Introduction
Lung cancer, additionally known as bronchial cancer,
is the leading cause of cancer-associated mortality in males, and
is among the most common types of female malignancies (1,2). In
China, approximately two-thirds of adult males are smokers,
representing one-third of all smokers worldwide (3). Cigarette smoking and second-hand
smoke inhalation are the primary causes of lung cancer (4). For this reason, proven
population-based tobacco prevention strategies used in the US
should be implemented in China, to reduce the lung cancer
incidence. Despite advances in surgical, radiotherapeutic and
chemotherapeutic strategies, lung cancer is an aggressive and
heterogeneous disease, and therefore the long-term survival rate
remains low (5–7). Lung cancer treatment is complex for
numerous reasons, including hard-to-detect early symptoms, early
metastasis and vascular factors that mediate drug-resistance and
disease progression (8–10). Previous studies have investigated
inhibiting angiogenesis and antagonizing vascular endothelial
growth factors as potential therapeutic targets for the treatment
of lung cancer (11,12); however, the potential of this
strategy remains unclear.
Genistein, a natural phytoestrogen found in soy, has
demonstrated the potential to inhibit numerous types of cancer,
including breast, pancreatic and colorectal cancers (13–15).
Previous studies have indicated that this effect may be due to its
ability to induce cancer cell apoptosis, arrest the cell cycle and
inactivate critical signaling pathways in human cancer cells
(16,17). However, the underlying mechanisms
of genistein, and its potential therapeutic effects in lung cancer,
remain to be determined. The present study therefore investigated
the anti-tumor effects of genistein on the A549 lung cancer cell
line and its underlying molecular mechanisms.
Materials and methods
Cell culture
A549 human lung carcinoma cells were obtained from
the American Type Culture Collection (Manassas, VA, USA),
maintained in RPMI-1640 medium (HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% fetal calf serum
(HyClone; GE Healthcare Life Sciences), 2 mM glutamine, 100 U/ml
penicillin and 100 µg/ml streptomycin at 37°C in 5% CO2
and passaged twice a week. Cells were cultured at a density of
5×103 cells/well in 6-well culture plates for 24 h. Following this,
A549 cells were transferred to serum-free RPMI-1640 medium for
overnight serum starvation prior to each experiment.
Assessment of cell viability
The cytotoxicity of the genistein was determined
using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay as previously described by Mossman (18). Cells were seeded at 1×105 cells/ml
in 96-well plates and treated with 0–200 µmol/l genistein for 24,
48 and 72 h. Following incubation, 10 µl 5 mg/ml MTT dye
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) was added to
the cells for 4 h followed by incubation with dimethyl sulfoxide
for 10 min. The absorbance at a wavelength of 570 nm was measured
using a microplate reader (Asys UVM340; Bichrom, Ltd., Cambridge,
UK). Cell viability was determined as the ratio of the signal
obtained from treated and control cultures.
Analysis of apoptosis
Cells were cultured and harvested by trypsinization,
then washed twice with cold PBS and centrifuged at room temperature
for 8 min, at 800 × g. About 1×105–1×106 cells were then
resuspended in 300 µl 1X binding buffer and centrifuged again at
1,000 rpm for 5 min. Cells were resuspended in 300 µl 1X binding
buffer and transferred to a sterile flow cytometry glass tube. A
total of 10 µl Annexin V-FITC Annexin was added and incubated in a
dark at room temperature for 30 min. Then cells were incubated in
the dark with 5 µl propidium iodide. Cells were analyzed by a flow
cytometer (FACS Calibur, BD Biosciences, San Jose, CA, USA).
Cellular apoptosis was determined using the Annexin V-FITC
Apoptosis Detection kit I (Clontech Laboratories Inc.,
Mountainview, CA, USA).
Detection of intracellular reactive
oxygen species (ROS)
ROS detection was performed using the ROS assay kit
(Beyotime Institute of Biotechnology, Haimen, China) according to
the manufacturer's protocol. A549 cells were treated with 0, 25, 50
and 100 µmol/l genistein for 24, 48 and 72 h. Following this, 5×106
cells were incubated with 10 µmol/l
2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA; Beyotime
Institute of Biotechnology) at 37°C for 30 min, and washed three
times with PBS to remove the residual dye. DCF-DA fluorescence was
detected using a flow cytometer (BD Biosciences) and the results
were analyzed using Quantity One software version 4.62 (Bio-Rad
Laboratories Inc., Hercules, CA, USA).
Western blot analysis
Cells were centrifuged at 125 × g for 10 min at 4°C
and washed twice with ice-cold PBS. They were subsequently lysed
with Triton X-100 in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic
acid buffer (Sigma-Aldrich; Merck Millipore). Lysed cells were
sonicated and centrifuged for 5 min at room temperature, at a speed
of 6,000 × g. The total protein concentration measurement was
performed using the Bradford method. Total protein (50 µg) was
loaded onto gels and separated by 10% SDS-PAGE. The proteins were
subsequently transferred onto a polyvinylidene difluoride membrane
using a Bio-Rad apparatus (Bio-Rad Laboratories, Inc.) for 2 h at
4°C and 100 V. Following this, membranes were blocked with 5%
skimmed milk in Tris-buffered saline containing 0.1% Tween-20 for 1
h at room temperature. They were subsequently incubated at 4°C
overnight with the following primary antibodies: Mouse monoclonal
anti-B-cell lymphoma 2-associated X protein (Bax; 1:400; catalog
no. sc-20067; Santa Cruz Biotechnology, Inc., Dallas, TX, USA);
mouse monoclonal anti-apoptosis inducing factor (AIF; 1:400;
catalog no. sc-13116; Santa Cruz Biotechnology, Inc.); mouse
monoclonal anti-cytochrome c (cyto-c; 1:400; catalog
no. sc-13561; Santa Cruz Biotechnology, Inc.); rabbit monoclonal
anti-caspase-3 (1:400; catalog no. 9664; Cell Signaling Technology,
Inc., Danvers, MA, USA); mouse monoclonal anti-total (t)-protein
kinase B (AKT; 1:400; catalog no. sc-377457; Santa Cruz
Biotechnology, Inc.); rabbit polyclonal anti-phosphorylated (p)-AKT
(1:400; catalog no. sc-135650; Santa Cruz Biotechnology, Inc.);
mouse monoclonal anti-hypoxia-inducible factor-1α (HIF-1α; 1:400;
catalog no. sc-53546; Santa Cruz Biotechnology, Inc.) and mouse
monoclonal anti-β-actin (1:400; catalog no. sc-47778; Santa Cruz
Biotechnology, Inc.). Following this, the membranes were incubated
with horseradish peroxidase-conjugated polyclonal goat anti-mouse
(catalog no. sc-2005) and goat anti-rabbit (catalog no. sc-2004)
IgG secondary antibodies (1:5,000; Santa Cruz Biotechnology, Inc.)
for 2 h at room temperature and the resulting protein bands were
visualized using an Enhanced Chemiluminescence reagent (Pierce;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. Densitometry was performed using the
Quantity One software version 4.62 (Bio-Rad Laboratories,
Inc.).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was isolated using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). RNase-free
DNase I was used in order to eliminate genomic DNA contamination in
the RNA samples. The 260/280 absorbance ratio was measured for
verification of the purity of RNA. The sequences of the nuclear
factor-κB (NF-κB), cyclooxygenase (COX-2) and GAPDH genes were
obtained from the GenBank database and specific primers were
designed using Primer Premier software version 5.0 (Premier Biosoft
International, Palo Alto, CA, USA). The primer sequences were as
follows: Forward, 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′ and reverse,
5′-CGGAGTCAACGGATTTGGTCGTAT-3′, for GAPDH; forward,
5′-CTGAACCAGGGCATACCTGT-3′ and reverse, 5′-GAGAAGTCCATGTCCGCAAT-3′
for NF-κB; and forward, 5′-TGAAACCCACTCCAAACACA-3′ and reverse,
5′-TGGAACAACTGCTCATCACC-3′ for COX-2. PCR reactions were performed
with PrimeScript™ RT-PCR kit RR014A (Takara Bio, Inc, Otsu, Japan),
using a GeneAmp® PCR system 9700 (PerkinElmer, Inc.,
Waltham, MA, USA) and amplified. The reaction conditions were as
follows: 94°C for 4 min; 94°C for 40 sec, 50°C for 45 sec, and 72°C
for 45 sec, for 35 cycles; and followed by extension at 72°C for 10
min before ending.
The amplified products were separated by
electrophoresis on a 2% agarose gel and visualized by ethidium
bromide staining. IPLab software, version no: 2.8 (Scanalytics,
Fairfax, VA, USA) was sued for densitometry and image density was
quantified using a FluoroImager™ SI scanner (GE Healthcare Life
Sciences, Chalfont, UK).
Statistical analysis
Data are expressed as the mean ± standard error. The
significance of differences between groups was assessed by one-way
analysis of variance. Data was analyzed using SPSS software version
18 (SPSS, Inc., Chicago, IL, USA). Individual comparisons were
subsequently performed using the Tukey's post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of genistein on the apoptosis
of A549 cells
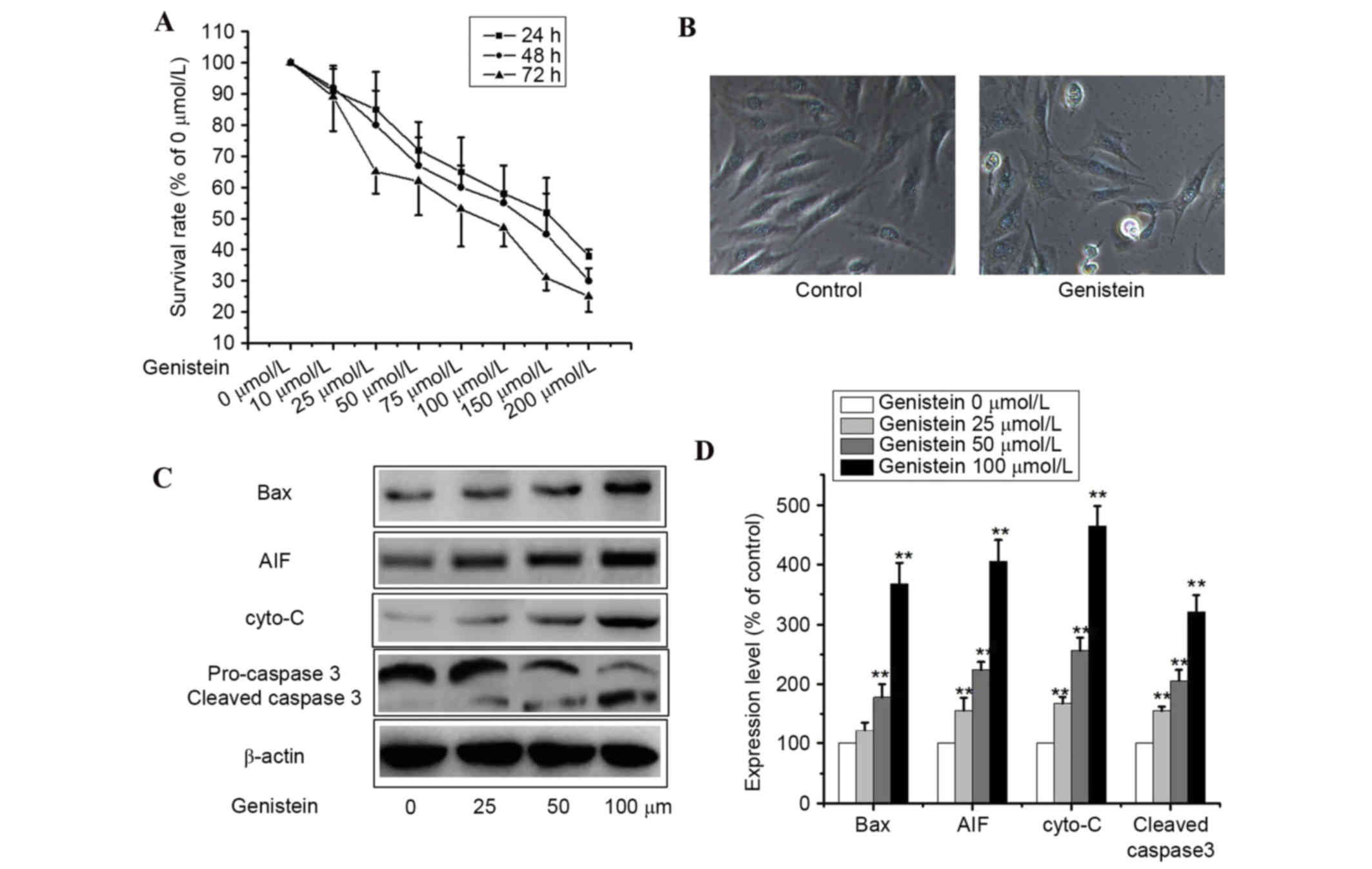
Cell viability assays were performed to assess the
inhibition of cell growth by genistein. A549 cells were treated
with various doses of genistein (0–200 µmol/l) for 24, 48 and 72 h.
Cell viability assays revealed that genistein inhibited cell growth
in a dose- and time-dependent manner (Fig. 1A). Furthermore, genistein induced
apoptosis most effectively at a concentration of 200 µl mol/l,
however, concentrations that induced a reversible level of
apoptosis were selected for experimentation.
A549 cells incubated with 50 µmol/l genistein for 48
h lost their original morphological shape and additional floating
cells appeared, as observed by the CX22 microscope (Olympus
Corporation, Tokyo, Japan; Fig.
1B; P<0.01). To examine genistein-induced apoptosis, western
blotting was performed to detect the expression levels of the
apoptosis-associated proteins AIF, cyto-c, Bax and
caspase-3. As presented in Fig. 1C and
D, genistein markedly upregulated the protein expression levels
of cleaved caspase-3, Bax, cyto-c and AIF in A549 cells
(P<0.01). These data indicate that genistein inhibited cell
viability via the induction of apoptosis in A549 cells.
Effects of genistein on intracellular
ROS production in A549 cells
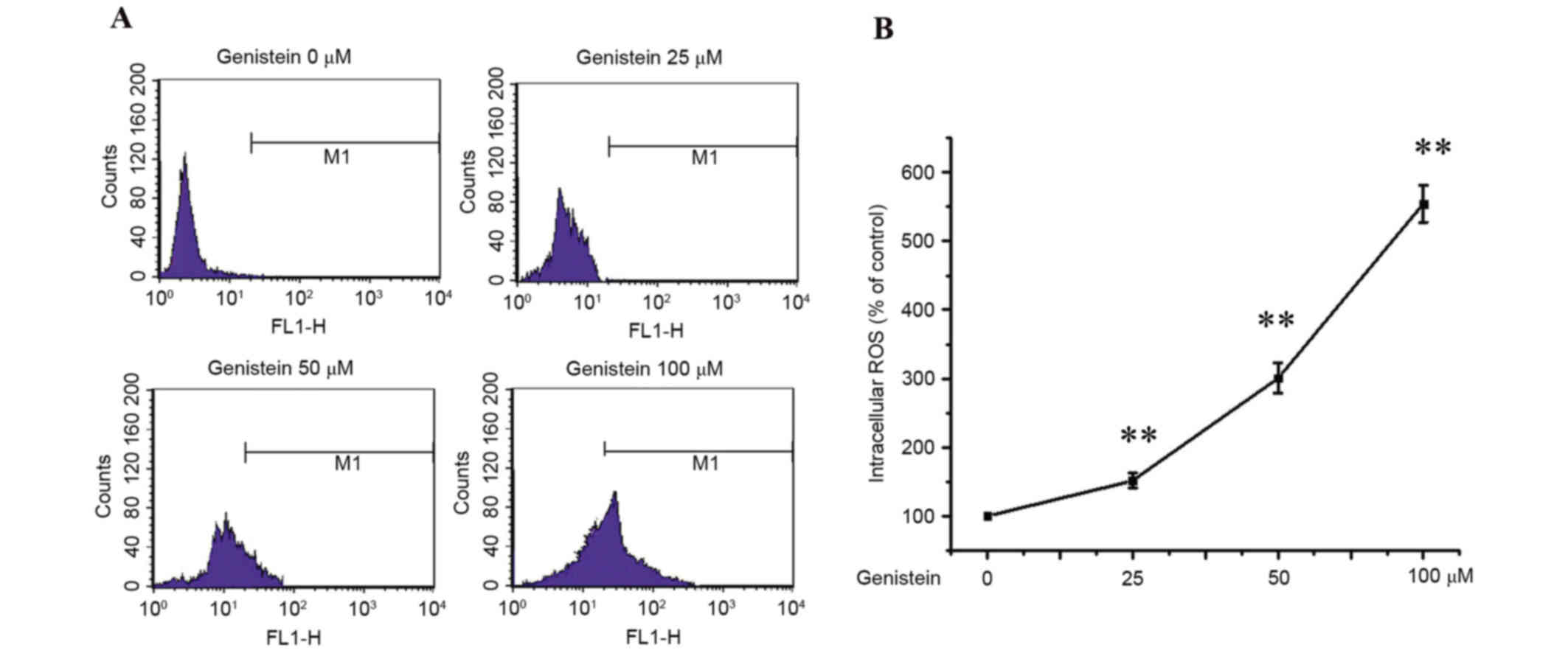
Apoptosis is, at least partially, mediated by
oxidative stress (19). Thus,
intracellular ROS levels in A549 cells were examined by DCF-DA
staining. A549 cells were treated with 0, 25, 50 and 100 µmol/l
genistein for 48 h, and intracellular ROS levels were detected by
flow cytometry. As presented in Fig.
2, these results demonstrated that ROS production increased
following genistein treatment in a dose-dependent manner
(P<0.01).
Effects of genistein on the
phosphatidylinositol-4,5-biphosphate 3-kinase (PI3K)/AKT and HIF-1α
signaling pathways in A549 cells
The PI3K/AKT and HIF-1α signaling pathways have been
demonstrated to be involved in cell viability and tumor
angiogenesis, and mediate cell survival (20,21).
To investigate the underlying molecular mechanisms by which
genistein exerts its apoptotic effects, the PI3K/AKT and HIF-1α
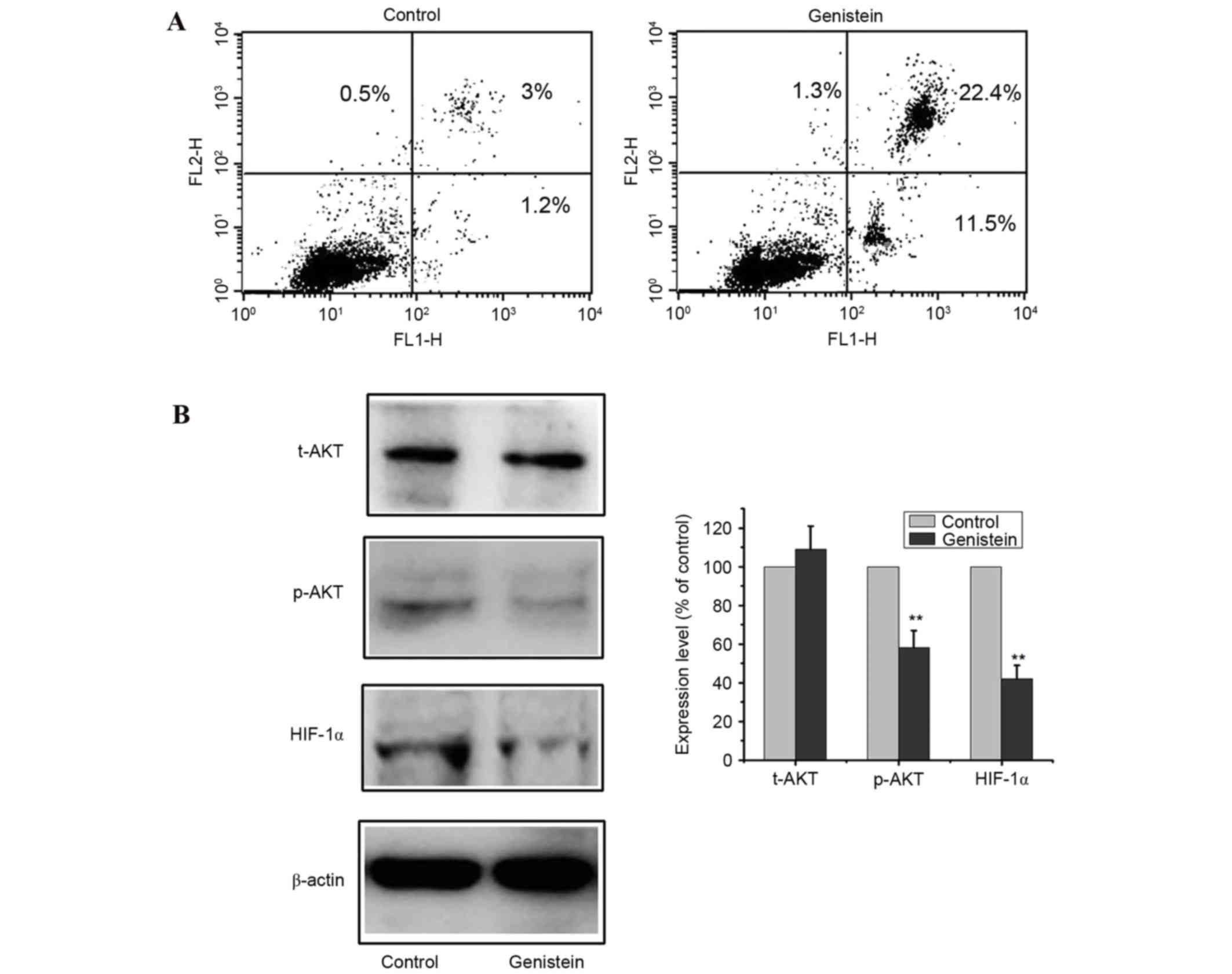
signaling pathways were examined. A549 cells were treated with 0 or
50 µmol/l genistein for 48 h and apoptosis was determined by flow
cytometry. Flow cytometry assays revealed a marked increase in
apoptosis in cells treated with genistein (P<0.01; Fig. 3A). Additionally, p-AKT, t-AKT and
HIF-1α protein expression levels were determined by western blot
analysis (Fig. 3B). Following
exposure to 50 µmol/l genistein for 48 h, p-AKT and HIF-1α protein
expression levels decreased compared with the control (P<0.01),
while t-AKT levels were not significantly altered. These results
suggested that genistein induced cell apoptosis by suppressing the
PI3K/AKT and HIF-1α signaling pathways.
Effects of genistein on the
NF-κB/COX-2 signaling pathway in A549 cells
The NF-κB and COX-2 signaling pathways serve
important roles in cancer cell growth, tumor angiogenesis and
invasion (22,23). To investigate the underlying
molecular mechanisms by which genistein contributes to these
malignant features, the present study examined the effect of
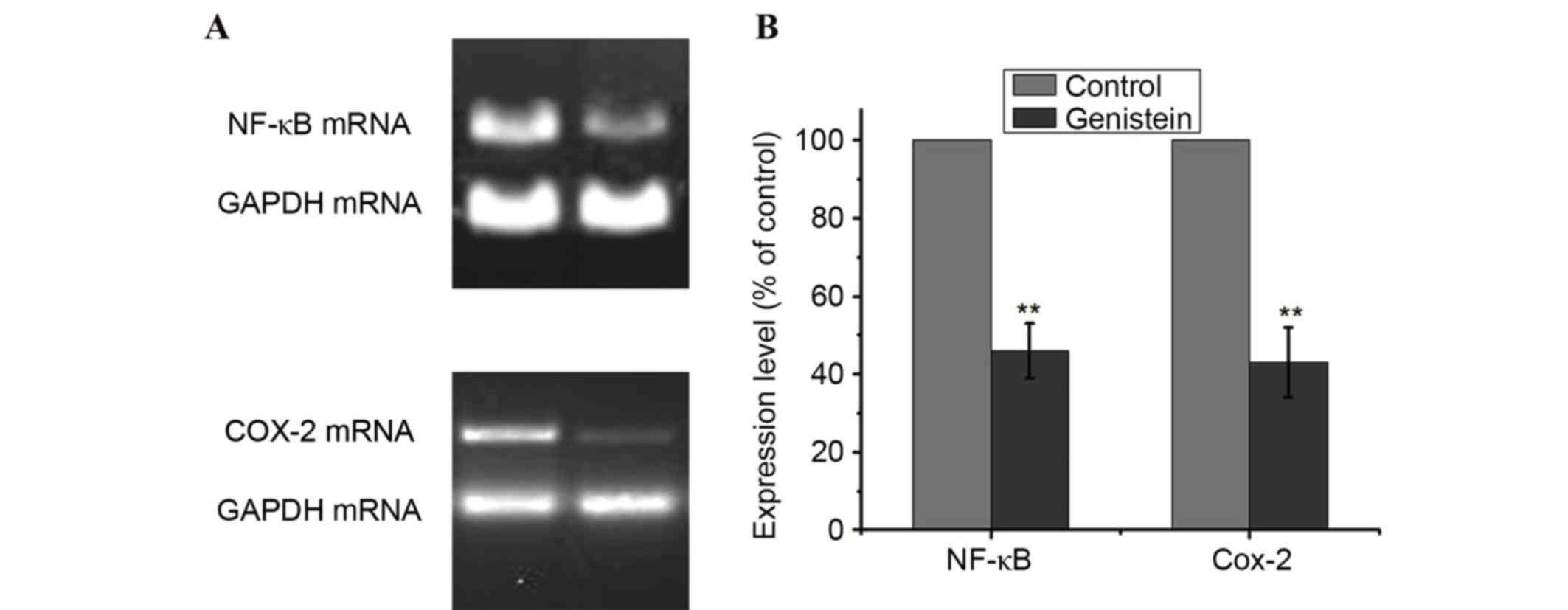
genistein on the NF-κB and COX-2 signaling pathways. A549 cells
were treated with 0 or 50 µmol/l genistein for 48 h, and the mRNA
expression levels of NF-κB and COX-2 were detected by RT-PCR. As
presented in Fig. 4, treatment
with genistein decreased the mRNA expression levels of NF-κB and
COX-2 in A549 cells, compared with the control (P<0.01).
Therefore, this indicates that genistein-induced A549 cell
apoptosis was partly dependent on the inhibition of the NF-κB/COX-2
signaling pathways.
Discussion
Lung cancer is the most common type of malignant
tumor, yet effective treatments remain to be developed. Intravenous
chemotherapy supplemented with chest radiation is the most common
method of treatment, with surgery only rarely performed (24). However, genetic mutations in cancer
cells and multidrug resistance, in addition to insensitivity to
radiotherapy, often result in poor outcomes (25).
The lungs are supplied with blood by the systemic
and pulmonary circulations. The blood supply of lung cancer is
primarily provided by the bronchial artery; however, whether the
pulmonary blood supply is involved remains to be determined. A
previous study addressed this issue using clinical pulmonary
angiography, and indicated that the pulmonary artery does not
supply lung cancer with blood (26). However, the dual blood supply of
non-small cell lung cancer may depend on tumor size and
histological subtype (27). A
previous study demonstrated that the blood supply of the pulmonary
artery serves important roles in the nourishment, development,
metastasis, prognosis and angiogenesis of lung cancer (28). Furthermore, a previous tumor study
suggested that tumor cell proliferation and angiogenesis are
closely associated (29). Growth
factors, including vascular endothelial, fibroblast, transforming
and platelet-derived growth factors, have been demonstrated to
induce tumor angiogenesis (30).
Previous reports have indicated that genistein, a
naturally occurring isoflavonoid, possesses anticancer properties
(13–17). However, the underlying mechanisms
of inhibition remain unclear. The present study demonstrated that
genistein decreased A549 cell viability in a dose- and
time-dependent manner, and induced apoptosis. Treatment of A549
cells with genistein significantly increased ROS formation,
activation of caspase-3, cyto-c leakage and protein
expression levels of Bax and AIF, which are involved in the
mitochondrial apoptosis pathway. Additionally, the current study
investigated the underlying mechanisms of the anti-cancer effects
of genistein. Previous studies have demonstrated that the PI3K/AKT
and HIF-1α signaling pathways may regulate critical steps in
apoptosis and cancer cell survival (29,31).
Therefore, activation of these pathways may mediate angiogenesis,
resulting in accelerated cancer cell growth. Genistein was
demonstrated to significantly inhibit cell apoptosis via
downregulation of the PI3K/AKT and HIF-1α signaling pathways.
Furthermore, due to the importance of the NF-κB and COX-2 signaling
pathways in apoptosis (32,33),
the present study hypothesized that genistein may inhibit these
pathways in A549 cells and antagonize apoptosis. These results
revealed that genistein significantly inhibited apoptosis via
suppressing the NF-κB and COX-2 signaling pathways in lung cancer
cells.
The mitogen-activated protein kinase (MAPK)
signaling pathway serves important roles in tumorigenesis, cell
growth, differentiation, proliferation, apoptosis, migration and
angiogenesis. It has been demonstrated in previous studies that the
MAPK signaling pathway is overactive in cancer, and that its
activation is associated with angiogenesis (34). Additionally, previous studies have
revealed that genistein has effects on the MAPK signaling pathway
(35,36). The MAPK and PI3K/AKT signaling
pathways are important for cell membrane receptor signal
transduction, which regulates apoptosis, cell growth and the
expression of numerous genes. Computer simulations have
demonstrated that interactions between the two pathways are
context-dependent, and that they may activate or inhibit each other
(37). Typically, NF-κB and COX-2
appear as downstream pathways (38). The interaction of these pathways
suggests that the effects of genistein on signal transduction
requires further investigation.
In conclusion, the present study demonstrated that
genistein induced apoptosis in A549 cells. Genistein appeared to
exert its pro-apoptotic effects via inhibition of the
PI3K/AKT/HIF-1α and NF-κB/COX-2 signaling pathways. This implicates
genistein as a potential chemopreventive agent for the treatment of
lung cancer. Therefore, future clinical studies investigating the
long-term benefits of genistein are required, in addition to
investigation into the mechanism of action of genistein and in
vitro animal studies.
Acknowledgements
The present study was supported by the Xiangyang
Science and Technology Bureau (grant no. 20136811).
References
|
1
|
Henley SJ, Richards TB, Underwood JM,
Eheman CR, Plescia M and McAfee TA: Centers for Disease Control and
Prevention (CDC): Lung cancer incidence trends among men and
women-United States, 2005–2009. MMWR Morb Mortal Wkly Rep. 63:1–5.
2014.PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Zeng H and Zhang S: The
updated incidences and mortalities of major cancers in China, 2011.
Chin J Cancer. 34:502–207. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo H and Sa Z: Socioeconomic
differentials in smoking duration among adult male smokers in
China: Result from the 2006 China Health and Nutrition Survey. PLoS
One. 10:e01173542015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lubin JH, Caporaso N, Wichmann HE,
Schaffrath-Rosario A and Alavanja MC: Cigarette smoking and lung
cancer: Modeling effect modification of total exposure and
intensity. Epidemiology. 18:639–648. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stinchcombe TE: Recent advances in the
treatment of non-small cell and small cell lung cancer. F1000Prime
Rep. 6:1172014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hensing T, Chawla A, Batra R and Salgia R:
A personalized treatment for lung cancer: Molecular pathways,
targeted therapies, and genomic characterization. Adv Exp Med Biol.
799:85–117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Izbicki JR, Passlick B, Hosch SB,
Kubuschock B, Schneider C, Busch C, Knoefel WT, Thetter O and
Pantel K: Mode of spread in the early phase of lymphatic metastasis
in non-small-cell lung cancer: Significance of nodal
micrometastasis. J Thorac Cardiovasc Surg. 112:623–630. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guldbrandt LM: The effect of direct
referral for fast CT scan in early lung cancer detection in general
practice. A clinical, cluster-randomised trial. Dan Med J.
61:B50272015.
|
|
10
|
Shen H, Feng G, Cui J, Du Q, Qin Y, Cai J,
Shen L and Zhu Y: Clinical implications of serum hypoxia inducible
factor-1α and vascular endothelial growth factor in lung cancer.
Tumori. 101:404–411. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakade J, Takeuchi S, Nakagawa T, Ishikawa
D, Sano T, Nanjo S, Yamada T, Ebi H, Zhao L, Yasumoto K, et al:
Triple inhibition of EGFR, Met, and VEGF suppresses regrowth of
HGF-triggered, erlotinib-resistant lung cancer harboring an EGFR
mutation. J Thorac Oncol. 9:775–783. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu L, Chen W, Guo W, Wang J, Tian Y, Shi
D, Zhang X, Qiu H, Xiao X, Kang T, et al: Berberine targets
AP-2/hTERT, NF-κB/COX-2, HIF-1α/VEGF and cytochrome-c/caspase
signaling to suppress human cancer cell growth. PLoS One.
8:e692402013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pons DG, Nadal-Serrano M,
Blanquer-Rossello MM, Sastre-Serra J, Oliver J and Roca P:
Genistein modulates proliferation and mitochondrial functionality
in breast cancer cells depending on ERalpha/ERbeta ratio. J Cell
Biochem. 115:949–958. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pavese JM, Krishna SN and Bergan RC:
Genistein inhibits human prostate cancer cell detachment, invasion,
and metastasis. Am J Clin Nutr. 100:431S–436S. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao X, Liu Z, Wang R, Wang J, Zhang S,
Cai X, Wu K, Bergan RC, Xu L and Fan D: Genistein suppresses FLT4
and inhibits human colorectal cancer metastasis. Oncotarget.
6:3225–3239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim IG, Kim JS, Lee JH and Cho EW:
Genistein decreases cellular redox potential, partially suppresses
cell growth in HL-60 leukemia cells and sensitizes cells to
γ-radiation-induced cell death. Mol Med Rep. 10:2786–2792.
2014.PubMed/NCBI
|
|
17
|
Prietsch RF, Monte LG, da Silva FA, Beira
FT, Del Pino FA, Campos VF, Collares T, Pinto LS, Spanevello RM,
Gamaro GD and Braganhol E: Genistein induces apoptosis and
autophagy in human breast MCF-7 cells by modulating the expression
of proapoptotic factors and oxidative stress enzymes. Mol Cell
Biochem. 390:235–242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kannan K and Jain SK: Oxidative stress and
apoptosis. Pathophysiology. 7:153–163. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Joshi S, Singh AR, Zulcic M and Durden DL:
A macrophage-dominant PI3K isoform controls hypoxia-induced HIF1α
and HIF2α stability and tumor growth, angiogenesis, and metastasis.
Mol Cancer Res. 12:1520–1531. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee H, Jung KH, Jeong Y, Hong S and Hong
SS: HS-173, a novel phosphatidylinositol 3-kinase (PI3K) inhibitor,
has anti-tumor activity through promoting apoptosis and inhibiting
angiogenesis. Cancer Lett. 328:152–159. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang M, Zou J, Zhu H, Liu S, Wang H, Bai P
and Xiao X: Paris saponin II inhibits human ovarian cancer
cell-induced angiogenesis by modulating NF-κB signaling. Oncol Rep.
33:2190–2198. 2015.PubMed/NCBI
|
|
23
|
Ma JX, Sun YL, Wang YQ, Wu HY, Jin J and
Yu XF: Triptolide induces apoptosis and inhibits the growth and
angiogenesis of human pancreatic cancer cells by downregulating
COX-2 and VEGF. Oncol Res. 20:359–368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maguire J, Khan I, McMenemin R, O'Rourke
N, McNee S, Kelly V, Peedell C and Snee M: SOCCAR: A randomised
phase II trial comparing sequential versus concurrent chemotherapy
and radical hypofractionated radiotherapy in patients with
inoperable stage III non-small cell lung cancer and good
performance status. Eur J Cancer. 50:2939–2949. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun FF, Hu YH, Xiong LP, Tu XY, Zhao JH,
Chen SS, Song J and Ye XQ: Enhanced expression of stem cell markers
and drug resistance in sphere-forming non-small cell lung cancer
cells. Int J Clin Exp Pathol. 8:6287–6300. 2015.PubMed/NCBI
|
|
26
|
Xiao XS, Yu H, Li HM, Liu SY, Li CZ and
Liu J: Impact of multi-layer spiral CT angiography of bronchial
artery and pulmonary artery in assessment of the main blood supply
to the primary lung cancer. Zhonghua Zhong Liu Za Zhi. 28:302–305.
2006.(In Chinese). PubMed/NCBI
|
|
27
|
Nguyen-Kim TD, Frauenfelder T, Strobel K,
Veit-Haibach P and Huellner MW: Assessment of bronchial and
pulmonary blood supply in non-small cell lung cancer subtypes using
computed tomography perfusion. Invest Radiol. 50:179–186. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang X, Zhang Y, Hosaka K, Andersson P,
Wang J, Tholander F, Cao Z, Morikawa H, Tegnér J, Yang Y, et al:
VEGF-B promotes cancer metastasis through a VEGF-A-independent
mechanism and serves as a marker of poor prognosis for cancer
patients. Proc Natl Acad Sci USA. 112:E2900–E2909. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yao L, Nie X, Shi S, Song S, Hao X, Li S
and Zhu D: Reciprocal regulation of HIF-1α and 15-LO/15-HETE
promotes anti-apoptosis process in pulmonary artery smooth muscle
cells during hypoxia. Prostaglandins Other Lipid Mediat. 99:96–106.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Raica M, Mogoantă L, Kondylis A and
Cîmpean AM: Angiogenesis in the human thymoma assessed by
subclassification of tumor-associated blood vessels and endothelial
cells proliferation. Rom J Morphol Embryol. 51:627–631.
2010.PubMed/NCBI
|
|
31
|
Zhang HB, Lu P, Guo QY, Zhang ZH and Meng
XY: Baicalein induces apoptosis in esophageal squamous cell
carcinoma cells through modulation of the PI3K/Akt pathway. Oncol
Lett. 5:722–728. 2013.PubMed/NCBI
|
|
32
|
Mohankumar K, Sridharan S, Pajaniradje S,
Singh VK, Ronsard L, Banerjea AC, Somasundaram DB, Coumar MS,
Periyasamy L and Rajagopalan R: BDMC-A, an analog of curcumin,
inhibits markers of invasion, angiogenesis, and metastasis in
breast cancer cells via NF-κB pathway-A comparative study with
curcumin. Biomed Pharmacother. 74:178–186. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mena MP, Papiewska-Pajak I, Przygodzka P,
Kozaczuk A, Boncela J and Cierniewski CS: NFAT2 regulates COX-2
expression and modulates the integrin repertoire in endothelial
cells at the crossroads of angiogenesis and inflammation. Exp Cell
Res. 324:124–136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao JH, Wang CH, Tong H, Wen SL, Huang ZY
and Tang CW: Targeting inhibition of extracellular signal-regulated
kinase kinase pathway with AZD6244 (ARRY-142886) suppresses growth
and angiogenesis of gastric cancer. Sci Rep. 16:163822015.
View Article : Google Scholar
|
|
35
|
Jin CY, Park C, Kim GY, Lee SJ, Kim WJ and
Choi YH: Genistein enhances TRAIL-induced apoptosis through
inhibition of p38 MAPK signaling in human hepatocellular carcinoma
Hep3B cells. Chem Biol Interact. 180:143–150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang X, Chen S, Xu L, Liu Y, Deb DK,
Platanias LC and Bergan RC: Genistein inhibits p38 map kinase
activation, matrix metalloproteinase type 2, and cell invasion in
human prostate epithelial cells. Cancer Res. 65:3470–3478.
2005.PubMed/NCBI
|
|
37
|
Aksamitiene E, Kiyatkin A and Kholodenko
BN: Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt
pathways: A fine balance. Biochem Soc Trans. 40:139–146. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
N'Guessan PD, Hippenstiel S, Etouem MO,
Zahlten J, Beermann W, Lindner D, Opitz B, Witzenrath M, Rosseau S,
Suttorp N and Schmeck B: Streptococcus pneumoniae induced p38 MAPK-
and NF-kappaB-dependent COX-2 expression in human lung epithelium.
Am J Physiol Lung Cell Mol Physiol. 290:L1131–L1138. 2006.
View Article : Google Scholar : PubMed/NCBI
|