Introduction
Non-alcoholic fatty liver disease (NAFLD) represents
a range of liver diseases, between steatosis and non-alcoholic
steatohepatitis and liver cirrhosis (1), and is considered a public health
problem (2). Evidence suggests
that NAFLD is more common in patients with diabetes compared with
the general population by approximately threefold (3). Prospective studies have revealed that
diabetes mellitus (DM) is an independent risk factor for NAFLD
development and liver-associated mortality (4,5).
A number of mechanisms are shared between NAFLD and
DM, including insulin resistance, hyperinsulinemia, and increased
oxidative stress and inflammation. Among them, oxidative stress and
inflammation serve an important role (6). In DM with insulin resistance,
increased lipolysis occurs, generating more free fatty acids
(FFAs). FFAs may be oxidized in the mitochondria, and increased FFA
oxidation leads to the generation of more reactive oxygen species
(ROS), which can trigger oxidative stress injuries. Therefore, the
study and development of novel antioxidant drugs to neutralize free
radicals and to reduce their biomolecular effects may provide
potential options for the prevention and treatment of NAFLD.
Molecular hydrogen (H2) is a colorless,
odorless and tasteless diatomic gas. It has previously been
reported that H2 could ameliorate oxidative stress
injuries by selectively scavenging peroxynitrite (ONOO−)
and hydroxyl radicals (•OH), the two most cytotoxic ROS (7). The therapeutic effects of
H2 have been demonstrated in ischemia-reperfusion
injuries (8,9), transplantation injuries (10) and other injuries associated with
oxidative stress. H2-rich saline has been widely studied
in atherosclerosis, stress-induced nerve damage, type 2 DM (T2DM),
cisplatin-induced renal injury and Parkinson's disease (11–14).
In 2011, Kamimura et al (15) reported that H2 was
beneficial for obesity, DM and energy metabolism in mice.
Considering the roles of oxidative stress in NAFLD injury, the
present study hypothesized that, as a specific free radical
scavenger, H2 could improve NAFLD induced by a
high-sugar and high-fat diet, and evaluated the effects of
H2 on NAFLD as well as the possible underlying
mechanisms.
Materials and methods
Ethics statement
All animal experimental protocols were approved by
the ethics committee of Changhai Hospital affiliated to the Second
Military Medical University (Shanghai, China) and were conducted in
accordance with their guidelines.
Animals and grouping
A total of 24 male Sprague-Dawley rats (age, 10
weeks; weight, 250–280 g) were purchased from the Shanghai
Laboratory Animal Center of the Chinese Academy of Sciences
(Shanghai, China). Rats were housed at 24±2°C under a 12-h
light/dark cycle, and fed ad libitum. All rats received humane care
according to the Guide for the Care and Use of Laboratory Animals
(16). The animals were randomly
divided into three groups: i) The control group (n=8), which was
fed a normal diet (15% kcal from fat), and normal saline (5 ml/kg)
was administered intraperitoneally twice daily; ii) the model group
(n=8), which was fed a high-sugar and high-fat diet, and normal
saline (5 ml/kg) was administered intraperitoneally twice daily;
and iii) the H2-rich saline treatment group (n=8), which
was fed a high-sugar and high-fat diet, and H2-rich
saline (5 ml/kg) was administered intraperitoneally twice daily.
The high-fat diet was prepared according to Li et al
(17) and contained 2%
cholesterol, 7% lard, 8.3% yolk, 16.7% sucrose and 66% standard
diet, which provided 4.66 kcal/g with an energy composition of
31.59% from fat, 51.73% from carbohydrate and 16.68% from
protein.
Animal model establishment
Lipotoxicity was induced by a high-fat diet, and
streptozotocin (STZ; Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) was injected to cause glucotoxicity, which subsequently
led to islet β-cell failure and apoptosis (18). Briefly, a single injection of STZ
(25 mg/kg) was administered via the tail vein in 0.1 mol/l citrate
buffer (pH 4.2) followed by a continuous high-fat and high-sugar
diet for 8 weeks. Subsequently, an oral glucose tolerance test was
performed as described previously (19). Rats from the control and treated
groups received 2 g/kg glucose orally; a 2 h blood glucose result
measuring >1.2 mmol/l or a random blood glucose result measuring
>16.8 mmol/l in rats was considered successful model
establishment.
H2-rich saline
preparation
H2-rich saline was prepared as previously
described (16). Briefly,
H2 gas was dissolved in normal saline for 2 h under 0.4
MPa pressure to saturation. The concentration was measured with gas
chromatography to ensure the hydrogen level was >0.6 mmol.
H2-rich saline was prepared each week and stored at 4°C
in aluminum bags until ready for use.
Determination of serum biochemical
markers, and insulin sensitivity and resistance
Blood was collected from the tail vein at 8 weeks,
and serum alanine transferase (ALT), total bilirubin (TBIL), total
cholesterol (TC), triglycerides (TG), fasting blood glucose (FBG)
and fasting insulin (FINS) levels were determined using a
biochemistry analyzer. In addition, the insulin sensitivity index
(ISI) and homeostasis model assessment-insulin resistance (HOMA-IR)
were calculated as follows: HOMA-IR = (fasting blood glucose ×
fasting insulin) / 22.5; ISI = 1/(fasting blood glucose × fasting
insulin). Insulin tolerance tests were conducted on the three rat
groups as described previously (20).
Hematoxylin and eosin (H&E)
staining
After 8 weeks, the rats were anesthetized using by
an intraperitoneal injection of 10% aqueous solution (0.3 ml/100 g)
of chloral hydrate (Huai'an Xingzhi Biological Technology Co.,
Ltd., Huaian, China) and their livers were harvested, sectioned,
fixed in 4% paraformaldehyde and embedded in paraffin. The paraffin
blocks were then sliced into 4 µm sections and deparaffinized
according to Shi et al (21).
Sections were stained with H&E and images were
captured using a light microscope. A pathologist that was blinded
to the animal groups evaluated the slides and scored each liver
tissue specimen for steatosis, inflammation and fibrosis based on
the criteria proposed by previous studies (22–24).
Briefly, for steatosis: Score 0, none present; score 1, steatosis
<33% of the parenchyma; score 2, steatosis 34–66% of the
parenchyma; score 3, steatosis >67% of the parenchyma. For
inflammation: Score 0, no foci of inflammation; score 1, <1 foci
per two 200x fields; score 2, one foci per two 200x fields to one
foci per one 200x field; score 3, one to two foci per one 200x
field; score 4, >2 foci per one 200x field. For fibrosis: Score
1, zone-3 perisinusoidal fibrosis; score 2, zone-3 perisinusoidal
fibrosis with portal fibrosis; score 3, zone-3 perisinusoidal
fibrosis and portal fibrosis with bridging fibrosis; and score 4,
cirrhosis. The total score (steatosis + inflammation + fibrosis) of
each rat was calculated and the average score for each group was
determined.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) staining
TUNEL staining was performed using an In Situ
Cell Death Detection kit (Roche Diagnostics, Basel, Switzerland).
Liver sections were heated to 60°C for dewaxing and were then
rehydrated according to standard protocols and as previously
reported (21). After cooling to
room temperature, the sections were incubated with 20 µg/ml
proteinase K solution for 20 min followed by incubation in TUNEL
reaction mixture for 1 h at 37°C. Converter-alkaline phosphatase
antibody was then added to the samples for 1 h at 37°C.
Subsequently, sections were washed with PBS three times for 5 min,
followed by color development in the dark with nitroblue
tetrazolium and 5-bromo-4-chloro-3-indolylphosphate. Ten fields
were randomly chosen (x200). The number of apoptotic hepatocytes
was counted in each field and an average was calculated.
Determination of tumor necrosis factor
alpha (TNF-α) and interleukin-1 beta (IL-1β)
Rat livers were collected and washed in normal
saline, and were then homogenized immediately on ice in 1 ml normal
saline at 2–8°C. The homogenates were centrifuged at 3,000 × g at
4°C for 15 min. The expression levels of TNF-α (catalog no. H052)
and IL-1β (catalog no. H002) were measured using commercial ELISA
kits (Nanjing Jianchen Bioengineering Institute, Nanjing, China)
according to the manufacturer's protocol.
Determination of caspase-3 (CASP-3)
activity
Similar methods were used to determine CASP-3
activity as previously described (21). Livers were harvested at 8 weeks,
and CASP-3 activity was determined with the CASP-3/CPP32
Fluorometric Assay kit (BioVision, Inc., Milpitas, CA, USA).
Briefly, hepatocyte lysates were incubated with 10 µg/ml proteinase
K for 30 min at 37°C in a 96-well plate. The peptide substrate
DEVD-AFC (5 µl) was then added to initiate the reaction. After
incubation in the dark at 37°C, a fluorometer was used to read the
plate using a 400-nm excitation filter and a 505-nm emission
filter. Fold increase in CASP-3 activity was calculated by
comparing with the level of the control.
Determination of 3-nitrotyrosine
(3-NT) and 8-hydroxy-2′-deoxyguanine (8-OHdG)
Similar methods were employed as previously reported
(25). Liver sections were
deparaffinized in xylene, rehydrated with ethanol and pretreated
with 10 µg/ml proteinase K for 30 min at 37°C. Subsequently, the
sections were incubated in 10% bovine serum albumin (Nanjing
Jianchen Bioengineering Institute) for 20 min. After overnight
incubation at room temperature with anti-8-OHdG (1:200; catalog no.
ZY-1131R) and anti-3-NT antibodies (1:200; catalog no. 28-60252P)
from Heyzer Ye Biological Technology Co., Ltd. (Shanghai, China).
An alkaline phosphatase-conjugated secondary antibody (1:500;
catalog no. E030210; Shanghai Yanhua Bio-Tech Co., Ltd., Shanghai,
China) were added for 1 h at 37°C, and the sections were incubated
with diaminobenzidine. The number of 8-OHdG- and 3-NT-positive
cells were counted under a light microscope, and integral optical
densities were calculated with Image-Pro Plus software version 6
(Media Cybernetics, Inc., Rockville, MD, USA).
The concentrations of 3-NT and 8-OHdG in the liver
were determined using commercial ELISA kits [catalog nos.
JK-(a)-5053 and JK-(a)-1571; Shanghai Jinkang Medicine Technology
Co., Ltd., Shanghai, China], according to the manufacturer's
protocol. Liver tissues were homogenized in 2 ml 10 mM
phosphate-buffered saline (pH 7.4). After centrifugation at 10,000
× g for 30 min at 37°C, the levels of 3-NT and 8-OHdG in the
supernatant were measured using the corresponding kits.
Determination of the expression levels
of peroxisome proliferator-activated receptor (PPAR)α and
PPARγ
The protein expression levels of PPARα and PPARγ in
the liver were examined using immunohistochemistry. The
transcription of PPARα and PPARγ were determined by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
Total RNA was extracted using TRIzol® reagent according
to the manufacturer's protocol (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The RT reaction was performed
using the Superscript II Reverse Transcriptase kit (Invitrogen;
Thermo Fisher Scientific, Inc.) in a 20 µl volume with 1 µg total
RNA; RT was conducted at 16°C for 30 min, 42°C for 42 min and 85°C
for 5 min. The RT product (1 µl cDNA, corresponding to 6.25 ng RNA)
was used to conduct subsequent qPCR analyses in an ABI Prism 7300
Sequence Detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.), with the SYBR® Green PCR kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Each experiment was
performed in duplicate for each gene under the following cycling
conditions: Initial template denaturation at 95°C for 5 min,
followed by 40 cycles at 95°C for 10 sec, 60°C for 20 sec, 72°C for
20 sec and 78°C for 20 sec, and a final 10 min extension step at
72°C. The primer sequences were as follows: PPARα, forward
5′-GGTCTTAACCGGCCC-3′, reverse 5′-AAACGCAACGTAGAG-3′; PPARγ,
forward 5′-CTGTTTTATGCTGTTATGGGTGAAA-3′, and reverse
5′-GCACCATGCTCTGGGTCAA-3′; and GAPDH, forward
5′-TGGGTGTGAACCACGAGAA-3′, and reverse 5′-GGCATGGACTGTGGTCATGA-3′.
The ΔΔCq method was used to to normalize mRNA expression levels to
those of GAPDH (26).
Statistical analysis
All data are presented as the mean ± standard
deviation (n=8) and were analyzed with SPSS 9.1 software (SPSS,
Inc., Chicago, IL, USA). Differences between groups were compared
with one-way analysis of variance followed by the
Student-Newman-Keuls post hoc test. Glucose tolerance and insulin
tolerance test results were analyzed by mixed model for repeatedly
measured data for multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
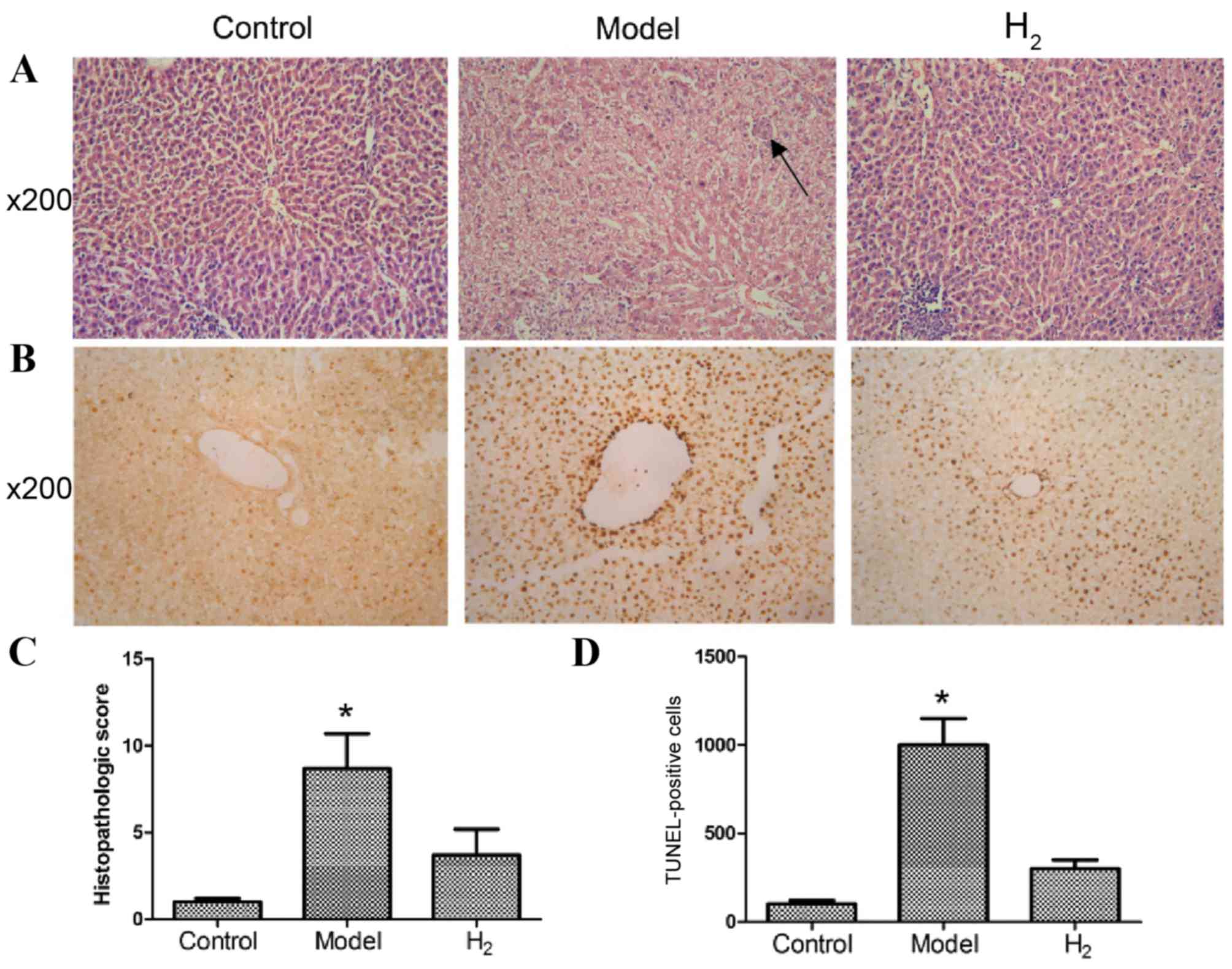
H&E staining of the liver
H&E staining was used to observe liver
morphology by microscopy (Fig. 1).
As presented in Fig. 1A, rats in
the control group demonstrated a normal architecture with
hepatocytes arranged in plates aligned to sinusoids converging to
centrilobular veins. Rats in the model group, which were fed a
high-sugar and high-fat diet, presented with moderate to severe
steatosis, ballooning degeneration, piecemeal necrosis and
inflammatory cell infiltration. In addition, early fibrosis was
detected. In the H2 group, a relatively normal
histologic architecture was observed, with little steatosis,
ballooning degeneration, necrosis and inflammatory cell
infiltration. The average histopathological scores are presented in
Fig. 1C. Scores in the model group
were significantly higher than those in the control and
H2 groups (P<0.05 model group vs. control and
H2 groups).
TUNEL staining
For analysis of apoptosis, TUNEL staining results
are presented in Fig. 1B. At a
magnification of ×200, the nuclei of hepatocytes were clearly
stained. Some occasional TUNEL-positive cells were detected in the
control group. Significantly more apoptotic cells were present in
the model group, in which the apoptotic cells were significantly
shrunken and the nuclei were strongly stained. In the H2
group, the number of apoptotic cells was significantly smaller than
that in the model group (Fig. 1D;
P<0.05 model group vs. control and H2 groups).
Serum biochemical markers
To assess lipid metabolism and liver function, serum
levels of ALT, TBIL, TC and TG were measured at 8 weeks. ALT and
TBIL levels in the model group were slightly higher compared with
the control and H2 groups (Fig. 2A and B); however, the differences
were not statistically significant (P=0.08). The TC and TG levels
in the model group were significantly higher compared with those in
the control and H2 groups (Fig. 2C and D; P<0.05 model group vs.
control and H2 groups).
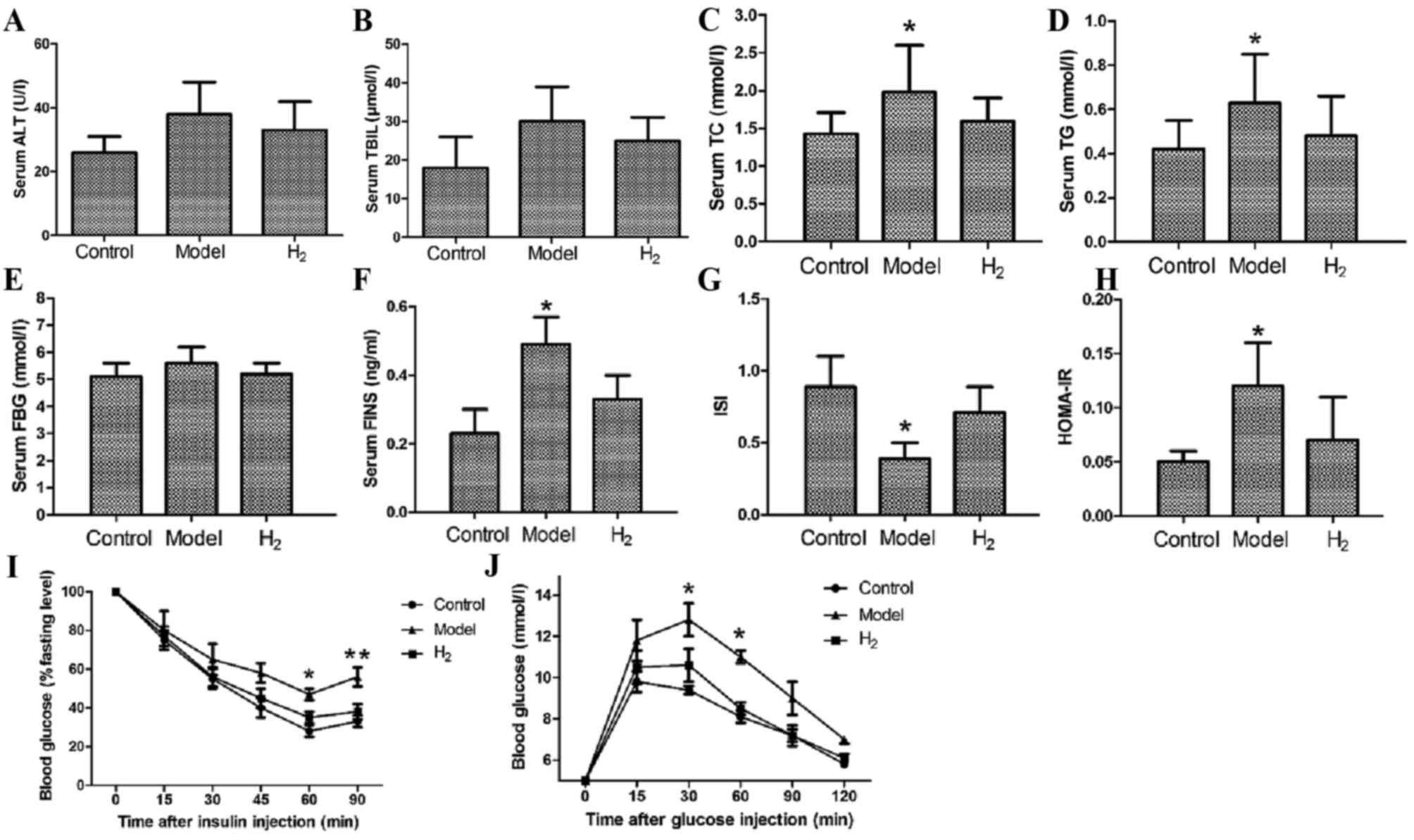 | Figure 2.Biochemical marker analysis, insulin
tolerance and glucose tolerance tests. (A) Serum ALT and (B) TBIL
levels were higher in the model group than in the control and
H2 groups. The difference was not statistically
significant. H2 significantly decreased serum levels of
(C) TC and (D) TG compared with the model group. Serum levels of
(E) FBG and (F) FINS. (G) Results of the ISI and HOMA-IR
measurements. (I) Insulin tolerance test; after insulin
administration, blood glucose gradually decreased. (J) Glucose
tolerance test; after glucose intake, blood glucose gradually
increased and reached its peak at 30 min and then decreased.
*P<0.05 vs. control and H2 groups; **P<0.01 vs.
control and H2 groups. ALT, alanine transferase; FBG,
fasting blood glucose; FINS, fasting insulin; H2,
molecular hydrogen; HOMA-IR, homeostasis model assessment-insulin
resistance; ISI, insulin sensitivity index; TBIL, total bilirubin;
TC, total cholesterol; TG, triglycerides. |
At 8 weeks, the serum FBG and FINS levels were also
examined to assess the function of pancreas islets (Fig. 2E and F). For FBG, the differences
between the model group and the control or H2 groups
were not statistically significant; for FINS, the difference was
statistically significant (P<0.05 model group vs. control and
H2 groups).
Finally, the ISI and HOMA-IR were calculated to
assess insulin resistance, and it was revealed that in the
H2 group, when compared to the model group, the ISI was
significantly elevated and the HOMA-IR was significantly reduced
(P<0.05 H2 vs. model group).
Insulin sensitivity and glucose
tolerance tests
At 8 weeks, the insulin tolerance test and glucose
tolerance test were performed. The insulin tolerance test was
performed to assess pituitary function and adrenal function, and as
presented in Fig. 2I, following
injection of insulin serum glucose levels gradually decreased over
time. The rate of decrease in the model group was significantly
lower than that in the other two groups. At 60 and 90 min after the
injection, the difference was statistically significant (P<0.05
model group vs. control and H2 groups, P<0.01 model
group vs. control and H2 groups, respectively). The
glucose tolerance test was performed to diagnose diabetes, insulin
resistance and impaired beta cell function. As presented in
Fig. 2J, following glucose intake
blood glucose gradually increased, reached a peak at 30 min and
subsequently decreased (*P<0.05 model vs. control and
H2 groups).
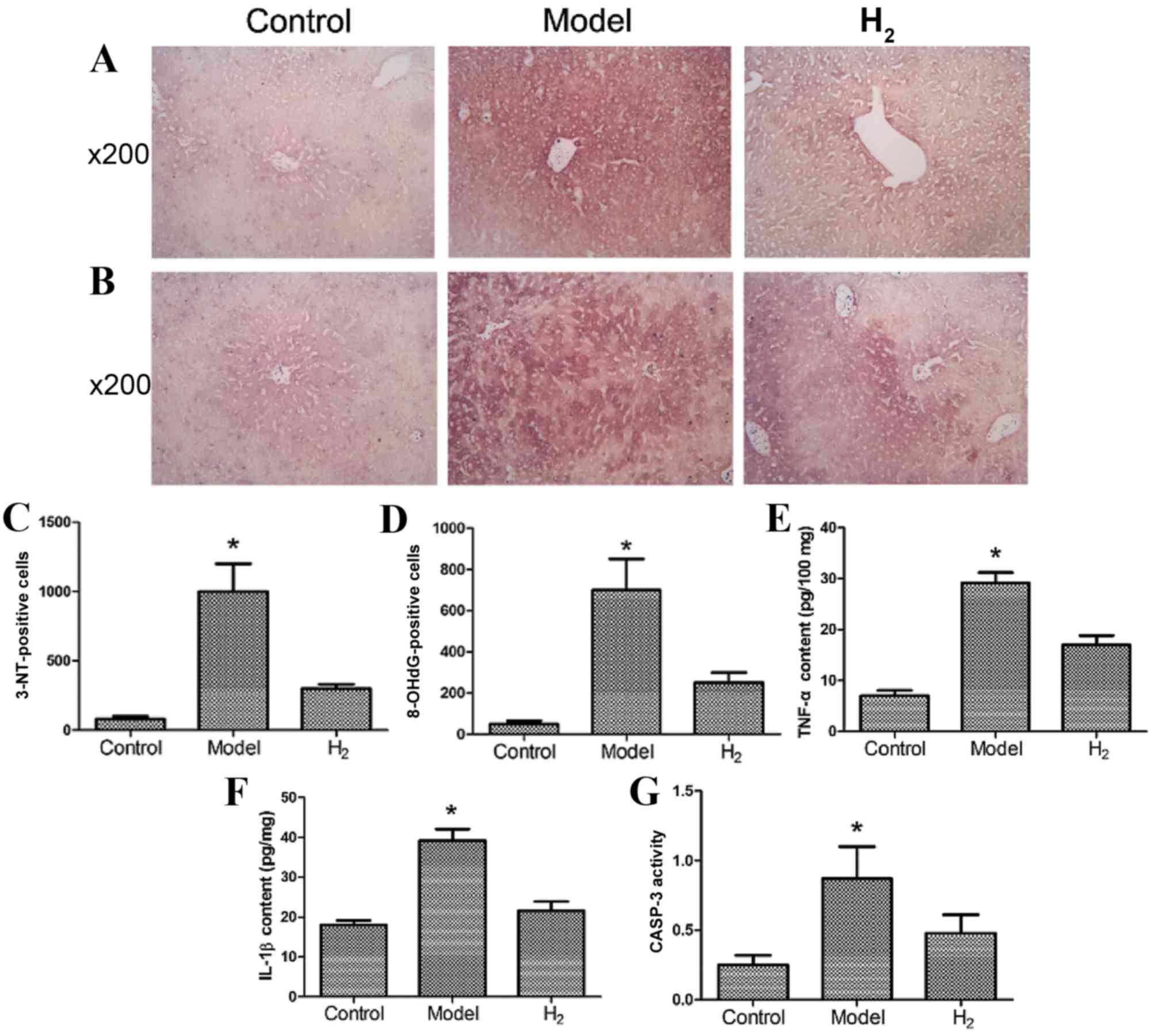
Determination of 3-NT and 8-OHdG in
liver cells
3-NT, a biochemical marker of peroxynitrite, and
8-OHdG, a product of free radical oxidative damage to DNA, have
been reported to be ameliorated by H2 (25). The number of 3-NT- and
8-OHdG-positive cells were detected and are presented in Fig. 3A-D. The model group had more
3-NT-positive cells (Fig. 3A and
C) and more 8-OHdG-positive cells (Fig. 3B and D) compared with the control
and H2 groups (P<0.05 model group vs. control and
H2 groups).
TNF-α, IL-1β and CASP-3 in the
liver
The levels of the inflammatory cytokines TNF-α
(Fig. 3E) and IL-1β (Fig. 3F) in the model group were
significantly greater compared with the control and H2
groups. CASP-3 activity in the model group was significantly
greater compared with the control and H2 groups
(Fig. 3G; P<0.05 model group
vs. control and H2 groups).
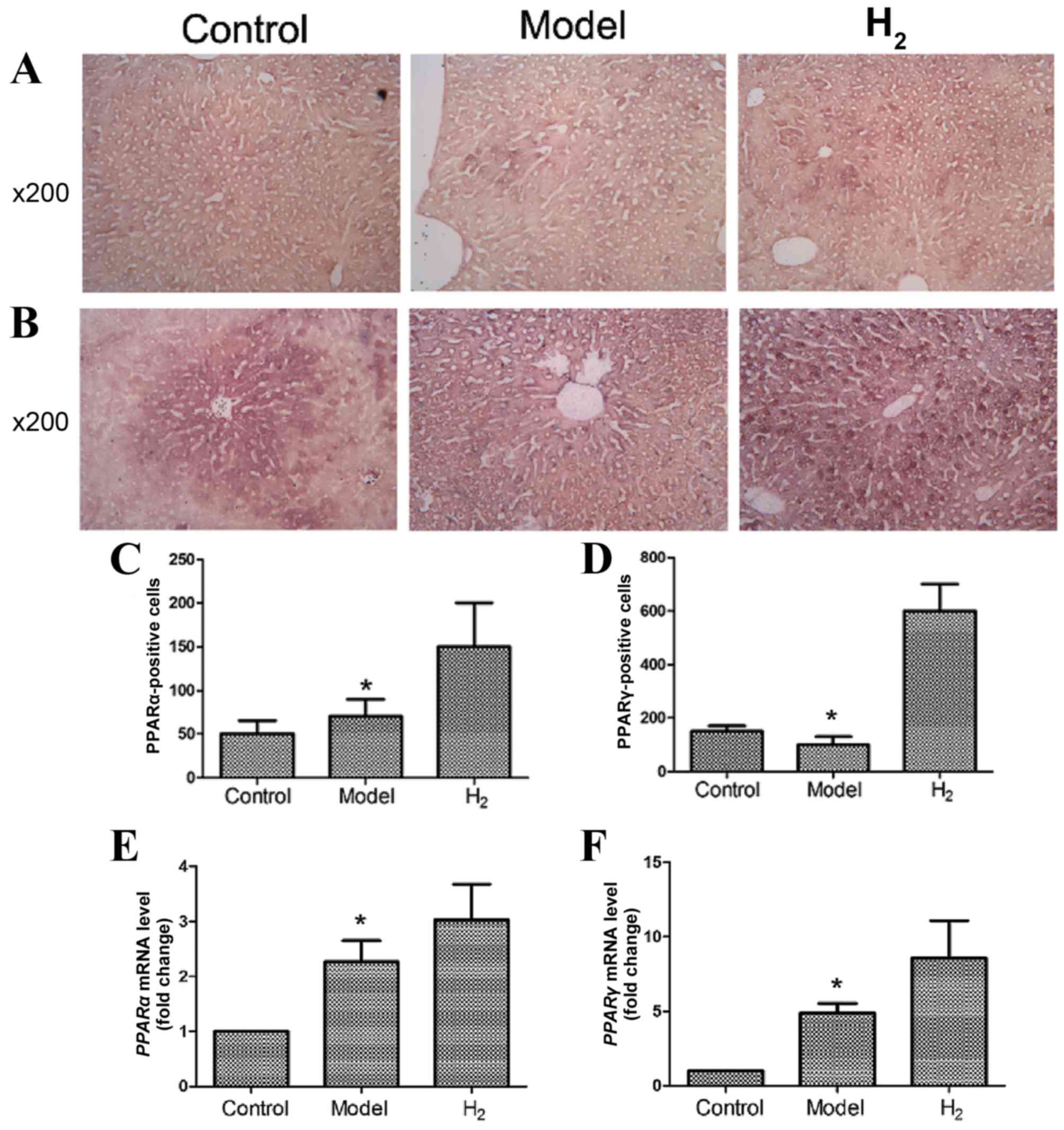
PPARα and PPARγ expression in the
liver
PPARα and PPARγ protein expression was detected
using immunohistochemistry (Fig.
4A-D). As shown in Fig 4A and
C, PPARα protein expression was enhanced in the H2
group compared with the control and model groups. As shown in
Fig. 4B and D, PPARγ protein
expression was also increased in the H2 group compared
with the control and model groups. These differences were
statistically significant. The immunohistochemical results were
confirmed by RT-qPCR (Fig. 4E and
F; P<0.05 model vs. H2 group).
Discussion
The present study demonstrated that
H2-rich saline significantly ameliorated NAFLD, as
demonstrated by reduced serum ALT, TBIL, TC, TG, FBG and FINS
levels, improved insulin sensitivity and glucose tolerance, and
reduced hepatocyte apoptosis, inflammation and oxidative stress.
The mechanism may possibly function by upregulating the expression
of PPARα and PPARγ.
The key pathophysiological process associated with
NAFLD is insulin resistance, which is common in T2DM. Insulin
resistance within adipocytes causes lipolysis, which subsequently
results in the generation of excess FFAs that are released into
circulation and finally delivered to the liver. When the import and
synthesis of FFAs exceeds the capacity of the liver to use them,
hepatic steatosis occurs. Excessive FFAs are metabolized within the
mitochondria, peroxisomes and microsomal system, which leads to
lipid peroxidation, production of excess ROS and initiation of the
inflammatory response. At present, the ‘two-hit’ theory of
steatohepatitis pathogenesis, proposed by Day and James in 1998, is
widely accepted (27). The theory
separates the pathogenesis into two ‘hits’. The first hit is lipid
accumulation due to insulin resistance and hyperlipidemia, which
leaves the hepatocytes more vulnerable to injuries. Oxidative
stress and the inflammatory response serve an important role in the
second hit, which directly leads to hepatocyte injury. Therefore,
NAFLD is a downstream effect of oxidative stress (28). Reduction of the overproduced ROS
may theoretically improve NAFLD damage.
As an antioxidant, H2 has been reported
to scavenge •OH and ONOO−, the two most toxic ROS in
cells. In addition, H2 protects organs from tissue
damage caused by severe oxidative stress induced by inflammation,
intense exercise, cardiac infarction, cessation of blood flow and
organ transplantation, among others (17). H2 is highly flammable,
and it is not safe to preserve and use H2 for clinical
practice and laboratory experiments. Therefore, H2 gas
is often dissolved in normal saline under high pressure to produce
H2-rich saline, which exerts similar protective effects
(29). Notably, it is safer and
more convenient in practice.
The pathophysiological effects of DM are closely
associated with oxidative stress (30), and several studies have examined
the antidiabetic effects of H2. Kim and Kim (31) reported that electrolyzed reduced
water with ROS scavenging ability had a potential effect on
diabetic animals, significantly reducing blood glucose
concentration and improving glucose tolerance. Kajiyama et
al (12) demonstrated that in
patients with T2DM, intake of H2-rich water was
associated with significant decreases in the levels of modified
low-density lipoprotein (LDL) cholesterol, small dense LDL and
urinary 8-isoprostanes by 15.5, 5.7 and 6.6%, respectively, and
concluded that supplementation with H2-rich water may
have a beneficial role in the prevention and treatment of T2DM and
insulin resistance. Kamimura et al (15) reported that H2 improved
obesity and DM by inducing the expression of hepatic fibroblast
growth factor 21 (FGF21) and improving energy metabolism in
diabetic db/db mice, which is an important finding in the
study of the mechanism of antioxidative effects of H2.
The present study revealed that H2-rich saline
ameliorated hepatic oxidative stress, which was demonstrated by a
reduction in the levels of TNF-α, IL-1β, 3-NT and 8-OHdG in the
liver.
The three PPAR subtypes (PPARα, PPARβ and PPARγ)
belong to the nuclear receptor superfamily of transcription
factors, and their response elements are located in the promoter
region of FGF21. PPARα and PPARγ are two important upstream
regulators of FGF21. PPARα is predominantly expressed in the liver
and regulates lipid metabolism through activating the expression of
various proteins, such as lipoprotein lipase and diacylglycerol
acyltransferase. PPARγ is mainly found in adipose and hepatic
tissue, and its activation serves a major role in increasing
insulin sensitivity (32).
The present study demonstrated that
H2-rich saline significantly increased the expression
levels of PPARα and PPARγ, and significantly improved glucose and
lipid metabolism, as demonstrated by reduced serum levels of TC and
TG, and improved insulin sensitivity and glucose tolerance. The
present study also examined the serum levels of ALT and TBIL.
Although H2 lowered the levels of ALT and TBIL, the
difference was not statistically significant compared with the
model group. The present study attributed this result to the
powerful compensatory capacity of the liver and the relatively
short experimental time. It is expected that, if the experiment
were conducted for a longer period of time and the damages to the
liver went beyond the hepatic compensatory ability, liver function
may be compromised.
Upon activation, PPARα and PPARγ synergistically
inhibit fatty acid synthesis by regulating the expression of sterol
regulatory element-binding protein-1c and its target gene fatty
acid synthase (33). PPARα and
PPARγ activation exerts anti-inflammatory effects on the liver by
reducing hepatic steatosis, downregulating the expression of
inflammatory genes and attenuating inflammation in adipose tissue
(34). Furthermore, PPARα and
PPARγ could regulate the production of free radicals by increasing
the expression of superoxide dismutase and reducing NADPH oxidase
activity (35,36).
In conclusion, the protective effects of
H2 on high-sugar and high-fat diet-induced NAFLD may be
attributed to its direct antioxidative properties, as well as its
activation of PPARα and PPARγ. With regards to PPAR activation,
H2-rich saline shares the same mechanism as
rosiglitazone, which is a potent PPARγ receptor activator. Although
further studies are required, H2-rich saline may be
considered a potential agent in the prevention and treatment of
NAFLD.
Acknowledgements
The authors would like to thank Professor Yan Zhang
from the Department of Foreign Language Teaching of the Second
Military Medical University for her assistance with the grammar and
readability of the manuscript. This study is supported by the
Changhai 1255 Scientific Research Project (grant no.
CH125541900).
References
|
1
|
Adams LA, Lymp JF, St Sauver J, Sanderson
SO, Lindor KD, Feldstein A and Angulo P: The natural history of
nonalcoholic fatty liver disease: A population-based cohort study.
Gastroenterology. 129:113–121. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Angulo P: Nonalcoholic fatty liver
disease. N Engl J Med. 346:1221–1231. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clark JM: The epidemiology of nonalcoholic
fatty liver disease in adults. J Clin Gastroenterol. 40:(Suppl 1).
S5–S10. 2006.PubMed/NCBI
|
|
4
|
Ekstedt M, Franzén LE, Mathiesen UL,
Thorelius L, Holmqvist M, Bodemar G and Kechagias S: Long-term
follow-up of patients with NAFLD and elevated liver enzymes.
Hepatology. 44:865–873. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Porepa L, Ray JG, Sanchez-Romeu P and
Booth GL: Newly diagnosed diabetes mellitus as a risk factor for
serious liver disease. CMAJ. 182:E526–E531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giacco F and Brownlee M: Oxidative stress
and diabetic complications. Circ Res. 107:1058–1070. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohsawa I, Ishikawa M, Takahashi K,
Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S
and Ohta S: Hydrogen acts as a therapeutic antioxidant by
selectively reducing cytotoxic oxygen radicals. Nat Med.
13:688–694. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hayashida K, Sano M, Ohsawa I, Shinmura K,
Tamaki K, Kimura K, Endo J, Katayama T, Kawamura A, Kohsaka S, et
al: Inhalation of hydrogen gas reduces infarct size in the rat
model of myocardial ischemia-reperfusion injury. Biochem Biophys
Res Commun. 373:30–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fukuda KI, Asoh S, Ishikawa M, Yamamoto Y,
Ohsawa I and Ohta S: Inhalation of hydrogen gas suppresses hepatic
injury caused by ischemia/reperfusion through reducing oxidative
stress. Biochem Biophys Res Commun. 361:670–674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Buchholz BM, Kaczorowski DJ, Sugimoto R,
Yang R, Wang Y, Billiar TR, McCurry KR, Bauer AJ and Nakao A:
Hydrogen inhalation ameliorates oxidative stress in transplantation
induced intestinal graft injury. Am J Transplant. 8:2015–2024.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ohsawa I, Nishimaki K, Yamagata K,
Ishikawa M and Ohta S: Consumption of hydrogen water prevents
atherosclerosis in apolipoprotein E knockout mice. Biochem Biophys
Res Commun. 377:1195–1198. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kajiyama S, Hasegawa G, Asano M, Hosoda H,
Fukui M, Nakamura N, Kitawaki J, Imai S, Nakano K, Ohta M, et al:
Supplementation of hydrogen-rich water improves lipid and glucose
metabolism in patients with type 2 diabetes or impaired glucose
tolerance. Nutr Res. 28:137–143. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sato Y, Kajiyama S, Amano A, Kondo Y,
Sasaki T, Handa S, Takahashi R, Fukui M, Hasegawa G, Nakamura N, et
al: Hydrogen-rich pure water prevents superoxide formation in brain
slices of vitamin C-depleted SMP30/GNL knockout mice. Biochem
Biophys Res Commun. 375:346–350. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fu Y, Ito M, Fujita Y, Ito M, Ichihara M,
Masuda A, Suzuki Y, Maesawa S, Kajita Y, Hirayama M, et al:
Molecular hydrogen is protective against 6-hydroxydopamine-induced
nigrostriatal degeneration in a rat model of Parkinson's disease.
Neurosci Lett. 453:81–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kamimura N, Nishimaki K, Ohsawa I and Ohta
S: Molecular hydrogen improves obesity and diabetes by inducing
hepatic FGF21 and stimulating energy metabolism in db/db mice.
Obesity (Silver Spring). 19:1396–1403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garber J, Barbee R, Bielitzki J, Clayton
L, Donovan J, Hendriksen C, Kohn D, Lipman N, Locke P, Melcher J,
et al: Guide for the Care and Use of Laboratory Animals. 8th.
Washington (DC): National Academies Press (US); 2011, PubMed/NCBI
|
|
17
|
Li L, Chen L, Hu L, Liu Y, Sun HY, Tang J,
Hou YJ, Chang YX, Tu QQ, Feng GS, et al: Nuclear factor
high-mobility group box 1 mediating the activation of Toll-like
receptor 4 signaling in hepatocytes in the early stage of
nonalcoholic fatty liver disease in mice. Hepatology. 54:1620–1630.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nugent DA, Smith DM and Jones HB: A review
of islet of Langerhans degeneration in rodent models of type 2
diabetes. Toxicol Pathol. 36:529–551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Agwaya MS, Vuzi PC and Nandutu AM:
Hypoglycemic activity of aqueous root bark extract zanthoxylum
chalybeum in alloxan-induced diabetic rats. J Diabetes Res.
2016:87275902016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De Oliveira JC, Ludemann Camargo R,
Barella LF, Chaves Souto Branco R, Gravena C, Grassiolli S,
Torrezan R and De Cezar Freitas Mathias P: Anesthetic-induced
transient hyperglycemia and insulin resistance do not depend on the
sympathoadrenal axis. Minerva Endocrinol. 38:379–388.
2013.PubMed/NCBI
|
|
21
|
Shi J, Yao F, Zhong C, Pan X, Yang Y and
Lin Q: Hydrogen saline is protective for acute lung
ischaemia/reperfusion injuries in rats. Heart Lung Circ.
21:556–563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brunt EM, Janney CG, Di Bisceglie AM,
Neuschwander-Tetri BA and Bacon BR: Nonalcoholic steatohepatitis: A
proposal for grading and staging the histological lesions. Am J
Gastroenterol. 94:2467–2474. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Knodell RG, Ishak KG, Black WC, Chen TS,
Craig R, Kaplowitz N, Kiernan TW and Wollman J: Formulation and
application of a numerical scoring system for assessing
histological activity in asymptomatic chronic active hepatitis.
Hepatology. 1:431–435. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee GS, Yan JS, Ng RK, Kakar S and Maher
JJ: Polyunsaturated fat in the methionine-choline-deficient diet
influences hepatic inflammation but not hepatocellular injury. J
Lipid Res. 48:1885–1896. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhai X, Chen X, Shi J, Shi D, Ye Z, Liu W,
Li M, Wang Q, Kang Z, Bi H and Sun X: Lactulose ameliorates
cerebral ischemia-reperfusion injury in rats by inducing hydrogen
by activating Nrf2 expression. Free Radic Biol Med. 65:731–741.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C (T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Day CP and James OF: Steatohepatitis: A
tale of two ‘hits’? Gastroenterology. 114:842–845. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leite NC, Villela-Nogueira CA, Cardoso CR
and Salles GF: Non-alcoholic fatty liver disease and diabetes: From
physiopathological interplay to diagnosis and treatment. World J
Gastroenterol. 20:8377–8392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cai J, Kang Z, Liu K, Liu W, Li R, Zhang
JH, Luo X and Sun X: Neuroprotective effects of hydrogen saline in
neonatal hypoxia-ischemia rat model. Brain Res. 1256:129–137. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Suzuki H, Kayama Y, Sakamoto M, Iuchi H,
Shimizu I, Yoshino T, Katoh D, Nagoshi T, Tojo K, Minamino T, et
al: Arachidonate 12/15-lipoxygenase-induced inflammation and
oxidative stress are involved in the development of Diabetic
Cardiomyopathy. Diabetes. 64:618–630. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim MJ and Kim HK: Anti-diabetic effects
of electrolyzed reduced water in streptozotocin-induced and genetic
diabetic mice. Life Sci. 79:2288–2292. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao X, Xue J, Wang XL, Zhang Y, Deng M
and Xie ML: Involvement of hepatic peroxisome
proliferator-activated receptor α/γ in the therapeutic effect of
osthole on high-fat and high-sucrose-induced steatohepatitis in
rats. Int Immunopharmacol. 22:176–181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Konig B, Koch A, Spielmann J, Hilgenfeld
C, Hirche F, Stangl GI and Eder K: Activation of PPARalpha and
PPARgamma reduces triacylglycerol synthesis in rat hepatoma cells
by reduction of nuclear SREBP-1. Eur J Pharmacol. 605:23–30. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stienstra R, Mandard S, Patsouris D, Maass
C, Kersten S and Muller M: Peroxisome proliferator-activated
receptor alpha protects against obesity-induced hepatic
inflammation. Endocrinology. 148:2753–2763. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim JC, Lee YH, Yu MK, Lee NH, Park JD,
Bhattarai G and Yi HK: Anti-inflammatory mechanism of PPARγ on
LPS-induced pulp cells: Role of the ROS removal activity. Arch Oral
Biol. 57:392–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Inoue I, Goto S, Matsunaga T, Nakajima T,
Awata T, Hokari S, Komoda T and Katayama S: The ligands/activators
for peroxisome proliferator-activated receptor alpha (PPARalpha)
and PPARgamma increase Cu2+,Zn2+-superoxide dismutase and decrease
p22phox message expressions in primary endothelial cells.
Metabolism. 50:3–11. 2001. View Article : Google Scholar : PubMed/NCBI
|