Introduction
Diabetes is a ‘coronary heart disease (CHD)
equivalent’ disease, as epidemiological data has previously
demonstrated that patients with diabetes without any prior evidence
of CHD are at greater risk of death from CHD than nondiabetic
patients with prior evidence of CHD (1,2).
Atherosclerosis (AS) is a disease of arterial lipid deposition
leading to a number of biological responses, including a chronic,
macrophage-dominated inflammatory reaction (3), and is the pathological basis of CHD.
The hexosamine biosynthesis pathway (HBP), a normal pathway for
glucose metabolism, is activated excessively in patients with
diabetes, resulting in increased cellular glucosamine (4). Endoplasmic reticulum (ER) is a
membranous network to synthesize, modify, fold and assemble
proteins. When the ER function, particularly folding capacity, is
challenged, the unfolded protein response (UPR) is executed as a
protective mechanism in ER. Failure of this mechanism to fold newly
synthesized proteins shows unique damage to the cell and is termed
‘ER stress’ (5,6). Numerous studies have highlighted that
ER stress may link hyperglycemia to AS (4,7,8).
Important ER stress markers, including protein kinase-like ER
kinase (PERK), glucose regulated protein 78 (GRP78) and C/EBP
homologous protein (CHOP), were expressed in the arterial wall of
streptozotocin-induced hyperglycemic apolipoprotein
(apoE)-deficient mice (4).
Glucosamine levels and the expression of GRP78 were increased
following hyperglycemia and prior to the early stages of fatty
streak formation in aortic endothelial cells of hyperglycemic
apoE−/− mice (7). In
vitro studies have demonstrated that incubation of hepatic
cells (9) and adipocytes (10) with 5 mM glucosamine resulted in
lipid accumulation, impaired insulin-stimulated glucose transport
and elevated levels of ER stress markers. High-dose glucosamine
may, therefore, increase the inflammatory response and induce lipid
metabolic abnormalities, further aggravate endothelial cell injury
and, ultimately, accelerate the development of AS (11). However, as an effective nutritional
supplement in human osteoarthritis, orally administered glucosamine
sulfate demonstrated an anti-atherosclerotic effect in rabbits with
AS aggravated by chronic arthritis (12). Incubation of human umbilical vein
endothelial cells (HUVECs) with 0.5 mM glucosamine has previously
been demonstrated to inhibit tumor necrosis factor-α-induced
inflammation (13), and
glucosamine significantly suppressed mesangial cell viability at a
concentration of 15 mM (14). The
concentration of glucosamine that induces cell injury, and the
mechanism by which ER stress leads to cell injury under conditions
of high glucosamine is, therefore, unclear.
Persistent and serious ER stress causes apoptosis,
which results in a series of pathophysiological changes, including
increased phosphorylation of protein kinase-like ER kinase (PERK),
a trans-ER-membrane factor, which results in increased levels of
C/EBP homologous protein (CHOP) (15). CHOP can induce transcriptional
activation of endoplasmic reticulum oxidoreductase-1 (Ero1α) and
Ero1α can activate the inositol triphosphate receptor (IP3R). IP3R
can subsequently stimulate excess Ca2+ transport from
the ER to the mitochondria, triggering cell death (16). CHOP also can inhibit Bcl-2
transcription directly to initiate apoptosis (16,17).
Diabetes and AS are characterized by low-grade, chronic
inflammation (11,18). Previous studies have suggested that
c-Jun N-terminal kinase (JNK) linked ER stress and apoptosis, and
was also the link between ER stress and inflammation (19) and insulin resistance (20). Thus, the use of anti-inflammatory
and anti-apoptotic agents against inflammation, apoptosis and ER
stress may contribute to the prevention of AS in patients with
diabetes.
Quercetin is a widely distributed plant flavonoid,
which has been reported to possess biological activities against
cardiovascular disease and associated risk factors (21). Epidemiological (22) and clinical studies (23) suggest that there is an inverse
correlation between flavonoid supplementation and cardiovascular
risk, and numerous clinical and animal studies have reported the
anti-inflammatory and anti-oxidative functions of quercetin
(23–29). Furthermore, quercetin alleviates AS
development in rabbits (30) and
mice (31). Quercetin has also
been demonstrated to control blood glucose levels, and improve
glucose uptake and insulin sensitivity in vitro (32,33).
Suganya et al (34) also
demonstrated that quercetin prevents tunicamycin-induced ER stress
through modulation of GRP78 and CHOP levels in endothelial cells.
Chao et al (35)
demonstrated that the inhibitory effect of 300 nM quercetin
sulfate/glucuronide (the metabolite of quercetin in blood) on
apoptosis and JNK activity under conditions of high glucose was
similar to that of 100 µM ascorbic acid, an antioxidant commonly
used to improve vascular function. However, limited research has
been performed to investigate the effect of quercetin on ER stress
under diabetic conditions, and the relevant mechanisms.
As vascular endothelial cell injury is the initial
step of AS (36), the present
study hypothesized that high levels of glucosamine, a HBP
metabolite, would mimic the early stages of vascular endothelial
cell injury in diabetes. The present study aimed to investigate the
protective effect of quercetin on inflammation and apoptosis in
HUVECs treated with high-dose glucosamine, and to determine whether
this protective effect was associated with inhibition of ER
stress.
Materials and methods
Reagents and antibodies
High-glucose Dulbecco's modified Eagle's medium
(DMEM) and fetal bovine serum (FBS) were obtained from Gibco;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Trypsin,
dimethyl sulfoxide (DMSO) and MTT were purchased from MAC Gene
Technology (Beijing, China; http://macgene.com/cart/). Quercetin, (≥95%, HPLC),
N-acetyl-D-glucosamine and tunicamycin (from Streptomyces spp.)
were purchased from Sigma-Aldrich, Merck Millipore (Darmstadt,
Germany). Endothelial cell growth supplement (ECGS; cat. no. 1052)
was obtained from Sciencell Research Laboratories (Carlsbad, CA,
USA). Human soluble intercellular adhesion molecule-1 (ICAM-1)/CD54
Quantikine ELISA kit (cat. no. DCD540), human soluble vascular cell
adhesion molecule-1 (VCAM-1)/CD106 Quantikine ELISA kit (cat. no.
DVC00) and endothelin-1 (ET-1) Quantikine ELISA kit (cat. no.
DET100) were obtained from R&D Systems, Inc. (Minneapolis, MN,
USA). One-step terminal-deoxynucleotidyl transferase dUTP nick end
labeling (TUNEL) apoptosis in situ detection kit was
purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China).
Polyclonal antibody to GRP78 (cat. no. 3183) and monoclonal
antibodies to CHOP (cat. no. 2895), PERK (cat. no. 5683) and
β-actin (cat. no. 4970) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Phosphorylated (p)-PERK (cat.
no. sc-32577), JNK D-2 (cat. no. sc-7345), p-JNK G-7 (cat. no.
sc-6254), VCAM-1 H276 (cat. no. sc-8304) and caspase-3 (cat. no.
sc-7148) antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Goat anti-mouse immunoglobulin G (IgG;
H+L)-horseradish peroxidase (HRP; cat. no. ZB-2305), goat
anti-rabbit IgG (H+L)-HRP (cat. no. ZB-2301) and goat anti-rat IgG
(H+L)-HRP (cat. no. ZB-2307) antibody conjugates were obtained from
OriGene Technologies, Inc. (Beijing, China).
Cell culture and treatments
HUVECs (no. CRL-1730) were obtained from the
American Type Culture Collection (Manassas, VA, USA). Cells were
cultured in high-glucose DMEM supplemented with 10% FBS and 0.05
mg/ml ECGS at 37°C in a humidified, 5% CO2 atmosphere.
Cells were subcultured in culture flasks (Corning Incorporated,
Corning, NY, USA) and passaged every 3 days. The cells were used at
their fourth passage. All experiments were performed at the
logarithmic phase of cell growth, and it took 24 h to grow to
logarithmic phase of cell. At 70% confluence, the cells were
cultured for 12 h in serum-free medium. The cells incubated in
normal medium (high-glucose DMEM with 10% FBS) for 24 h were used
as the vehicle group and other HUVECs in high-glucose DMEM and 10%
FBS were subsequently divided into seven groups: ‘HG’ group cells
were cultured in 15 mM glucosamine; 5, 10, 20 and 50 µM quercetin
group cells were cultured in 15 mM glucosamine and various doses of
quercetin (5, 10, 20 and 50 µM, respectively); mannitol group cells
were cultured in 15 mM mannitol (subsequently referred to as
osmolarity control); and another HUVECs were cultured in
high-glucose DMEM and 10% FBS with 50 µM quercetin (subsequently
referred to as quercetin control). The aforementioned cells were
cultured for a further 24 h. In another group of experiments, the
cells were treated with 5 µg/ml tunicamycin for 4 h as positive
control. The concentration of quercetin used in previous in
vitro studies into cardiovascular diseases ranged from 10–80 µM
(30,35,37),
while the highest concentration of quercetin observed in murine
plasma was 27.6 µM (31). However,
the highest concentration of quercetin observed in human plasma was
4.1 µM, which was observed 10 h after ingestion of 1,095 mg
quercetin (38). Due to the
limited availability of information regarding quercetin cell
cytotoxicity, concentrations of 0, 5, 10, 20 and 50 µM quercetin
were utilized in the present study.
MTT assay
Cell viability was detected by MTT assay as
previously described (14,37,39).
Following treatment in 96-well plates for 24 h, 100 µl MTT (1
mg/ml) solutions was added to each well, then cells were incubated
for 4 h at 37°C. The MTT solution was then discarded, and 100 µl
DMSO was added to each well. Absorbance at 490 nm was then read
using a microplate reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). Values were normalized to those of cells in the vehicle
group.
TUNEL assays
DNA fragmentation was observed by TUNEL as
previously described (14). Cells
were treated on cover slips in 6-well plates at a density of 3×105
cells/ml. Following intervention, cells were fixed in 4%
paraformaldehyde for 30 min at room temperature, then washed 3
times in phosphate-buffered saline (PBS). Triton X-100 (1%) was
added for 3–5 min to promote permeability, then cells were washed
again in PBS. Cells were blocked with 3% H2O2
for 10 min at room temperature and incubated with 50 µl terminal
deoxynucleotidyl transferase enzyme reaction solution (45 µl
equilibration buffer, 1 µl tetramethyl rhodamine
isothiocyanate-5-dUTP and 4 µl terminal deoxynucleotidyl
transferase enzyme) for 60 min at 37°C in the dark. Fluorescence
intensity was measured using an Eclipse TE2000-S fluorescence
microscope (Nikon Corporation, Tokyo, Japan) using wavelengths of
543 nm (excitation) and 571 nm (detection). Red fluorescence
indicated the presence of apoptotic cells. Apoptotic cells were
counted in three random high-power fields (HPF) of three different
slides.
ELISA
Following 24-h treatment in 24-well plates,
concentrations of VCAM-1, ICAM-1, and ET-1 were determined by ELISA
according to manufacturer's instructions (40,41).
Supernatant was collected and centrifuged at 1,000 × g for 10 min.
The lyophilised quantikine standard was reconstituted in distilled
water and serially diluted 1:2 in kit standard diluent to produce
standard curve samples. Prior to the experiment, the samples
required a 5-fold dilution (a suggested 5-fold dilution is 20 µl of
samples and 80 µl of Calbrator Diluent). A total of 100 µl human
ICAM-1 or VCAM-1 conjugate was added to each well. Next, 100 µl
standard, control (recombinant human sICAM-1 and VCAM-1 provided
with the kits as positive controls) and experimental samples were
added to the designated wells in a 96-well polystyrene microplate
(provided with the kit). The plate was covered with the adhesive
strip provided and incubated for 1.5 h at room temperature on a
horizontal orbital microplate shaker (0.12′' orbit). Following
this, the wells were washed with 400 µl wash buffer three times.
After the final wash, for ET-1, 150 µl of assay diluent was added
to each well. A total of 75 µl standard, control (Synthetic
Endothelin-1 provided with the kits as positive controls) and
experimental samples were subsequently added to each well. The
plate was covered with an adhesive strip and incubated for 1 h at
room temperature on a horizontal orbital microplate shaker. The
plates were washed as before four times. The plates were the
incubated for 3 h at room temperature with 200 µl HRP-conjugated
secondary antibody. The wells were washed, as before. Substrate
solution (200 µl for ICAM-1 and ET-1, 100 µl for VCAM-1, chromogen
solution A and chromogen solution B were mixed together in equal
volumes) was then added to each well, and the plates were protected
from light and incubated for 30 min at room temperature. Finally,
50 µl stop solution was added to the wells and the colored products
were measured at 450 nm within 30 min, with the wavelength
correction set at 570 nm, on a multi-detection microplate reader
(Bio-Rad Laboratories, Inc.). The standard curve, experimental and
control samples were assayed in duplicate.
Western blotting
Cells from each group were treated for 24 h in 10 mm
culture dishes, and then cells (1×107) were washed three times in
ice-cold PBS, then lysed for 30 min in lysis buffer [20 mM Tris-HCl
(pH 7.5), 150 mM NaCl, 1 mM Na2 EDTA, 1 mM EGTA, 1 mM
Na3VO4, 11 mM β-mercaptoethanol, 0.1% Triton
X-100, 2.5 mM Na4P2O7, 1 µg/ml
leupeptin, 1 µg/ml aprotinin, and 1 µg/ml pepstatin] with 1 mM
phenylmethylsulfonyl fluoride (Sigma-Aldrich) (14,39).
Lysates were centrifuged at 12,000 × g for 5 min at 4°C, and the
concentration of total protein in the supernatant was quantified by
bicinchoninic acid protein assay. Protein samples (20 µg) were
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis on 8, 10 or 12% gels, then transferred onto 0.45 µm
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked with 5% (v/v) nonfat dried milk in
Tris-buffered saline containing 0.05% Tween-20 (TBS-T) at room
temperature for 1 h, then incubated with the primary antibody at
4°C overnight. GRP78, CHOP and PERK antibodies were used at a
dilution of 1:1,000, β-actin antibody at 1:2,000, and VCAM-1,
p-PERK, JNK, p-JNK and caspase-3 antibodies at 1:200. Membranes
were then washed 3 times in TBS-T, and incubated in HRP-conjugated
secondary antibodies (1:4,000) for 1 h at 37°C. Protein complexes
were detected using enhanced chemiluminescence western blotting
detection reagents (MAC Gene Technology Ltd.). Digital images of
the blots were analyzed using Image Pro Plus 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
All experimental data are presented as the mean ±
standard deviation of at least three independent experiments.
One-way analysis of variance was performed using SPSS 13.0 for
Windows (SPSS, Inc., Chicago, IL, USA) to compare variances. If
variances were equal, Bonferroni multiple comparison tests were
performed using with SPSS 13.0; otherwise, Tamhane's T2 test was
performed by SPSS 13.0. P<0.05 was considered to indicate a
statistically significant difference.
Results
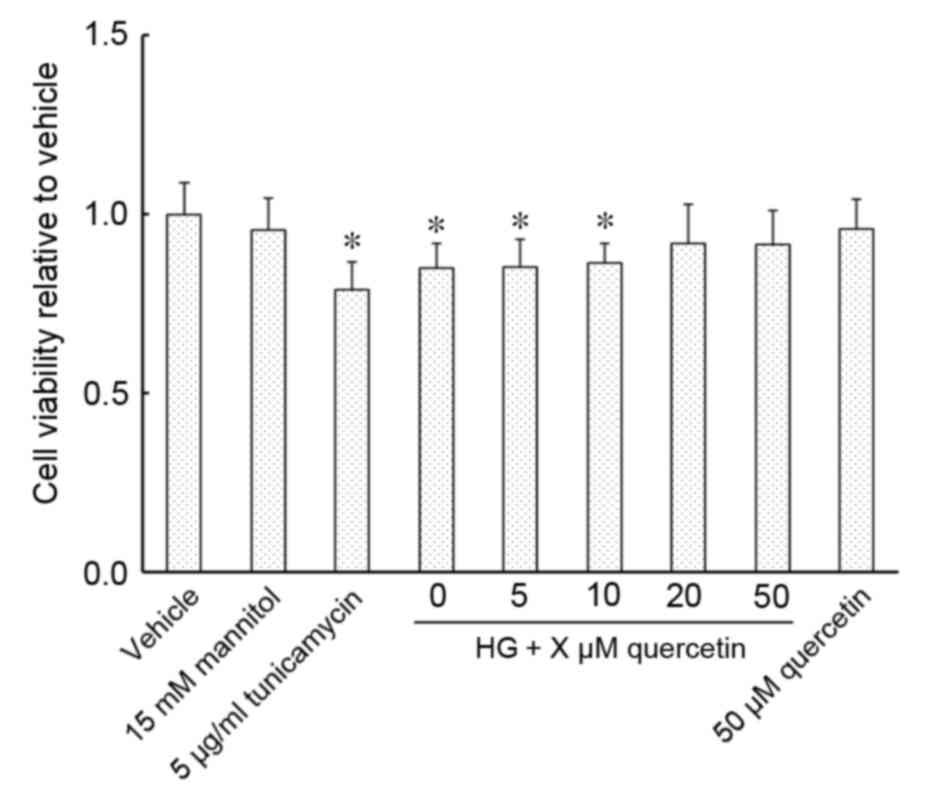
Effect of quercetin on the viability
of HUVECs treated with high-dose glucosamine
Exposure of HUVECs to an ER stress inducer,
tunicamycin, and 15 mM glucosamine resulted in a significant
decrease in cell viability compared with the vehicle control
(P<0.001 for 5 µg/ml tunicamycin group and P=0.002 for HG group;
Fig. 1). Cell viability was
restored in cells cultured in 15 mM glucosamine following treatment
with 20 or 50 µM quercetin, with no significant changes detected
compared with vehicle (Fig. 1).
Cell viability compared with the vehicle control was not
significantly altered in cells treated with 50 µM quercetin,
whether cultured with or without 15 mM glucosamine (Fig. 1).
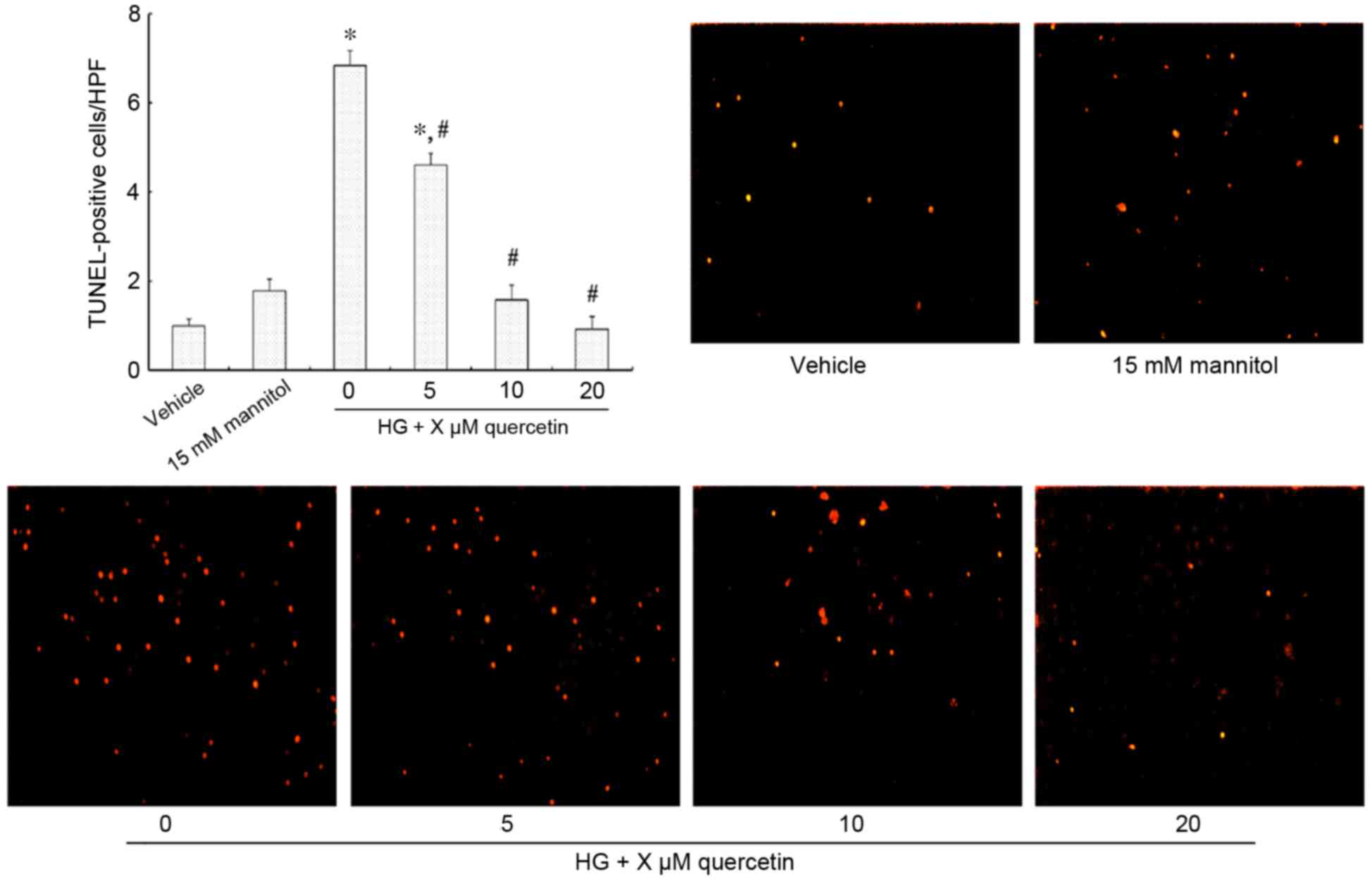
Effect of quercetin on the apoptosis
of HUVECs treated with high-dose glucosamine
Fig. 2 demonstrates
TUNEL-positive HUVECs (strong red fluorescence) that are undergoing
apoptosis. There was no significant difference observed between the
vehicle and mannitol-treated groups (Fig. 2). Treatment with 15 mM glucosamine
resulted in increased apoptosis compared with the vehicle group
(P<0.001; Fig. 2). However, a
dose-dependent effect was observed in cells also treated with
quercetin, with no significant difference in the number of
TUNEL-positive cells when treated with 10 and 20 µM quercetin
compared with the vehicle control (Fig. 2).
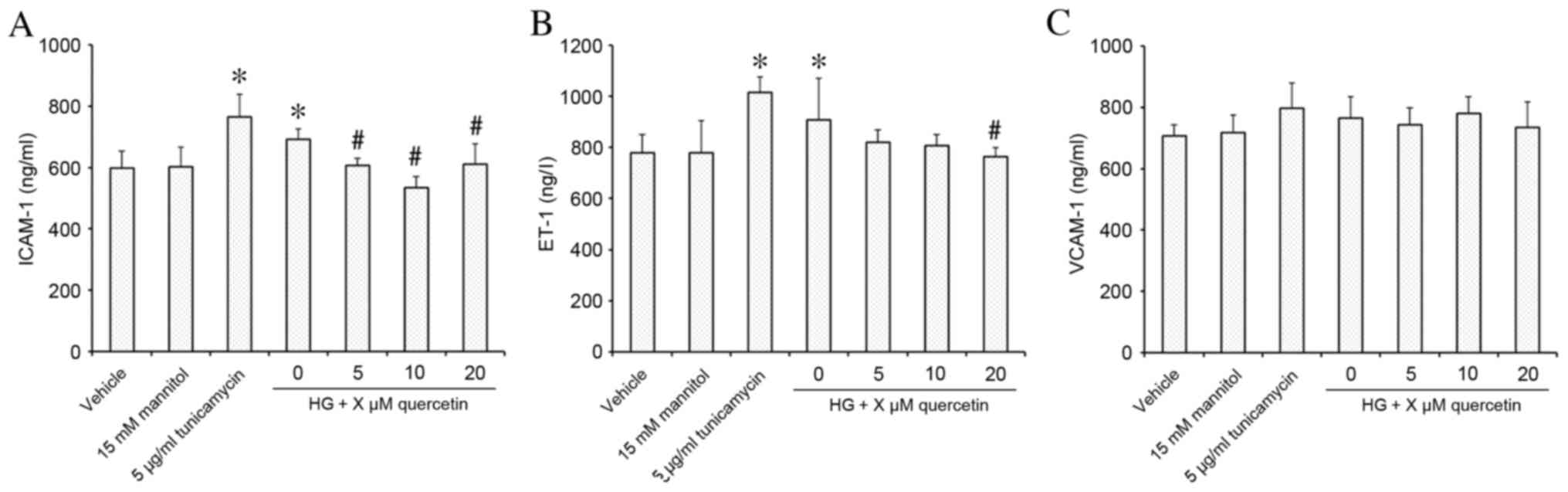
Effect of quercetin on expression of
markers of endothelial dysfunction in HUVECs treated with high-dose
glucosamine
Stimulation of HUVECs with 15 mM glucosamine
resulted in significantly increased expression of ICAM-1 and ET-1
compared with the vehicle control group (P=0.009 for ICAM-1 and
P=0.049 for ET-1, respectively; Fig.
3A and B, respectively). Following quercetin treatment, ICAM-1
expression was decreased significantly at all concentrations of
quercetin treatment compared with the HG group (P=0.016, P<0.001
and P=0.022 for 5, 10, 20 µM quercetin, respectively; Fig. 3A), and demonstrated no significant
difference compared with the vehicle control. ET-1 expression was
significantly decreased compared with the HG group at 20 µM
(P=0.030; Fig. 3B), and
demonstrated no significant difference compared with the vehicle
control at all concentrations of quercetin treatment (Fig. 3B). No effect on VCAM-1 expression
was observed in response to treatment with 15 mM glucosamine or
quercetin compared with the vehicle control (Fig. 3C).
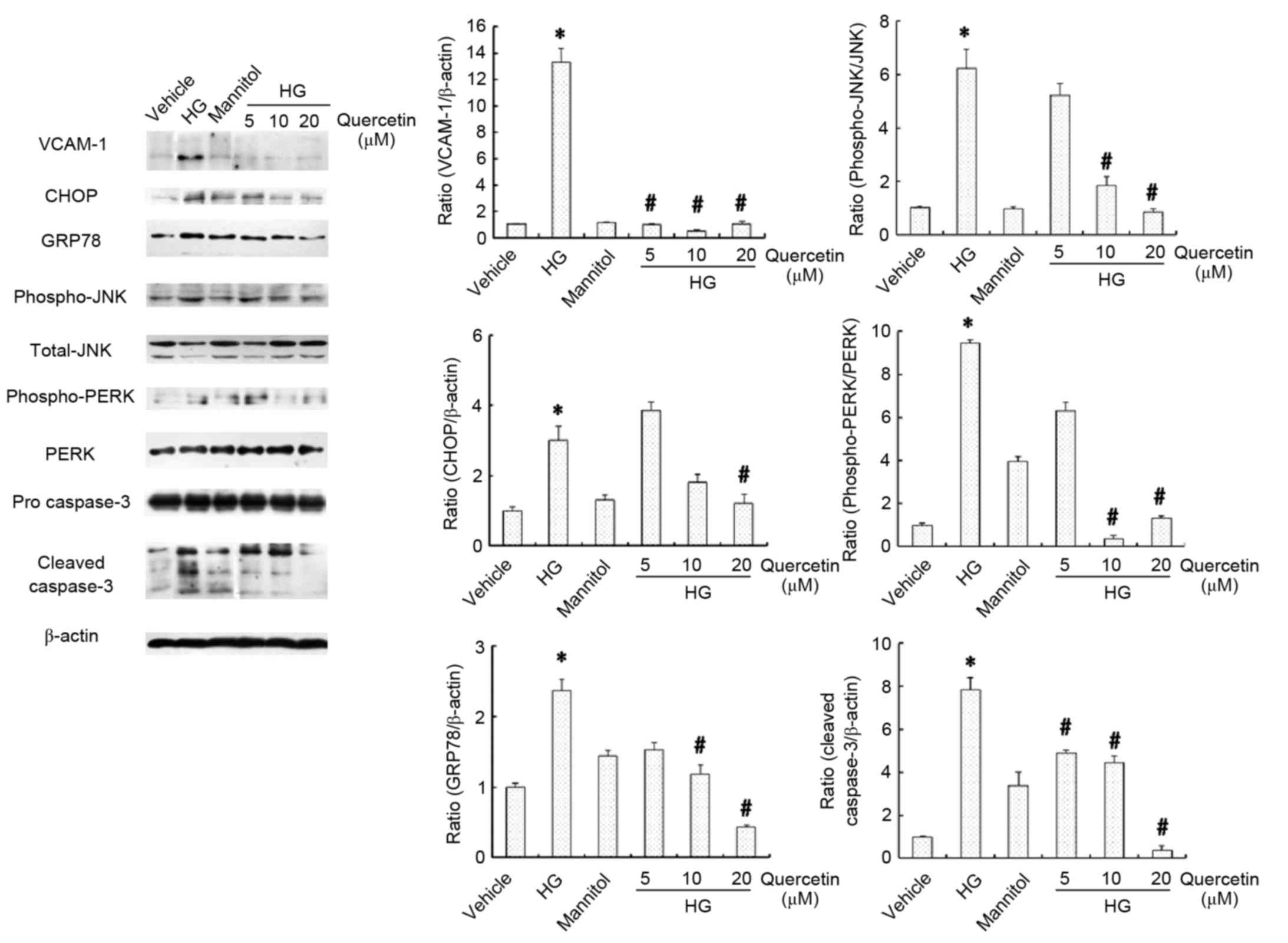
Effects of quercetin on the expression
of inflammation, apoptosis and ER stress markers in HUVECs treated
with high-dose glucosamine
Inflammation, apoptosis and ER stress in HUVECs was
evaluated by assessment of the protein expression levels of VCAM-1,
CHOP, GRP78, JNK, PERK and caspase-3 (Fig. 4). No significant differences were
observed between the vehicle and mannitol-treated groups with
respect to these parameters (Fig.
4). Protein expression levels of VCAM-1, CHOP and GRP78 were
increased in cells cultured in 15 mM glucosamine compared with the
vehicle control group (P<0.05; Fig.
4). Furthermore, the ratios of p-JNK/JNK, p-PERK/PERK and
cleaved caspase-3 at 20, 17 and 11 kDa/pro-caspase-3 were also
significantly higher in cells cultured in 15 mM glucosamine
compared with the vehicle control group (P<0.05; Fig. 4). Treatment with quercetin
(particularly 10 and 20 µM) significantly attenuated this effect
compared with cells cultured in 15 mM glucosamine (P<0.05
following treatment with 20 µM quercetin; Fig. 4), suggesting that quercetin may
protect against ER stress, thus suppressing glucosamine-induced
inflammation and apoptosis in HUVECs.
Discussion
To the best of our knowledge, the present study is
the first to report that quercetin ameliorates glucosamine-induced
apoptosis and inflammation of HUVECs in vitro. Furthermore,
these effects may be partially attributed to the alleviation of ER
stress pathways in HUVECs.
Quercetin absorption depends on its form, and its
solubility in the vehicle used for administration (42). Quercetin glycoside is the
predominant form of quercetin, and the majority of the quercetin
metabolites in plasma are sulfate/glucuronide conjugates of
quercetin (43). In a study
investigating the fate of quercetin, 14C-quercetin was
administered orally (100 mg, 330 µM) to healthy volunteers
(44). The study observed that the
oral absorption ranged from 36.4 to 53%, and the biological
half-life was 20–72 h. The maximum concentration of quercetin in
the plasma of mice has been demonstrated to be 27.6 µM (31), however, in certain population
studies the highest concentration of quercetin in plasma was <5
µM (23,38). Further studies are, therefore,
required to determine the pharmacokinetics of quercetin.
The effect of glucosamine on diabetic AS and
osteoarthritis management remains controversial. At a concentration
of 5 mM in vitro, glucosamine has been demonstrated to
induce insulin resistance (10)
and promote pro-apoptotic and pro-inflammatory factors in HUVECs at
a concentration of 7.5 mM (45).
However, another study showed that glucosamine, up to 20 mM, fully
protected the chondrocytes from IL-1-induced expression of
inflammatory cytokines (46).
In vivo studies have demonstrated glucosamine to be both
pro- (11) and
anti-atherosclerotic (12). In the
present study, glucosamine induced apoptosis in HUVECs at a
concentration of 15 mM, while no significant difference in
apoptosis was observed with 15 mM mannitol, an osmolarity control,
compared with vehicle. As demonstrated in a previous study,
quercetin could effectively inhibit the apoptosis of HUVECs induced
by tunicamycin (34). The present
study demonstrated that in HUVECs cultured in 15 mM glucosamine, 24
h treatment with quercetin significantly reduces apoptosis compared
with untreated cells. These results demonstrate that quercetin can
prevent glucosamine-induced apoptosis, and may represent a novel
approach to inhibition of vascular endothelial cell apoptosis in
diabetic AS.
Inflammation is an important in the process of AS
(47,48). Hyperglycemia induces ICAM-1 and
VCAM-1 expression in HUVECs (49);
however, Azcutia et al (50) suggested that high levels of
extracellular D-glucose alone are not sufficient to promote
vascular inflammation. The present study demonstrated that
glucosamine significantly elevates ICAM-1 and VCAM-1 protein
expression levels. Quercetin has previously been demonstrated to be
a powerful anti-inflammatory agent to alleviate AS in vivo
(31) and in vitro
(30,51). The present study indicated that
protein expression levels of ICAM-1 and VCAM-1 were significantly
reduced following treatment with quercetin. These data, therefore,
suggest that vascular inflammation may be partially due to the
elevated glucosamine levels present in patients with diabetes;
quercetin may represent an effective, novel therapy to resist
HUVECs glucosamine-induced inflammation.
The HBP is associated with vasodilation and the
ET-1-induced vasoconstriction response (52,53).
HBP activated by excess glucosamine causes endothelial nitric oxide
synthase uncoupling to decrease nitric oxide production in isolated
mouse aortas, this effect impaired endothelium-dependent
relaxations finally (52).
Furthermore, ET-1 increases glycosylation with
β-N-acetylglucosamine in vascular smooth muscle cells, which
increases vascular contractile responses (53). Both decreased vasodilation, and
increased vasoconstriction associated with the HBP, could result in
vascular endothelial dysfunction. ET-1 induced glucose uptake dose
dependently in neonatal rat cardiomyocytes when cells were cultured
in normal medium. However, when cells were cultured in 15 mM
glucosamine based on 5 mM glucose, the increased glucose-uptake
effect of ET-1 on glucose-uptake was completely abolished (54). The present study demonstrated a
significant increase in ET-1 protein expression in cells cultured
in 15 mM glucosamine treatment, compared with vehicle. However,
treatment of glucosamine-stimulated cells with quercetin, resulted
in a significant decrease in ET-1 expression. This suggests that
quercetin intervention may improve vascular endothelial
dysfunction.
ER stress results in dissociation of GRP78, an ER
chaperone protein, from trans-ER-membrane factors, including
activating transcriptional factor-6, PERK and inositol requiring
enzyme-1. This results in their activation, and the subsequent
activation of CHOP, JNK or caspase cascades, causing apoptosis and
inflammation (6). Glucosamine has
previously been demonstrated to significantly increase GRP78 levels
in HUVECs (45). Qiu et al
(55) demonstrated that
glucosamine-induced ER stress was associated with increased
phosphorylation of PERK. JNK is the down-stream effector of ER
stress, responsible for induction of apoptosis (56); it also mediates the process between
ER stress and inflammation (19).
Suganya et al (34)
demonstrated that pre-treatment of tunicamycin-stimulated HUVECs
with 25 and 50 µM quercetin could modulate GRP78 and CHOP levels,
reduce expression of B cell lymphoma 2 apoptosis regulator (Bcl-2),
increase expression of the pro-apoptotic regulator Bcl-2 associated
protein X apoptosis regulator (Bax) and prevent apoptosis,
therefore demonstrating the potential of quercetin to combat ER
stress. In the present study, the effects of quercetin that
contribute to reduced vascular endothelial cell injury were
hypothesized to have a common upstream target: ER stress. This was
confirmed by assessment of GRP78 and p-PERK protein expression
levels in HUVECs; elevated expression of GRP78 and an increased
p-PERK/PERK ratio were induced by supplementation with 15 mM
glucosamine. Significant increases in CHOP, p-JNK and cleaved
caspase-3 expression levels were also observed in cells cultured in
15 mM glucosamine. Treatment with quercetin reduced the expression
of GRP78, p-PERK, CHOP, cleaved caspase-3 and p-JNK in
glucosamine-supplemented cells, thus restoring ER homeostasis.
Tunicamycin is a typical ER stress inducer by
interfering with N-linked protein glycosylation in ER (57). Whether tunicamycin can abolish the
beneficial effects of quercetin on glucosamine-induced HUVECs
damage remains to be investigate. It can further confirm that the
ER stress pathway is involved in the beneficial effects of
quercetin on glucosamine-induced HUVECs damage. Although animal
studies have demonstrated a maximum concentration of 27.6 µM
quercetin in mouse plasma (31),
human clinical studies have observed a maximum plasma concentration
of 5 µM (23,38). In the present study, 20 µM
quercetin was identified as the concentration at which positive
effects were observable. As this is likely to be a difficult
concentration to achieve in the human diet, further experiments are
required to determine a safe and effective quercetin dose in
vivo. In addition, further experiments using animal models of
diabetic AS and human clinical studies will be essential to further
understand the mechanism of action.
In conclusion, the present study suggests that
quercetin suppresses the glucosamine-induced inflammatory response
and apoptosis in HUVECs, in vitro, and that this effect may
be partially due to the inhibition of ER stress. The ER-CHOP and
ER-JNK pathways may be involved in the protective effects of
quercetin against glucosamine-induced HUVEC injury, and PERK may be
a critical factor in the molecular mechanism involved in its
protective effects. These results provide further evidence that
quercetin may be a potential therapeutic agent for diabetic AS, and
ER stress may be one of the possible targets.
Acknowledgements
The present study was supported by a research grant
from the National Natural Science Foundation of China (grant no.
81172652).
Glossary
Abbreviations
Abbreviations:
|
AS
|
atherosclerosis
|
|
CHD
|
coronary heart disease
|
|
CHOP
|
C/EBP homologous protein
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
DMSO
|
dimethyl sulfoxide
|
|
ET-1
|
endothelin-1
|
|
FBS
|
fetal bovine serum
|
|
GRP78
|
glucose regulated protein 78
|
|
HBP
|
hexosamine biosynthesis pathway
|
|
HUVEC
|
human umbilical vein endothelial
cell
|
|
ICAM-1
|
intercellular adhesion molecule-1
|
|
JNK
|
c-Jun N-terminal kinase
|
|
PERK
|
protein kinase-like ER kinase
|
|
TUNEL
|
terminal-deoxynucleotidyl transferase
mediated dUTP nick end labeling
|
|
VCAM-1
|
vascular cell adhesion molecule-1
|
References
|
1
|
Juutilainen A, Lehto S, Rönnemaa T,
Pyörälä K and Laakso M: Type 2 diabetes as a ‘coronary heart
disease equivalent’: An 18-year prospective population-based study
in Finnish subjects. Diabetes Care. 28:2901–2907. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haffner SM, Lehto S, Rönnemaa T, Pyörälä K
and Laakso M: Mortality from coronary heart disease in subjects
with type 2 diabetes and in nondiabetic subjects with and without
prior myocardial infarction. N Engl J Med. 339:229–234. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Han S, Liang CP, DeVries-Seimon T,
Ranalletta M, Welch CL, Collins-Fletcher K, Accili D, Tabas I and
Tall AR: Macrophage insulin receptor deficiency increases ER
stressinduced apoptosis and necrotic core formation in advanced
atherosclerotic lesions. Cell Metab. 3:257–266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Werstuck GH, Khan MI, Femia G, Kim AJ,
Tedesco V, Trigatti B and Shi Y: Glucosamine-induced endoplasmic
reticulum dysfunction is associated with accelerated
atherosclerosis in a hyperglycemic mouse model. Diabetes.
55:93–101. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marciniak SJ and Ron D: Endoplasmic
reticulum stress signaling in disease. Physiol Rev. 86:1133–1149.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hotamisligil GS: Endoplasmic reticulum
stress and atherosclerosis. Nat Med. 16:396–399. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khan MI, Pichna BA, Shi Y, Bowes AJ and
Werstuck GH: Evidence supporting a role for endoplasmic reticulum
stress in the development of atherosclerosis in a hyperglycaemic
mouse model. Antioxid Redox Signal. 11:2289–2298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou J, Lhoták S, Hilditch BA and Austin
RC: Activation of the unfolded protein response occurs at all
stages of atherosclerotic lesion development in apolipoprotein
E-deficient mice. Circulation. 111:1814–1821. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim AJ, Shi Y, Austin RC and Werstuck GH:
Valproate protects cells from ER stress-induced lipid accumulation
and apoptosis by inhibiting glycogen synthase kinase-3. J Cell Sci.
118:89–99. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen H, Ing BL, Robinson KA, Feagin AC,
Buse MG and Quon MJ: Effects of overexpression of glutamine:
Fructose-6-phosphate amidotransferase (GFAT) and glucosamine
treatment on translocation of GLUT4 in rat adipose cells. Mol Cell
Endocrinol. 135:67–77. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Defronzo RA: Insulin resistance,
lipotoxicity, type 2 diabetes and atherosclerosis: The missing
links. The Claude Bernard Lecture 2009. Diabetologi. 53:1270–1287.
2010. View Article : Google Scholar
|
|
12
|
Largo R, Martínez-Calatrava MJ,
Sánchez-Pernaute O, Marcos ME, Moreno-Rubio J, Aparicio C, Egido J
and Herrero-Beaumont G: Effect of a high dose of glucosamine on
systemic and tissue inflammation in an experimental model of
atherosclerosis aggravated by chronic arthritis. Am J Physiol Heart
Circ Physiol. 297:H268–H276. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rajapakse AG, Ming XF, Carvas JM and Yang
Z: O-linked beta-N-acetylglucosamine during hyperglycemia exerts
both anti-inflammatory and pro-oxidative properties in the
endothelial system. Oxid Med Cell Longev. 2:172–175. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bao L, Cai X, Zhang Z and Li Y: Grape seed
procyanidin B2 ameliorates mitochondrial dysfunction and inhibits
apoptosis via the AMP-activated protein kinase-silent mating type
information regulation 2 homologue 1-PPARγ co-activator-1α axis in
rat mesangial cells under high-dose glucosamine. Br J Nutr.
113:35–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sano R and Reed JC: ER stress-induced cell
death mechanisms. Biochim Biophys Acta. 1833:3460–3470. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bahar E, Kim H and Yoon H: ER
stress-mediated signaling: Action potential and Ca(2+) as key
players. Int J Mol Sci. 17:E15582016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garg R, Tripathy D and Dandona P: Insulin
resistance as a proinflammatory state: Mechanisms, mediators, and
therapeutic interventions. Curr Drug Targets. 4:487–492. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang K and Kaufman RJ: From
endoplasmic-reticulum stress to the inflammatory response. Nature.
454:455–462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ozcan U, Yilmaz E, Ozcan L, Furuhashi M,
Vaillancourt E, Smith RO, Görgün CZ and Hotamisligil GS: Chemical
chaperones reduce ER stress and restore glucose homeostasis in a
mouse model of type 2 diabetes. Science. 313:1137–1140. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Russo M, Spagnuolo C, Tedesco I, Bilotto S
and Russo GL: The flavonoid quercetin in disease prevention and
therapy: Facts and fancies. Biochem Pharmacol. 83:6–15. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hertog MG, Kromhout D, Aravanis C,
Blackburn H, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A,
Nedeljkovic S, et al: Flavonoid intake and long-term risk of
coronary heart disease and cancer in the seven countries study.
Arch Intern Med. 155:381–386. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Egert S, Bosy-Westphal A, Seiberl J,
Kürbitz C, Settler U, Plachta-Danielzik S, Wagner AE, Frank J,
Schrezenmeir J, Rimbach G, et al: Quercetin reduces systolic blood
pressure and plasma oxidised low-density lipoprotein concentrations
in overweight subjects with a high-cardiovascular disease risk
phenotype: A double-blinded, placebo-controlled cross-over study.
Br J Nutr. 102:1065–1074. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Egert S, Boesch-Saadatmandi C, Wolffram S,
Rimbach G and Müller MJ: Serum lipid and blood pressure responses
to quercetin vary in overweight patients by apolipoprotein E
genotype. J Nutr. 140:278–284. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duarte J, Pérez-Palencia R, Vargas F,
Ocete MA, Pérez-Vizcaino F, Zarzuelo A and Tamargo J:
Antihypertensive effects of the flavonoid quercetin in
spontaneously hypertensive rats. Br J Pharmacol. 133:117–124. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Juźwiak S, Wójcicki J, Mokrzycki K,
Marchlewicz M, Białecka M, Wenda-Rózewicka L, Gawrońska-Szklarz B
and Droździk M: Effect of quercetin on experimental hyperlipidemia
and atherosclerosis in rabbits. Pharmacol Rep. 57:604–609.
2005.PubMed/NCBI
|
|
27
|
Motoyama K, Koyama H, Moriwaki M, Emura K,
Okuyama S, Sato E, Inoue M, Shioi A and Nishizawa Y:
Atheroprotective and plaque-stabilizing effects of enzymatically
modified isoquercitrin in atherogenic apoE-deficient mice.
Nutrition. 25:421–427. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chuang CC, Martinez K, Xie G, Kennedy A,
Bumrungpert A, Overman A, Jia W and McIntosh MK: Quercetin is
equally or more effective than resveratrol in attenuating tumor
necrosis factor-{alpha}-mediated inflammation and insulin
resistance in primary human adipocytes. Am J Clin Nutr.
92:1511–1521. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ortega MG, Saragusti AC, Cabrera JL and
Chiabrando GA: Quercetin tetraacetyl derivative inhibits
LPS-induced nitric oxide synthase (iNOS) expression in J774A.1
cells. Arch Biochem Biophys. 498:105–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lara-Guzman OJ, Tabares-Guevara JH,
Leon-Varela YM, Álvarez RM, Roldan M, Sierra JA, Londoño-Londoño JA
and Ramirez-Pineda JR: Proatherogenic macrophage activities are
targeted by the flavonoid quercetin. J Pharmacol Exp Ther.
343:296–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kleemann R, Verschuren L, Morrison M,
Zadelaar S, van Erk MJ, Wielinga PY and Kooistra T:
Anti-inflammatory, anti-proliferative and anti-atherosclerotic
effects of quercetin in human in vitro and in vivo models.
Atherosclerosis. 218:44–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li YQ, Zhou FC, Gao F, Bian JS and Shan F:
Comparative evaluation of quercetin, isoquercetin and rutin as
inhibitors of alpha-glucosidase. J Agric Food Chem. 57:11463–11468.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dai X, Ding Y, Zhang Z, Cai X, Bao L and
Li Y: Quercetin but not quercitrin ameliorates tumor necrosis
factor-alpha-induced insulin resistance in C2C12 skeletal muscle
cells. Biol Pharm Bull. 36:788–795. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Suganya N, Bhakkiyalakshmi E,
Suriyanarayanan S, Paulmurugan R and Ramkumar KM: Quercetin
ameliorates tunicamycin-induced endoplasmic reticulum stress in
endothelial cells. Cell Prolif. 47:231–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chao CL, Hou YC, Chao PD, Weng CS and Ho
FM: The antioxidant effects of quercetin metabolites on the
prevention of high glucose-induced apoptosis of human umbilical
vein endothelial cells. Br J Nutr. 101:1165–1170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao B, Zhang Y, Liu B, Nawroth P and
Dierichs R: Endothelial cells injured by oxidized low density
lipoprotein. Am J Hematol. 49:250–252. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yao S, Sang H, Song G, Yang N, Liu Q,
Zhang Y, Jiao P, Zong C and Qin S: Quercetin protects macrophages
from oxidized low-density lipoprotein-induced apoptosis by
inhibiting the endoplasmic reticulum stress-C/EBP homologous
protein pathway. Exp Biol Med (Maywood). 237:822–831. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Larson A, Witman MA, Guo Y, Ives S,
Richardson RS, Bruno RS, Jalili T and Symons JD: Acute,
quercetin-induced reductions in blood pressure in hypertensive
individuals are not secondary to lower plasma
angiotensin-converting enzyme activity or endothelin-1: Nitric
oxide. Nutr Res. 32:557–564. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ding Y, Zhang ZF, Dai XQ and Li Y:
Myricetin protects against cytokine- induced cell death in RIN-m5f
β cells. J Med Food. 15:733–740. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Siawaya JF Djoba, Roberts T, Babb C, Black
G, Golakai HJ, Stanley K, Bapela NB, Hoal E, Parida S, van Helden P
and Walzl G: An evaluation of commercial fluorescent bead-based
luminex cytokine assays. PLoS One. 3:e25352008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Matěj R, Smětáková M, Vašáková M, Nováková
J, Sterclová M, Kukal J, Olejár Tětáková M, Vašáková M, Nováková J,
Sterclová M, Kukal J and Olejár TT: PAR-2, IL-4R, TGF-β and TNF-α
in bronchoalveolar lavage distinguishes extrinsic allergic
alveolitis from sarcoidosis. Exp Ther Med. 8:533–538.
2014.PubMed/NCBI
|
|
42
|
Kelly GS: Pantothenic acid. Monograph.
Altern Med Rev. 16:263–274. 2011.PubMed/NCBI
|
|
43
|
Azuma K, Ippoushi K, Ito H, Horie H and
Terao J: Enhancing effect of lipids and emulsifiers on the
accumulation of quercetin metabolites in blood plasma after the
short-term ingestion of onion by rats. Biosci Biotechnol Biochem.
67:2548–2555. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Walle T, Walle UK and Halushka PV: Carbon
dioxide is the major metabolite of quercetin in humans. J Nutr.
131:2648–2652. 2001.PubMed/NCBI
|
|
45
|
Fiorentino TV, Procopio T, Mancuso E,
Arcidiacono GP, Andreozzi F, Arturi F, Sciacqua A, Perticone F,
Hribal ML and Sesti G: SRT1720 counteracts glucosamine-induced
endoplasmic reticulum stress and endothelial dysfunction.
Cardiovasc Res. 107:295–306. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gouze JN, Gouze E, Popp MP, Bush ML,
Dacanay EA, Kay JD, Levings PP, Patel KR, Saran JP, Watson RS and
Ghivizzani SC: Exogenous glucosamine globally protects chondrocytes
from the arthritogenic effects of IL-1beta. Arthritis Res Ther.
8:R1732006. View
Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ross R: Atherosclerosis-an inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Altannavch TS, Roubalová K, Kucera P and
Andel M: Effect of high glucose concentrations on expression of
ELAM-1, VCAM-1 and ICAM-1 in HUVEC with and without cytokine
activation. Physiol Res. 53:77–82. 2004.PubMed/NCBI
|
|
50
|
Azcutia V, Abu-Taha M, Romacho T,
Vázquez-Bella M, Matesanz N, Luscinskas FW, Rodríguez-Mañas L, Sanz
MJ, Sánchez-Ferrer CF and Peiró C: Inflammation determines the
pro-adhesive properties of high extracellular d-glucose in human
endothelial cells in vitro and rat microvessels in vivo. PLoS One.
5:e100912010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Panicker SR, Sreenivas P, Babu MS,
Karunagaran D and Kartha CC: Quercetin attenuates Monocyte
Chemoattractant Protein-1 gene expression in glucose primed aortic
endothelial cells through NF-kappaB and AP-1. Pharmacol Res.
62:328–336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wu Z, Xiong Y, Gajanayake T, Ming XF and
Yang Z: p38 Mitogen-activated protein kinase is required for
glucosamine-induced endothelial nitric oxide synthase uncoupling
and plasminogen-activator inhibitor expression. Circ J.
76:2015–2022. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lima VV, Giachini FR, Carneiro FS,
Carvalho MH, Fortes ZB, Webb RC and Tostes RC: O-GlcNAcylation
contributes to the vascular effects of ET-1 via activation of the
RhoA/Rho-kinase pathway. Cardiovasc Res. 89:614–622. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wu-Wong JR, Berg CE and Dayton BD:
Endothelin-stimulated glucose uptake: Effects of intracellular
Ca(2+), cAMP and glucosamine. Clin Sci (Lond). 103:418S–423S. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Qiu W, Su Q, Rutledge AC, Zhang J and
Adeli K: Glucosamine-induced endoplasmic reticulum stress
attenuates apolipoprotein B100 synthesis via PERK signaling. J
Lipid Res. 50:1814–1823. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Urano F, Wang X, Bertolotti A, Zhang Y,
Chung P, Harding HP and Ron D: Coupling of stress in the ER to
activation of JNK protein kinases by transmembrane protein kinase
IRE1. Science. 287:664–666. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xu C, Bailly-Maitre B and Reed JC:
Endoplasmic reticulum stress: Cell life and death decisions. J Clin
Invest. 115:2656–2664. 2005. View Article : Google Scholar : PubMed/NCBI
|