Introduction
Hypertension is a multifactorial disease that
results from the combined effects of genetic, environmental and
behavioral factors (1). Data
obtained from the Chinese National Center for Cardiovascular
Diseases (Ministry of Health, Beijing, China) indicate that
approximately 18.8% of Chinese adults (aged ≥18 years) were
diagnosed with hypertension in 2015 (2). In addition, data obtained from a
suburban town of Shanghai demonstrated that the prevalence,
awareness, treatment and control rates of hypertension in an
elderly Chinese population (aged ≥60 years) from 2006 to 2008 were
59.4, 72.5, 65.8 and 24.4%, respectively (3). In addition, the Global Burden of
Disease Study 2010 revealed that blood pressure is one of the
leading risk factors for the global disease burden (4). In Africa, hypertension is the leading
cause of heart failure (5).
Globally, hypertension is responsible for >50% of mortalities
due to stroke (5). Therefore,
studies that aim to identify effective treatments for hypertension
are important for the prevention of heart failure and stroke.
β-blockers are commonly prescribed for the treatment of
cardiovascular diseases, such as hypertension. The selective
β-blocker, known as metoprolol (Met), is considered to be an
effective therapy as it has been demonstrated to reduce mortality
rates and adverse cardiac events among patients with coronary
artery disease, heart failure and hypertension (6,7).
However, only ~50% of patients with hypertension respond to
β-blocker treatment (8).
In previous studies, polymorphisms in the
adrenoreceptor β1 gene (Adrb1) and cytochrome P4502D6
(CYP2D6) genes are reportedly involved in the
inter-individual differences in the response to Met treatment
(9,10). Approximately 70–80% of Met is known
to be metabolized by CYP2D6, and CYP2D6 polymorphisms have
been demonstrated to affect the rate of Met metabolism (11). Previous studies investigating
Adrb1 polymorphisms have focused primarily on the Ser49Gly
and Arg389Gly polymorphisms. For instance, Wu et al
(12) demonstrated that Chinese
patients with essential hypertension that were homozygous for a
mutant Adrb1 genotype (Arg389), exhibited an increased
sensitivity to Met treatment. By contrast, an additional study
demonstrated that subjects with the same CYP2D6 and
Adrb1 genotypes exhibited varying responses to Met, which
suggests that additional mechanisms responsible for the
inter-individual differences in Met responses may exist (13).
The β1 adrenergic receptor (β1-AR) is the primary
β-AR subtype in the heart and is the target of Met (14). A previous study conducted by our
group demonstrated a correlation between reduced Adrb1
promoter methylation levels in the myocardium and enhanced
Met-mediated antihypertensive activity in spontaneously
hypertensive rats (SHR), and increased β1-AR expression in H9C2
cells (15). Thus, the authors
hypothesized that the expression levels of myocardial β1-AR may
affect the antihypertensive activity of Met, and may be responsible
for the observed inter-individual variations in the response to Met
treatment in patients with hypertension (16).
In the present study, a recombinant adeno-associated
virus type 9 (rAAV9) vector containing a short-hairpin RNA (shRNA)
sequence against Adrb1, was used to inhibit β1-AR expression
in the myocardium, in order to investigate the effects of reduced
cardiac β1-AR expression on the antihypertensive efficacy of Met in
SHR. The data suggest a novel mechanism underlying the observed
inter-individual differences in the response to Met treatment.
Materials and methods
Plasmids and cell culture
Plasmids containing shRNA sequences against rat
β1-AR or non-targeting controls were purchased from Changsha
Yingrun Biotechnology Co., Ltd. (Changsha, China). Adeno-associated
virus (AAV) packaging mixtures were obtained from the Institute of
Biomedicine and Biotechnology (Shenzhen Institutes of Advanced
Technology, Chinese Academy of Sciences, Shenzhen, China). The
HEK293 human embryonic kidney cell line was obtained from Vector
Gene Technology Company Ltd. (Beijing, China) and cultured in
Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal
bovine serum (FBS).
Preparation of rAAV9 vectors
rAAV9 vectors containing Adrb1 shRNA
(rAAV9-Adrb1-shRNA-ZsGreen) or negative control (rAAV9-NC-ZsGreen)
shRNA sequences were prepared in HEK293 cells as described
previously (17,18). Briefly, polyethylenimine (1 µg/µl;
Helixgen, Co., Ltd., Guangzhou, China) was added to the AAV
Packaging Plasmid, target plasmid and Ad Helper (1.5 ml; 20 µg: 10
µg: 30 µg), mixed and incubated at room temperature for 15 min to
generate the transfection mixture. The transfection mixture was
then added to HEK293 cells (9×106) and incubated for
8–12 h. The medium was replaced with fresh 10% FBS-DMEM and
incubated for 72 h, before cells and rAAV9 virus particles were
harvested. The mixture was purified using chloroform, precipitated
using polyethylene glycol/NaCl and extracted with chloroform as
described previously (19). The
purity of rAAV-ZsGreen was evaluated by gel electrophoresis using
12% SDS-PAGE gels. To achieve this, the viral stock solution was
mixed with loading buffer (2X; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and incubated in boiling water for 10 min.
Samples (15 µl) were then loaded and run at 20 mA for 2.5 h. The
gel was stained with Coomassie Brilliant Blue R250 (Amresco, LLC,
Solon, OH, USA) for 1 h and destained, until clear bands with a low
background were evident. The viral titer, expressed as viral
genomes (v.g.)/ml, was determined using dot blot hybridization as
described previously (20). The
efficiency of infection was validated by infecting HEK293 cells.
Briefly, 10 µl purified virus was added to HEK293 cells (5×105) and
incubated for 48 h at 37°C. Fluorescence signals were then
visualized using an Olympus IX71 fluorescence microscope (Olympus
Corporation, Tokyo, Japan).
Experimental animals
A total of 36 male SHR (age, 20 weeks; weight, ~300
g) were purchased from Vital River Laboratory Animal Technology
Co., Ltd. (Beijing, China). Rats were acclimated to laboratory
conditions for one week before experimental procedures were
performed according to the Guidelines for the Care and Use of
Laboratory Animals (National Institutes of Health, publication
86–23, revised 1986) and the animal regulations of the Department
of Science and Technology of Hunan Province (Changsha, China). Rats
were maintained at the Animal Experiment Center of Central South
University (Changsha, China), and housed in individual cages at
22±2°C and 55±5% humidity with 12-h environmental light/dark cycles
with free access to standard laboratory chow and tap water during
the experimental period.
The rats were randomly divided into the following
three groups of 12: i) Sham-operated; ii) rAAV9-NC; and iii)
rAAV9-Adrb1. Rats in the rAAV9-NC and rAAV9-Adrb1groups were
injected with a single dose of 1×1012 v.g./kg rAAV9-NC
or rAAV9-Adrb1 into the myocardium via the pericardial cavity using
the methods described previously (21,22).
Briefly, SHR were anesthetized with 10% chloral hydrate (300
mg/kg), and each rat was placed in a supine position before
connecting to an endotracheal tube. The tidal volume was set at 10
ml, and the ventilator rate was set at 75-breaths/min. A 2-cm skin
incision was made over the fourth intercostal space, which
separated the subcutaneous tissue and the underlying muscle,
thereby permitting entrance to the thorax via the fourth
intercostal space. The pericardial cavity was injected with 200 µl
vector using an insulin syringe. The muscle and the skin were
closed following injection. The rats received penicillin for three
days via intramuscular injections to prevent infection. 50% of rats
in each group received saline (10 ml/kg), while the remaining rats
received Met (50 mg/kg/day) via oral gavage twice a day for 4
weeks. The systolic blood pressure (SBP) of the rats was monitored
weekly using a tail blood pressure meter. The rats were sacrificed
by intraperitoneal injection of 10% chloral hydrate (300 mg/kg;
Hunan Xiangya Medicine Co., Ltd., Changsha, China) 4 weeks after
the initial treatment.
Transfection efficiency of rAVV9
vectors
The transfection efficiency of
rAAV9-shRNA-Adrb1-ZsGreen was determined at 4 weeks following rAAV9
injection, at which point the hearts were harvested and myocardial
tissue (150 mg) was collected. The heart tissue was washed with
ice-cold saline, immersed in 4% paraformaldehyde overnight,
dehydrated in consecutive 15% and 30% sucrose solutions and dried
with filter paper. Tissue sections were then embedded in paraffin
at room temperature, and divided into 10-µm frozen sections. Cell
nuclei were visualized by staining tissue sections with DAPI, and
blue and green fluorescence signals were visualized using an
Olympus IX-71 fluorescence microscope (Olympus Corporation).
Evaluation of β1-AR mRNA expression
using reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
Following excision of the heart from rats in each
experimental group, a portion of the heart was used to measureβ1-AR
mRNA expression levels by RT-qPCR. Briefly, total RNA was extracted
from each heart using the RNA-Solv Reagent (Omega Bio-Tek, Inc.,
Norcross, GA, USA) according to the manufacturer's instructions.
Total RNA (1 µg) was then reverse transcribed to generate cDNA
using the to Revert Aid First Strand cDNA Synthesis kit (Thermo
Fisher Scientific, Inc.). qPCR reaction mixtures (10 µl) consisted
of 5 µl SYBR Green (Omega Bio-Tek, Inc.), 0.8 µl cDNA and 0.2 µl
each primer. The following primers were used: Adrb1 (Rattus
norvegicus) forward, 5′-CGCTGCCCTTTCGCTACCAG-3′ and reverse,
5′-CCGCCACCAGTGCTGAGGAT-3′; β-actin forward,
5′-CGTAAAGACCTCTATGCCAA-3′, and reverse, 5′-GGTGTAAAACGCAGCTCAGT-3′
(Nanjing GenScript Biotechnology Co., Ltd., Nanjing, China). The
thermal cycling parameters were as follows: 95°C for 15 min,
followed by 40 cycles of 95°C for 15 sec, 60°C for 30 sec and 72°C
for 30 sec. Target gene expression levels were quantified relative
to β-actin expression using the 2-ΔΔCq method (23).
Evaluation of β1-AR protein expression
by western blot analysis
Heart tissue (50 mg) was lysed in lysis buffer
consisting of phenylmethylsulfonyl fluoride and
radioimmunoprecipitation assay buffer (1:100; Applygen Technologies
Inc., Beijing, China) and centrifuged at 16.2 × g for 40 min at
4°C, before total protein was quantified using a bicinchoninic acid
assay kit (Beijing Kawin Biotech Co., Ltd., Beijing, China). For
the western blot analysis, 80 µl sample (~50 µg protein) was
separated by 8% SDS-PAGE, and proteins were transferred to
polyvinylidene difluoride membranes. After blocking with 5% non-fat
milk in phosphate-buffered saline for 5 h at room temperature,
membranes were incubated with polyclonal rabbit antibodies against
Adrb1 (dilution, 1:1,000; cat. no. ab3442; Abcam, Cambridge, MA,
USA) or GAPDH (dilution, 1:3,000; cat. no. 10494-1-AP, ProteinTech
Group, Inc., Chicago, IL, USA) overnight at 4°C. Membranes were
then incubated with horseradish peroxidase-conjugated goat
anti-rabbit secondary antibody (dilution, 1:8,000; cat. no.
10285-1-AP; ProteinTech Group, Inc.) for 1 h at room temperature.
Chemiluminescence was detected using the ECL Western Blotting
Substrate kit (Abnova Corporation, Taipei, Taiwan), and the
relative intensity of the bands of interest were analyzed using
ImageJ image analysis software (version, 1.44p; National Institutes
of Health, Bethesda, MD, USA). The expression of β1-AR was
calculated relative to GAPDH.
Evaluation of β1-AR protein expression
by immunohistochemical analysis
Following excision of rat hearts, they were fixed in
4% paraformaldehyde and embedded in paraffin. Heart tissue sections
were blocked using 5 ml horse serum (ZSGB-Bio, Beijing, China) at
room temperature for 1 h. β1-AR expression was detected by staining
with a polyclonal rabbit anti-β1-AR antibody (dilution, 1:100; cat.
no. 10285-1-AP; ProteinTech Group Inc.) overnight at 4°C, followed
by staining with hematoxylin. The integrated optical density of the
positive expression area of the myocardial tissue sections was
calculated using Image-Pro Plus software (version, 6.0; Media
Cybernetics, Inc., Rockville, MD, USA).
Measurement of heart rate and SBP
The heart rate and SBP in conscious, resting rats
was measured weekly using a tail-cuff method with an
electrosphygmomanometer attached to a computerized recorder
(Shanghai Alcott Biotech Co., Ltd., Shanghai, China) from
14:30-17:30 p.m. by the same investigator (Miss Xiao-Li Liu, The
Second Affiliated Hospital of Hunan University of Traditional
Chinese Medicine, Changsha, China). Each rat was held in a silent,
dark and sizeable cylinder. Blood pressure measurements were
performed in a blind manner, and the mean of three repeated
measurements for each rat during each session was recorded.
Statistical analysis
Data are expressed as mean ± standard deviation.
Levene's test was used to assess the equality of variances. One-way
analysis of variance with the least significant difference test was
used to compare the differences among the experimental and control
groups. Data analyses were performed using the SPSS software
program (version, 17.0; SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Validating the purity and titer of
rAAV9 vectors and the transfection efficiency in HEK293 cells
The purity of rAVV9-shRNA-Adrb1-ZsGreen plasmid
vectors was assessed using SDS-PAGE followed by Coomassie brilliant
blue (CBB) staining. Three characteristic protein bands for viral
protein (VP) 1, VP2 and VP3, with molecular weights of 87, 73 and
62 KDa, respectively, were observed, indicating the purity of the
rAAV9 vector (Fig. 1A). Dot blot
results demonstrated that the vector titer was 1.5×1012 v.g./ml
(Fig. 1B). Transfection efficiency
was assessed by infecting HEK293 cells and visualizing green
fluorescence signals with a fluorescence microscope. As shown in
Fig. 1C, approximately 90% of the
cells exhibited green fluorescence.
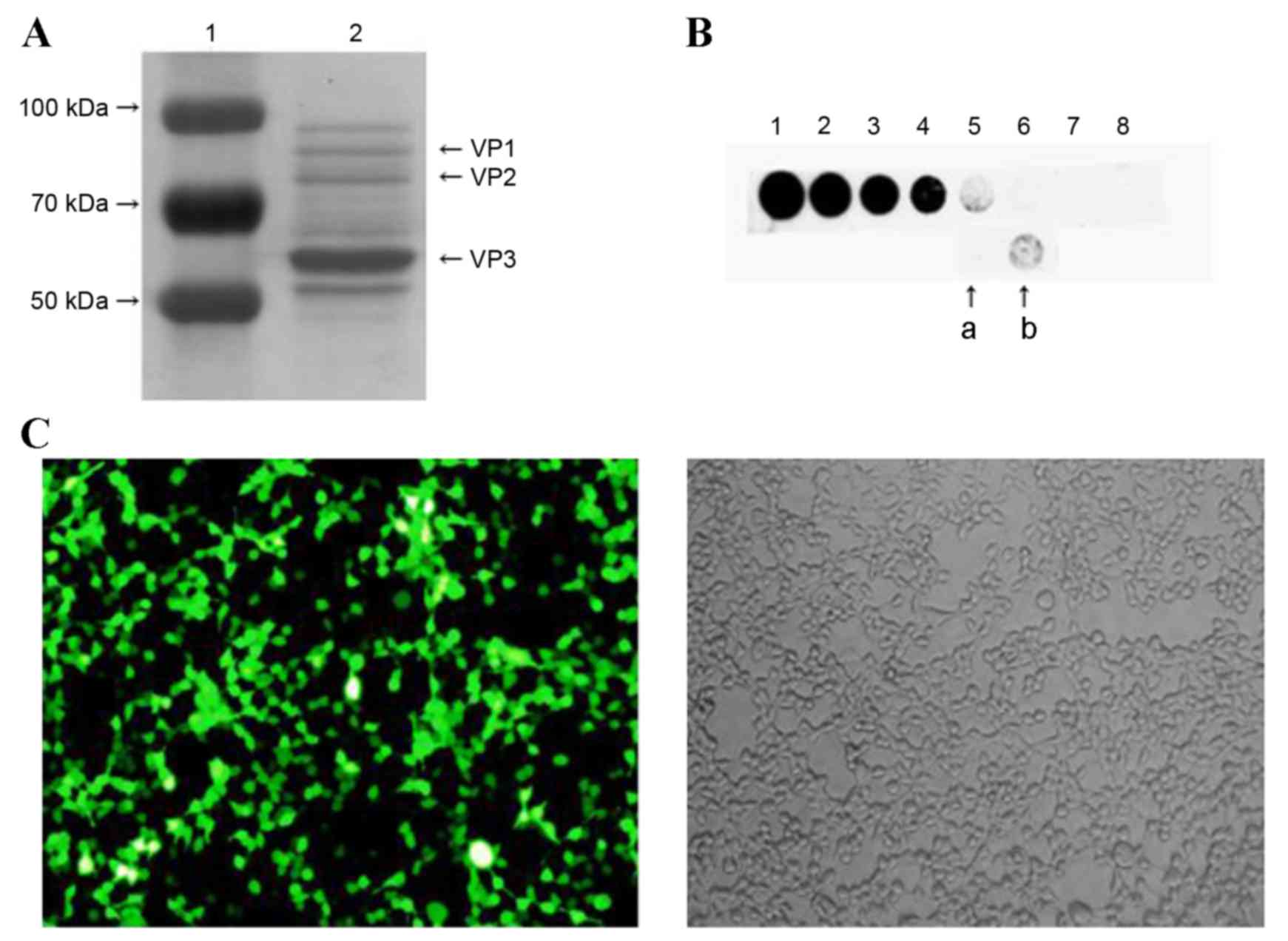 | Figure 1.The purity, titer and transfection
efficiency of rAAV9-shRNA-Adrb1-ZsGreen vectors in HEK293 cells.
(A) The purity of rAAV9-shRNA-Adrb1-ZsGreen vectors as determined
by SDS-PAGE followed by Coomassie Brilliant Blue staining. The left
lane indicates the protein maker (1), and the right lane indicates the
purified vector sample (2). (B)
The titer of the purified vectors. Spots 1–8 indicate 50, 25, 12.5,
6.25, 3.125, 1.56, 0.78, and 0.39×109 v.g./ml purified
vector, respectively (a, 2-fold dilution of purified vectors; b,
purified vectors). (C) Fluorescence (left) and light (right)
microscope images of the transfection efficiency of
rAAV9-shRNA-Adrb1-ZsGreen vectors in HEK293 cells (magnification,
×200). rAAV9, recombinant adeno-associated virus type 9 vector;
shRNA, short-hairpin RNA; Adrb1, adrenergic receptor β1; v.g.,
viral genomes. |
Determination of
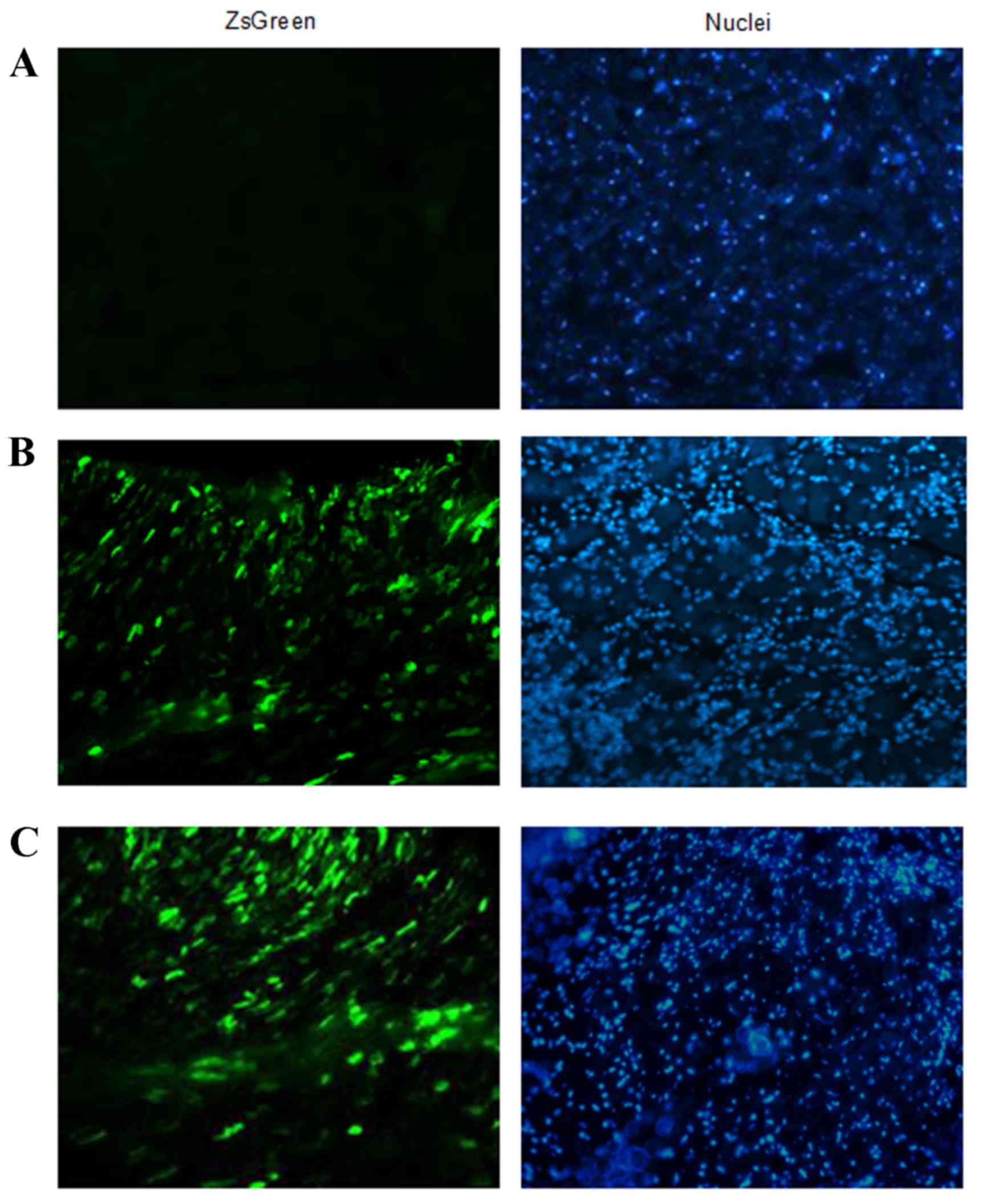
rAAV9-Adrb1-shRNA-ZsGreen expression in vivo
In order to investigate the efficiency of
rAAV9-Adrb1-shRNA-ZsGreen delivery in vivo, the expression
of ZsGreen in heart tissue sections of rats from the sham-operated,
rAAV9-Adrb1 and rAAV9-NC groups at 4 weeks following injection of
rAAV9-Adrb1-shRNA-ZsGreen and rAAV9-NC-ZsGreen vectors into the
myocardium was examined by fluorescence microscopy. As shown in
Fig. 2B and C, green fluorescence
was observed in heart tissues of rAAV9-NC and rAAV9-Adrb1 groups.
By contrast, no ZsGreen expression was detected in the heart
tissues from the sham group (Fig.
2A).
Inhibitory effects of
rAAV9-Adrb1-shRNA-ZsGreen on β1-AR expression
In order to assess the inhibitory effects of the
injection of rAAV9-Adrb1-shRNA-ZsGreen vectors on β1-AR expression
in the myocardium, the β1-AR mRNA expression levels in all
experimental groups were examined. Compared with the rAAV9-NC
group, cardiomyocytes in the rAAV9-Adrb1 group exhibited a
significant decrease in β1-AR mRNA expression (0.42±0.06 vs.
1.3±0.08; P<0.001), whereas no significant difference in β1-AR
mRNA expression was observed between the sham and rAAV9-NC groups
(1.3±0.08 vs. 1.3±0.06; P>0.05; Fig. 3A). Immunohistochemical and western
blot analyses revealed that cardiomyocytes in the rAAV9-Adrb1 group
displayed a significant reduction in β1-AR protein expression
levels when compared with the rAAV9-NC group (immunohistochemistry,
50.02±4.4 vs. 80.05±3.7, P<0.001; western blot, 0.52±0.12 vs.
0.83±0.17, P=0.032; Fig. 3B-E).
Western blot analysis demonstrated no significant difference
between β1-AR protein expression levels between the rAAV9-NC and
sham groups (80.05±3.7 vs. 79.30±2.8; P>0.05; Fig. 3D and E). These data indicated that
β1-AR expression in the myocardium of SHR was downregulated upon
infection with rAAV9-Adrb1-shRNA-ZsGreen but not rAAV9-NC.
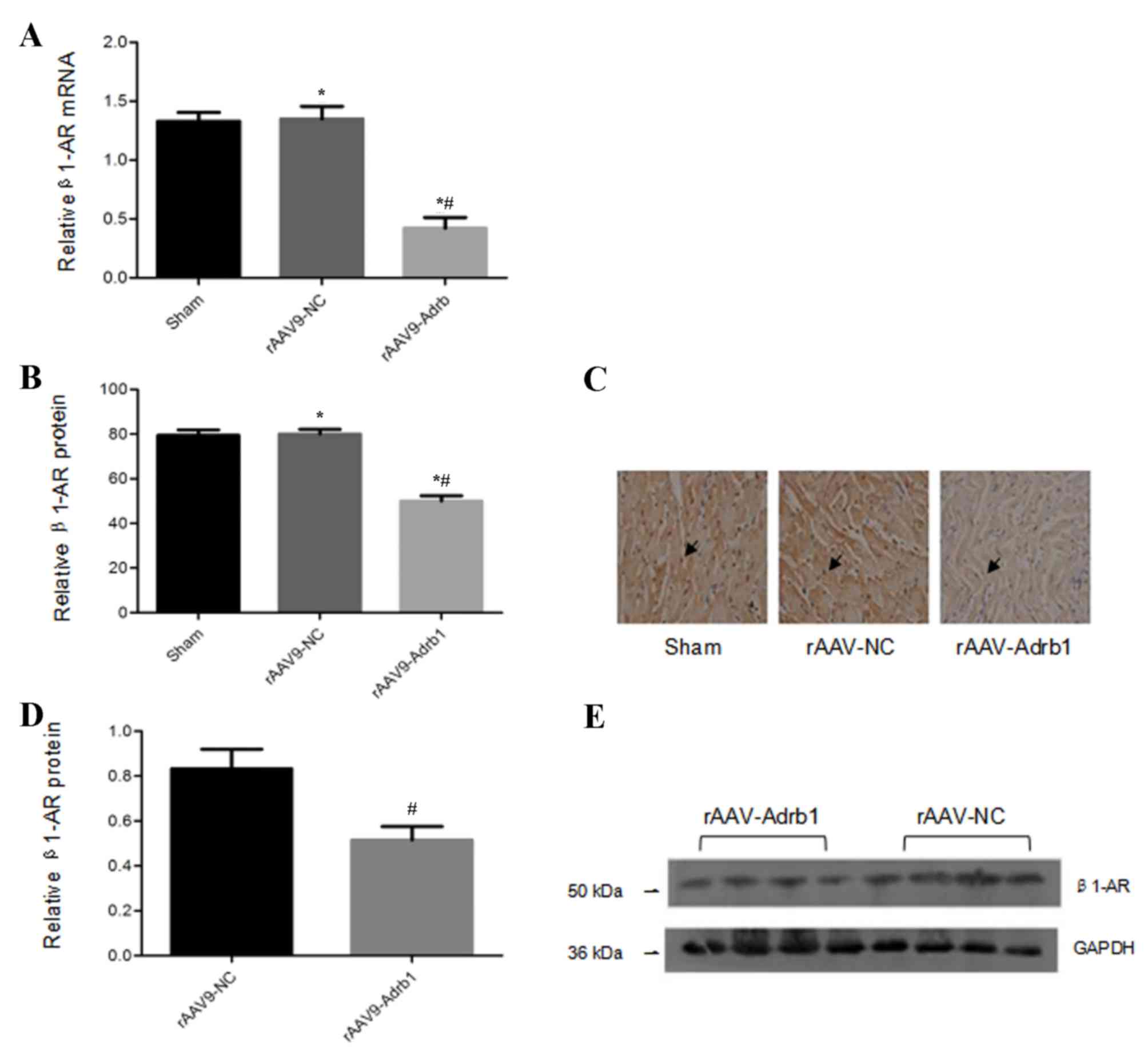 | Figure 3.β1-AR mRNA and protein expression
levels in the myocardium of SHR from sham, rAAV9-NC and rAAV9-Adrb1
groups at 4 weeks following injection with saline, rAAV9-NC-ZsGreen
or rAAV9-shRNA-Adrb1-ZsGreen vectors, respectively. (A) β1-AR mRNA
levels as determined by RT-qPCR. (B) The integrated optical density
of β1-AR protein levels as determined by immunohistochemical
analysis. (C) Representative microscope images (magnification,
×400) of β1-AR protein levels as determined by immunohistochemical
analysis. The black arrows indicate positive protein particles. (D)
Relative β1-AR protein levels as determined by western blot
analysis. (E) Representative western blot image of β1-AR protein
levels. GAPDH was used as a loading control. *P<0.05 vs. sham
group; #P<0.05 vs. rAAV9-NC group. β1-AR,
β1-adrenergic receptor; SHR, spontaneously hypertensive rats;
rAAV9, recombinant adeno-associated virus type 9 vector; NC,
negative control; Adrb1, adrenergic receptor β1; shRNA,
short-hairpin RNA; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction. |
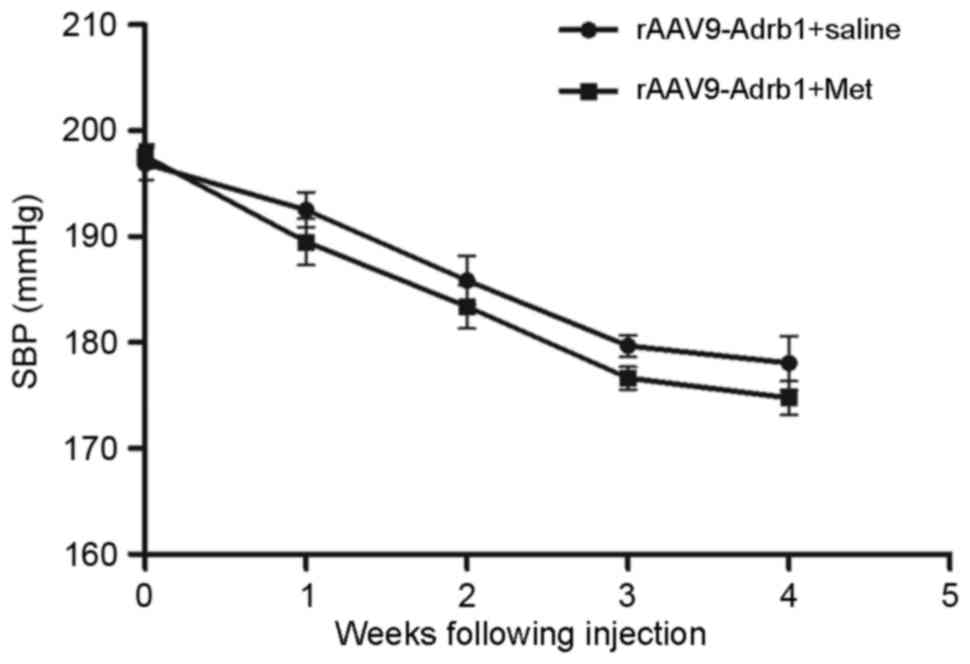
Downregulating β1-AR expression in the
myocardium results decreased sensitivity to Met
In order to investigate the effects of β1-AR
downregulation on the response to Met treatment in SHR, the SBP of
rats in the sham, rAAV9-NC and rAAV9-Adrb1 groups was examined
using a tail blood pressure meter. As shown in Fig. 4, no significant difference in blood
pressure was observed among all three experimental groups prior to
Met treatment (P>0.05). Following 4 weeks of Met treatment, a
decrease in SBP was observed in all experimental groups (Fig. 4). In addition, a significantly
greater reduction in SBP was observed in the sham and rAAV9-NC
groups when compared with the rAAV9-Adrb1 group (ΔSBP, 22.84±1.7,
28.78±2.7 and 30.16±5.7 mmHg for rAAV9-Adrb1, rAAV9-NC and sham
groups, respectively; P=0.035; Fig.
4). As shown in Table I, no
significant differences in heart rate among the three experimental
groups was observed before or after 4 weeks of Met treatment
(P>0.05). However, the heart rate of rats in all groups
significantly decreased following 4 weeks of Met treatment
(P<0.001; Table I).
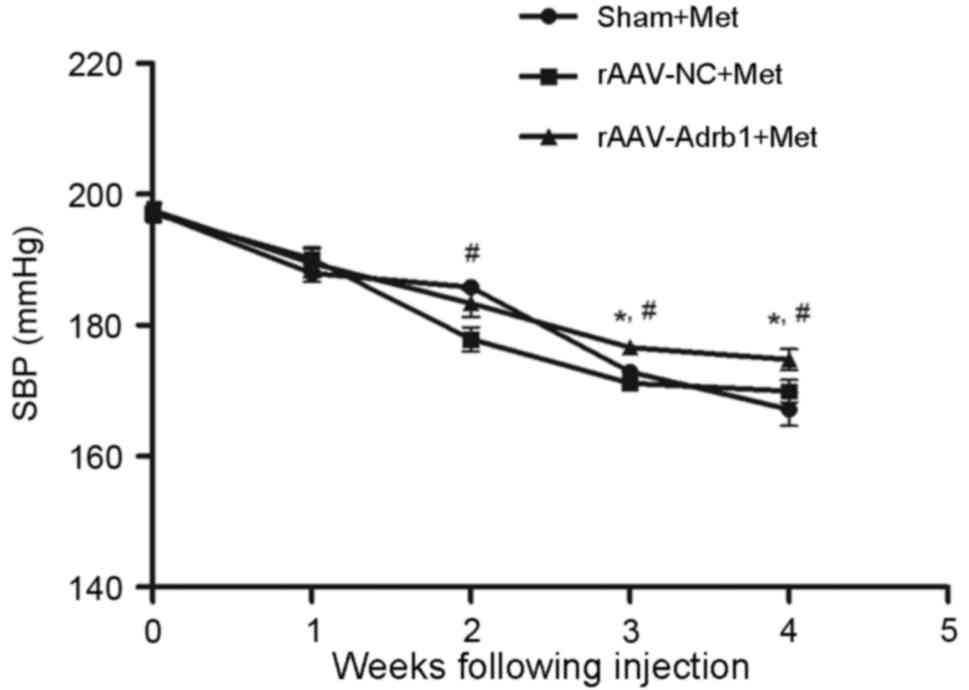 | Figure 4.Effect of cardiac β1-AR
downregulation on the SBP in SHR from sham, rAAV9-NC and
rAAV9-Adrb1 groups up to 4 weeks following injection with saline,
rAAV9-NC-ZsGreen and rAAV9-shRNA-Adrb1-ZsGreen vectors,
respectively, and treatment with Met. *P<0.05 vs. rAAV9-NC+Met
group; #P<0.05 vs. the sham+Met group. β1-AR,
β1-adrenergic receptor; SBP, systolic blood pressure; rAAV9,
recombinant adeno-associated virus type 9 vector; NC, negative
control; Adrb1, adrenergic receptor β1; shRNA, short-hairpin RNA;
Met, metoprolol. |
 | Table I.Effect of Met on the heart rate of
SHR following downregulation of cardiac β1-AR expression. |
Table I.
Effect of Met on the heart rate of
SHR following downregulation of cardiac β1-AR expression.
| Group | No. of samples | Heart rate prior to
Met intervention (bmp) | Heart rate
following Met intervention (bmp) |
|---|
| Sham+Met | 4 | 461.7±3.7 |
435.7±3.3a |
| rAAV9-NC+Met | 6 | 459.9±6.0 |
434.1±2.6a |
|
rAAV9-Adrb1+Met | 6 | 459.0±3.0 |
437.2±2.3a |
Downregulation of β1-AR in the
myocardium reduces blood pressure
In addition to the assessment of Met treatment
responses, the authors observed an unexpected phenomenon regarding
the effects of cardiac β1-AR downregulation on blood pressure in
SHR. As shown in Fig. 5, SBP of
SHR in the rAAV9-Adrb1 group was significantly lower than that of
the rAAV9-NC group at 4 weeks following injection (178.1±6.3 vs.
199.4±3.1 mmHg; P<0.001). By contrast, no significant difference
in SBP was observed between the rAAV9-NC and sham groups (199.4±3.1
vs. 198.3±4.5 mmHg; P>0.05; Fig.
5). As shown in Table II, no
significant differences in heart rate of SHR among all treatment
groups were observed at 0 or 4 weeks following injection of saline
or the rAAV9 vectors (P>0.05). In addition, no significant
difference in the SBP of SHR in the rAAV9-Adrb1 group was observed
following treatment with Met or saline at 4 weeks following
injection of rAAV9-shRNA-Adrb1-ZsGreen vectors (174.8±3.9 vs.
178.1±6.3 mmHg; P>0.05; Fig.
6).
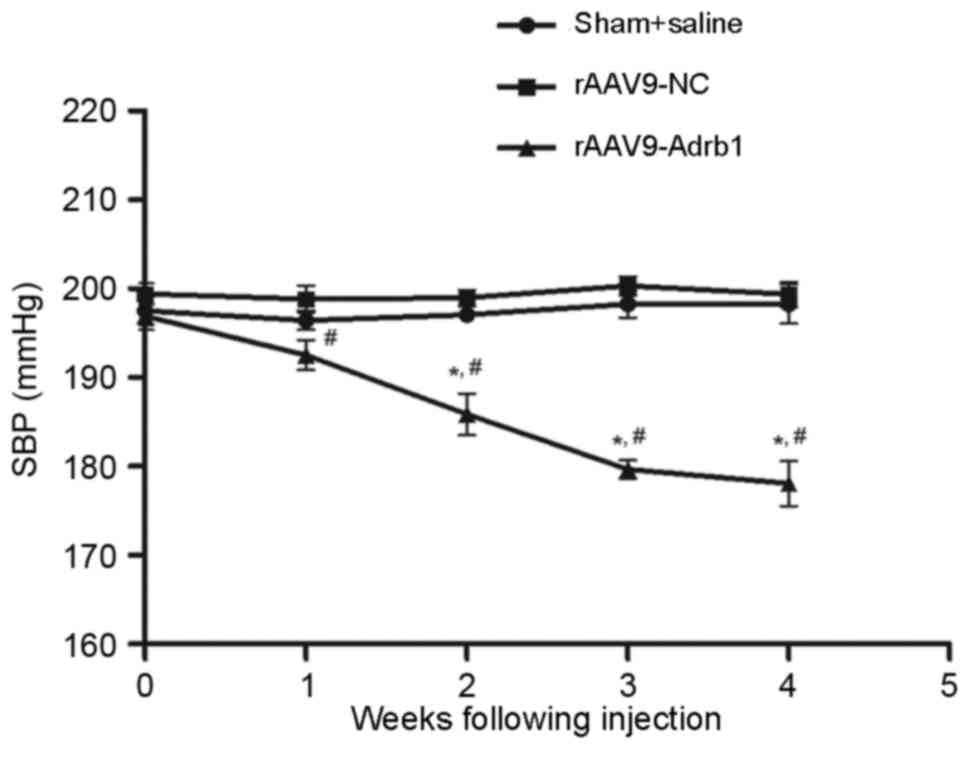 | Figure 5.Effect of cardiac β1-AR
downregulation on the SBP in SHR from sham+saline, rAAV9-NC and
rAAV9-Adrb1 groups up to 4 weeks following injection with saline,
rAAV9-NC-ZsGreen and rAAV9-shRNA-Adrb1-ZsGreen vectors,
respectively. *P<0.05 vs. rAAV9-NC group; #P<0.05
vs. sham+saline group. β1-AR, β1-adrenergic receptor; SBP, systolic
blood pressure; SHR, spontaneously hypertensive rats; rAAV9,
recombinant adeno-associated virus type 9 vector; NC, negative
control; Adrb1, adrenergic receptor β1; shRNA, short-hairpin
RNA. |
 | Table II.Effect of cardiac β1-AR suppression
on heart rate in SHR. |
Table II.
Effect of cardiac β1-AR suppression
on heart rate in SHR.
| Groups | No. of samples | Heart rate prior to
β1-AR suppression (bmp) | Heart rate
following β1-AR suppression (bmp) |
|---|
| Sham+saline | 4 | 461.7±3.7 | 460.4±3.3 |
| rAAV9-NC | 5 | 459.9±6.0 | 455.1±2.6 |
| rAAV9-Adrb1 | 6 | 459.0±3.0 | 456.5±2.3 |
Discussion
Met is commonly prescribed for the treatment of
hypertension. Although its efficacy is extremely variable among
patients, the presence of CYP2D6 and Adrb1
polymorphisms only provide a partial explanation for this
variability (10–12). A previous study investigating
Adrb1 promoter methylation suggested that the level of
Adrb1 promoter methylation influences the efficacy of Met
via the regulation of β1-AR expression (14). In addition, β1-AR is the target of
Met and its expression has been demonstrated to vary among
individuals (23,24). Therefore, the authors of the
present study hypothesized that the expression of myocardial β1-AR
may present a novel mechanism underlying the inter-individual
differences in response to Met treatment (15). In order to test this notion, the
effect of downregulated cardiac β1-AR expression in the response to
Met treatment in SHR was investigated in the present study. The
results indicated that SHR injected with rAAV9-Adrb1 vectors into
the myocardium, exhibited a lower reduction in SBP following Met
treatment when compared with negative controls. Naya et al
(24) demonstrated that patients
with idiopathic dilated cardiomyopathy and decreased myocardial
β-AR expression exhibited a greater sensitivity to the
antiadrenergic drug carvedilol in clinical trails. Together, these
findings suggest that alterations in myocardial β-AR expression
levels are associated with differential responses to
β-blockers.
A number of factors, including age, drugs and
comorbidities are known to influence β1-AR expression (25,26).
Oliver et al (27) revealed
that β1-AR expression levels were increased in the circulating
lymphocytes of hypertensive patients. Lymphocytes are considered to
be a practical surrogate for myocardial or vascular cells. Patients
with heart failure exhibit a downregulation and desensitization of
β1-AR, which leads to a markedly diminished β1-AR-mediated
contractile response (28). In
this way, β1-AR expression is altered under different physiological
conditions, and may potentially be used as a marker of response to
different treatments. Therefore, if the hypothesis presented by the
authors (15) is accepted by
future clinical trials, β1-AR expression may be considered as a
potential biomarker when establishing a quantitative
pharmacological model of patient response to Met for individual
therapy.
In addition to the original study objective, the
results of the present study demonstrated an unexpected effect of
suppressed β1-AR expression on blood pressure in SHR.
shRNA-mediated reduction of cardiac β1-AR expression was associated
with a significant decrease in SBP of SHR when compared with
negative and sham-treated controls. In addition, this effect on SBP
was comparable to Met-treated rAAV9-Adrb1 rats. Several studies
have demonstrated that β1-AR expression serves a role in the
development of cardiovascular diseases, such as hypertension
(29–31). The intravenous injection of β1-AR
antisense-oligodeoxy nucleotides delivered in cationic liposomes in
an animal model of hypertension, demonstrated a significant
reduction in cardiac β1-AR density of ~30–50% for 18 days, and a
reduction in the blood pressure of SHR for 20 days, with a maximum
decrease of 38 mmHg (30). Arnold
et al (29) developed a
small interfering RNA targeted to β1-AR that significantly reduced
β1-AR mRNA levels to 33.3% of control levels, and lowered diastolic
blood pressure in SHR by a maximum of 30 mmHg for >12 days.
Using gene knockout technology, the 24 h mean artery pressure was
decreased by 10% (102.02±1.81 vs. 92.11±2.62 mmHg) in
β1/β2-AR−/− compared with wild-type mice
(31). Consistent with these
results, a decrease in cardiac β1-AR expression was associated with
a reduction in SBP in the present study.
Since β1-AR is a member of the autonomic nervous
system, the effect of cardiac β1-AR expression on blood pressure
regulation may be comprehensible. The β1-AR subtype is often
classified as the ‘cardiac’ β-AR subtype, due to the observation
that the in vivo stimulation of this receptor by its
agonists increases heart rate and contractility. It is known that
stimulation of β1-AR induces robust chronotropic and inotropic
effects via the Gs-protein adenylate cyclase-cyclic adenosine
monophosphate-protein kinase A signaling pathway (32). Following stimulation of β1-AR,
protein kinase A is activated and L-type Ca2+ channels
in ventricular myocytes are phosphorylated, which leads to
increased myocardial contractility through increased
Ca2+ influx and the release of Ca2+ from the
sarcoplasmic reticulum. Zhang et al (30) observed a marked attenuation of the
β1-AR-mediated positive inotropic response in isolated perfused
hearts in vitro and in conscious SHR following myocardial
β1-AR downregulation. Through the targeted deletion of β1-AR, mice
lacking β1-AR were demonstrated to exhibit increased heart rate
variability and decreased resting heart rate (33). Therefore, myocardial β1-AR
expression may influence blood pressure regulation by affecting
heart rate and cardiac output through altering cardiac inotropy and
chronotropy.
In the present study, decreasing β1-AR expression in
the myocardium decreased blood pressure but had no significant
effect on heart rate in SHR. Which is consistent with several
previous studies (29–30). It is therefore possible that β2-AR
may serve a more important role in regulating heart rate compared
with β1-AR (29). However, a
previous study revealed that β1-AR may also function to control
heart rate (33). Ecker et
al (33) demonstrated that
resting heart rate (in beats/min) was decreased in β1-knockout (KO)
and β1/β2-double KO mice when compared with wild-type mice. The
possible reasons underlying these contradictory results require
further investigation in future studies.
The present study was limited by the small sample
size and β1-AR was downregulated only in the myocardium by
injection of lentiviral shRNA vectors directly into the pericardial
cavity (34). The results
demonstrated that β1-AR expression in the myocardium affects the
efficacy of Met in SHR. In addition, cardiac β1-AR expression may
be associated with blood pressure regulation, however, the effect
of β1-AR expression on cardiac physiology was not investigated in
the present study. Therefore, the clinical applications or
mechanisms involved in β1-AR-mediated regulation of blood pressure
and response to Met treatment warrant attention in future studies.
The rAAV9 vector, used to deliver shRNA to the myocardium in
vivo in the present study, is a valuable, safe and efficacious
means of cardiac gene transfer (18). However, its potential effects on
cardiac function and the immune system were not examined. This will
be an important aim in future studies.
Acknowledgements
The present study was supported by grants from The
National Natural Science Foundation of China (grant no. 81470535),
The National Science and Technology Major Projects for ‘Major New
Drugs Innovation and Development’ (grant no. 2012ZX09303014001) and
The National Key Technology Research and Development Program (grant
no. 2012BAI137B05).
References
|
1
|
Chen S: Essential hypertension:
Perspectives and future directions. J Hypertens. 30:42–45. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chinese National Center for Cardiovascular
Diseases (CNCCD), . Report on Cardiovascular Diseases in China.
2013.
|
|
3
|
Sheng CS, Liu M, Kang YY, Wei FF, Zhang L,
Li GL, Dong Q, Huang QF, Li Y and Wang JG: Prevalence, awareness,
treatment and control of hypertension in elderly Chinese. Hypertens
Res. 36:824–828. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lim SS, Vos T, Flaxman AD, Danaei G,
Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee
M, et al: A comparative risk assessment of burden of disease and
injury attributable to 67 risk factors and risk factor clusters in
21 regions, 1990–2010: A systematic analysis for the Global Burden
of Disease Study 2010. Lancet. 380:2224–2260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tibazarwa KB and Damasceno AA:
Hypertension in developing countries. Can J Cardiol. 30:527–533.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yancy CW, Jessup M, Bozkurt B, Butler J,
Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi
JL, et al: 2013 ACCF/AHA guideline for the management of heart
failure: A report of the American College of Cardiology
Foundation/American Heart Association Task Force on Practice
Guidelines. J Am Coll Cardiol. 62:e147–e239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McAlister FA, Wiebe N, Ezekowitz JA, Leung
AA and Armstrong PW: Meta-analysis: Beta-blocker dose, heart rate
reduction, and death in patients with heart failure. Ann Intern
Med. 150:784–794. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Materson BJ, Reda DJ, Cushman WC, Massie
BM, Freis ED, Kochar MS, Hamburger RJ, Fye C, Lakshman R and
Gottdiener J: Single-drug therapy for hypertension in men. A
comparison of six antihypertensive agents with placebo. The
Department of Veterans Affairs Cooperative Study Group on
Antihypertensive Agents. N Engl J Med. 328:914–921. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johnson JA and Liggett SB: Cardiovascular
pharmacogenomics of adrenergic receptor signaling: Clinical
implications and future directions. Clin Pharmacol Ther.
89:366–378. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bijl MJ, Visser LE, van Schaik RH, Kors
JA, Witteman JC, Hofman A, Vulto AG, van Gelder T and Stricker BH:
Genetic variation in the CYP2D6 gene is associated with a lower
heart rate and blood pressure in beta-blocker users. Clin Pharmacol
Ther. 85:45–50. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou SF: Polymorphism of human cytochrome
P450 2D6 and its clinical significance: Part II. Clin
Pharmacokinet. 48:761–804. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu D, Li G, Deng M, Song W, Huang X, Guo
X, Wu Z, Wu S and Xu J: Associations between ADRB1 and CYP2D6 gene
polymorphisms and the response to β-blocker therapy in
hypertension. J Int Med Res. 43:424–434. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuan H, Huang Z, Yang G, Lv H, Sang H and
Yao Y: Effects of polymorphism of the beta(1) adrenoreceptor and
CYP2D6 on the therapeutic effects of metoprolol. J Int Med Res.
36:1354–1362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rockman HA, Koch WJ and Lefkowitz RJ:
Seven- transmembrane-spanningreceptors and heart function. Nature.
415:206–212. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang Q, Yuan H, Xing X, Liu J, Huang Z
and Du X: Methylation of adrenergic β1 receptor is a potential
epigenetic mechanism controlling antihypertensive response to
metoprolol. Indian J Biochem Biophys. 48:301–307. 2011.PubMed/NCBI
|
|
16
|
Liu H, Xing X, Huang L, Huang Z and Yuan
H: The expression level of myocardial β1-adrenergic receptor
affects metoprolol antihypertensive effects: A novel mechanism for
interindividual difference. Med Hypotheses. 81:71–72. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vandenberghe LH, Xiao R, Lock M, Lin J,
Korn M and Wilson JM: Efficient serotype-dependent release of
functional vector into the culture medium during adeno-associated
virus manufacturing. Hum Gene Ther. 21:1251–1257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mueller C, Ratner D, Zhong L, Esteves-Sena
M and Gao G: Production and discovery of novel recombinant
adeno-associated viral vectors. Curr Protoc Microbiol. Chapter 14:
Unit14D.1. 2012. View Article : Google Scholar
|
|
19
|
Wu XB, Dong XY, Wu ZJ, Cao H, Niu DB, Qu
JG, Wang H and Hou YD: A novel method for purification of
recombinant adenoassociated virus vectors on a large scale. Chinese
Sci Bull. 46:pp485–488. 2001. View Article : Google Scholar
|
|
20
|
McClure C, Cole KL, Wulff P, Klugmann M
and Murray AJ: Production and titering of recombinant
adeno-associated viral vectors. J Vis Exp. e33482011.PubMed/NCBI
|
|
21
|
Bish LT, Sweeney HL, Müller OJ and
Bekeredjian R: Adeno-associated virus vector delivery to the heart.
Methods Mol Biol. 807:219–237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Katz MG, Swain JD, Tomasulo CE, Sumaroka
M, Fargnoli A and Bridges CR: Current strategies for myocardial
gene delivery. J Mol Cell Cardiol. 50:766–776. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Naya M, Tsukamoto T, Morita K, Katoh C,
Nishijima K, Komatsu H, Yamada S, Kuge Y, Tamaki N and Tsutsui H:
Myocardial beta-adrenergic receptor density assessed by
11C-CGP12177 PET predicts improvement of cardiac function after
carvedilol treatment in patients with idiopathic dilated
cardiomyopathy. J Nucl Med. 50:220–225. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ferrara N, Komici K, Corbi G, Pagano G,
Furgi G, Rengo C, Femminella GD, Leosco D and Bonaduce D:
β-adrenergic receptor responsiveness in aging heart and clinical
implications. Front Physiol. 4:3962014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hellgren I, Sylven C and Magnusson Y:
Study of the beta1 adrenergic receptor expression in human tissues:
Immunological approach. Biol Pharm Bull. 23:700–703. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oliver E, Rovira E, Montó F, Valldecabres
C, Julve R, Muedra V, Ruiz N, Barettino D and D'Ocon P:
beta-Adrenoceptor and GRK3 expression in human lymphocytes is
related to blood pressure and urinary albumin excretion. J
Hypertens. 28:1281–1289. 2010.PubMed/NCBI
|
|
28
|
Hamdani N and Linke WA: Alteration of the
beta-adrenergic signaling pathway in human heart failure. Curr
Pharm Biotechnol. 13:2522–2531. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arnold AS, Tang YL, Qian K, Shen L,
Valencia V, Phillips MI and Zhang YC: Specific beta1-adrenergic
receptor silencing with small interfering RNA lowers high blood
pressure and improves cardiac function in myocardial ischemia. J
Hypertens. 25:197–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang YC, Bui JD, Shen L and Phillips MI:
Antisense inhibition of beta(1)-adrenergic receptor mRNA in a
single dose produces a profound and prolonged reduction in high
blood pressure in spontaneously hypertensive rats. Circulation.
101:682–688. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim SM, Huang Y, Qin Y, Mizel D,
Schnermann J and Briggs JP: Persistence of circadian variation in
arterial blood pressure in beta1/beta2-adrenergic
receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol.
294:R1427–R1434. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yoo B, Lemaire A, Mangmool S, Wolf MJ,
Curcio A, Mao L and Rockman HA: Beta1-adrenergic receptors
stimulate cardiac contractility and CaMKII activation in vivo and
enhance cardiac dysfunction following myocardial infarction. Am J
Physiol Heart Circ Physio. l297:H1377–H1386. 2009. View Article : Google Scholar
|
|
33
|
Ecker PM, Lin CC, Powers J, Kobilka BK,
Dubin AM and Bernstein D: Effect of targeted deletions of beta1-
andbeta2-adrenergic-receptor subtypes on heart rate variability. Am
J Physiol Heart Circ Physiol. 290:H192–H199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen SJ, Johnston J, Sandhu A, Bish LT,
Hovhannisyan R, Jno-Charles O, Sweeney HL and Wilson JM: Enhancing
the utility of adeno-associated virus gene transfer through
inducible tissue-specific expression. Hum Gene Ther Methods.
24:270–278. 2013. View Article : Google Scholar : PubMed/NCBI
|