Introduction
Colorectal cancer (CRC) is the third most prevalent
cancer and the third most common cause of cancer-associated
mortality in males and females worldwide (1). Despite rapid advances in diagnosis
and treatment methods, including surgery and chemotherapy,
prognosis remains poor. Certain patients suffer drug resistance,
which is partially due to the presence of cancer stem cells (CSCs)
(2).
MicroRNAs (miRNAs) are a class of small,
evolutionarily conserved, noncoding RNAs. miRNAs regulate various
target genes by binding to the 3′ untranslated region (3′UTR) of
target mRNA, resulting in translational repression at the
posttranscriptional level, via mRNA degradation, or through
altering gene expression by interactions with gene promoters
(3). miRNAs are important in
biological and cellular processes, including proliferation,
apoptosis, metabolism and differentiation (4,5).
Aberrant expression of miRNAs has been observed in numerous human
diseases, including cancer, metabolic disease, autoimmune disease,
cardiovascular disease and neurological disorders. Currently, it is
widely accepted that miRNAs may act as oncogenes or suppressor
genes during tumor development. For example, miR-27b has been
demonstrated to inhibit CRC progression and angiogenesis via
targeting vascular endothelial growth factor C, and miR-130b
promotes liver CSC growth and self-renewal through targeting tumor
protein P53 inducible nuclear protein 1 (6).
Considering that CSCs may be critical for tumor
development and drug resistance, the present study aimed to
investigate molecular differences between CSCs and differentiated
cancer cells, including in miRNA expression. Numerous surface
markers have been identified for CSC sorting, including cluster of
differentiation CD133, CD24, CD44 and CD166 (7,8), of
which CD133 is the most widely used. CD133 is a five-transmembrane
glycoprotein with a molecular weight of 120 kDa. It has been
demonstrated to identify CSCs in various human tumors, including
prostate cancer, pancreatic cancer, leukemia, brain tumor,
hepatocellular carcinoma, breast cancer and colon cancer (9–15).
Our previous study have established the Y cell line,
which is enriched in CD133+ cells and exhibits distinct
stemness characteristics, including sphere formation and the
capacity for self-renewal (16).
Using miRNA microarrays, the present study revealed that miR-141
expression is decreased in CSC-like Y cells and in CRC tissues.
miR-141 is located on chromosome 12 and belongs to the same cluster
as the miR-200 family. miR-141 has been observed to be
downregulated in gastric cancer, renal cell carcinoma and breast
cancer (17). However, the
functions and potential targets of miR-141 in CRC cells remain to
be fully elucidated. The present study demonstrated that miR-141
inhibited CRC cell proliferation via targeting cyclin D2, which is
involved in cell cycle regulation, and inhibited the maintenance of
CSC stemness, thereby enhancing drug susceptibility. Targeting
miR-141 may therefore be a potential strategy for the treatment of
CRC.
Materials and methods
Clinical colorectal cancer tissue
sample collection and analysis
Tissue samples (n=8) were collected between January
2008 and December 2010 at the Second Affiliated Hospital, Zhejiang
University School of Medicine (Hangzhou, China) and confirmed to be
colorectal cancer pathologically. All participants provided written
consent of their information to be stored in the hospital database
and for samples to be used for research. This research was approved
by the Institutional Review Boards of the Second Affiliated
Hospital, Zhejiang University School of Medicine.
Cell lines
The Y human colorectal cancer cell line was
established in our laboratory, as described previously (2). The HT29 and SW620 human colorectal
adenocarcinoma cell line was obtained from the Cell Bank of the
China Academy of Medical Sciences (Beijing, China). Y and HT29
cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.). SW620
cells were cultured in Leibovitz L15 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with FBS (Gibco; Thermo Fisher
Scientific, Inc.). All cells were maintained at 37°C in a
humidified 5% CO2 atmosphere.
Flow cytometry
CRC cells (<2×107) were labeled with an
anti-human monoclonal CD133 antibody conjugated to R-phycoerythrin
(R-PE; cat. no. 130-098-826; 10 µl per 107 cells; Miltenyi Biotec
GmbH, Bergisch Gladbach, Germany) in PBS containing 2% FBS for 10
min in the dark at 4°C. A blank control and mouse IgG1-R-PE isotype
control (cat. no. 130-098-845; 10 µl per 107 cells; Miltenyi Biotec
GmbH) served as controls. CD133+ cells were identified and sorted
using a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA,
USA).
miRNA expression microarray
analysis
Total RNA was isolated from CD133+ and CD133- CRC
cells using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
quantity and the quality of RNA were evaluated using a Nanodrop
spectrophotometer (Thermo Fisher Scientific, Inc.). The miRNA
expression profile of CD133+ and CD133- CRC cells was assessed
using an Affymetrix miRNA array (Affymetrix GeneChip miRNA 2.0
array; Affymetrix, Inc., Santa Clara, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from cell lines and tissues
using TRIzol reagent according to the manufacturer's protocol. The
quantity and the quality of RNA were evaluated using a Nanodrop
spectrophotometer. The RNA was reverse-transcribed to cDNA using
Moloney Murine Leukemia Virus Reverse Transcriptase (Promega
Corporation, Madison, WI, USA). The expression of miR-141, sex
determining region Y-box 2 (Sox2), octamer-binding transcription
factor 4 (Oct4), nanog homeobox (Nanog) and B cell-specific Moloney
murine leukemia virus integration site 1 (Bmi1) was measured using
the SYBR®-Green PCR Master mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.) and the StepOnePlus Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). All
samples were run in triplicate. The miR-141 expression levels were
normalized to those of U6, which served as an internal control. The
Sox2, Oct4, Nanog and Bmi1 expression levels were normalized to
those of GAPDH, which served as an internal control. The relative
expression of target genes were calculated using the
2−ΔΔCq method (18).
The primer sequences used were as follows: SOX2, F
5′-AACCCCAAGATGCACAACTC-3′, R 5′-CGGGGCCGGTATTTATAATC-3′; Oct4, F
5′-TTCTCAGGGGGACCAGTGTC-3′; R 5′-CCCATTCCTAGAAGGGCAGG-3′; Nanog, F
5′-CCAGTGACTTGGAGGCTGC-3′, R 5′-AAGGATTCAGCCAGTGTCC-3′; Bmi1, F
5′-TGACAAATGCTGGAGAACTG-3′, R 5′-AAGATTGGTGGTTACCGCT-3′; GADPH, F
5′-ACAGTCAGCCGCATCTTCTT-3′; R 5′-TGGAAGATGGTGATGGGATT-3′. Results
were expressed as relative quantitation.
Proliferation assay
CRC cells were transfected with the negative control
mimic (5′-UUCUCCGAACGUGUCACGUTT-3′), an miR-141 mimic
(5′-UAACACUGUCUGGUAAAGAUGG-3′) or an anti-miR-141 inhibitor
(5′-CCAUCUUUACCAGACAGUGUUA-3′), all obtained from Shanghai
GenePharma Co., Ltd. (Shanghai, China) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) and incubated for 12 h. Cells were subsequently
seeded at a density of 3×103 cells per well in 0.2 ml RPMI 1640
medium containing 10% FBS in a 96-well plate. MTS reagent (20 µl;
Promega Corporation) was added to each well and the cells were
incubated at 37°C for 4 h. The absorbance values were measured at a
wavelength of 490 nm on a microplate reader (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) and assessed every day for 3 consecutive
days.
Cell viability assay
CRC cells were transfected as described above. After
a further incubation of 24 h, cells were reseeded in 96-well plates
and treated with various concentrations of 5-fluorouracil (5-FU;
Nantong Jinghua Pharmaceutical Co., Ltd., Nantong, China) or
oxaliplatin (Sanofi S.A., Gentilly, France) for 48 h. A
proliferation assay was subsequently performed as described above,
to determine the individual inhibitory concentration 50 values (50%
cell growth inhibitory concentrations).
Cell cycle analysis
Cells were trypsinized and fixed in 70% ethanol. The
DNA content was assessed by analyzing incorporated propidium iodide
(PI) (10 µl PI in 1.5 ml 1×106 cells; Multi Sciences (Lianke)
Biotech Co., Ltd., Hangzhou, China) using a FACScanto II. The cell
populations in G0/G1, S and G2/M phases were determined using
ModFit LT software v2.0 (Verity Software House, Inc., Topsham, ME,
USA).
Western blotting
Total protein was extracted from cells lysed with
the M-PER Mammalian Protein Extraction Reagent (Thermo Fisher
Scientific, Inc.) supplemented with a protease inhibitor cocktail
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany). Protein
extracts (50 µl extract from 1×106 cells) were separated
by SDS-PAGE and transferred to a PVDF membrane. Following blocking
with 5% non-fat milk in TBS containing Tween-20 (TBST) for 60 min,
the membrane was incubated with anti-human-cyclin D2 (cat. no.
3741S; 1:2,000; Cell Signaling Technology, Inc., Danvers, MA, USA)
or anti-human GAPDH (cat no. KC-5G5; 1:2,000; Shanghai Kang-Chen
Biotech, Shanghai, China). Primary antibodies were diluted in 5%
bovine serum albumin (Amresco, LLC, Solon, OH, USA) in TBST and
incubated overnight at 4°C. Membranes were incubated in secondary
antibody [anti-rabbit horseradish peroxidase conjugated; cat. no.
70-GAR007; 1:5,000; Multi Sciences (Lianke) Biotech Co., Ltd.) for
1 h at room temperature. ECL chromogenic substrate [cat no.
70-P1421; Multi Sciences (Lianke) Biotech Co., Ltd.] was used to
visualize the proteins.
Statistical analysis
Data are presented as the mean ± standard error
using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). The RT-qPCR results
from paired clinical samples were analyzed using a two-tailed
paired Student's t-test and the remaining data were analyzed using
a two-tailed unpaired Student's t-test or analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-141 expression levels decrease in
colorectal CSCs
CSCs are involved in tumor initiation, recurrence,
metastasis and chemotherapy resistance (7,19).
The present study assessed miRNA expression profiles of CD133+ and
CD133- cells to identify differentially expressed miRNAs
potentially involved in tumor progression. Microarray analysis
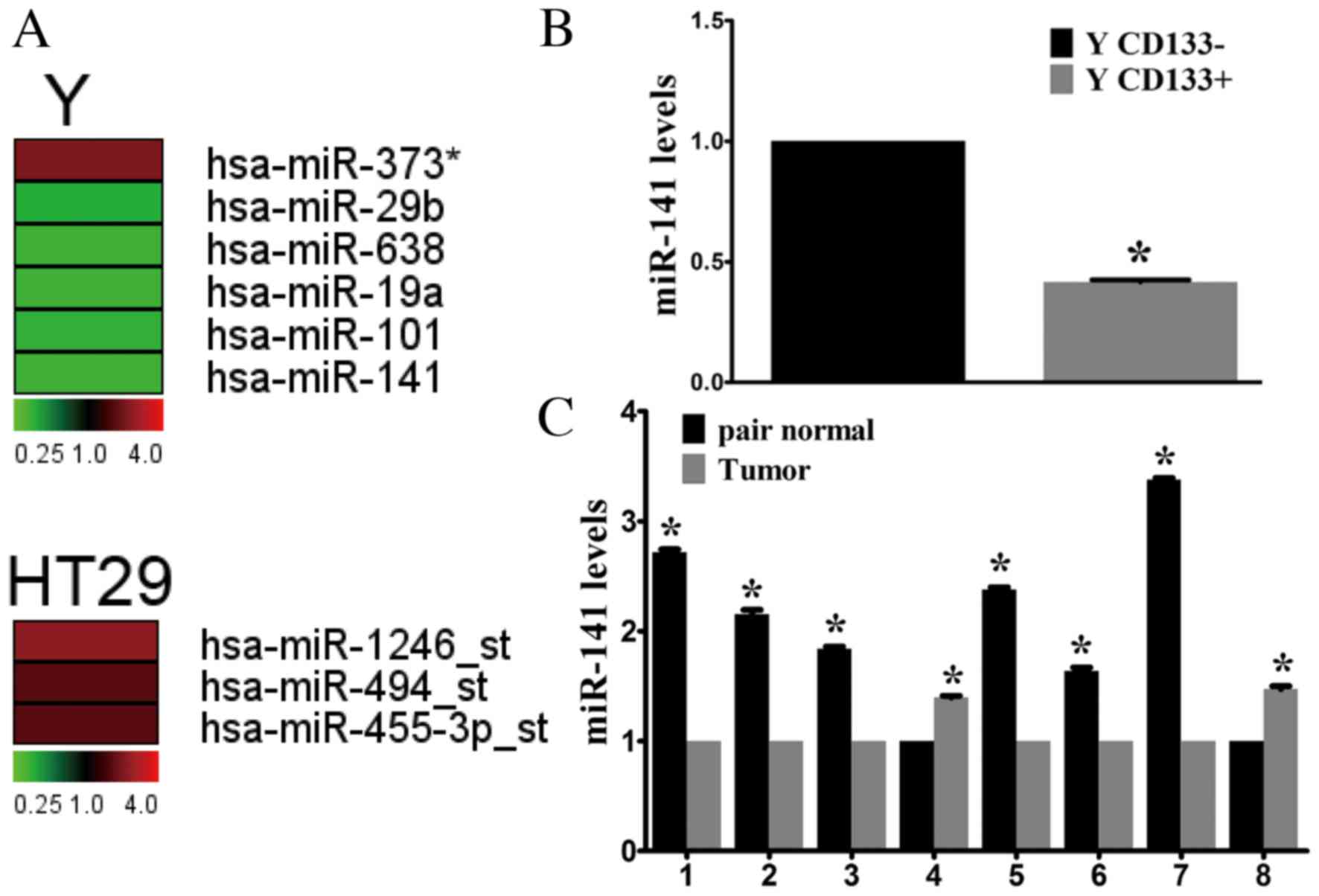
identified four miRNAs that were upregulated (miR-373,
miR-1246-star, miR-494-star and miR-455-3p-star) and 5 that were
downregulated (miR-29b, miR-638, miR-19a, miR-101 and miR-141) in
CD133+ cells compared with CD133- cells (Fig. 1A). Of these, miR-141 underwent the
greatest decrease. In addition, the importance of the miR-200
family in cancer development has been demonstrated, therefore
miR-141 was selected for further investigation. RT-qPCR confirmed
the microarray results, demonstrating a 2.33-fold decrease in
miR-141 expression levels in CD133+ cells compared with CD133-
cells sorted from the Y cell line (Fig. 1B).
miR-141 expression levels were analyzed in CRC
tissue samples. In the limited number of fresh tissue samples
available (n=8), miR-141 expression was downregulated in the
majority of CRC samples compared with adjacent healthy tissues
(Fig. 1C). Thus, downregulation of
miR-141 may be important during CRC development.
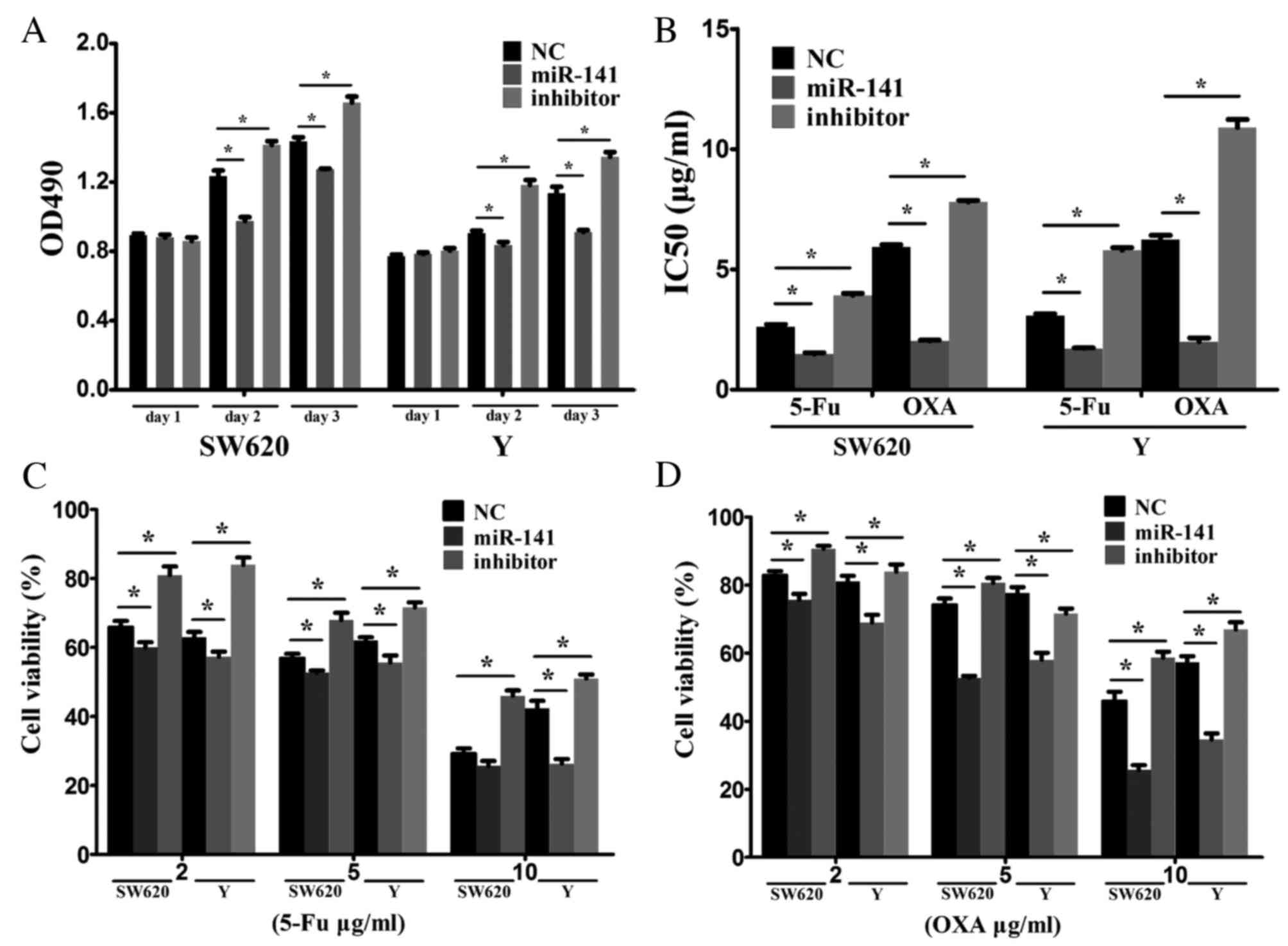
miR-141 inhibits tumor cell
proliferation and increases chemotherapy sensitivity
CRC cells were transfected with a negative control
mimic, an miR-141 mimic or an anti-miR-141 inhibitor to investigate
the biological functions of miR-141. RT-qPCR confirmed the
transfection efficiency. Overexpression of miR-141 suppressed cell
proliferation, whereas inhibiting miR-141 via an anti-miR-141
promoted cell proliferation (Fig.
2A).
The effect of miR-141 on drug resistance was
examined in vitro. 5-FU or oxaliplatin as typical antitumor
drugs were added into the culture medium of SW620 and Y cells.
Transfection with anti-miR-141 enhanced drug resistance (Fig. 2B). Following treatment of SW620 and
Y cells with various concentrations (2, 5 or 10 ug/ml) of 5-FU
(Fig. 2C) or oxaliplatin (Fig. 2D), cell viability was markedly
reduced in the miR-141 mimic-transfected, compared with the
negative control, group. Transfection with anti-miR-141
demonstrated the opposite effect. These results indicated that
miR-141 suppresses CRC proliferation and enhances drug
sensitivity.
miR-141 promotes stem cell
differentiation in CRC
Sox2, Oct4, Nanog and Bmi1 are stem cell-associated
genes, which are involved in self-renewal and the maintenance of
stemness in stem cells (20).
Following transfection of SW620 cells with anti-miR-141, Sox2,
Oct4, Nanog and Bmi1 mRNA expression levels were elevated (Fig. 3A) indicating that miR-141 may
promote stem cell differentiation.
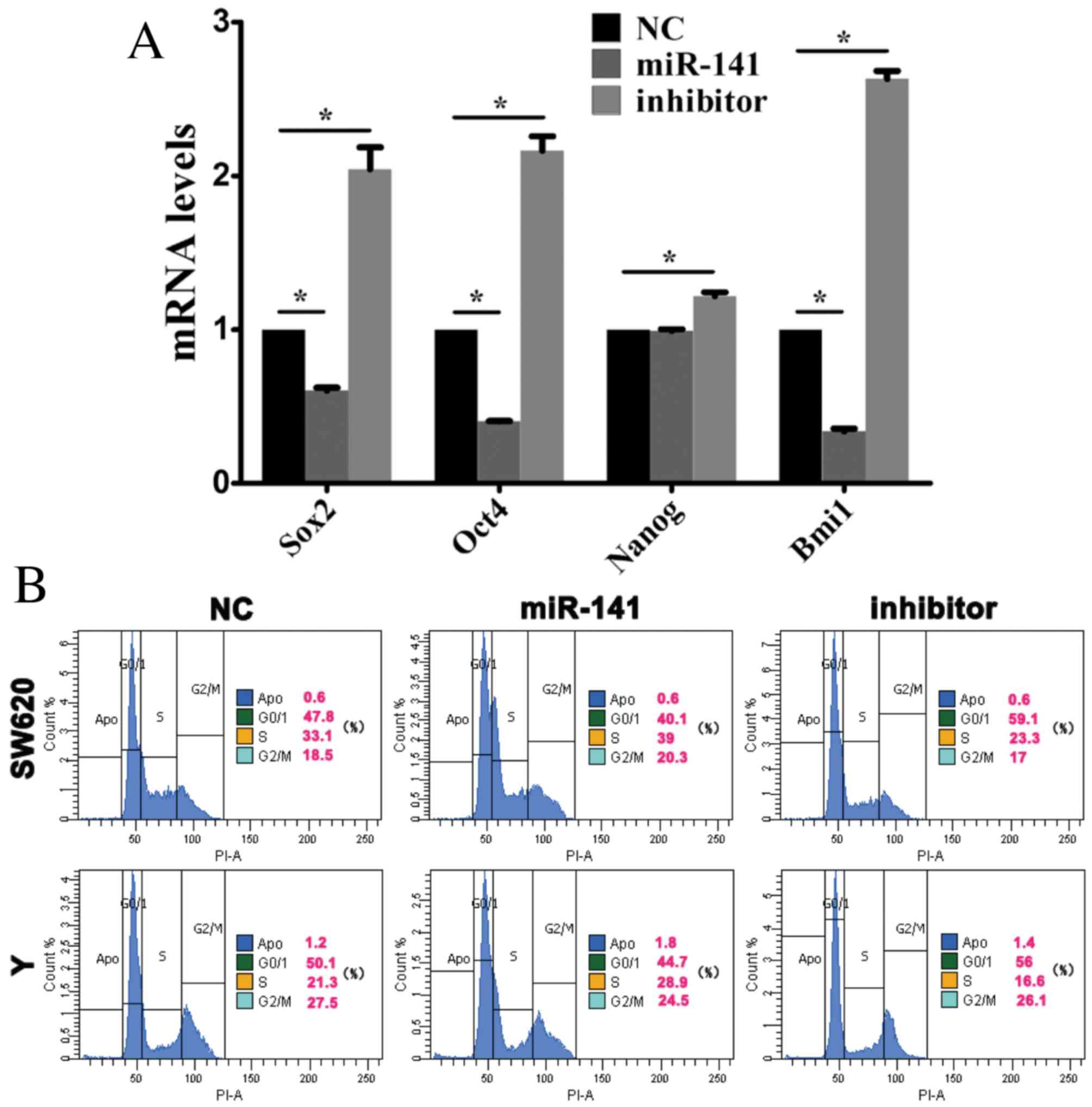 | Figure 3.miR-141 inhibits the maintenance of
cancer stem cell stemness. Cells were transfected with a negative
control mimic, an miR-141 mimic or an anti-miR-141 inhibitor. (A)
Sox2, Oct4, Nanog and Bmi1 mRNA expression levels in CRC cells were
assessed by reverse transcription-quantitative polymerase chain
reaction analysis. *P<0.05. (B) Cell cycle progression of CRC
cells was assessed by flow cytometry. miR, microRNA; CRC,
colorectal cancer; Sox2, sex determining region Y-box 2; Oct4,
octamer-binding transcription factor 4; Nanog, nanog homeobox;
Bmi1, B cell-specific Moloney murine leukemia virus integration
site 1; NC, negative control. |
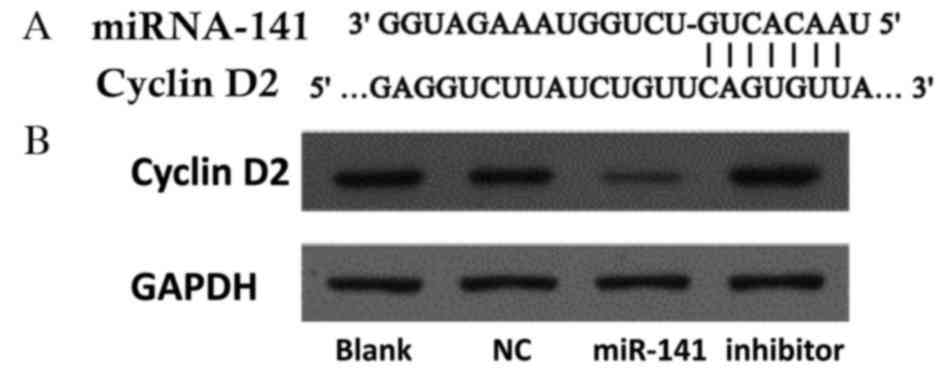
Cyclin D2 is a novel target of miR-141
in CRC
Stem cell differentiation is associated with cell
cycle progression. Flow cytometry revealed that, in SW620 and Y
cells transfected with an miR-141 mimic, the proportion of cells in
the G0/1 phase decreased, whereas the percentage in S phase
increased (Fig. 3B).
Potential gene targets of miR-141 were analyzed
using the TargetScan database (www.targetscan.org). Hundreds of potential miR-141
targets were further analyzed using the Kyoto Encyclopedia of Genes
and Genomes pathway database (www.genome.jp/kegg/). Cyclin D2 was identified as a
functional downstream target of miR-141. The cyclin D2 3′ UTR
contains highly conserved miR-141 binding sites (Fig. 4A). Western blotting revealed that
cyclin D2 protein expression levels were decreased in cells
following transfection with an miR-141 mimic, and increased
following transfection with an anti-miR-141 inhibitor (Fig. 4B).
Discussion
miRNAs are currently undergoing extensive
investigation due to their importance in the regulation of various
developmental processes. They have been implicated in diverse
diseases, including cancer. The present study investigated miRNAs
that were differentially expressed in CSCs and differentiated
cancer cells. Microarray analysis identified four miRNAs that were
upregulated (miR-373, miR-1246-star, miR-494-star and
miR-455-3p-star) and five that were downregulated (miR-29b,
miR-638, miR-19a, miR-101 and miR-141) in CSCs compared with
differentiated cancer cells. miR-373 has been revealed to be
upregulated in breast cancer and to promote tumor invasion and
metastasis (21). miR-1246-star
promotes angiogenesis in CRC (22). miR-494-star has previously been
demonstrated to be upregulated in CRC, and promotes cell migration
and invasion by targeting the phosphatase and tensin homolog gene
(23). miR-29b is downregulated in
CRC and suppresses tumor growth and metastasis by inhibiting
epithelial-mesenchymal transition (24); miR-638 is also downregulated and
regulates the cell cycle by targeting tetraspanin 1 (25). miR-19a targets tissue factor to
inhibit CRC migration and invasion (26). miR-101 exerts its antitumor effect
via downregulating sphingosine kinase 1 (27). Low expression of miR-141 was
identified in CSCs; however, its specific underlying mechanisms
remained to be fully elucidated. In the present study, miR-141
inhibited the proliferation of CRC cells and the maintenance of
stemness in CSC, enhancing drug susceptibility.
CSCs constitute a very small proportion of cancer
cells that possess the capacity for self-renewal and
differentiation. CSCs are hypothesized to be responsible for tumor
recurrence, metastasis and chemotherapy resistance. CD133 is a
well-established cell marker used to identify CSCs in colorectal
cancer cell lines, including Y and HT29. These two cell lines were
therefore selected to investigate the differential expression of
miRNAs between CSCs and differentiated cancer cells. miR-141
expression was decreased in CSCs, and this finding was confirmed by
RT-qPCR. In addition, expression levels were decreased in CRC
tissues compared with adjacent healthy tissues.
The function of miR-141 in CRC was subsequently
investigated. miR-141 was demonstrated to inhibit cell
proliferation and enhance chemotherapeutic drug sensitivity.
Furthermore, cyclin D2 was identified as a target of miR-141 in
CRC. Cyclin D2 is a member of the D-type cyclin protein family that
regulates cell cycle progression. In mammalian cells, cyclin D
forms a complex with cyclin-dependent kinase (CDK) 4 and CDK6 and
regulates G1/S phase progression. Overexpression of cyclin D2 has
been identified in various cancers and is associated with the
proliferation of cancer cells (28). Increased expression of cyclin D2
has been detected in advanced CRC and was associated with reduced
disease-free survival. In addition, cyclin D2 is regarded as an
important regulator of self-renewal of human embryonic stem cells
(hES) (29). Cyclin D2 is
prominently expressed in pluripotent hES cells. The present study
demonstrated that following inhibition of miR-141, cyclin D2
protein expression levels and the mRNA expression levels of stem
cell-associated genes were elevated. These results indicated that
miR-141 may affect the maintenance of stemness in CSC via cyclin
D2.
Conventional cancer chemotherapy targets only
differentiated cancer cells, which constitute the majority of the
tumor. Although CSCs account for only a small fraction of total
tumor cells, they remain quiescent in the G0 phase and are
insensitive to chemotherapy (30).
The present study revealed that miR-141 may promote CSC
differentiation by reducing the expression of stem cell-associated
genes, and thereby enhance their chemosensitivity.
In conclusion, the results of the present study
demonstrated that miR-141 suppresses the growth of CRC cells via
targeting cyclin D2, and inhibits the maintenance of CSC stemness,
thereby enhancing drug susceptibility. Targeting miR-141 may
therefore be a potential strategy for the treatment of CRC.
Acknowledgements
The present study was supported by the Zhejiang
Provincial Natural Science Foundation of China (grant no.
LY14H160027), and the Health and Family Planning Commission of
Zhejiang Province (grant nos. 2015115856 and 2013KYA093).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Todaro M, Alea MP, Di Stefano AB,
Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G,
Medema JP and Stassi G: Colon cancer stem cells dictate tumor
growth and resist cell death by production of interleukin-4. Cell
Stem Cell. 1:389–402. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma S, Tang KH, Chan YP, Lee TK, Kwan PS,
Castilho A, Ng I, Man K, Wong N, To KF, et al: miR-130b Promotes
CD133(+) liver tumor-initiating cell growth and self-renewal via
tumor protein 53-induced nuclear protein 1. Cell Stem Cell.
7:694–707. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dalerba P, Dylla SJ, Park IK, Liu R, Wang
X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, et al:
Phenotypic characterization of human colorectal cancer stem cells.
Proc Natl Acad Sci USA. 104:10158–10163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yin S, Li J, Hu C, Chen X, Yao M, Yan M,
Jiang G, Ge C, Xie H, Wan D, et al: CD133 positive hepatocellular
carcinoma cells possess high capacity for tumorigenicity. Int J
Cancer. 120:1444–1450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wright MH, Calcagno AM, Salcido CD,
Carlson MD, Ambudkar SV and Varticovski L: Brca1 breast tumors
contain distinct CD44+/CD24- and CD133+ cells with cancer stem cell
characteristics. Breast Cancer Res. 10:R102008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Olempska M, Eisenach PA, Ammerpohl O,
Ungefroren H, Fandrich F and Kalthoff H: Detection of tumor stem
cell markers in pancreatic carcinoma cell lines. Hepatobiliary
Pancreat Dis Int. 6:92–97. 2007.PubMed/NCBI
|
|
14
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bhatia M: AC133 expression in human stem
cells. Leukemia. 15:1685–1688. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang YK, Zhu YL, Qiu FM, Zhang T, Chen ZG,
Zheng S and Huang J: Activation of Akt and MAPK pathways enhances
the tumorigenicity of CD133+ primary colon cancer cells.
Carcinogenesis. 31:1376–1380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu L, Li Q, Xu D, Wang Q, An Y, Du Q,
Zhang J, Zhu Y and Miao Y: hsa-miR-141 downregulates TM4SF1 to
inhibit pancreatic cancer cell invasion and migration. Int J Oncol.
44:459–466. 2014.PubMed/NCBI
|
|
18
|
Grimholt RM, Urdal P, Klingenberg O and
Piehler AP: Rapid and reliable detection of alpha-globin copy
number variations by quantitative real-time PCR. BMC Hematol.
14:42014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: Models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Z, Oron E, Nelson B, Razis S and
Ivanova N: Distinct lineage specification roles for NANOG, OCT4 and
SOX2 in human embryonic stem cells. Cell Stem Cell. 10:440–454.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang Q, Gumireddy K, Schrier M, le Sage
C, Nagel R, Nair S, Egan DA, Li A, Huang G, Klein-Szanto AJ, et al:
The microRNAs miR-373 and miR-520c promote tumour invasion and
metastasis. Nat Cell Biol. 10:202–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamada N, Tsujimura N, Kumazaki M,
Shinohara H, Taniguchi K, Nakagawa Y, Naoe T and Akao Y: Colorectal
cancer cell-derived microvesicles containing microRNA-1246 promote
angiogenesis by activating Smad 1/5/8 signaling elicited by PML
down-regulation in endothelial cells. Biochim Biophys Acta.
1839:1256–1272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun HB, Chen X, Ji H, Wu T, Lu HW, Zhang
Y, Li H and Li YM: miR-494 is an independent prognostic factor and
promotes cell migration and invasion in colorectal cancer by
directly targeting PTEN. Int J Oncol. 45:2486–2494. 2014.PubMed/NCBI
|
|
24
|
Wang B, Li W, Liu H, Yang L, Liao Q, Cui
S, Wang H and Zhao L: miR-29b suppresses tumor growth and
metastasis in colorectal cancer via downregulating Tiam1 expression
and inhibiting epithelial-mesenchymal transition. Cell Death Dis.
5:e13352014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Fei B, Wang Q, Song M, Yin Y,
Zhang B, Ni S, Guo W, Bian Z, Quan C, et al: MicroRNA-638 inhibits
cell proliferation, invasion and regulates cell cycle by targeting
tetraspanin 1 in human colorectal carcinoma. Oncotarget.
5:12083–12096. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu G, Li H, Wang X, Wu T, Zhu J, Huang S,
Wan Y and Tang J: MicroRNA-19a targets tissue factor to inhibit
colon cancer cells migration and invasion. Mol Cell Biochem.
380:239–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen MB, Yang L, Lu PH, Fu XL, Zhang Y,
Zhu YQ and Tian Y: MicroRNA-101 down-regulates sphingosine kinase 1
in colorectal cancer cells. Biochem Biophys Res Commun.
463:954–960. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meyyappan M, Wong H, Hull C and Riabowol
KT: Increased expression of cyclin D2 during multiple states of
growth arrest in primary and established cells. Mol Cell Biol.
18:3163–3172. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Becker KA, Ghule PN, Lian JB, Stein JL,
van Wijnen AJ and Stein GS: Cyclin D2 and the CDK substrate p220
(NPAT) are required for self-renewal of human embryonic stem cells.
J Cell Physiol. 222:456–464. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang J, Yang M, Li Y and Han B: The role
of MicroRNAs in the chemoresistance of breast cancer. Drug Dev Res.
76:368–374. 2015. View Article : Google Scholar : PubMed/NCBI
|