E, Schmidt J,
Cardoso M, Pereira CM, Carvalho AL, Oliveira CR and Rego AC: Aβ and
NMDAR activation cause mitochondrial dysfunction involving ER
calcium release. Neurobiol Aging. 36:680–692. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao Y, Liang G, Chen Q, Joseph DJ, Meng
Q, Eckenhoff RG, Eckenhoff MF and Wei H: Anesthetic induced
neurodegeneration mediated via inositol 1,4,5-trisphosphate
receptors. J Pharmacol Exp Ther. 333:14–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Timmins JM, Ozcan L, Seimon TA, Li G,
Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Aderson ME
and Tabas I: Calcium/calmodulin-dependent protein kinase II links
ER stress with Fas and mitochondrial apoptosis pathways. J Clin
Invest. 119:2925–2941. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Su TR, Tsai FJ, Lin JJ, Huang HH, Chiu CC,
Su JH, Yang YT, Chen JY, Wong BS and Wu YJ: Induction of apoptosis
by 11-dehydrosinulariolide via mitochondrial dysregulation and ER
stress pathways in human melanoma cells. Mar Drugs. 10:1883–1898.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Presti MF, Schmeichel AM, Low PA, Parisi
JE and Benarroch EE: Degeneration of brainstem respiratory neurons
in dementia with Lewy bodies. Sleep. 37:373–378. 2014.PubMed/NCBI
|
|
22
|
Thomas T, Miners S and Love S: Post-mortem
assessment of hypoperfusion of cerebral cortex in Alzheimer's
disease and vascular dementia. Brain. 138:1059–1069. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gordienko DV and Bolton TB: Crosstalk
between ryanodine receptors and IP(3) receptors as a factor shaping
spontaneous Ca(2+)-release events in rabbit portal vein myocytes. J
Phsyiol. 542:743–762. 2002. View Article : Google Scholar
|
|
24
|
Zhang GH, Dong YL, Zhang B, Ichinose F, Xu
Wu, Culley DJ, Crosby G, TanziR E and Xie Z: Isoflurane-induced
caspase-3 activation is dependent on cytosolic calcium and can be
attenuated bymemantine. J Neurosci. 28:4551–4560. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karch CM, Jeng AT and Goate AM: Calcium
phosphatase calcineurin influences tau metabolism. Neurobio Aging.
34:374–386. 2013. View Article : Google Scholar
|
|
26
|
Sepulveda-Falla D, Barrera-Ocampo A, Hagel
C, Korwitz A, Vinueza-Veloz MF, Zhou K, Schonewille M, Zhou H,
Velazquez-Perez L, Rodriguez-Labrada R, et al: Familial Alzheimer's
disease-associated presenilin-1 alters cerebellar activity and
calcium homeostasis. J Clin Invest. 124:1552–1567. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yefimova MG, Shcherbakova IS and
Shushakova ND: Transferrin and ferritin modulate the activity of
brain calcium-calmodulin-dependent phosphodiesterase. Biochemistry
(Mosc). 62:165–170. 1997.PubMed/NCBI
|
|
28
|
Liang G, Wang Q, Li Y, Kang B, Eckenhoff
MF, Eckenhoff RG and Wei H: A presenilin-1 mutation renders neurons
vulnerable to isoflurane toxicity. Anesth and Analg. 106:492–500.
2008. View Article : Google Scholar
|