Introduction
Chemotherapeutic agents frequently produce
peripheral neuropathic pain as their primary side effect.
Vincristine, which is one of the most common chemotherapeutic
drugs, is used to treat a variety of types of cancer. However, the
development of neuropathy induced by vincristine chemotherapy not
only leads to dose reduction or discontinuation of treatment, but
also decreases the quality of life in cancer survivors (1).
Conventional analgesics, including tricyclic
antidepressants, anticonvulsants, opioids, nonsteroidal
anti-inflammatory drugs, and α2-adrenoceptor agonists have been
prescribed for the treatment of neuropathic pain (2,3).
However, these medications have also been found to exhibit a wide
spectrum of adverse effects, which limit their full clinical
exploitation. Various herbal medicines have been documented to have
therapeutic potential for the management of neuropathic pain
(4). Clinical studies have also
reported the beneficial effects of herbal medicines in cases of
neuropathic pain (5,6). Therefore, the investigation of novel
herbal medicines in the management of neuropathic pain is urgently
required.
Fucoidan is a complex sulfated polysaccharide,
derived from marine brown seaweed, which has been reported to have
anticoagulant, antithrombotic, antiviral antitumor, antioxidant and
anti-inflammatory effects (7,8). It
is readily available from several marine algae species and is
considered as a functional food, which may exert systemic effects
following oral administration. Our previous investigations
demonstrated that fucoidan attenuates allodynia and hyperalgesia in
spinal nerve ligation-induced neuropathic pain (9). However, it is unclear whether it
would relieve chemotherapy-induced neuropathic pain. In the present
study, it was observed that fucoidan exerted an analgesic effect in
an animal model of vincristine-induced neuropathic pain. It was
also found that the GABAB receptor is possibly involved in the
antinociceptive effects of fucoidan on neuropathic pain.
Materials and methods
Animals
Male SPF Sprague-Dawley rats (200–250 g) were
obtained from the Experimental Animal Center of Guangdong Medical
University (Guangdong, China). All animals were maintained on a 12
h light/dark cycle at a room temperature of 22±1°C with food and
water available ad libitum. All experimental protocols described in
the present study were approved by the Animal Care and Use
Committee at Guangdong Medical University and conformed to the NIH
guidelines (10).
Induction of neuropathic pain by
vincristine
Peripheral neuropathic pain was induced in the rats
via the administration of 50 µg/kg vincristine sulfate
intraperitoneally (i.p.) for 10 consecutive days, as described by
Siau and Bennett (11).
Intrathecal implantation
Animals were anesthetized with 60 mg/kg,
intraperitoneal injection (i.p.) of sodium pentobarbital. A
polyethylene (PE)-10 tube (BD Biosciences, San Jose, CA, USA) was
implanted into the subarachnoid space of the lumbar enlargement for
intrathecal drug administration. The catheter placement was
verified by observing transient hindpaw paralysis induced by
intrathecal injection of 2% lidocaine (10 µl). Only those rats
showing complete paralysis of both hind limbs and the tail
following the administration of lidocaine were used for the
subsequent experiments. At the end of each experiment, the position
of the PE tubing in the intrathecal space at the lumbar enlargement
was visually verified by exposing the lumbar spinal cord.
Drug administration
Vincristine sulfate (Hisun Pharmaceutical Co., Ltd.,
Zhejiang, China) was dissolved in normal saline (0.9% NaCl).
Fucoidan from Fucus vesiculosus (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) was dissolved in normal saline (0.9% NaCl).
Different doses of fucoidan (50, 100 and 200 mg/kg) were injected
i.p. The day prior to the first administration of vincristine was
considered to be day 0. The investigation was divided into three
experiments. In the single treatment experiment, fucoidan (50, 100
or 200 mg/kg) or normal saline was administered to the
vincristine-treated rats once on day 14 (n=6 rats per treatment
group). Behavioral assessments were performed on day 14 immediately
prior to administration of the drug, and at 2, 12 h, 1 day, 3 days
and 7 days post-administration.
In the repetitive treatment experiment, the animals
were divided into six groups for administration: Rats were
administered with normal saline for 14 consecutive days as a
control group; vincristine-treated rats were administered with
fucoidan (50, 100 or 200 mg/kg i.p.) or normal saline once daily
for 14 consecutive days; pregabalin (Sigma-Aldrich; Merck
Millipore) was dissolved in normal saline and 10 mg/kg was injected
(i.p.) for 14 consecutive days in the vincristine-treated rats as a
positive control (n=6 rats per treatment group). Behavioral
assessments were performed on days 0, 1, 3, 7, 14 and 21.
To evaluate the involvement of GABAB receptor in the
analgesic effect of fucoidan, a third experiment was performed.
Based on the results obtained in the repetitive treatment
experiment, a 200 mg/kg/day dose of fucoidan was selected for
further investigations. The vincristine-treated rats were
administered with 200 mg/kg fucoidan or normal saline once daily
for 14 consecutive days. Saclofen (Sigma-Aldrich; Merck Millipore)
was diluted to a concentration of 1 µg/µl in saline for intrathecal
injection. Between days 15 and 21, 10 µl of saclofen or saline were
administered once daily for 7 consecutive days. A total of 24 rats
were equally randomized into four groups: i)
Vincristine-saline-saline treatment; ii)
vincristine-fucoidan-saline treatment; iii)
vincristine-saline-saclofen treatment; (4) vincristine-fucoidan-saclofen
treatment. Behavioral assessments were performed on days 14 and
21.
Mechanical paw withdrawal
assessment
Mechanical allodynia was assessed using von Frey
filaments (Stoelting, Kiel, WI, USA) by experimenters who were
blinded to the group assignment. The ipsilateral hind paw was
pressed with one of a series of von Frey filaments with gradually
increasing rigidity (2, 4, 6, 8, 10, 15 and 20 g), each of which
was applied to the plantar surface for 5–6 sec. A positive paw
withdrawal response was recorded if the animal briskly lifted the
hindpaw. The mechanical withdrawal threshold was determined using
the up-down method (12).
Cold allodynia assessment
Cold allodynia was assessed by placing 100 µl of
acetone on the planter surface of the left hind paw of the rat. A
cold chemical sensitive reaction with respect to either paw
licking, shaking or rubbing the left hind paw was observed and
recorded as the paw withdrawal threshold. The cut-off duration of
20 sec was maintained.
Western blot analysis
Following the behavioral assessments on day 21, the
rats were anesthetized with chloral hydrate (300 mg/kg, i.p.),
perfused intracardially with 250 ml cold saline and then sacrificed
by decapitation. The L4-6 spinal cord was promptly removed onto an
ice-cold plate, frozen and stored at −80°C until use. All collected
tissue samples were homogenized in an SDS sample buffer containing
a mixture of proteinase and phosphatase inhibitors (Sigma-Aldrich;
Merck Millipore). Each individual rat spinal cord protein was
processed separately. The protein concentrations were estimated
using the bicinchoninic acid method. Protein samples (30 µg) were
separated by 10% SDS-PAGE gel and transferred onto a nitrocellulose
membrane. The membranes were blocked in 10% skimmed milk for 1 h at
room temperature, and then incubated with the following primary
antibodies overnight at 4°C: Guinea pig polyclonal anti-GABAB2
(1:2,000; cat. no. AB2255; Chemicon, Temecula, CA, USA) and mouse
monoclonal anti-β-actin (1:3,000; cat. no. A1978; Sigma-Aldrich;
Merck Millipore). The blots were further incubated for 1 h at room
temperature with HRP-conjugated secondary antibody (anti-guinea pig
1:3,000; cat. no. 61_4620; anti-mouse cat. no. 31430; 1:5,000; GE
Healthcare Life Sciences, Chalfont, UK), and washed three times in
TBS for 30 min. The immune complexes were detected using the
enhanced chemiluminescence detection method (GE Healthcare Life
Sciences) and exposure to film. A square of the same size was drawn
around each band to measure the density, and the background
adjacent to that band was subtracted. Levels of the target protein
were normalized against the levels of β-actin and expressed as
relative fold changes.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. For behavioral data, comparisons were made using two-way
repeated measures analysis of variance (ANOVA). For others,
comparisons were performed using two-way ANOVA followed by
Bonferroni tests. Data were analyzed using SPSS 13.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
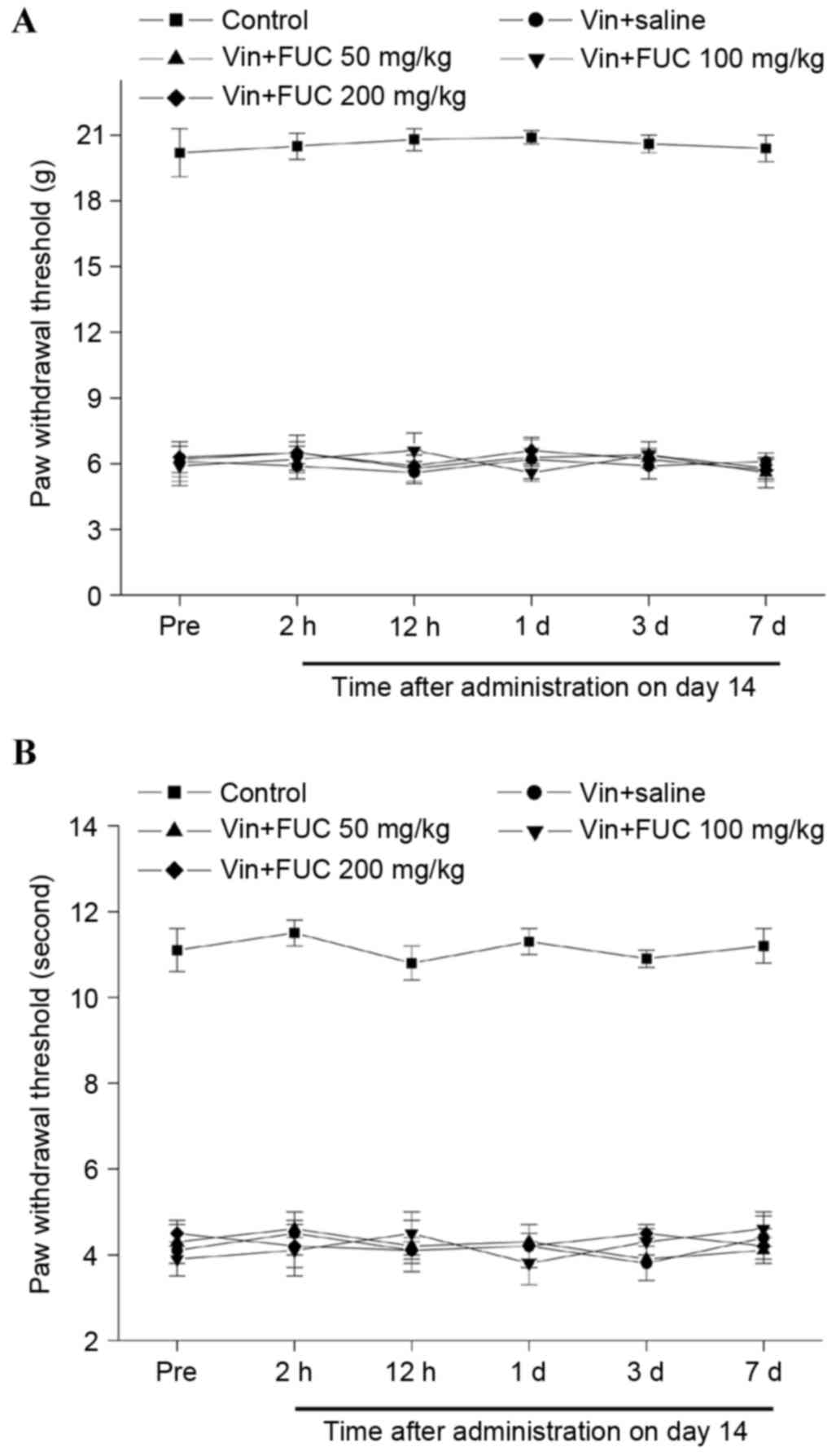
Single treatment of fucoidan on
vincristine-induced mechanical and cold allodynia
Vincristine administration resulted in the
development of mechanical and cold allodynia, as reflected by the
significant reductions in the mechanical and cold withdrawal
thresholds, compared with the normal control group. To determine
the effects of a single injection of fucoidan on
vincristine-induced allodynia, fucoidan was injected i.p. on day
14, when the neuropathic pain had been maintained. The behavioral
responses 2, 12 h, 1 day, 3 days and 7 days following
administration were then observed. No differences in mechanical or
cold allodynia were detected between the rat treated with fucoidan
and normal saline, at any doses or time points, following injection
(Fig. 1A and B).
Repeated injection of fucoidan on
vincristine-induced mechanical and cold allodynia
The effects of repeated fucoidan treatment on
vincristine-induced neuropathic pain were also examined. As shown
in Fig. 2A and B, repeated
administration of fucoidan attenuated vincristine-induced
mechanical and cold allodynia in a dose-dependent manner. A lower
dose of fucoidan (50 mg/kg) only marginally elevated the mechanical
and cold paw withdrawal threshold. Higher doses of fucoidan (100
and 200 mg/kg) markedly and significantly increased the mechanical
and cold paw withdrawal threshold. Similar effects were observed
with pregabalin administration. In addition, a higher dose of
fucoidan ameliorated vincristine-induced neuropathic pain for at
least 1 week following the final treatment.
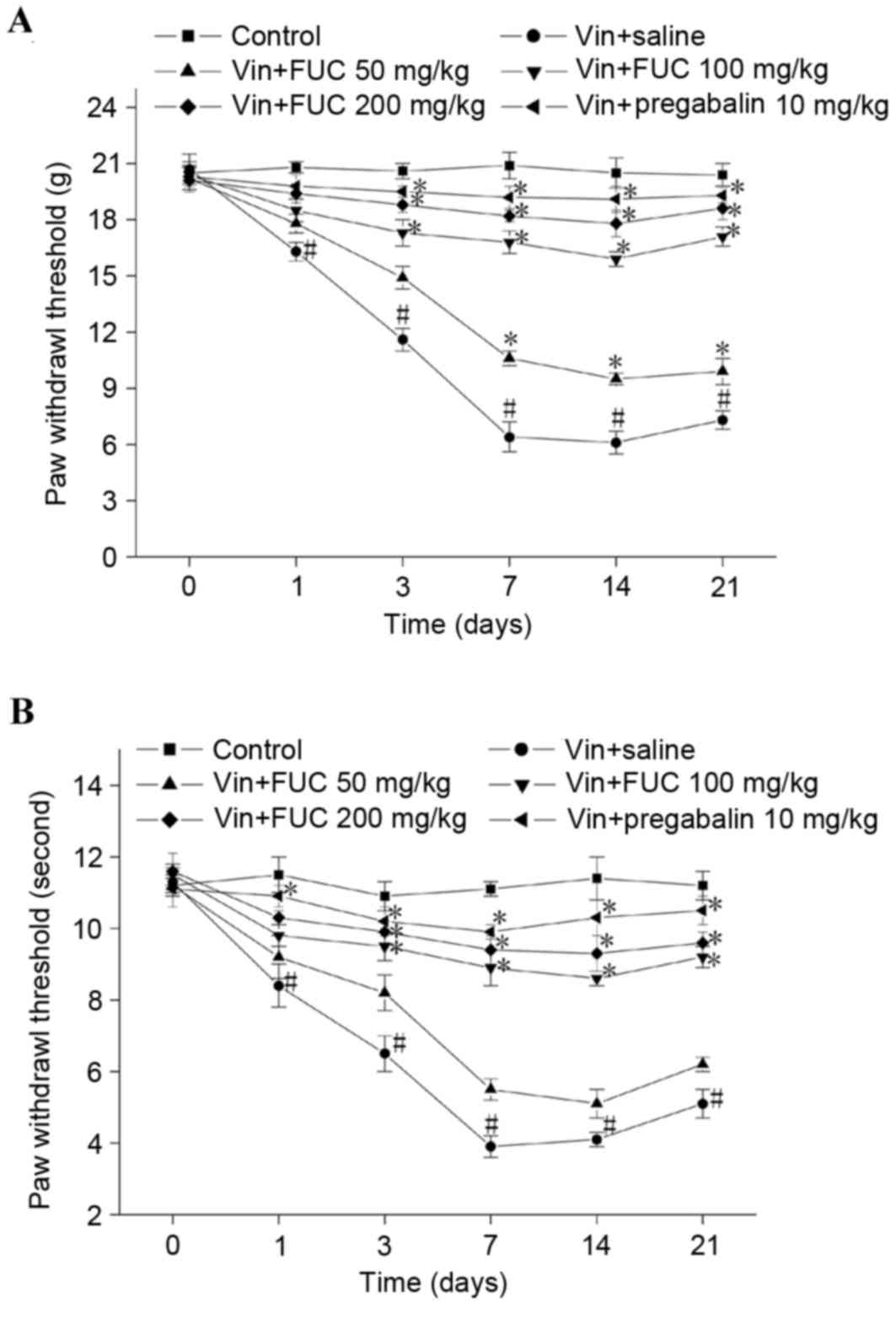 | Figure 2.Effects of repeated treatment with
fucoidan on vincristine-induced mechanical and cold allodynia.
Vincristine (50 µg/kg) was injected (i.p.) for 10 consecutive days.
Fucoidan (50, 100 or 200 mg/kg) and normal saline or pregabalin (10
mg/kg) were administered once daily for 14 consecutive days in
vincristine-treated rats. Rats were administered with normal saline
for 14 consecutive days as a control. Behavioral assessments were
performed on days 0, 1, 3, 7, 14 and 21. Following treatment with
fucoidan, (A) mechanical and (B) cold paw withdrawal threshold in
the Vin+FUC group were significantly higher, compared with those of
the Vin+saline group at each relative time point. The effect of
fucoidan administration on vincristine-induced neuropathic pain was
dose-dependent (n=6 in each group). *P<0.05, compared with the
Vin+saline group; #P<0.05, compared with the control.
Vin, vincristine; FUC, fucoidan. |
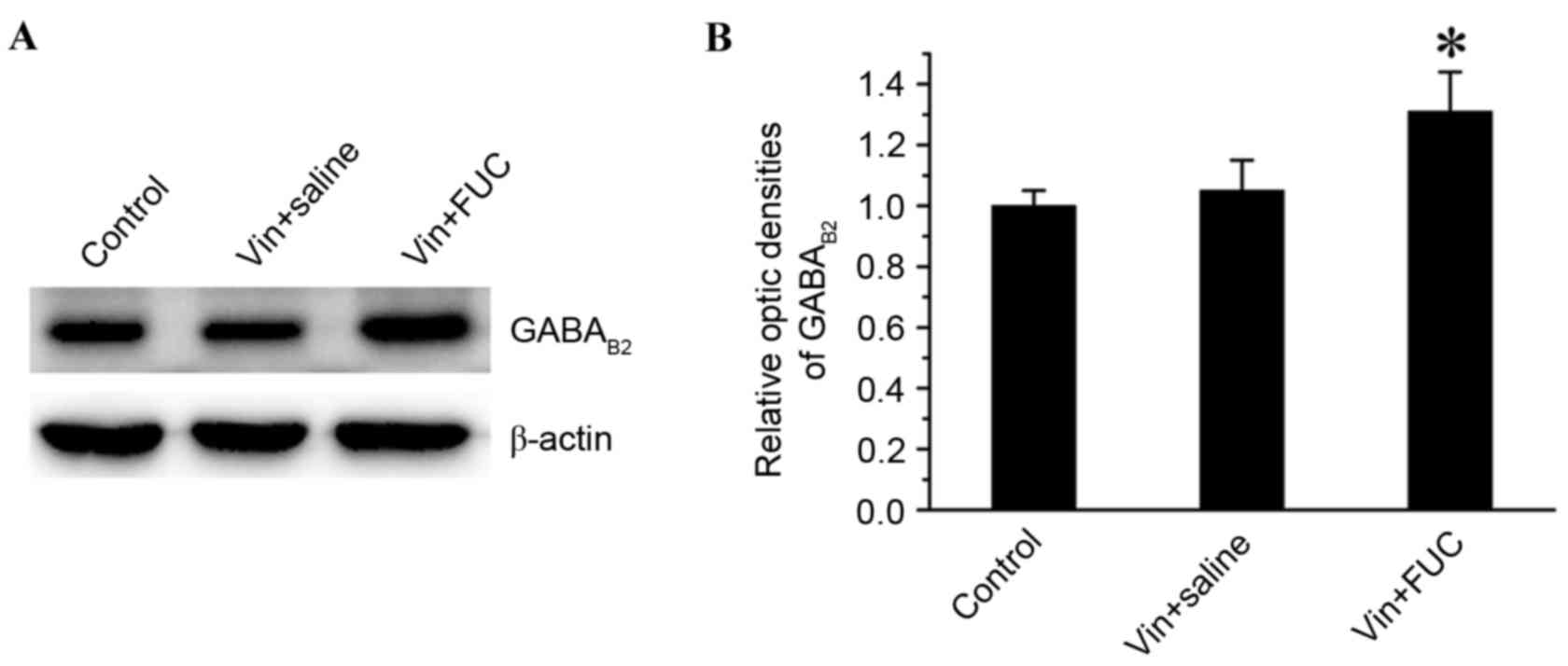
Effects of repeated fucoidan
administration on the expression of GABAB receptor in the spinal
cord of vincristine-treated rats
GABAB receptor is densely expressed in the spinal
cord dorsal horn. Activation of spinal GABAB receptor produces
antinociception in acute and chronic pain (13,14).
A study by Thibault et al (15) reported that the GABAB receptor is
functionally required for the alleviating effect of oxycodone in
vincristine-induced neuropathic pain. The analgesic effect of
fucoidan on vincristine-induced neuropathic pain may be associated
with its upregulation of the expression of GABAB receptor. To
confirm this hypothesis, the present study examined the expression
levels of GABAB2 receptor using western blot analysis. The data
showed that there was an upregulation in the expression of GABAB2
receptor in the vincristine-fucoidan treated animals, compared with
the expression in the animals in the vincristine-saline treated
animals or the saline-treated animals on 21 (Fig. 3A and B).
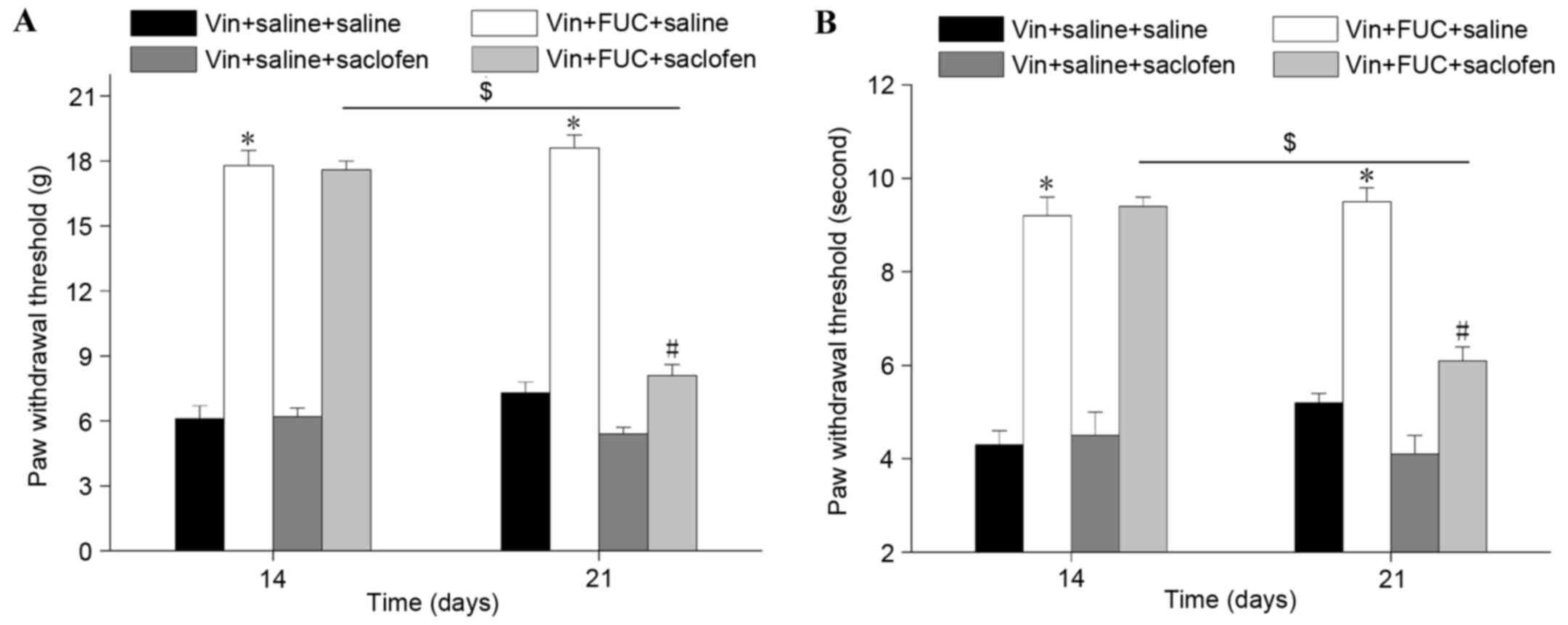
Effect of the GABAB receptor on the
analgesic effect of repeated fucoidan injections determined by
intrathecal administration of saclofen
The present study directly examined the role of
GABAB receptor in the analgesic effect of fucoidan by assessing the
effect of the intrathecal administration of saclofen, a selective
GABAB receptor antagonist. As shown in Fig. 4A and B, the alleviating effect of
repeated fucoidan administration on vincristine-induced mechanical
and cold allodynia was completely reversed by the antagonist.
Furthermore, saclofen eliminated the effect of fucoidan on the
upregulated expression of GABAB2 receptor in the spinal cord
(Fig. 5). Of note, the
antinoticeptive effect of fucoidan (200 mg/kg) was maintained 7
days following the final administration. These results revealed
that fucoidan had a prolonged effect via the upregulation of GABAB
receptor, which can be observed even when fucoidan treatment is
terminated.
Discussion
GABAB receptors are located both pre- and
post-synaptically throughout the central nervous system. GABAB
receptor stimulation affects neurotransmission by directly reducing
neurotransmitter release or hyperpolarizing postsynaptic neurons
(16). Increasing GABAergic tonus
decreases neuronal excitation and has an antinociceptive property.
For example, Baclofen, the prototypic, orthosteric GABAB receptor
agonist, produces antinociception in neuropathic pain models
whether administered intrathecally or intraperitoneally and has
been used clinically as an adjuvant therapy for managing certain
types of pain (13,17,18).
In the present study, it was observed that repeated administration
of fucoidan significantly attenuated vincristine-induced mechanical
and cold allodynia. Of note, the antinociceptive effect of fucoidan
was not detected following a single injection of fucoidan. In
addition, fucoidan treatment induced an increase in GABAergic tone
in the spinal cord, which was mediated by the GABAB2 receptor. As
the effect of this Chinese herb extract usually has a long duration
to onset, repetitive treatments of fucoidan are required to exert
the effect of antinociception. The present study hypothesized that
the upregulated expression of GABAB2 receptor following fucoidan
administration led to a decrease in excitatory transmission and
counteracted the increased neuronal excitability induced by
vincristine treatment. This hypothesis was confirmed by the use of
saclofen, a selective GABAB receptor antagonist, which reversed the
effects of fucoidan (200 mg/kg) against vincristine-induced
neuropathy. Therefore, the alleviating effect of fucoidan in
neuropathic pain appeared to involve the spinal GABAergic tonus
through the GABAB receptor. However, how fucoidan increases the
expression of GABAB receptor remains to be elucidated. Glutamate is
a major excitatory transmitter in the spinal cord and
N-methyl-D-aspartate receptor (NMDAR) is known to be involved in
painful neuropathy. Activation of the GABAB receptor can
downregulate NMDAR in the spinal cord in diabetic neuropathy
(19), whereas NMDAR is
upregulated in chemotherapeutic drug oxaliplatin-induced
neuropathic pain (20). Thus it
was hypothesized that the fucoidan-induced increase in GABAB
receptor occurred through the downregulation of NMDAR. However,
further investigations are required to confirm this hypothesis.
Previous studies have shown that a decline in the
expression of GABAB receptor in the spinal cord is associated with
certain types of neuropathic pain. For example, nerve
injury-induced neuropathic pain has been found to cause
upregulation, downregulation, and no effect on the expression of
GABAB receptor (21–23). GABAB receptor is downregulated in
the spinal dorsal horn in the rat models of streptozotocin-induced
diabetic neuropathic pain (24).
However, vincristine treatment was found not to effect the
expression of GABAB2 receptor in the spinal dorsal horn of rats
with chemotherapy-induced neuropathic pain (14). The present study also observed no
alteration in the expression of GABAB2 in vincristine-treated rats.
These data suggested that an association between the development of
neuropathic pain and a decline in spinal cord GABAergic activity
was not always observed, possibly due to differences in the animal
models or in the route or pattern of drug administration. There is
substantial evidence indicating that the systemic administration of
orthosteric GABAB receptor agonist generally produces
antinociception in animal models of acute and persistent pain
(13,25,26).
Therefore, agents that activate GABAB receptor may provide
antinociceptive effects. The results of the present study suggested
that the repeated administration of fucoidan significantly
ameliorated vincristine-induced mechanical and cold allodynia,
possibly through activation of the GABAB receptor. Therefore,
fucoidan may be a promising drug for the treatment of
chemotherapeutic drug-induced neuropathic pain.
Acknowledgements
The present study was supported by the Science and
Technology Projects of Guangdong Province (grant no.
2014A020212507).
References
|
1
|
Dougherty PM, Cata JP, Burton AW, Vu K and
Weng HR: Dysfunction in multiple primary afferent fiber subtypes
revealed by quantitative sensory testing in patients with chronic
vincristine-induced pain. J Pain Symptom Manage. 33:166–179. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dworkin RH, O'Connor AB, Audette J, Baron
R, Gourlay GK, Haanpää ML, Kent JL, Krane EJ, Lebel AA, Levy RM, et
al: Recommendations for the pharmacological management of
neuropathic pain: An overview and literature update. Mayo Clin
Proc. 85 Suppl 3:S3–S14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee J and Nandi P: Improving the
management of neuropathic pain. Practitioner. 254:27–30, 3.
2010.PubMed/NCBI
|
|
4
|
Kim YS, Park HJ, Kim TK, Moon DE and Lee
HJ: The effects of Ginkgo biloba extract EGb 761 on mechanical and
cold allodynia in a rat model of neuropathic pain. Anesth Analg.
108:1958–1963. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nurmikko TJ, Serpell MG, Hoggart B, Toomey
PJ, Morlion BJ and Haines D: Sativex successfully treats
neuropathic pain characterised by allodynia: A randomised,
double-blind, placebo-controlled clinical trial. Pain. 133:210–220.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ellis RJ, Toperoff W, Vaida F, van den
Brande G, Gonzales J, Gouaux B, Bentley H and Atkinson JH: Smoked
medicinal cannabis for neuropathic pain in HIV: A randomized,
crossover clinical trial. Neuropsychopharmacology. 34:672–680.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Zhang Q, Zhang Z, Song H and Li P:
Potential antioxidant and anticoagulant capacity of low molecular
weight fucoidan fractions extracted from Laminaria japonica. Int J
Biol Macromol. 46:6–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aisa Y, Miyakawa Y, Nakazato T, Shibata H,
Saito K, Ikeda Y and Kizaki M: Fucoidan induces apoptosis of human
HS-sultan cells accompanied by activation of caspase-3 and
down-regulation of ERK pathways. Am J Hematol. 78:7–14. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu CY, Zhang GP and Zhao YT: Fucoidan
attenuates the existing allodynia and hyperalgesia in a rat model
of neuropathic pain. Neurosci Lett. 571:66–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
National Institutes of Health (NIH), .
Office of Laboratory Animal Welfare (OLAW). http://olaw.nih.govMay 20–2016.
|
|
11
|
Siau C and Bennett GJ: Dysregulation of
cellular calcium homeostasis in chemotherapy-evoked painful
peripheral neuropathy. Anesth Analg. 102:1485–1490. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Smith GD, Harrison SM, Birch PJ, Elliott
PJ, Malcangio M and Bowery NG: Increased sensitivity to the
antinociceptive activity of (+/−)-baclofen in an animal model of
chronic neuropathic, but not chronic inflammatory hyperalgesia.
Neuropharmacology. 33:1103–1108. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dirig DM and Yaksh TL: Intrathecal
baclofen and muscimol, but not midazolam, are antinociceptive using
the rat-formalin model. J Pharmacol Exp Ther. 275:219–227.
1995.PubMed/NCBI
|
|
15
|
Thibault K, Calvino B, Rivals I, Marchand
F, Dubacq S, McMahon SB and Pezet S: Molecular mechanisms
underlying the enhanced analgesic effect of oxycodone compared to
morphine in chemotherapy-induced neuropathic pain. PLoS One.
9:e912972014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brenowitz S, David J and Trussell L:
Enhancement of synaptic efficacy by presynaptic GABA(B) receptors.
Neuron. 20:135–141. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hwang JH, Hwang KS, Kim JU, Choi IC, Park
PH and Han SM: The interaction between intrathecal neostigmine and
GABA receptor agonists in rats with nerve ligation injury. Anesth
Analg. 93:1297–1303. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Slonimski M, Abram SE and Zuniga RE:
Intrathecal baclofen in pain management. Reg Anesth Pain Med.
29:269–276. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bai HP, Liu P, Wu YM, Guo WY, Guo YX and
Wang XL: Activation of spinal GABAB receptors normalizes
N-methyl-D-aspartate receptor in diabetic neuropathy. J Neurol Sci.
341:68–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu XY, Zhang GF, Dong L, Wang X, Sun H,
Shen J, Li W and Xu J: Repeated administration of mirtazapine
attenuates oxaliplatin-induced mechanical allodynia and spinal NR2B
up-regulation in rats. Neurochem Res. 38:1973–1979. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McCarson KE, Ralya A, Reisman SA and Enna
SJ: Amitriptyline prevents thermal hyperalgesia and modifications
in rat spinal cord GABA(B) receptor expression and function in an
animal model of neuropathic pain. Biochem Pharmacol. 71:196–202.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Castro-Lopes JM, Malcangio M, Pan BH and
Bowery NG: Complex changes of GABAA and GABAB receptor binding in
the spinal cord dorsal horn following peripheral inflammation or
neurectomy. Brain Res. 679:289–297. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Engle MP, Gassman M, Sykes KT, Bettler B
and Hammond DL: Spinal nerve ligation does not alter the expression
or function of GABA(B) receptors in spinal cord and dorsal root
ganglia of the rat. Neuroscience. 138:1277–1287. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang XL, Zhang Q, Zhang YZ, Liu YT, Dong
R, Wang QJ and Guo YX: Downregulation of GABAB receptors in the
spinal cord dorsal horn in diabetic neuropathy. Neurosci Lett.
490:112–115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McCarson KE and Enna SJ: GABA
pharmacology: The search for analgesics. Neurochem Res.
39:1948–1963. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thomas DA, Navarrete IM, Graham BA,
McGowan MK and Hammond DL: Antinociception produced by systemic
R(+)-baclofen hydrochloride is attenuated by CGP 35348 administered
to the spinal cord or ventromedial medulla of rats. Brain Res.
718:129–137. 1996. View Article : Google Scholar : PubMed/NCBI
|