Introduction
Osteosarcoma is one of the most common malignant
bone tumors that affects adolescents and children worldwide.
Frequent acquisition of drug resistance is often associated with
the chemotherapeutic treatment of osteosarcoma (1). Therefore, there is an urgent
requirement to elucidate the underlying molecular mechanisms of
chemoresistance in osteosarcoma, in order to facilitate the
development of therapeutic strategies for patients.
Previous studies have demonstrated that autophagy is
usually activated as a protective mechanism in tumor cells against
numerous chemotherapeutics. Limited efficacy of doxorubicin (Dox),
cisplatin (DDP) and methotrexate due to the induction of autophagy
has been certified in osteosarcoma cells (2–4).
Autophagy is a highly conserved process that entails the
degradation of intracellular components to regenerate metabolites
for energy and growth through the lysosomal machinery (5). However, the molecular mechanisms
underlying autophagy-mediated chemotherapy resistance of
osteosarcoma cells remain largely unknown.
MicroRNAs (miRNAs; miRs) are single-stranded, small
noncoding RNAs ~22 nucleotides in length, which negatively regulate
gene expression through base-pairing to the 3′-untranslated region
(3′UTR) of target mRNA (6,7). miRNAs are involved in crucial
biological processes, and the dysregulation of miRNAs is associated
with tumorigenesis and the development of numerous malignancies,
including osteosarcoma (8).
Previous studies have investigated the role of miR-410 in several
types of cancer (9–11); however, the role of miR-410 in
osteosarcoma remains unknown.
The present study demonstrated that miR-410
expression was downregulated in osteosarcoma cell lines and
tissues. Target prediction algorithms (TargetScan and miRanda)
indicated that autophagy related 16-like 1 (ATG16L1) was a
potential target gene of miR-410. ATG16L1 is a component of a large
protein complex essential for autophagy, and autophagy is a tightly
regulated intracellular catabolic pathway involving the lysosomal
degradation of cytoplasmic organelles and proteins. Previous
studies have demonstrated miRNAs mediate the regulation of proteins
in the autophagy pathway, and autophagy is commonly activated as a
protective mechanism when cancer cells were exposed to three
commonly used anticancer drugs (12–14).
In the current study, it was demonstrated that miR-410
overexpression is able to improve the therapeutic response of cells
to chemotherapy [including rapamycin (Rap), Dox and DDP].
Furthermore, miR-410 could enhance the chemosensitivity of those
drugs via autophagy inhibition through targeting ATG16L1 in
osteosarcoma cells. The present study provided an insight into the
biological function of miR-410 in osteosarcoma and offered a
promising approach for future osteosarcoma treatment.
Materials and methods
Osteosarcoma tissues, cell lines and
transfection
U2OS and MG-63 human osteosarcoma cell lines, the
hFOB 1.19 human osteoblast cell line, and human embryonic kidney
(HEK)293T cells were purchased from the Cell Resource Center of
Institute of Basic Medical Sciences, Chinese Academy of Medical
Sciences and Peking Union Medical College (Beijing, China). The
cells were cultured in Dulbecco's modified Eagle's medium
(GlutaMAX™; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (12483–020; Thermo
Fisher Scientific, Inc.), 100 mg/ml streptomycin and 100 IU/ml
penicillin (Thermo Fisher Scientific, Inc.) at 37°C in an
atmosphere containing 5% CO2. Transfection was performed
when the cells had grown to 80% confluence, using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. A total of 40 paired tumor tissues and
matched non-tumor tissues were obtained from patients with
osteosarcoma at Renmin Hospital of Wuhan University (Wuhan, China)
between 2013 and 2015. All tissues were immediately stored in
liquid nitrogen until further use. Histological diagnosis of the
tumors was made and agreed upon by two senior pathologists at the
Department of Pathology (Renmin Hospital of Wuhan University) based
on World Health Organization criteria. The present study was
approved by the Institutional Review Board and Human Ethics
Committee of Renmin Hospital of Wuhan University.
Reagents and antibodies
Reagents used were as follows:
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
M5655; Sigma-Aldrich, St. Louis, MO, USA) and Hoechst 3342 (B2261;
Sigma-Aldrich), both of which were dissolved in phosphate-buffered
saline (PBS). The following antibodies were obtained:
Anti-microtubule-associated protein 1A/1B-light chain 3 (LC3)I/II
(1:1,000; ab58610; Abcam, Cambridge, MA, USA), anti-caspase 3
(1:1,000; ab2171; Abcam), anti-ATG161L (1:1,000; 8089S; Cell
Signaling Technology, Inc., Danvers, MA, USA) and anti-β-actin
(1:2,000; ab6276; Abcam). 3-methyladenine (3-MA; M9281;
Sigma-Aldrich); Rap (37094; Sigma-Aldrich); Dox (D1515;
Sigma-Aldrich). DDP (Chengdu Organic chemicals Co., Ltd., Wuhou,
China). All drugs were dissolved in dimethyl sulfoxide and were
stored at −20°C until the cells were treated with the drugs for 48
h (Rap, 20 nM; Dox, 0.1 µg/ml; DDP, 10 µM).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Tissues were homogenized prior to RNA extraction.
Total RNA was extracted from the cells and tissues using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), and was reverse transcribed using Revert Aid First Strand
cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. qPCR was performed using the Maxima
SYBR Green/Fluorescein qPCR Master Mix (Thermo Fisher Scientific,
Inc.) on the Real-Time PCR Detection system (iQ5; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The cDNA templates were
diluted to 1:10 and 20 µl per reaction (SYBR Green Mix 10 µl,
forward primer 2 µl, reverse primer 2 µl, RNase Free
dH2O 4.5 µl) was analyzed. The PCR conditions were as
follows: DNA denaturation at 94°C for 4 min, followed by 40 cycles
of amplification at 94°C for 40 sec, 52°C for 40 sec and 72°C for
40 sec for data collection. The Bulge-Loop™ hsa-miR-410 qRT-PCR
primer set (miRQ0002171-1-2) was purchased from Guangzhou RiboBio
Co., Ltd (Guangzhou, China). The qPCR experiments were run three
times on separate days, each with independent biological samples
(n=4 per group); within each experiment run, relative expression
values were normalized to the standard deviations from the mean.
The relative expression levels of miR-410 were normalized to those
of the internal control gene U6 using the 2-ΔΔCq cycle
quantification method (15).
Cell transfection
The sequences of miR-410 were retrieved from the
miRNA database miRBase (www.mirbase.org) (AAUAUAACACAGAUGGCCUGU). miR-410
(miR-410) and negative control (NC) mimics were purchased from
Guangzhou RiboBio Co., Ltd. and were transfected into the U2OS and
MG-63 cells using Lipofectamine 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) or 24 h or 48 h in different experiments.
qPCR assays were used to detect the expression levels of miR-410
subsequent to each transfection.
Cell proliferation and apoptosis
assays
Cells were seeded in 96-well culture plates at 30%
confluence 1 day prior to transfection. Post-transfection with
miR-410 or NC the cells were evaluated using an MTT assay. The MTT
assay was used to determine relative cell growth, according to the
manufacturer's protocol. Subsequently, medium was replaced with 0.1
ml dimethyl sulfoxide (Sigma-Aldrich) and the 96-well culture
plates were agitated at room temperature for 1 min. The absorbance
was subsequently measured at 490 nm. In addition, a Hoechst assay
was used to detect cell apoptosis. Cells were seeded in 6-well
culture plates at 40% confluence 1 day prior to transfection. After
24 h, the cells were washed with PBS and were fixed in 4%
paraformaldehyde for 15 min. Subsequently, Hoechst 33342 diluted in
PBS (final concentration, 5%) was added to each well for 15 min,
the cells were washed twice with PBS (10 min/wash), the
blue-stained nuclei were observed and the apoptotic rate was
calculated under fluorescence microscopy (Olympus Corporation,
Hamburg, Germany).
Luciferase reporter assay
The 3′UTR of the human ATG16L1 gene was predicted to
interact with miR-410. A 360 bp-fragment of the ATG16L1 3′-UTR,
which contained a putative binding site of miR-410, was subcloned
into the 3′-UTR region of the pMIR-REPORT firefly luciferase
construct (Ambion; Thermo Fisher Scientific, Inc.). The empty
vector and the PCR products were digested with SacI and
XbaI, and subjected to T4 DNA ligation (Promega Corporation,
Madison, WI, USA) at 16°C overnight. The ligation product was then
transformed. Single colonies were picked and the construct was
confirmed by sequencing, yielding pMIR-REPORT ATG16L1. Mutations
within the potential miR-410 binding sites were generated by
nucleotide replacement of the wild-type sequence to inhibit miR-410
binding (Guangzhou RiboBio Co., Ltd.). HEK293T cells were seeded
into 96-well plates (40% cell density). After a 24 h incubation,
the cells were co-transfected with 0.4 mg of the reporter construct
or 0.2 mg of the control vector, and miR-410 or NC. Luciferase
values were subsequently determined using the Dual-Luciferase
Reporter Assay system (Promega Corporation). All transfection
experiments were performed in triplicate and repeated at least
three times.
Detection of green fluorescent protein
(GFP)-LC3 autophagic dots
pcDNA3.1-GFP-LC3 vectors were provided as a gift by
Professor Shi Wu (Tsinghua University, Beijing, China). U2OS and
MG-63 cells, which stably expressed high levels of GFP-LC3 protein,
were established by pcDNA3.1-GFP-LC3 transfection and were selected
using G418 (10131027; Thermo Fisher Scientific, Inc.). The
U2OS-GFP-LC3 and MG-63-GFP-LC3 stably expressing cells were
detected and confirmed by fluorescence microscopy, and used for the
following experiments. Post-transfection with miR-410 or NC, the
percentage of GFP-LC3 punctate-positive cells was quantified and
analyzed using automated image acquisition (DMIRE2 Fluorescence
Microscope; Leica Microsystems, Wetzlar, Germany) with a threshold
of ≥5 dots/cell. Data are presented as the mean ± standard
deviation and are representative of three independent
experiments.
Western blotting
Protein concentrations were quantified using the DC
Protein Assay kit (500–0121; Bio-Rad Laboratories, Inc.) following
cell protein extraction in radioimmunoprecipitation assay buffer
(EMD Millipore, Billerica, MD, USA). The proteins (20 µg) were then
subjected to 10% sodium dodecyl sulfate-polyacrylimide gel
electrophoresis and were transferred onto nitrocellulose membranes.
The membranes were blocked with 5% milk for 1 h at room
temperature. The membranes were then incubated with the
aforementioned primary rabbit antibodies at 4°C for 18 h. The
membranes were then incubated with horseradish
peroxidase-conjugated rabbit IgG secondary antibodies (1:3,000;
cat. no. 7074; Cell Signaling Technology, Inc.) at room temperature
for 60 min. Immunoreactivities were visualized using enhanced
chemiluminescence reagents (WBKLS0500; EMD Millipore). The relative
expression levels of the proteins of interest were obtained by
normalizing their band densities to that of β-actin.
Immunofluorescence
Cells were plated on coverslips, and were fixed with
4% paraformaldehyde for 30 min, permeabilized with 0.1% Triton
X-100 for 15 min, and blocked with 5% bovine serum albumin
(Sigma-Aldrich) for 1 h at room temperature post-treatment. After
being rinsed three times with Tris-buffered saline containing 1%
Tween-20, the cells were incubated with anti-ATG16L1 overnight at
4°C, followed by an incubation with conjugated secondary antibody
for 2 h at 25°C, with 0.1 M 4′6-diamidino-2-phenylindole in PBS
staining as the control. Images were acquired using the Leica
DMIRE2 fluorescence microscope.
Statistical analysis
All statistical analyses were performed using SPSS
13.0 software (SPSS, Inc., Chicago, IL, USA). All experiments were
repeated three times and data are presented as the mean ± standard
deviation. Student's t-test was used to determine significant
differences between results. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-410 is downregulated in
osteosarcoma tissues and cell lines
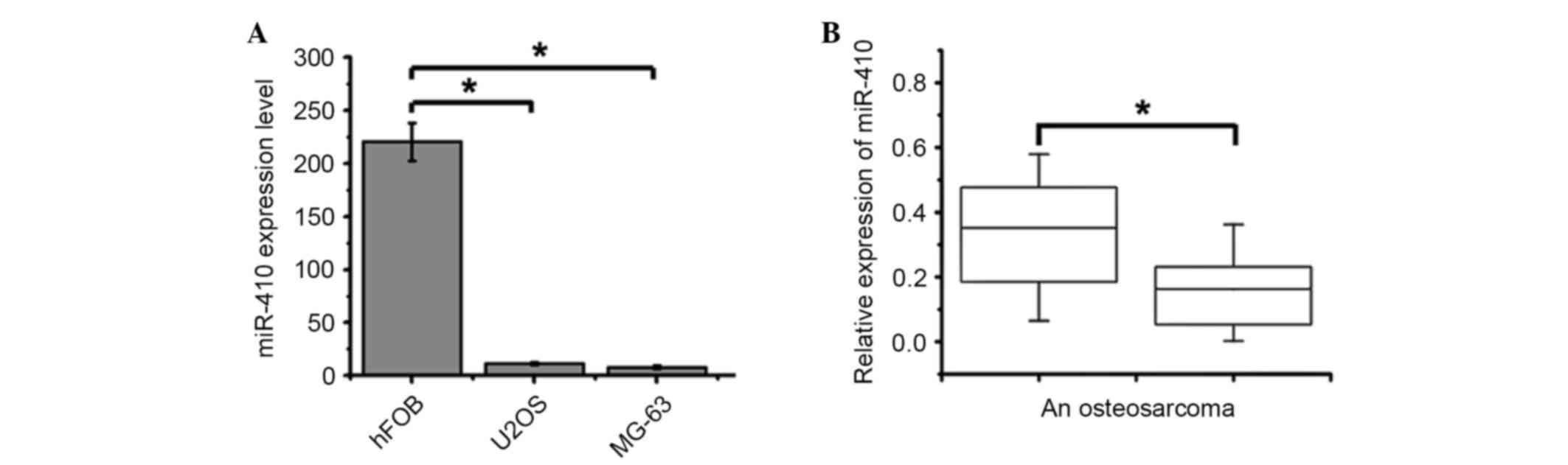
qPCR was used to detect the expression levels of
miR-410 in U2OS and MG-63 human osteosarcoma cell lines. The
expression levels of miR-410 were significantly downregulated in
U2OS and MG-63 cells compared with in the hFOB 1.19 human
osteoblast cell line (Fig. 1A). In
addition, the expression levels of miR-410 were significantly
downregulated in 34 out of 40 osteosarcoma tissues compared with in
the matched normal non-tumor tissues (Fig. 1B). Further investigations indicated
that miR-410 expression was not associated with age, gender or
clinical stage; however, it had a close association with response
to chemotherapy. Osteosarcoma tissues with high miR-410 expression
exhibited a good response to chemotherapy (Table I; Chi-square test). Collectively,
these results suggest that miR-410 may exert an antitumor function
and be associated with chemoresistance in osteosarcoma.
 | Table I.Association between
clinicopathological features and miR-410 expression levels. |
Table I.
Association between
clinicopathological features and miR-410 expression levels.
|
|
| miR-410
expression |
|
|---|
|
|
|
|
|
|---|
| Factor | Cases (n) | High (6) | Low (34) | P-value |
|---|
| Age (years) |
|
|
| NS |
| ≥25 | 15 | 2 | 13 |
|
|
<25 | 25 | 4 | 21 |
|
| Gender |
|
|
| NS |
| Male | 24 | 3 | 21 |
|
|
Female | 16 | 3 | 13 |
|
| Tumor size (cm) |
|
|
| NS |
| ≥8 | 19 | 4 | 15 |
|
|
<8 | 21 | 2 | 19 |
|
| Serum level of
lactate dehydrogenase |
|
|
| NS |
|
Elevated | 26 | 4 | 22 |
|
|
Normal | 14 | 2 | 12 |
|
| Serum level of
alkaline dehydrogenase |
|
|
| NS |
|
Elevated | 28 | 5 | 23 |
|
|
Normal | 12 | 1 | 11 |
|
| Clinical stage |
|
|
| NS |
| IIA | 23 | 3 | 20 |
|
|
IIB/III | 17 | 3 | 14 |
|
| Response to
chemotherapy |
|
|
| <0.05 |
|
Good | 14 | 6 | 9 |
|
|
Poor | 26 | 0 | 26 |
|
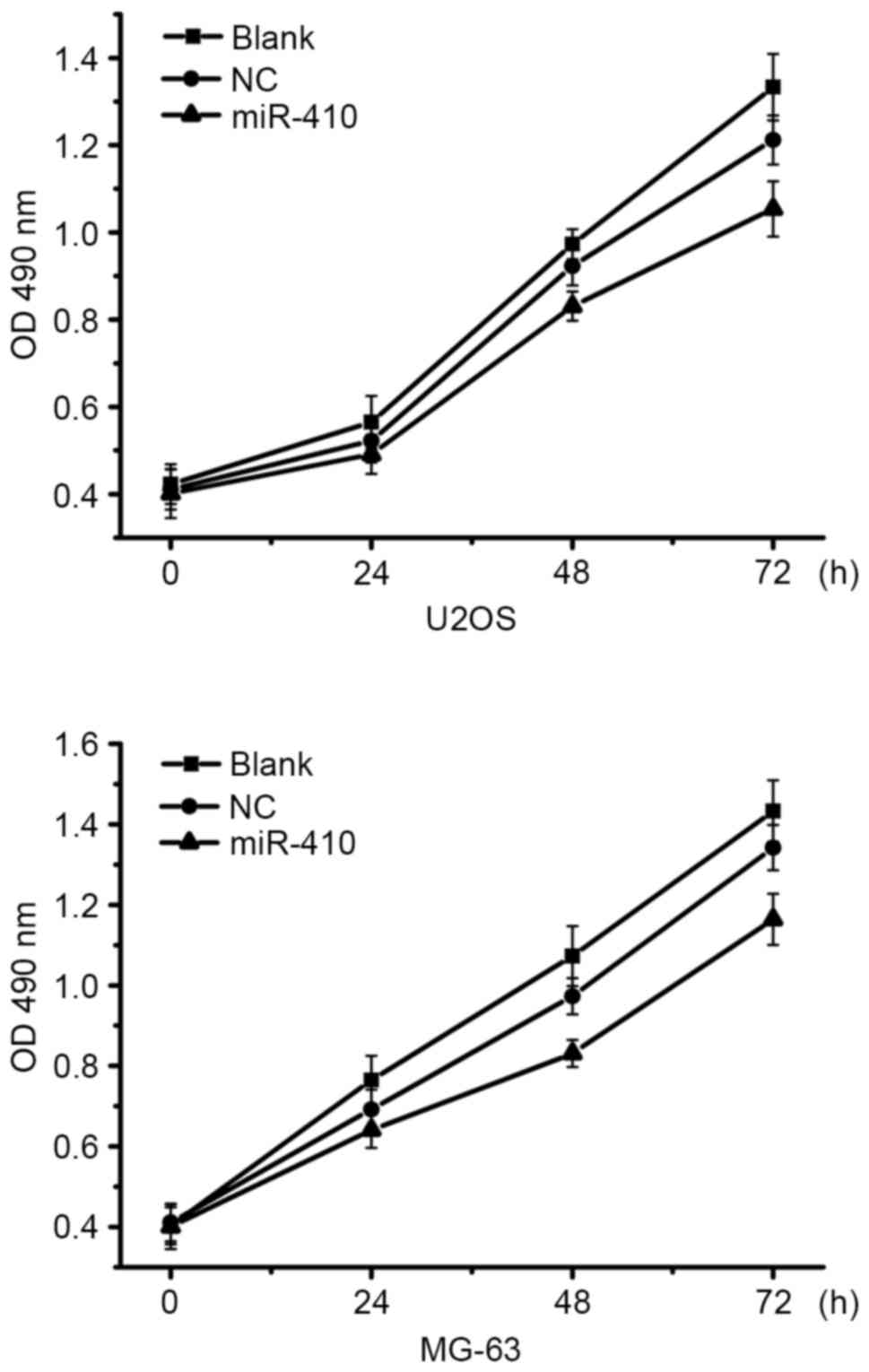
miR-410 exerts a limited influence on
the proliferation of osteosarcoma cells
To determine whether miR-410 was able to affect the
proliferation of osteosarcoma cells, proliferation assays were
conducted in U2OS and MG-63 cells transfected with miR-410 or NC
mimics. As shown in Fig. 2
overexpression of miR-410 had almost no affect on the viability of
U2OS and MG-63 cells. Therefore, further studies were conducted to
examine whether other mechanisms associated with antitumor function
were affected by miR-410.
miR-410 directly targets ATG161L in
osteosarcoma cell lines
Target prediction algorithms [TargetScan (http://www.targetscan.org/vert_71/) and miRanda
(http://www.microrna.org/microrna/home.do)] were used
to predict the targets of miR-410 in osteosarcoma cells. ATG16L1
was predicted to be a target gene of miR-410, and putative miR-410
binding sites were predicted to be present in the 3′UTR of ATG16L1
mRNA. ATG16L1 is a component of a large protein complex, which is
essential for autophagosome formation. Autophagy is generally
considered to be a pro-survival mechanism that preserves cell
viability in response to cancer therapy (16). To validate the prediction that
ATG16L1 is a direct target of miR-410, luciferase reporter vectors
containing wild-type (WT) or mutant ATG16L1 3′UTR were generated
(Fig. 3A). The luciferase assay
indicated that miR-410 inhibited luciferase activity in the ATG16L1
WT 3′UTR group; however, the inhibitory effects were abolished when
the ATG16L1 3′UTR binding sites were mutated (Fig. 3B). Furthermore, an
immunofluorescence assay demonstrated that overexpression of
miR-410 suppressed ATG16L1 expression in U2OS and MG-63 cells
(Fig. 3C). Concordantly, western
blot analysis revealed that miR-410 was able to suppress ATG16L1
protein expression levels. Notably, ATG16L1 expression levels were
stimulated by Rap (an autophagy activator), whereas miR-410
overexpression effectively decreased ATG16L1 expression (Fig. 3D). These results indicate that
miR-410 may directly target ATG16L1 in osteosarcoma cells.
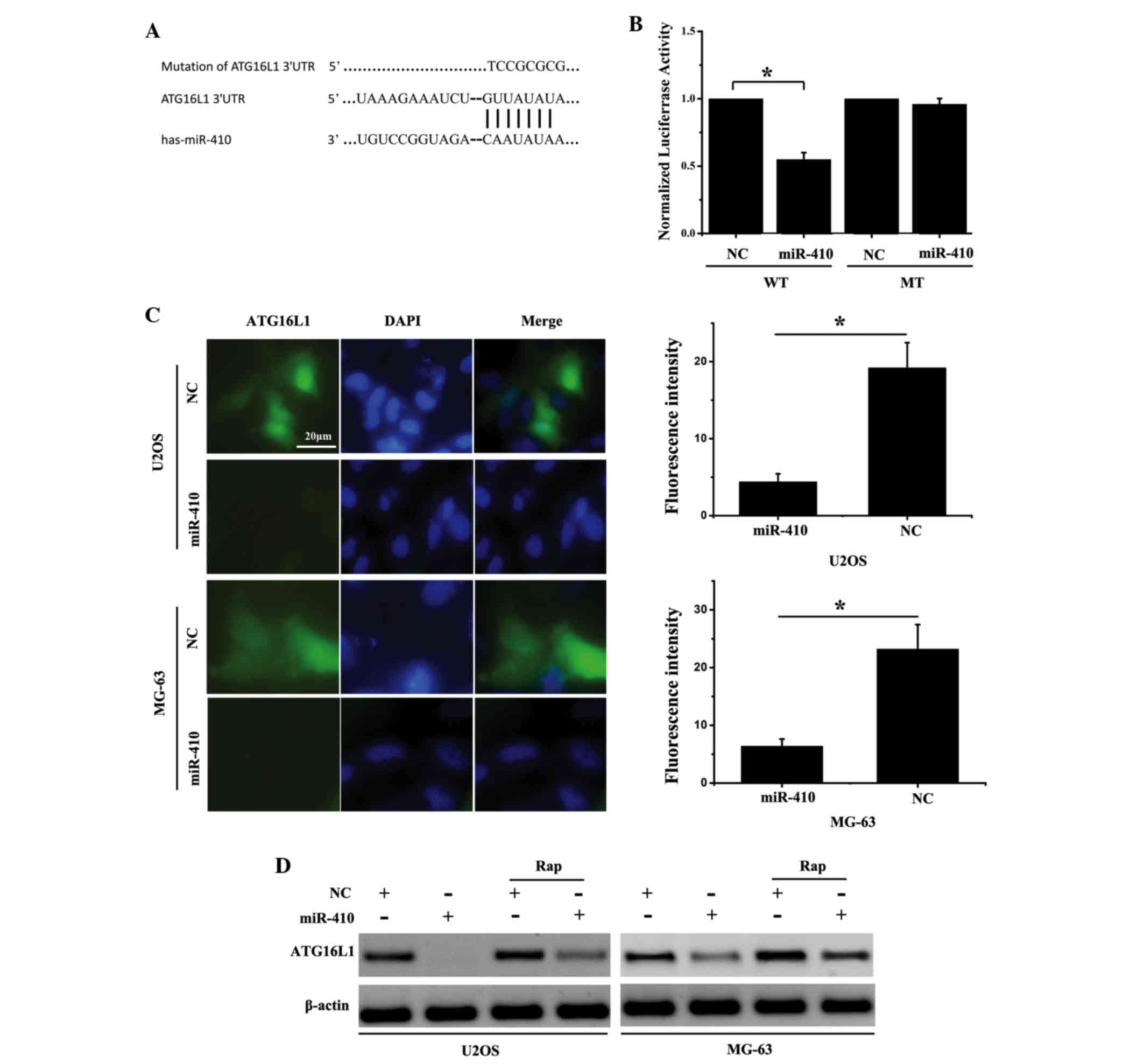 | Figure 3.miR-410 directly targets ATG161L in
osteosarcoma cell lines. (A) miR-410/ATG16L1 alignment by miRanda
analysis, and schematic diagram of the pMIR-ATG16L1/pMIR-
ATG16L1mut paired sequences for miR-410. (B) Normalized
luciferase activity of pMIR-ATG16L1/pMIR-ATG16L1mu
reporter in human embryonic kidney 293T cells transfected with
miR-410 or NC mimics. *P<0.05. (C) Immunofluorescent detection
of ATG16L1 expression in U2OS and MG-63 cells transfected with
miR-410 or NC for 48 h. (D) Western blotting was conducted to
detect the effects of miR-410 on ATG16L1 expression
post-transfection with miR-410 or NC for 48 h. Rapamycin (20 nM),
which is an activator of autophagy, increased ATG16L1 expression.
Data are presented as the mean ± standard deviation; *P<0.05.
ATG161L, autophagy related 16-like 1; miR-410, microRNA-410; NC,
negative control; WT, wild-type; mut/MT, mutant; Rap, rapamycin;
UTR, untranslated region; DAPI, 4′,6-diamidino-2-phenylindole. |
miR-410 inhibits autophagy in
osteosarcoma cell lines
In relation to tumorigenesis, the role of autophagy
is complex and depends on the environmental cues that cells are
exposed to. Accordingly, intrinsic resistance to Dox and DDP
through autophagic activity has been certified in osteosarcoma
cells (17). Therefore, since
miR-410 directly targeted ATG16L1 in osteosarcoma cancer cells, it
was hypothesized that miR-410 may be able to reverse
chemoresistance via autophagy inhibition. In order to verify this
hypothesis, three common chemotherapy drugs, Rap, Dox and DDP were
used to treat the cells in subsequent experiments. Initially, cell
strains that stably expressed GFP-LC3 were generated (U2OS-GFP-LC3
and MG-63-GFP-LC3) by transfection of U2OS and MG-63 cells with
GFP-LC3 (data not shown). U2OS-GFP-LC3 and MG-63-GFP-LC3 stably
expressed high levels of the GFP-LC3 protein. The cytosolic form of
LC3 (LC3-I) is conjugated to phosphatidylethanolamine to form the
LC3-phosphatidylethanolamine conjugate (LC3-II), which is recruited
to autophagosomal membranes and reflects autophagic activity. The
GFP-LC3 expressing cells were subsequently exposed to the drugs,
and autophagosomes (fluorescent dots) were accumulated due to the
GFP-LC3 translocation to the structural components of the
double-membrane autophagosome. As shown in Fig. 4A and B, the percentage of GFP-LC3
puncta-positive cells was significantly increased in the drug
treatment groups. Furthermore, 3-MA significantly enhanced the
sensitivity of cells to chemotherapy drugs in the drug treatment
groups (Fig. 4C). In order to
determine the effects of miR-410 on autophagy inhibition, U2OS and
MG-63 cells, which were transfected with miR-410 or NC, were
treated with Rap, Dox and DDP. The number of fluorescent dots and
GFP-LC3 puncta-positive cells was significantly reduced in the
cells transfected with miR-410 compared with in the NC group
(Fig. 4D and E). These results
suggest that miR-410 may function as a potential autophagy
inhibitor in U2OS and MG-63 cells. In addition, western blotting
was employed to detect the expression levels of ATG16L1 and the
autophagy marker LC3I/LC3II in the drug treatment groups. The
expression levels of ATG16L1 and LC3I/LC3II were markedly decreased
in cells transfected with miR-410 (Fig. 4F). Taken together, miR-410 may act
as a potential autophagy inhibitor in osteosarcoma cell lines.
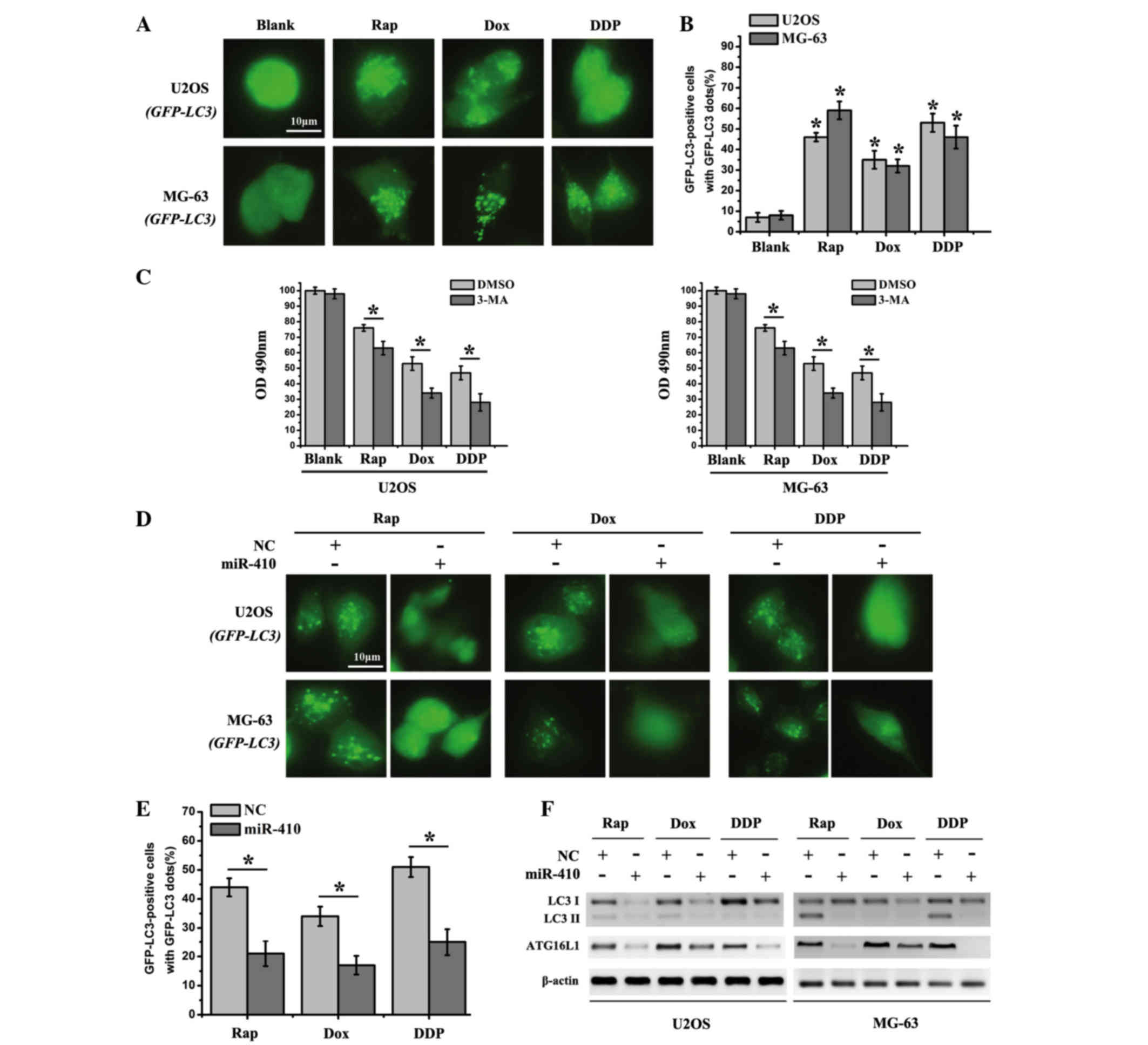 | Figure 4.miR-410 effectively inhibits autophagy
in osteosarcoma cell lines. (A) U2OS-GFP-LC3/MG-63-GFP-LC3 cells
which stably express GFP-LC3 were used to detect autophagy after
the cells were treated with various agents for 48 h (Rap, 20 nM;
Dox, 0.1 µg/ml; DDP, 10 µM). (B) Percentage of GFP-LC3
punctate-positive cells was quantified and analyzed by automated
image acquisition using a threshold of ≥5 dots/cell. (C) Cell
viability was detected after the cells were treated with various
agents for 48 h with the presence or absence of the autophagy
inhibitor 3-MA. (D) U2OS-GFP-LC3/MG-63-GFP-LC3 cells which stably
express GFP-LC3 were used to detect autophagy after the cells were
treated with Rap (20 nM), Dox (0.1 µg/ml) or DDP (10 µM), and were
transfected with miR-410 or NC mimics for 48 h. (E) Percentage of
GFP-LC3 punctate-positive cells was quantified and analyzed by
automated image acquisition using a threshold of ≥5 dots/cell
(U2OS-GFP-LC3). (F) Western blot analysis of LC3I/II and ATG16L1
expression from the lysates of U2OS and MG-63 cells transfected
with NC or miR-410 for 48 h. β-actin was used as an internal
control. Data are presented as the mean ± standard deviation and
are representative of three independent experiments (*P<0.05).
ATG161L, autophagy related 16-like 1; miR-410, microRNA-410; NC,
negative control; Rap, rapamycin; Dox, doxorubicin; DDP, cisplatin;
GFP, green fluorescent protein; LC3I/II, microtubule-associated
protein 1A/1B-light chain 3; OD, optical density; 3-MA,
3-methyladenine; DMSO, dimethyl sulfoxide. |
miR-410 enhances chemosensitivity of
cells to Rap, Dox and DDP via autophagy inhibition
To further determine the influence of
miR-410-induced autophagy inhibition on chemoresistance in
osteosarcoma cell lines, an MTT assay was used to detect cell
viability in U2OS and MG-63 cells. As shown in Fig. 5A, miR-410 sensitized U2OS and MG-63
cells to Rap, Dox and DDP, thus suggesting that the presence of
miR-410 could improve the therapeutic response to those agents.
Subsequently, cell apoptosis was detected by Hoechst assay. Cells
treated with miR-410 and chemotherapy agents exhibited enhanced
cell apoptosis compared with the cells treated with chemotherapy
agents alone (Fig. 5B). Notably,
Rap-, Dox- and DDP-treated groups all activated the cleavage of
caspase 3 (early molecular marker of apoptosis), whereas miR-410
enhanced the apoptotic-inducing ability of these agents (Fig. 5C). The cell apoptotic rate of the
aforementioned groups is presented in Fig. 5D. These results suggest that
miR-410 may reverse the resistance of U2OS and MG-63 cells to Rap,
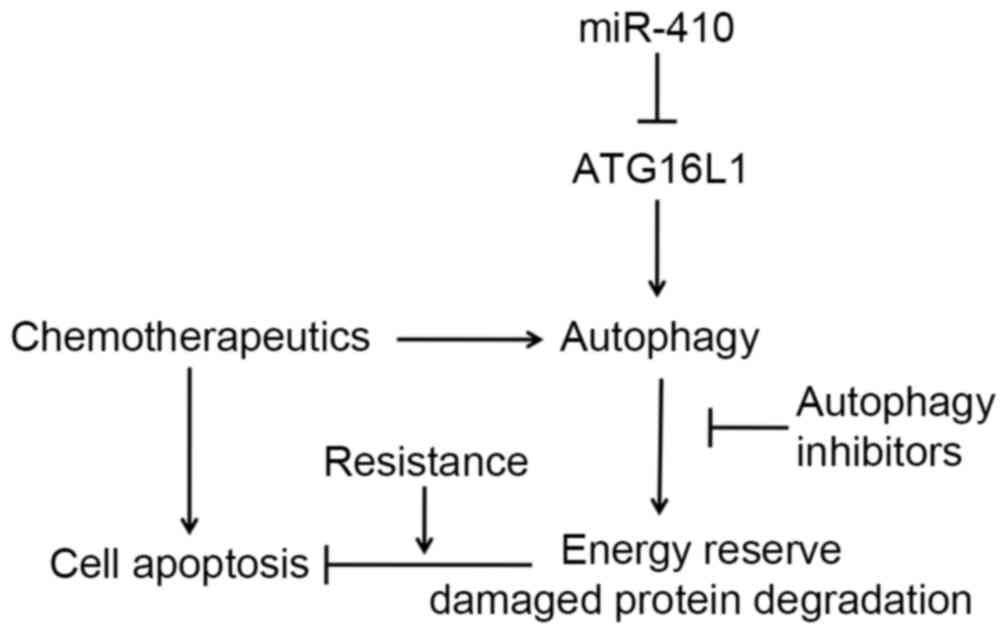
Dox and DDP through autophagy inhibition. In conclusion, miR-410
regulates ATG16L1 expression and enhances chemosensitivity of cells
via autophagy inhibition in osteosarcoma (Fig. 6).
Discussion
Osteosarcoma is the most common type of primary bone
tumor in children and adolescents, which accounts for 8% of the
incidence rate and is a leading cause of cancer-associated
mortality among young adults (18). Resistance to chemotherapeutic
agents remains a major clinical obstacle to effective therapy;
however, the mechanisms underlying osteosarcoma chemoresistance
remain largely unknown.
Previous studies have confirmed that autophagy, a
chief mechanism for bulk degradation of superfluous or aberrant
cytoplasmic components, functions as a protective mechanism that
degrades and enables reuse of abnormal proteins and organelles as
energy sources to promote cancer cell survival in response to
cancer treatment (5,19,20).
Mammalian target of rapamycin (mTOR) has generally been regarded as
a negative regulator of autophagy, and autophagy-related genes are
regulated by proteins upstream of mTOR signaling, including
phosphatase and tensin homolog, phosphoinositide-dependent
kinase-1, Akt and tuberous sclerosis 1/2 (20). In addition, upregulated autophagy
has been identified in various cancer cells following therapeutic
stress. Therefore, suppressing cancer cell autophagy is emerging as
a novel approach to enhance the efficiency of chemotherapy in
cancer treatment (21–23).
miRNAs have emerged as fundamental regulators of
gene expression, and are able to silence gene expression at the
post-transcriptional and translational levels (8). Dysregulation of miRNAs has been
demonstrated to be associated with the tumorigenesis and
progression of various types of tumor, including osteosarcoma
(24). Downregulation of miR-410
has been detected in several malignant tumors and overexpression of
miR-410 inhibits tumor growth and promotes cell apoptosis (11,25,26).
In the present study, the role of miR-410 in the progression of
osteosarcoma was investigated. The results demonstrated that
miR-410 expression was markedly downregulated in human osteosarcoma
tissues, and U2OS and MG-63 osteosarcoma cell lines; however, the
overexpression of miR-410 exhibited a limited effect on the
viability of U2OS and MG-63 cells. Furthermore, ATG16L1 was
identified as a direct target of miR-410, and overexpression of
miR-410 inhibited the expression of ATG16L1 at the mRNA and protein
level in osteosarcoma cell lines. It was also demonstrated that
miR-410 was able to markedly inhibit autophagy. Therefore, miR-410
may have the potential to reverse chemoresistance via autophagy
inhibition.
The regulatory mechanisms between miRNAs and
autophagy remain largely unknown. Chen et al reported that
increased miR-155 expression levels upregulated autophagy in
osteosarcoma cells, and ameliorated Dox-induced decreases in cell
viability (27). Li et al
demonstrated that overexpression of miR-22 targeted the 3′UTR of
high mobility group box 1 (HMGB1) and inhibited HMGB1-induced
autophagy, which contributed to chemotherapy resistance in
osteosarcoma in vitro (28). The present study revealed that
autophagy was activated after cells were treated with Rap, Dox and
DDP. Conversely, the presence of autophagy inhibitor 3-MA or
transfection with miR-410 was able to effectively reduce autophagic
activation. Further investigations indicated that, compared with
the Rap-, Dox- and DDP-treated groups, cell viability was
significantly decreased and apoptosis was significantly increased
in the drug-treated groups transfected with miR-410.
Taken together, the results of the present study
provided novel evidence suggesting that ATG16L1 is a direct target
of miR-410. Overexpression of miR-410 significantly sensitized
osteosarcoma cells to Rap, Dox and DDP. Understanding the
miR-410-mediated tumor suppressor pathways and the potential
ability to suppress the autophagic process in human osteosarcoma
may provide invaluable therapeutic targets for the treatment of
osteosarcoma.
The present study demonstrated that miR-410
expression was downregulated in osteosarcoma cell lines and
tissues; however, miR-410 overexpression exhibited limited effects
on the viability of U2OS and MG-63 cells. Furthermore, target
prediction algorithms identified ATG16L1 as a potential target gene
of miR-410, and luciferase reporter assays indicated that miR-410
directly suppressed ATG16L1 expression by targeting its 3′UTR.
Furthermore, miR-410 was shown to effectively inhibit autophagy.
Accordingly, autophagy was activated as a protective mechanism when
osteosarcoma cells were exposed to anticancer drugs, including Rap,
Dox and DDP (29,30). The present study demonstrated that
the autophagy inhibitor 3-MA and miR-410 expression were able to
improve the therapeutic response of cells to chemotherapy drugs
(Rap, Dox and DDP), thus indicating that miR-410 may reverse
chemoresistance via autophagy inhibition in osteosarcoma cells. The
studies conducted regarding the function of miR-410 on autophagy
provide insight into the biological function of miR-410 in
osteosarcoma, and offer a promising approach for osteosarcoma
treatment.
Acknowledgements
The authors would like to thank Professor Shi Wu for
providing kindly technological support assistance.
References
|
1
|
Tsai YC, Wu CT and Hong RL: Response of
refractory osteosarcoma to thalidomide and celecoxib. Lancet Oncol.
6:997–999. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang W, Li Q, Song C and Lao L: Knockdown
of autophagy-related protein 6, Beclin-1, decreases cell growth,
invasion, and metastasis and has a positive effect on
chemotherapy-induced cytotoxicity in osteosarcoma cells. Tumour
Biol. 36:2531–2539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie Z, Xie Y, Xu Y, Zhou H, Xu W and Dong
Q: Bafilomycin A1 inhibits autophagy and induces apoptosis in MG63
osteosarcoma cells. Mol Med Rep. 10:1103–1107. 2014.PubMed/NCBI
|
|
4
|
Kim HJ, Lee SG, Kim YJ, Park JE, Lee KY,
Yoo YH and Kim JM: Cytoprotective role of autophagy during
paclitaxel-induced apoptosis in Saos-2 osteosarcoma cells. Int J
Oncol. 42:1985–1992. 2013.PubMed/NCBI
|
|
5
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M
and Croce CM: Human microRNA genes are frequently located at
fragile sites and genomic regions involved in cancers. Proc Natl
Acad Sci USA. 101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Fu J, Jiang M, Zhang X, Cheng L,
Xu X, Fan Z, Zhang J, Ye Q and Song H: MiR-410 is overexpressed in
liver and colorectal tumors and enhances tumor cell growth by
silencing FHL1 via a direct/indirect mechanism. PLoS One.
9:e1087082014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Labialle S, Marty V, Bortolin-Cavaillé ML,
Hoareau-Osman M, Pradère JP, Valet P, Martin PG and Cavaillé J: The
miR-379/miR-410 cluster at the imprinted Dlk1-Dio3 domain controls
neonatal metabolic adaptation. EMBO J. 33:2216–2230. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen L, Zhang J, Feng Y, Li R, Sun X, Du
W, Piao X, Wang H, Yang D, Sun Y, et al: MiR-410 regulates MET to
influence the proliferation and invasion of glioma. Int J Biochem
Cell Biol. 44:1711–1717. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang J, Ni J, Liu K, Yu Y, Xie M, Kang R,
Vernon P, Cao L and Tang D: HMGB1 promotes drug resistance in
osteosarcoma. Cancer Res. 72:230–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lambert LA, Qiao N, Hunt KK, Lambert DH,
Mills GB, Meijer L and Keyomarsi K: Autophagy: A novel mechanism of
synergistic cytotoxicity between doxorubicin and roscovitine in a
sarcoma model. Cancer Res. 68:7966–7974. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Z, Tao L, Shen C, Liu B, Yang Z and
Tao H: Silencing of Barkor/ATG14 sensitizes osteosarcoma cells to
cisplatin-induced apoptosis. Int J Mol Med. 33:271–276.
2014.PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cadwell K, Patel KK, Komatsu M, Virgin HW
IV and Stappenbeck TS: A common role for Atg16L1, Atg5 and Atg7 in
small intestinal Paneth cells and Crohn disease. Autophagy.
5:250–252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao D, Yuan H, Yi F, Meng C and Zhu Q:
Autophagy prevents doxorubicin-induced apoptosis in osteosarcoma.
Mol Med Rep. 9:1975–1981. 2014.PubMed/NCBI
|
|
18
|
Mu Y, Zhang H, Che L and Li K: Clinical
significance of microRNA-183/Ezrin axis in judging the prognosis of
patients with osteosarcoma. Med Oncol. 31:8212014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu L, McPhee CK, Zheng L, Mardones GA,
Rong Y, Peng J, Mi N, Zhao Y, Liu Z, Wan F, et al: Termination of
autophagy and reformation of lysosomes regulated by mTOR. Nature.
465:942–946. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Behrends C, Sowa ME, Gygi SP and Harper
JW: Network organization of the human autophagy system. Nature.
466:68–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao F, Wang G, Wei W, Tu Y, Tong H and Sun
S: An autophagy inhibitor enhances the inhibition of cell
proliferation induced by a proteasome inhibitor in MCF-7 cells. Mol
Med Rep. 5:84–88. 2012.PubMed/NCBI
|
|
22
|
Yang C and Han LO: Knockdown of Beclin 1
inhibits vitamin K3-induced autophagy, but promotes apoptosis of
human hepatoma SMMC-7721 cells. Mol Med Rep. 3:801–807.
2010.PubMed/NCBI
|
|
23
|
Apel A, Herr I, Schwarz H, Rodemann HP and
Mayer A: Blocked autophagy sensitizes resistant carcinoma cells to
radiation therapy. Cancer Res. 68:1485–1494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang J, Yan YG, Wang C, Zhang SJ, Yu XH
and Wang WJ: MicroRNAs in osteosarcoma. Clin Chim Acta. 444:9–17.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen J, Niu W, Zhou M and Zhang H, Ma J,
Wang L and Zhang H: MicroRNA-410 suppresses migration and invasion
by targeting MDM2 in gastric cancer. PLoS One. 9:e1045102014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen N, Wang J, Hu Y, Cui B, Li W, Xu G,
Liu L and Liu S: MicroRNA-410 reduces the expression of vascular
endothelial growth factor and inhibits oxygen-induced retinal
neovascularization. PLoS One. 9:e956652014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen L, Jiang K, Jiang H and Wei P:
miR-155 mediates drug resistance in osteosarcoma cells via inducing
autophagy. Exp Ther Med. 8:527–532. 2014.PubMed/NCBI
|
|
28
|
Li X, Wang S, Chen Y, Liu G and Yang X:
miR-22 targets the 3′ UTR of HMGB1 and inhibits the
HMGB1-associated autophagy in osteosarcoma cells during
chemotherapy. Tumour Biol. 35:6021–6028. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shen C, Wang W, Tao L, Liu B, Yang Z and
Tao H: Chloroquine blocks the autophagic process in
cisplatin-resistant osteosarcoma cells by regulating the expression
of p62/SQSTM1. Int J Mol Med. 32:448–456. 2013.PubMed/NCBI
|
|
30
|
Wu W, Li W, Zhou Y and Zhang C: Inhibition
of beclin1 affects the chemotherapeutic sensitivity of
osteosarcoma. Int J Clin Exp Pathol. 7:7114–7122. 2014.PubMed/NCBI
|