Introduction
Neuropathic pain, defined as pain due to a lesion or
disease of the somatosensory system (1), is one of the most severe forms of
chronic pain. It may be induced by nerve trauma, infection,
metabolic disease, ischemia, radiotherapy or chemotherapy. It is
often refractory to current treatments, partially due to incomplete
understanding of the underlying mechanisms. Nerve injury or disease
may result in chronic central neuroinflammation in the dorsal horn
of the spinal cord and along the pain pathways to the thalamus and
the parietal cortex (2,3). Central neuroinflammation modulates
synaptic plasticity and facilitates nociceptive signal
transmission, which eventually develops into chronic pain (4,5).
Extensive evidence now indicates glial cells are critical in
central neuroinflammation following nerve injury (6–8). The
activation of astrocytes may explain the long-lasting behavioral
hypersensitivity (9,10).
Astrocytes are the most abundant glial cells in the
central nervous system (CNS), and were historically regarded as
support cells. However, previous data indicates that astrocytes
have multiple active roles in acute and chronic neuronal diseases,
including seizure, stroke, and ischemia (9,11,12).
Furthermore, accumulating evidence has demonstrated that astrocytes
are important for maintenance of neuropathic pain (7,9,10).
In all models of neuropathic pain, such as rhizotomy (8), chronic constriction injury (13) and spinal nerve ligation (14), proliferation and activation of
astrocytes has been demonstrated, and inhibiting astrocytes in the
spinal cord was demonstrated to reduce neuropathic pain (13,14).
However, the activation of astrocytes and how they mediate
neuropathic pain requires further elucidation.
Interleukin-17A (IL-17) is the first member of a
family of cytokines, designated IL-17A-F, which are predominantly
produced by activated cluster of differentiation (CD)4+
T cells. IL-17 has potent proinflammatory properties and it is
involved in modulating the immune response in inflammatory
disorders (15). Furthermore,
previous studies also demonstrated that IL-17 is involved in pain.
IL-17 or IL-17+ T cells have been observed in patients
suffering from arthritic pain (16), in the sciatic nerve (17,18),
or in optic nerve injury (19)
induced animal models of neuropathic pain. Pain-associated behavior
has been demonstrated to be reduced in IL-17 knockout (KO) mice in
inflammatory models with complete Freund's adjuvant (CFA) injection
into a plantar or the sciatic nerve (20) and in peripheral nerve injured
models (2,18). These previous studies suggest
peripheral IL-17 exerts an effect in inflammatory and neuropathic
pain. Intrathecal injection of recombinant IL-17 promotes thermal
hyperalgesia of normal mice (20),
which demonstrates that central IL-17 is a key factor in
inflammatory pain. The role of IL-17 in the CNS in models of
neuropathic pain was suggested by CD4+ T cell
infiltration (21), and its
elevated concentration in the spinal cord following nerve injury
(22). In addition, inflammatory
disorders that are associated with IL-17 and Th17 cells, including
multiple sclerosis (MS) or allergic encephalomyelitis, often result
in the development of neuropathic pain (23). However, the specific role of
central nerve system IL-17 in nerve injury-induced neuropathic pain
has not been extensively investigated.
The aim of the present study was to determine
whether IL-17 was highly expressed in the spinal cord and
investigate which cell produced it during the maintenance phase of
neuropathic pain. The association between IL-17 and astrocytes was
also investigated by analyzing the biological characteristics of
IL-17-stimulated astrocytes in cell proliferation and secretion of
proinflammatory cytokines in vitro.
Materials and methods
Animals
A total of 116 male Sprague-Dawley rats (age, 8–10
weeks; weight, 180–250 g) were obtained from the Experimental
Animal Center of Jiangsu University (Zhenjiang, China) and
acclimatized to the environment for at least 1 week prior to use in
the experiments. The environment was maintained at a constant room
temperature and humidity level (22°C; relative humidity, 40–60%).
All rats were housed with access to food and water ad
libitum and maintained in a 12:12 h light/dark cycle. The
well-being of the animals was monitored daily. All animal
experiments were approved by the Animal Care and Ethics Committee
of Jiangsu University.
Ligation of L5 and L6 spinal
nerves
Surgery was performed according to the method
described previously (24).
Briefly, rats were anesthetized with pentobarbital sodium (50 mg/kg
i.p.) and a longitudinal incision overlying the lumbar (L)4-sacral
(S)1 section was made and the left muscle tissue was separated. The
L6 transverse process and L6/S1 posterior interarticular process
were exposed and transected. The L5 and L6 spinal nerve was
carefully exposed and the distal nerve of L5 and L6 were gently
separated, firmly ligated with 5–0 silk suture and transected. The
wound was irrigated with sterile saline and inspected for
homeostasis, and closed. Sham surgery was performed identically,
however, the nerves were not ligated. The animals were allowed to
emerge from anesthesia in the warming place. The naive group did
not undergo an operation.
Testing mechanical sensitivity
The animals were habituated to the behavioral
testing apparatus for 30 min to 1 h prior to testing. The testing
environment was kept quiet and all tests were conducted in the
morning between 8:30 and 11:30. Mechanical sensitivity of the rats'
left feet was measured at 24 h prior to (baseline value) and at
days 1, 3, 5, 7, 10 and 14 after surgery. The midplantar surface of
the rats' left hind paws was stimulated using one of a series of
eight von Frey filaments (Stoelting Co., Wood Dale, IL, USA)
ranging from 0.4 to 15.0 g (log force 3.61, 3.84, 4.08, 4.31, 4.56,
4.74, 4.93, 5.18) using the Up-Down methods (25). The von Frey filament was presented
perpendicular to the plantar surface with sufficient force to
result in slight buckling against the paw for ≤8 sec. Paw
withdrawal was considered a positive response. The 50% mechanical
withdrawal threshold (50% MWT) was calculated. If the value of 50%
MWT exceeded 4.0 g at day 7 post-surgery, the animal model was
considered a failed preparation and excluded from the experiment. A
total of 8 rats were included in each group. The person performing
the behavioral tests was blinded to the experimental groups.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Rats were deeply anesthetized by inhalation of
isoflurane (4%) in 100% O2 and intracardially perfused
with 0.9% saline (between 200 and 250 ml per rat at 4°C), and the
spinal cords were subsequently harvested. The lumbar spinal dorsal
horns from the L5 to L6 ipsilateral to injury were dissected and
rapidly frozen. The tissues were homogenized and total RNA was
extracted with TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). In the in vitro
experiment, total RNA was extracted from astrocytes with 100 ng/ml
IL-17 (R&D Systems, Inc., Minneapolis, MN, USA) treatment for
three days or without. Interleukin-6 (IL-6), interleukin-1β
(IL-1β), tumor necrosis factor-α (TNF-α), and/or IL-17 mRNA were
measured by RT-qPCR. RT-qPCR was performed with the Verso 1-step
RT-qPCR kit, SYBR Green, low ROX (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol and the
thermocycling conditions were as follows: 5 min at 94°C; followed
by 35 cycles of 10 sec at 94°C, 5 sec at 50°C, and 10 sec at 72°C.
Relative expression was calculated with normalization to β-actin
values (26). The primers used are
presented in Table I.
 | Table I.Primers used in the present
study. |
Table I.
Primers used in the present
study.
| Gene | Sequence
(5′-3′) | Length (bp) |
|---|
| IL-6 | U:
CCGGAGAGGAGACTTCACAG | 161 |
|
| L: ACAGTGCATCATCG
CTGTTC |
|
| IL-1β | U:
AGGCTTCCTTGTGCAAGTGT | 230 |
|
| L:
TGAGTGACACTGCCTTC CTG |
|
| TNF-α | U: AGTCCGGGCAG
GTCTACTTT | 230 |
|
| L:
CGTGTGTTTCTGAGCATCGT |
|
| IL-17 | U:
ACAGTGAAGGCAGCGGTACT | 172 |
|
| L:
GAGTCCAGGGTGAAGTGGAA |
|
| β-actin | U:
CACGAAACTACCTTCAACTCC | 265 |
|
| L:
CATACTCCTGCTTGCTGATC |
|
Immunohistochemistry staining
Rats were deeply anesthetized by inhalation of
isoflurane (4%) in 100% O2 and intracardially perfused
with 0.9% saline (between 200 and 250 ml per rat at 4°C), followed
by 4% formaldehyde in 0.1 M phosphate-buffered saline (PBS; between
300 and 350 ml per rat). The L5 segment of lumbar spinal cord was
harvested, postfixed overnight in 4% formaldehyde and then
dehydrated in 30% sucrose for at least 72 h at 4°C. Tissue was then
freeze-mounted in optimal cutting temperature embedding medium
(Sakura Finetek USA, Inc., Torrance, CA, USA) on cork blocks for
cryostat sectioning. Spinal cords were sectioned transversely at a
thickness of 20 µm. The sections were washed three times with PBS.
Sections were then blocked for 1 h at 37°C with blocking solution
[PBS containing 5% bovine serum albumin (BSA; Shanghai Universal
Biotech Company, Shanghai, China) and 0.25% Triton X-100] followed
by incubation with the primary antibody diluted in PBS containing
1% BSA at 4°C overnight. Primary antibodies used were as follows:
Monoclonal purified goat anti-rat CD4 molecular complex antibody
(1:100; Santa Cruz Biotechnology, Inc., Dallas, TX, USA; cat. no.
sc-1140) for the detection of infiltrating CD4+ cells;
rabbit anti-rat IL-17 (1:100; Santa Cruz Biotechnology, Inc.; cat.
no. sc-7927); and mouse anti-rat glial fibrillary acidic protein
(GFAP, 1:1,000; Santa Cruz Biotechnology, Inc.; cat. no. sc-135921)
for the detection of astrocytes. The sections were then washed five
times with PBS and incubated with the appropriate secondary
antibody for 1 h at room temperature. Secondary antibodies used
were as follows: Fluorescein isothiocyanate (FITC)-conjugated
donkey anti-goat (1:100; Santa Cruz Biotechnology, Inc.; cat. no.
sc-2042), phycoerythrin (PE)-conjugated donkey anti-rabbit (1:100;
Santa Cruz Biotechnology, Inc.; cat. no. sc-3745), and
PE-conjugated goat anti-mouse (1:250; Invitrogen; Thermo Fisher
Scientific, Inc.; cat. no. M30004). Following washing with PBS, the
sections were stained with Hoechst 33258 at 37°C for 5 min and the
cells were examined with a fluorescence microscope (Olympus BX51;
Olympus Corporation, Tokyo, Japan).
Fluorescence-activated cell sorting
(FACS) was used to detect the infiltrated CD4+ T cells
in spinal cord
The ipsilateral (relative to injury) lumbar dorsal
horns of L5-L6 spinal cord were harvested as described above.
Mononuclear cells from the spinal cord tissue were obtained with
discontinuous Percoll gradients method following a previously
described method (27). Due to the
limited numbers of mononuclear cells from a single animal, each
FACS sample was prepared from three spinal cords pooled together.
The collected mononuclear cells was suspended in 100 µl PBS
containing anti-rat CD3-FITC (1:100; eBioscience, Inc., San Diego,
CA, USA; cat. no. 11–0030) and anti-rat CD4-PE (1:100; eBioscience,
Inc.; cat. no. 12–0040). Cells were incubated at 4°C for 30 min.
Following washing twice with PBS, all stained cells were
resuspended in 4% formaldehyde/PBS at 4°C overnight. Data were
acquired by flow cytometry (BD Biosciences, Franklin Lakes, NJ,
USA) and analyzed using CellQuest software (version 2.8; BD
Biosciences).
ELISA for serum IL-17
Serum IL-17 was measured by an ELISA kit (Rat IL-17A
Platinum ELISA; eBioscience, Inc.) following the manufacturer's
protocols. All samples were measured in triplicate, and the mean
concentration was calculated from the standard curve.
Astrocyte culture and cell
proliferation assay
As previously described (28), astrocyte cultures were prepared
from 1–2 day-old postnatal rats. Briefly, in a sterile environment,
spinal cords were dissected and cell suspensions were prepared.
These were subsequently transferred to culture flasks and incubated
at 37 °C in a CO2 balanced incubator. After 7 days, the
flask was agitated at 200 rpm at 37°C overnight to remove
oligodendrocytes, neurons, and microglial cells. Following three
passages, the astrocytes were divided into IL-17-stimulated and
naive groups. Briefly, 1×103 cells/well were seeded into
96-well plates in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.) and following cell adherence to the plate,
IL-17 was added to the wells at concentration of 10, 50 or 100
ng/ml, and incubated at 37°C for 24–72 h. Subsequently, 10 µl (5
mg/ml) MTT was added to each well and the plate was further
incubated for 4 h to deoxidize MTT under light-blocking conditions.
Following removal of the MTT dye solution, the cells were treated
with 100 µl DMSO and the absorbance at a wavelength of 490 nm was
measured using the ELx800 UV microplate reader (BioTek Instruments,
Inc., Winooski, VT, USA). Cells from the naive group were not
stimulated by IL-17.
Statistical analysis
All data were expressed as the mean ± standard
deviation. Figures and statistical analyses were made with GraphPad
Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). For
comparison of mechanical sensitivity, spinal inflammatory
cytokines, infiltrated-cellular phenotypes in the spinal cord and
the MTT assay, appropriate two-way analysis of variance was
performed followed by Bonferroni's multiple comparison post-hoc
test. Immunohistochemistry data were analyzed by t-test or one-way
analysis of variance. Inflammatory cytokines data in vitro
were evaluated using t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
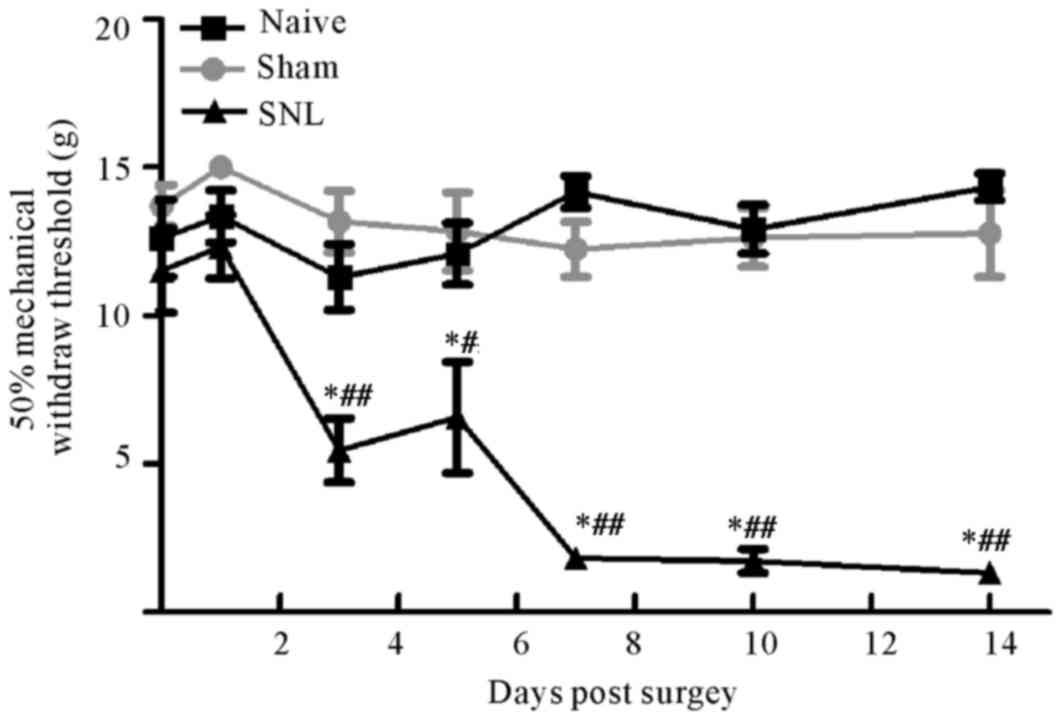
Behavioral mechanical sensitivity of
rats following L5/L6 spinal nerve ligation
The mechanical sensitivity (indicated by the 50%
threshold force for paw withdrawals) was measured in the injured
ipsilateral hind paws prior to (baseline values) and up to 2 weeks
after surgery. Baseline values were not different between the
groups. Following SNL, rats developed pain hypersensitivity at day
3 up to day 14 compared with the other two groups. Furthermore, no
significant differences were observed between the sham group and
the control group at any time point (Fig. 1).
CD4+ Th17 cell infiltration
was observed in the ipsilateral dorsal horn following L5-L6 spinal
cord ligation
Following spinal nerve ligation, IL-17 mRNA
increased significantly in the ipsilateral dorsal horn of L5-L6
spinal cord at day 7 (P<0.05). No differences were detected
between the sham and control group at any time (Fig. 2A). Serum IL-17 in naive,
sham-operated, and SNL rats at days 7 and 14 post-surgery was
analyzed by ELISA to determine whether the changes of IL-17 in
spinal cord was associated with the changes in the peripheral
serum. Though spinal nerve ligation induced a moderate increase in
IL-17 expression, no differences were determined amongst the three
groups at any time point (Fig.
2B). Furthermore, IL-17 localization was detected in the spinal
cord at day 7 after surgery. As IL-17 is preferentially expressed
by CD4+ T cells, double immunofluorescence labeling was
used to demonstrate that IL-17 immunoreactivity was colocalized
with CD4 in the ipsilateral dorsal horn, and the immunostaining
density of IL-17 and CD4 markedly increased when compared with the
sham-operated rats (Fig. 2C).
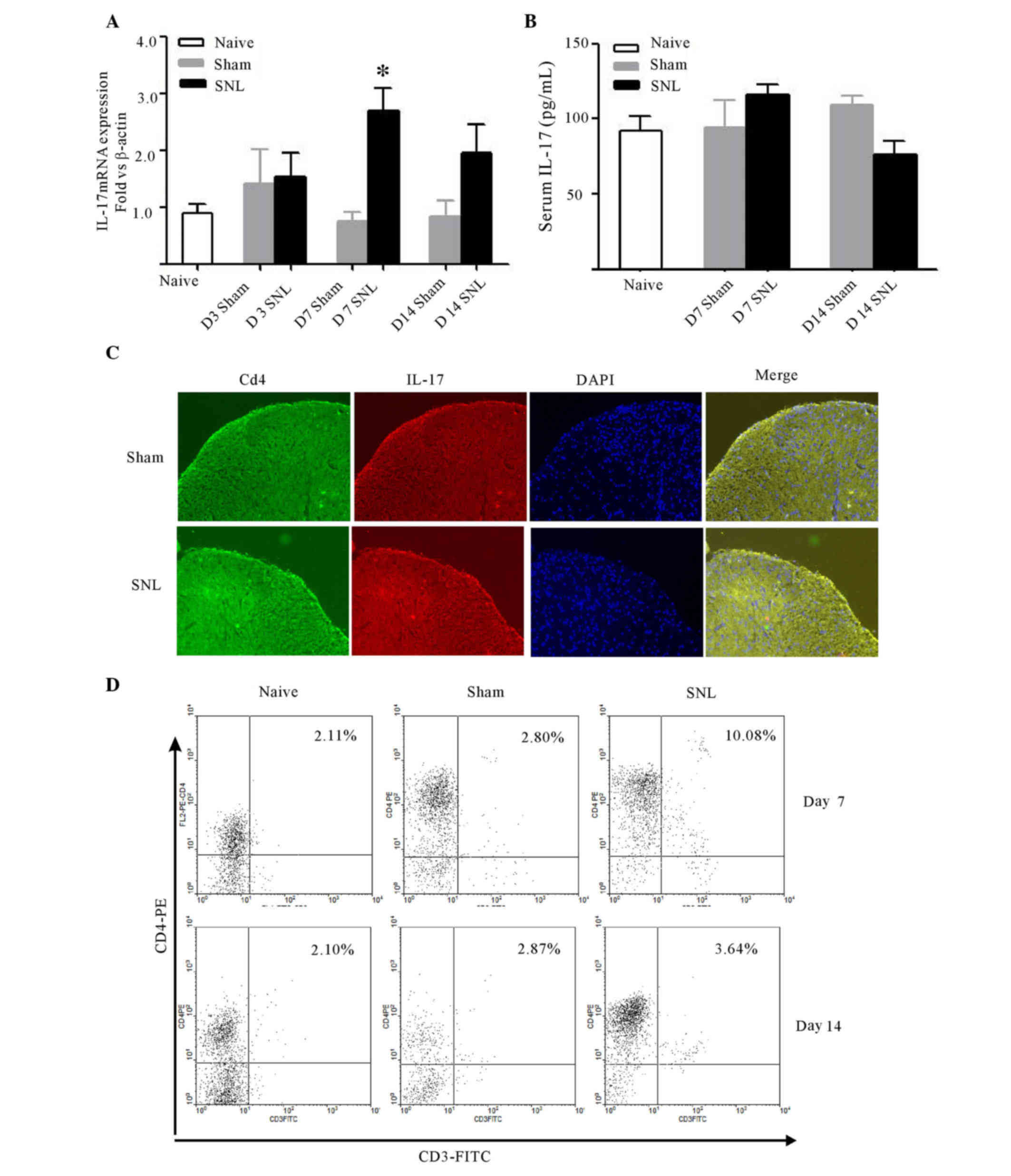 | Figure 2.Expression of IL-17 and CD4 in the
ipsilateral dorsal horn of L5-6 spinal cord following SNL, and
IL-17 in the serum. (A) Quantitative polymerase chain reaction
analysis of IL-17 mRNA expression in the rat spinal dorsal horn
over time following SNL compared with sham-operated and naive
animals, respectively. Data are expressed as mean fold ± SEM
(n=4/group). *P<0.05 vs. the sham-operated rats at day 7
post-surgery, as determined by two-way analysis of variance,
followed by Bonferroni's post-hoc test. (B) Serum IL-17 measured by
ELISA at days 7 and 14 post-surgery. Data are expressed as the mean
± SEM (n=6/group). (C) Double immunofluorescence staining with
antibodies against IL-17 and CD4 in the ipsilateral L5 spinal
dorsal horn at day 7 following SNL compared with the sham group.
The representative images from at least three independent
experiments depicted the IL-17A/CD4 overlay, indicating
colocalization of IL-17A immunoreactivity with CD4+
cells. The images are representative images of at least three
independent experiments. (D) CD4+ T cell infiltration
into the rats' ipsilateral lumbar spinal cord at L5-L6 at days 7
and 14 post-SNL, the sham operation or naive rats via FACS. Lumbar
spinal cord mononuclear cells were isolated and labeled with
anti-rat CD3-FITC and CD4-PE monoclonal antibodies at days 7 and
14, and then were analyzed in CD3 vs. CD4 dot plots. The images are
representative of three independent experiments at day 7 and 14,
respectively. Each FACS sample consisted of three animals.
Percentages of CD3hiCD4hi population within
the total mononuclear cells were analyzed. SNL, spinal nerve
ligation; SEM, standard error of the mean; CD, cluster of
differentiation; IL, interleukin; FITC, fluorescein isothiocyanate;
PE, phycoerythrin; FACS, fluorescence-activated cell sorting; D3,
day 3; D7, day 7; D14, day 14; L, lumbar. |
To further confirm IL-17 was produced by spinal
cord-infiltrating CD4+ T cells, CD4+ T cells
were detected in the lumbar spinal cord using FACS at days 7 and 14
post-surgery. SNL induced a significant increase in the
infiltration of CD4+ T cells into the ipsilateral side
of lumber spinal cord at day 7 (P<0.001), while no marked
changes were observed at day 14 compared with the naive and sham
groups (Fig. 2D).
Astrocyte activation was observed in
spinal dorsal horn accompanying proinflammatory cytokine increases
following SNL
SNL was demonstrated to induce marked expression of
GFAP at the ipsilateral side compared with either the contralateral
spinal cord or either side of the sham group. The level of GFAP in
the ipsilateral side was consistent with the contralateral side in
the sham group. GFAP activation was predominantly localized to the
superficial laminae of the lumbar spinal dorsal horn. The
immunoreactivity of GFAP notably increased compared with the
sham-operated rats (Fig. 3A).
Furthermore, the qPCR indicated significant increases in the
expression levels of IL-6 and IL-1β in the ipsilateral spinal
dorsal horn following SNL at day 7 (P<0.05 and P<0.01) and
IL-6 remained at high levels at day 14 (P<0.05; Fig. 3B and C). No change in expression of
TNF-α was demonstrated at days 7 and 14 in all groups (data not
shown).
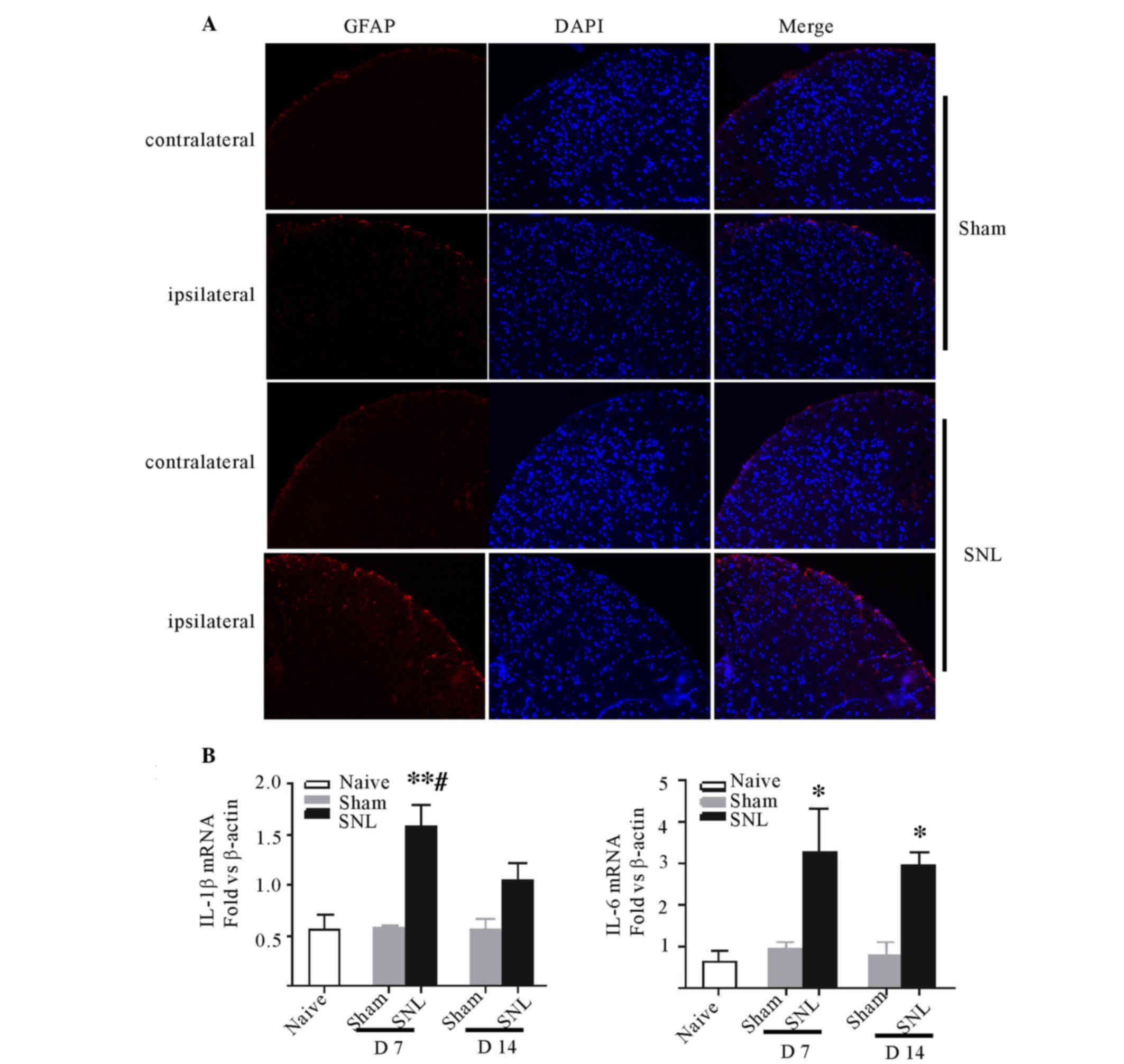 | Figure 3.Astrocytic responses and the
expression of proinflammatory cytokines in L5-L6 spinal cord
following L5 and L6 nerve ligation, sham surgery or naive rats. (A)
Fluorescent immunohistochemical staining for GFAP was performed on
day 7 post-SNL or sham surgery. GFAP expression markedly increased
in the ipsilateral dorsal horn following nerve ligation compared
with contralateral and sham-operated rats. Representative
photomicrographs of the ipsilateral dorsal horn of L5 lumbar spinal
cord are presented (magnification, ×20). (B) Quantitative
polymerase chain reaction analysis was used to determine the
expression of IL-1β, IL-6, tumor necrosis factor-α mRNA of the
ipsilateral L5-L6 spinal dorsal horn at day 7 and 14 in the rats
post-SNL compared with the sham-operated or naive rats. Data are
expressed as the mean fold ± standard error of the mean
(n=4/group). *P<0.05, **P<0.01 vs. the sham-operated/naive
rats; #P<0.05 vs. the SNL rats at day 14. GFAP, glial
fibrillary acidic protein; SNL, spinal nerve ligation; IL,
interleukin; D7, day 7; D14, day 14; L, lumbar. |
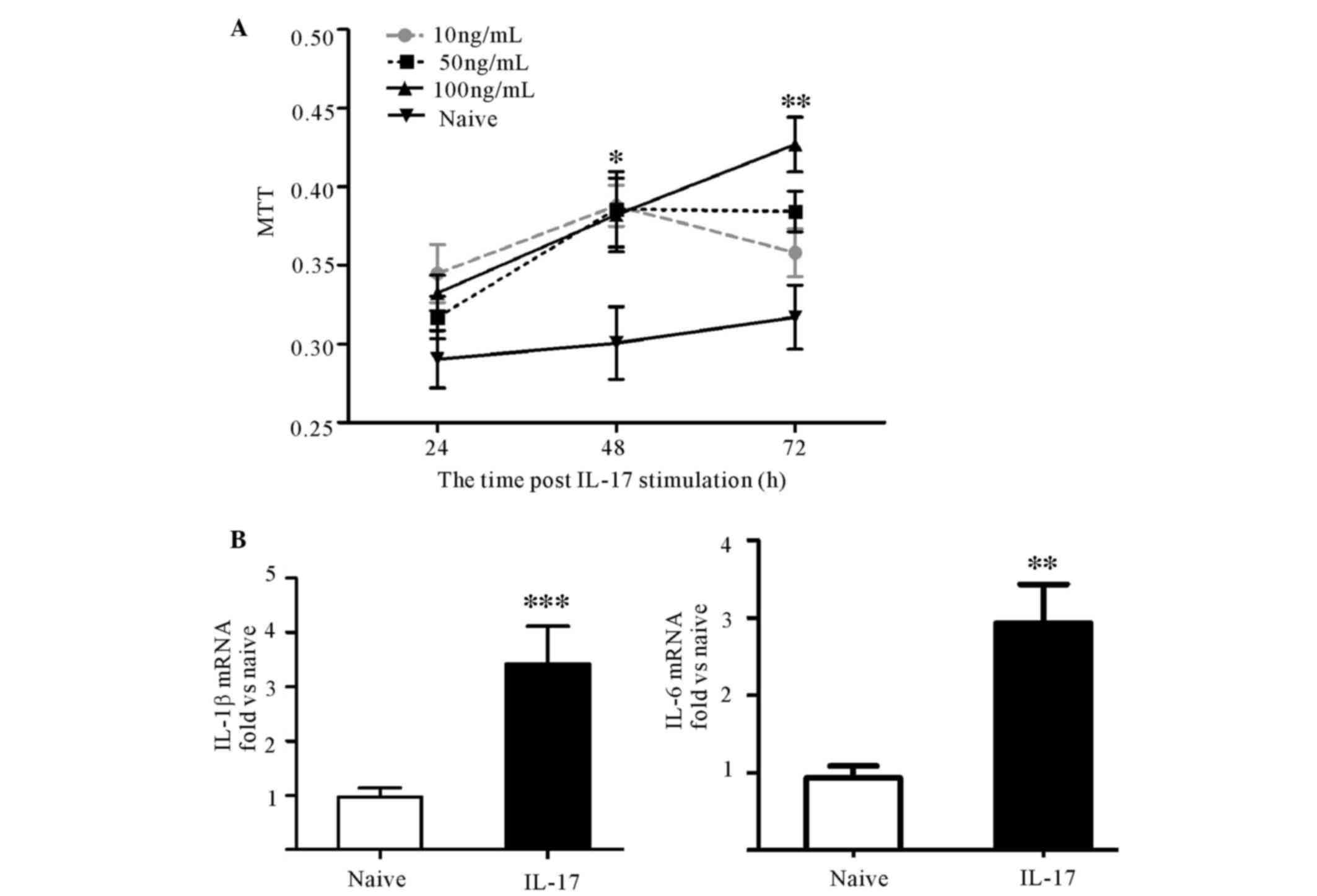
IL-17 promoted the proliferation of
astrocytes and the expression of proinflammatory cytokines in
vitro
To investigate the effect of IL-17 on astrocytes,
10, 50 or 100 ng/ml IL-17 was used to stimulate astrocytes in
vitro. The results of the MTT assay demonstrated that
astrocytes proliferated successively over time, but not
significantly higher than that at 24 h in each group. IL-17 induced
significant astrocyte proliferation at 48 h compared with the naive
group (P<0.05). However, at 72 h, only 100 ng/ml IL-17
significantly promoted proliferation (P<0.01; Fig. 4A). Thus, the expression levels of
IL-1β, IL-6 and TNF-α mRNA at 72 h between the group with 100 ng/ml
IL-17 stimulation and the naive group were investigated. Treatment
with 100 ng/ml IL-17 elevated IL-1β and IL-6 mRNA expression levels
(Fig. 4B), but not TNF-α (data not
shown). These results demonstrate that IL-17 enhanced astrocytic
activity.
Discussion
Damage to a peripheral nerve results in activity
from microglial and astrocytic cells, in addition to infiltration
of macrophages and T cells in the CNS. These non-neuronal cells can
sensitize neurons in nociceptive pathways by release of
pronociceptive factors, including cytokines and chemokines, which
is an important factor underlying the pathogenesis of neuropathic
pain (29). Nerve injury induced
behavioral hypersensitivity can be divided into at least two
phases, an initiation phase (from day 0 to day 5) and a maintenance
phase (after day 5) based on the pattern of nociceptive behaviors
and the animals' responses to selective treatment with therapeutic
agents. The spinal cord is an important gateway through which
peripheral pain signals are transmitted to the brain. The results
of the present study determined proinflammatory IL-17 contributed
to the persistence of tactile hypersensitivity following nerve
injury.
IL-17 is typically produced by immune cells,
including Th17, γδT cells and natural killer T cells,
CD8+ T cells (30). The
present study determined that IL-17 increases in the ipsilateral
spinal cord dorsal horn at day 7 post-SNL and observed IL-17 is
located in CD4+ cells via double immunostaining.
Previous studies have detected CD4+ cells in the lumbar
spinal cord or dorsal root ganglia in animal models of neuropathic
pain (21,31) and demonstrated these CD4-expressing
cells were predominantly CD4+ T cells (21). The current study confirmed
CD4+ T cell infiltration in the spinal cord at day 7
post-nerve injury. The temporal profile of CD4+ T cells
is consistent with IL-17 expression in the spinal cord.
Furthermore, ELISA demonstrated the high level of IL-17 in the
spinal cord following SNL did not come from the peripheral serum.
These results suggested IL-17 was produced by Th17 cells. However,
a previous study suggested spinal cord-infiltrating CD4+
T cells are Th1 cells (32). The
inconsistency between that and the present study may be associated
with the differences in species (rats in the present study, as
compared with mice) and method of detection. In addition, Th17
helper cells are not the only cells capable of producing IL-17. In
the CNS, expression of IL-17 was also demonstrated in astrocytes
located in the active areas of MS lesions (23), or in spinal dorsal horn during
CFA-induced inflammatory pain (19). To the best of our knowledge, there
are no previous studies regarding the expression of CD4 by
astrocytes in central neuroinflammation or post nerve injury.
Notably, a previous study reports damage-associated molecular
patterns activate Toll-like receptor 2 (TLR2) on microglia, which
upregulate interleukin-23 expression and subsequent IL-17 induction
following cerebral ischemia-reperfusion injury (33). Peripheral nerve injury may lead to
microglia activation via TLR2 (34), however, whether or not microglial
cells are the source of IL-17 requires further investigation.
Numerous experimental studies suggest that IL-17 is
important in a wide range of inflammatory diseases, including
autoimmune diseases, such as MS, and other nonimmune
neuroinflammatory processes, such as stroke and cerebral
ischemia-reperfusion injury (15–18,35).
However, its tissue-specific mechanism remains unclear. A previous
study demonstrated recombination-activating gene 1 KO mice lacking
functional IL-17-producing T lymphocytes exhibited reduced thermal
hyperalgesia due to impaired recruitment of macrophages and
expression of macrophage chemoattractant protein in the sciatic
nerve following chronic constriction injury, which provided initial
evidence of the involvement of IL-17 in neuropathic pain (36). In addition, a previous study
reported IL-17 KO mice presented with reduced mechanical allodynia
and decreased activation of the microglia and astrocytes in the
ipisilateral dorsal horn of the spinal cord following partial
sciatic nerve ligation indicates IL-17 contributes to neuropathic
pain likely via glial cells (2).
The results of the immunostaining in the present study indicated
that the majority of IL-17 is located in the superficial zone of
the ipisilateral spinal dorsal horn, which is also the region of
notable astrocytic activation. It is well known that astrocytes
constitutively express the receptor of IL-17A (37). Thus, it is reasonable to suggest
that IL-17 activates local astrocytes and induces glial response,
such as production of proinflammatory cytokines. This possibility
is supported by in vitro results from the present study,
which demonstrate, in addition to proliferation, stimulation of
resting astrocytes by IL-17 increases expression of IL-1β and IL-6,
which also occurs in vivo. These mediators lead to central
sensitization and pain hypersensitivity (29,38,39).
The finding that IL-17 stimulates astrocytes to produce
proinflammatory mediators is also demonstrated in MS, experimental
autoimmune encephalomyelitis and ischemic brain injury (12,40).
However, activation of spinal astrocytes subsequent to nerve injury
was not entirely explained by IL-17 and IL-6 expression remained
high at day 14 post-SNL and astroglia activation persisted for
longer than seven days (7,9,10),
which suggests that IL-17-mediated signaling, although important,
is not the only mechanism underlying astroglia activation and that
there may be independent and/or cooperative mechanisms involving
other signals. In addition, Meng et al (20) reported that spinal IL-17
facilitated inflammatory pain by enhancing phosphorylated-NR1 of
the N-methyl-D-aspartate receptor in CFA-injected rats.
Furthermore, IL-17 can stimulate microglia to upregulate the
production of proinflammatory mediators, such as CXCL2, IL-6, and
neurotrophic factors (41), and
increase the permeability of BBB in vitro to promote
infiltration of proinflammatory cytokines and CD4+ T
cells in the CNS. According to these findings, it is possible that
other mechanisms may be involved in spinal IL-17-mediated
neuropathic pain.
In conclusion, the present study demonstrated that
IL-17 is involved in maintaining neuropathic pain, due to
activation of astrocytes and the secretion of proinflammatory
cytokines. Elevated IL-17 may be produced by infiltrated
CD4+T cells. However, previous research on
IL-17-mediated disorders in the CNS demonstrated cross-interaction
between IL-17 and other factors is likely to be involved in the
pathogenesis of neuropathic pain. Future studies are required to
further investigate and design novel therapies for patients with
neuropathic pain.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81370084,
31201346 and 81502663).
References
|
1
|
Geber C, Baumgärtner U, Schwab R, Müller
H, Stoeter P, Dieterich M, Sommer C, Birklein F and Treede RD:
Revised definition of neuropathic pain and its grading system: An
open case series illustrating its use in clinical practice. Am J
Med. 122:(Supp 10). S3–S12. 2009.
|
|
2
|
Kim CF and Moalem-Taylor G: Interleukin-17
contributes to neuroinflammation and neuropathic pain following
peripheral nerve injury in mice. J Pain. 12:370–383. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saadé NE and Jabbur SJ: Nociceptive
behavior in animal models for peripheral neuropathy: Spinal and
supraspinal mechanisms. Prog Neurobiol. 86:22–47. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ellis A and Bennett DL: Neuroinflammation
and the generation of neuropathic pain. Br J Anaesth. 111:26–37.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ramesh G: Novel Therapeutic Targets in
Neuroinflammation and Neuropathic Pain. Inflamm Cell Signal.
1:pii–e111. 2014.
|
|
6
|
Liu F and Yuan H: Role of glia in
neuropathic pain. Front Biosci (Landmark Ed). 19:798–807. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chiang CY, Wang J, Xie YF, Zhang S, Hu JW,
Dostrovsky JO and Sessle BJ: Astroglial glutamate-glutamine shuttle
is involved in central sensitization of nociceptive neurons in rat
medullary dorsal horn. J Neurosci. 27:9068–9076. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu L, Rudin M and Kozlova EN: Glial cell
proliferation in the spinal cord after dorsal rhizotomy or sciatic
nerve transection in the adult rat. Exp Brain Res. 131:64–73. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chiang CY, Sessle BJ and Dostrovsky JO:
Role of astrocytes in pain. Neurochem Res. 37:2419–2431. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao YJ and Ji RR: Targeting astrocyte
signaling for chronic pain. Neurotherapeutics. 7:482–493. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kimelberg HK and Nedergaard M: Functions
of astrocytes and their potential as therapeutic targets.
Neurotherapeutics. 7:338–353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang Z, Altuntas CZ, Gulen MF, Liu C,
Giltiay N, Qin H, Liu L, Qian W, Ransohoff RM, Bergmann C, et al:
Astrocyte-restricted ablation of interleukin-17-induced
Act1-mediated signaling ameliorates autoimmune encephalomyelitis.
Immunity. 32:414–425. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin YC, Huang SY, Jean YH, Chen WF, Sung
CS, Kao ES, Wang HM, Chakraborty C, Duh CY and Wen ZH: Intrathecal
lemnalol, a natural marine compound obtained from Formosan soft
coral, attenuates nociceptive responses and the activity of spinal
glial cells in neuropathic rats. Behav Pharmacol. 22:739–750. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsuda M, Kohro Y, Yano T, Tsujikawa T,
Kitano J, Tozaki-Saitoh H, Koyanagi S, Ohdo S, Ji RR, Salter MW and
Inoue K: JAK-STAT3 pathway regulates spinal astrocyte proliferation
and neuropathic pain maintenance in rats. Brain. 134:1127–1139.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Isailovic N, Daigo K, Mantovani A and
Selmi C: Interleukin-17 and innate immunity in infections and
chronic inflammation. J Autoimmun. 60:1–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lubberts E: IL-17/Th17 targeting: On the
road to prevent chronic destructive arthritis? Cytokine. 41:84–91.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Noma N, Khan J, Chen IF, Markman S,
Benoliel R, Hadlaq E, Imamura Y and Eliav E: Interleukin-17 levels
in rat models of nerve damage and neuropathic pain. Neurosci Lett.
493:86–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Day YJ, Liou JT, Lee CM, Lin YC, Mao CC,
Chou AH, Liao CC and Lee HC: Lack of interleukin-17 leads to a
modulated micro-environment and amelioration of mechanical
hypersensitivity after peripheral nerve injury in mice. Pain.
155:1293–1302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng H, Zhang Z, Luo N, Liu Y, Chen Q and
Yan H: Increased Th17 cells and IL17 in rats with traumatic optic
neuropathy. Mol Med Rep. 10:1954–1958. 2014.PubMed/NCBI
|
|
20
|
Meng X, Zhang Y, Lao L, Saito R, Li A,
Bäckman CM, Berman BM, Ren K, Wei PK and Zhang RX: Spinal
interleukin-17 promotes thermal hyperalgesia and NMDA NR1
phosphorylation in an inflammatory pain rat model. Pain.
154:294–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao L and DeLeo JA: CNS-infiltrating CD4+
T lymphocytes contribute to murine spinal nerve transection-induced
neuropathic pain. Eur J Immunol. 38:448–458. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Costigan M, Moss A, Latremoliere A,
Johnston C, Verma-Gandhu M, Herbert TA, Barrett L, Brenner GJ,
Vardeh D, Woolf CJ and Fitzgerald M: T-cell infiltration and
signaling in the adult dorsal spinal cord is a major contributor to
neuropathic pain-like hypersensitivity. J Neurosci. 29:14415–14422.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Olechowski CJ, Truong JJ and Kerr BJ:
Neuropathic pain behaviours in a chronic-relapsing model of
experimental autoimmune encephalomyelitis (EAE). Pain. 141:156–164.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim SH and Chung JM: An experimental model
for peripheral neuropathy produced by segmental spinal nerve
ligation in the rat. Pain. 50:355–363. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beeton C and Chandy KG: Isolation of
mononuclear cells from the central nervous system of rats with EAE.
J Vis Exp. 5272007.PubMed/NCBI
|
|
28
|
Kerstetter AE and Miller RH: Isolation and
culture of spinal cord astrocytes. Methods Mol Biol. 814:93–104.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Clark AK, Old EA and Malcangio M:
Neuropathic pain and cytokines: Current perspectives. J Pain Res.
6:803–814. 2013.PubMed/NCBI
|
|
30
|
Iwakura Y, Ishigame H, Saijo S and Nakae
S: Functional specialization of interleukin-17 family members.
Immunity. 34:149–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu P, Bembrick AL, Keay KA and McLachlan
EM: Immune cell involvement in dorsal root ganglia and spinal cord
after chronic constriction or transection of the rat sciatic nerve.
Brain Behav Immun. 21:599–616. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Draleau K, Maddula S, Slaiby A,
Nutile-McMenemy N, De Leo J and Cao L: Phenotypic identification of
spinal cord-infiltrating CD4 T lymphocytes in a murine model of
neuropathic pain. J Pain Relief. (Suppl 3). 0032014.PubMed/NCBI
|
|
33
|
Zhang J, Takahashi HK, Liu K, Wake H, Liu
R, Maruo T, Date I, Yoshino T, Ohtsuka A, Mori S and Nishibori M:
Anti-high mobility group box-1 monoclonal antibody protects the
blood-brain barrier from ischemia-induced disruption in rats.
Stroke. 42:1420–1428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stokes JA, Cheung J, Eddinger K, Corr M
and Yaksh TL: Toll-like receptor signaling adapter proteins govern
spread of neuropathic pain and recovery following nerve injury in
male mice. J Neuroinflammation. 10:1482013.PubMed/NCBI
|
|
35
|
Knier B, Rothhammer V, Heink S, Puk O,
Graw J, Hemmer B and Korn T: Neutralizing IL-17 protects the optic
nerve from autoimmune pathology and prevents retinal nerve fiber
layer atrophy during experimental autoimmune encephalomyelitis. J
Autoimmun. 56:34–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kleinschnitz C, Hofstetter HH, Meuth SG,
Braeuninger S, Sommer C and Stoll G: T cell infiltration after
chronic constriction injury of mouse sciatic nerve is associated
with interleukin-17 expression. Exp Neurol. 200:480–485. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu S and Cao X: Interleukin-17 and its
expanding biological functions. Cell Mol Immunol. 7:164–174. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kawasaki Y, Zhang L, Cheng JK and Ji RR:
Cytokine mechanisms of central sensitization: Distinct and
overlapping role of interleukin-1beta, interleukin-6 and tumor
necrosis factor-alpha in regulating synaptic and neuronal activity
in the superficial spinal cord. J Neurosci. 28:5189–5194. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guo W, Wang H, Watanabe M, Shimizu K, Zou
S, LaGraize SC, Wei F, Dubner R and Ren K: Glial-cytokine-neuronal
interactions underlying the mechanisms of persistent pain. J
Neurosci. 27:6006–6018. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gelderblom M, Weymar A, Bernreuther C,
Velden J, Arunachalam P, Steinbach K, Orthey E, Arumugam TV,
Leypoldt F, Simova O, et al: Neutralization of the IL-17 axis
diminishes neutrophil invasion and protects from ischemic stroke.
Blood. 120:3793–3802. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kawanokuchi J, Shimizu K, Nitta A, Yamada
K, Mizuno T, Takeuchi H and Suzumura A: Production and functions of
IL-17 in microglia. J Neuroimmunol. 194:54–61. 2008. View Article : Google Scholar : PubMed/NCBI
|