Introduction
Osteoarthritis (OA) is the most prevalent chronic
form of arthritis among older people, and is characterized by
cartilage degradation, synovitis and remodeling of the subchondral
bone (1,2). Existing therapeutic options include
non-steroidal anti-inflammatory drugs and selective
cyclooxygenase-2 inhibitors. These drugs provide symptomatic relief
from the pain and inflammation associated with the later phases of
OA; however, they do not target the dysregulated molecular
processes responsible for the onset of OA. Furthermore,
pharmacological interventions fail to prevent cartilage damage and
the associated destruction of joint tissue, whereas they produce
extensive adverse effects (1,2).
Therefore, the development of agents that exhibit improved
therapeutic and safety profiles for the treatment of OA is of
critical importance.
OA is characterized by the enhanced degradation of
critical extracellular matrix (ECM) components, such as aggrecan
and collagen, in joint tissue (3).
It has previously been suggested that an excess of matrix
metalloproteinases (MMPs), such as MMP-1, MMP-2, MMP-3, MMP-8,
MMP-9 and MMP-13, may serve a central role in the breakdown of ECM
components, due to their ability to cleave various macromolecules,
including collagen and aggrecan (4). Numerous complex pathways and
mechanisms are involved in ECM degradation (5). It has previously been reported that
mitogen-activated protein kinases (MAPKs) serve a critical role in
the cytokine-mediated regulation of MMP expression, and consequent
cartilage degradation (6).
Furthermore, it has been demonstrated that the levels of
phosphorylated-MAPKs, including p38, c-Jun N-terminal kinase (JNK)
and extracellular signal-regulated kinase (ERK) are upregulated in
osteoarthritic cartilage. A number of inflammatory mediators have
been identified in OA joint tissues and fluids; interleukin (IL)-1β
expression was revealed to be mediated by the transcription factors
nuclear factor-κB and AP-1, resulting in the increase expression of
IL-1β in OA cartilage (1,7). In primary human chondrocytes and the
SW1353 human chondrosarcoma cell line, MMP-1, MMP-3 and MMP-13 were
strongly induced by IL-1β. IL-1β-stimulated human SW1353
chondrosarcoma cells appear to be a valuable in vitro
chondrocytic experimental system (8).
Icariin is a prenylated flavonol glycoside, and the
main active component of Herba Epimedii. It possesses antioxidant
and anti-inflammatory properties, and can also promote osteoblast
differentiation (9–11). In addition, Icariin has been
reported to interfere with the activation of MAPKs (12). Therefore, it may be hypothesized
that Icariin holds potential as a novel treatment for OA.
The present study investigated the effects of
Icariin on the expression of MMP-1, MMP-3 and MMP-13 in
IL-1β-stimulated SW1353 chondrosarcoma cells. In addition,
transcription factors possibly involved in the process, including
phosphorylated (P)-p38, P-JNK, and P-ERK, were also assessed.
Materials and methods
Cell cultures
Human SW1353 chondrosarcoma cells (Shanghai
Institute of Biochemistry and Cell Biology, Shanghai, China) were
cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% v/v fetal bovine serum (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA), 2 mM glutamine, 100 IU/ml penicillin and 100 µg/ml
streptomycin. The following treatments were applied: SB203580, a
p38 inhibitor (10 µM); PD98059, an ERK inhibitor (10 µM); SP600125,
a JNK inhibitor (10 µM) (Beyotime Institute of Biotechnology,
Haimen, China); U-46619, a p38 and ERK activator (50 µM; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA); Anisomycin, a p38 and JNK
activator (10 µg/ml; Santa Cruz Biotechnology, Inc.); or Icariin
(20 µM; Merck KGaA, Darmstadt, Germany). Control cells received no
treatment. After 1 h at 37°C in 5% CO2 cell incubator,
cells were then stimulated with the addition of IL-1β (10 ng/ml;
PeproTech, Inc., Rocky Hill, NJ, USA) for 24 h.
Enzyme-linked immunosorbent assay
(ELISA)
Culture media were centrifuged at 450 × g for 20
min, and the resultant supernatants were transferred to a clean
tube. The concentrations of MMP-1, MMP-3 and MMP-13 in the
cell-free supernatants were determined using ELISA kits (cat. nos.
SEA097HU, SEA101HU and SEA099HU; Uscn Life Sciences, Inc., Wuhan,
China) according to the manufacturer's protocols.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol®
reagent (cat. no. 15596018; Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Total RNA was
reverse transcribed into cDNA using the ReverTra Ace-α-kit (cat.
no. FSK-101; Toyobo Co., Ltd., Osaka, Japan). RT-qPCR was performed
using a 7500 Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.) with SYBR-Green Realtime PCR Master Mix
(cat. no. QPK-201; Toyobo Co., Ltd.). The following primers were
used for RT-qPCR: MMP-1, forward 5′-CCCCAAAAGCGTGTGACAGTAAG-3′,
reverse 5′-AAGGGATTTGTGCGCATGTAG-3′; MMP-3, forward
5′-CTCGGTTCCGCCTGTCTCAAG-3′, reverse
5′-GGAAGAGATGGCCAAAATGAAGAGA-3′; MMP-13, forward
5′-GCGTCATGCCAGCAAATTC-3′, reverse 5′-GTTCCAGCCACGCATAGTCAT-3′; and
β-actin, forward 5′-CTCCATCCTGGCCTCGCTGT-3′ and reverse
5′-GCTGTCACCTTCACCGTTCC-3′. Cycling conditions were as follows:
95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec, 59°C for
30 sec and 72°C for 1 min. Dissociation curves were obtained using
a thermal melting profile performed after the last PCR cycle as
follows: 59°C for 30 sec followed by a constant increase in the
temperature from 60 to 95°C. β-actin was used as an endogenous
control, and the 2−∆∆Cq method was used to calculate the
relative fold-changes in mRNA expression (13).
Western blot analysis
Proteins were isolated using an extraction kit
(Beyotime Institute of Biotechnology). Protein concentration was
determined by BCA Protein Assay kit (cat. no. 23227; Pierce; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Extracted protein samples (30 µg) were separated by 10% SDS-PAGE
and transferred onto a polyvinylidene difluoride membrane (EMD
Millipore, Billerica, MA, USA). After blocking in 5% non-fat milk
for 3 h at room temperature, the membrane was probed with the
following primary antibodies (all antibodies were used at 1:1,000)
overnight at 4°C: Anti-MMP-1 (cat. no. 26585-1-AP), anti-MMP-3
(cat. no. 17873-1-AP), anti-MMP-13 (cat. no. 18165-1-AP) and
anti-β-actin (cat. no. 20536-1-AP) were purchased from ProteinTech
Group, Inc. (Chicago, IL, USA); and anti-P-p38 (cat. no. YP0203),
anti-P-JNK (cat. no. YP0157) and anti-P-ERK (cat. no. YP0100) were
purchased from ImmunoWay Biotechnology Company (Plano, TX, USA).
Subsequently, membranes were washed with Tris-buffered saline
containing 0.05% Tween-20, and incubated with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (1:1000;
cat. no. A0208; Beyotime Institute of Biotechnology) for 1 h at
37°C. The bands were visualized using an enhanced chemiluminescence
kit (Beyotime Institute of Biotechnology). Optical density was
analyzed by Quantity One software version 4.6.2 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). β-actin was used to
normalize target protein expression.
Statistical analysis
All experiments were performed in triplicate using
independent samples. Data are expressed as the mean ± standard
deviation. The statistical significance of the difference between
groups was assessed by one-way analysis of variance and Tukey's
multiple comparisons test. P<0.05 was considered to indicate a
statistically significant difference. The analysis was performed
using SPSS software version 18.0 (SPSS, Inc., Chicago, IL,
USA).
Results
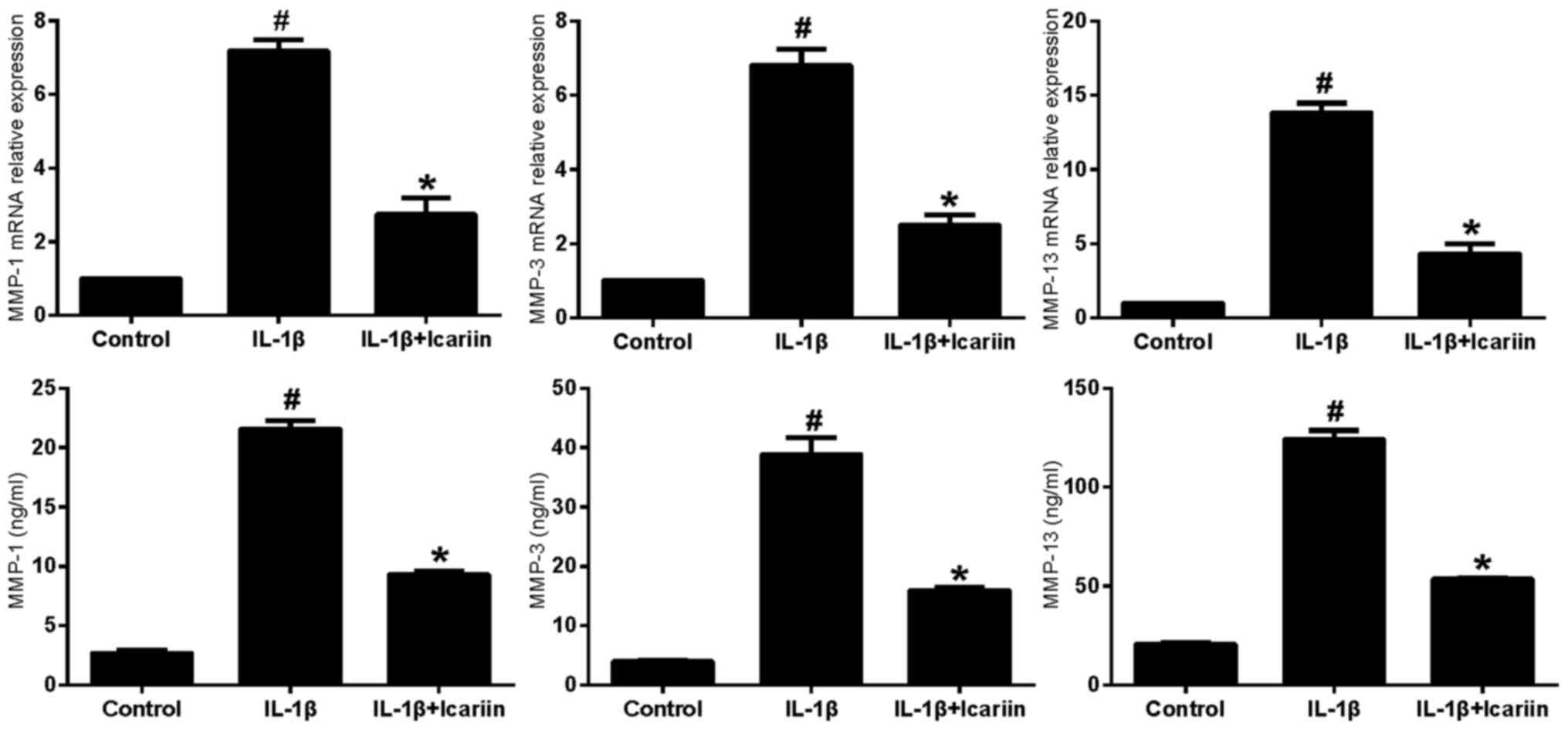
Effects of Icariin on MMP-1, MMP-3 and
MMP-13 expression in IL-1β-stimulated SW1353 cells
IL-1β-stimulated SW1353 cells were treated with
Icariin and the mRNA expression levels of MMP-1, MMP-3 and MMP-13
were assessed using RT-qPCR and ELISA. Treatment with 20 µM Icariin
was revealed to significantly inhibit the increase in MMP-1, MMP-3
and MMP-13 mRNA and protein expression levels in response to
stimulation with IL-1β compared with the control group (Fig. 1).
Effects of Icariin on P-p38, P-ERK and
P-JNK levels in IL-1β-stimulated SW1353 cells
In order to examine the effects of Icariin on the
MAPK signaling pathway, western blot analysis was used. The levels
of the phosphorylated forms of p38, ERK and JNK appeared to be
markedly increased in SW1353 cells stimulated with IL-1β.
Conversely, treatment with Icariin prior to stimulation appeared to
prevent the increase in P-p38, P-ERK and P-JNK levels (Fig. 2).
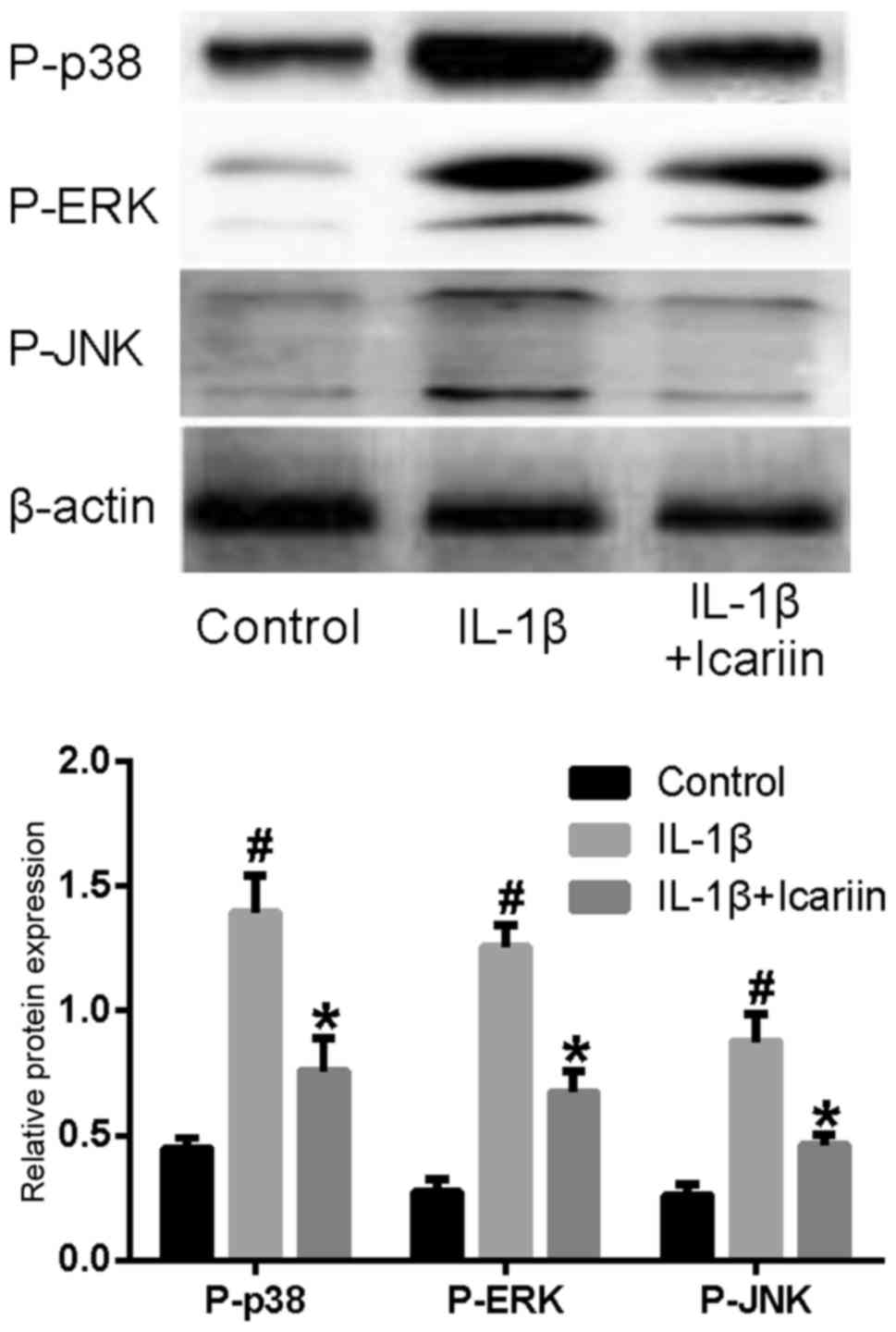 | Figure 2.Effects of Icariin on P-p38, P-ERK and
P-JNK levels. Human SW1353 chondrosarcoma cells were pretreated
with Icariin (20 µM) for 1 h, and were stimulated with 10 ng/ml
IL-1β. Control cells received no treatment and no stimulation.
After 24 h, protein expression levels of P-p38, P-ERK and P-JNK
were assessed using western blot analysis. The levels of P-p38,
P-ERK and P-JNK were increased following stimulation with IL-1β
compared with the control group. IL-1β-induced P-p38, P-ERK and
P-JNK upregulation was inhibited by treatment with 20 µM Icariin
compared with untreated IL-1β-stimulated cells. *P<0.05 compared
with IL-1β group; #P<0.05 compared with control
group. P-, phosphorylated; ERK, extracellular signal-regulated
kinase; JNK, c-Jun N-terminal kinase; IL, interleukin. |
Effects of MAPK pathway inhibitors and
Icariin on MMP-1, MMP-3 and MMP-13 in IL-1β-stimulated SW1353
cells
As aforementioned, Icariin treatment decreases the
IL1β-stimulated expression levels of MMP-1, MMP-3 and MMP-13
(Fig. 1). To further investigate
the role of Icariin in the regulation of MMP expression, inhibitors
of the MAPK signaling pathway were used. Treatment with Icariin
produced the greatest decrease in MMP-1 and MMP-3 levels, followed
by the p38 inhibitor and the ERK inhibitor (Fig. 3). The JNK inhibitor did not
significantly affect the expression of MMP-1 and MMP-3. In
addition, Icariin produced the greatest decrease in MMP-13 levels,
followed by the JNK inhibitor and the p38 inhibitor. However, the
ERK inhibitor did not significantly affect the expression of MMP-13
(Fig. 3). These results suggested
that, compared with the single MAPK inhibitor, Icariin had a better
inhibitory effect on the expression of MMP-1, MMP-3 and MMP-13. In
addition, the induction of MMP-1, MMP-3 and MMP-13 in
IL-1β-stimulated SW1353 cells may depend on different combinations
of MAPK signaling pathways, which is consistent with previous
results (14).
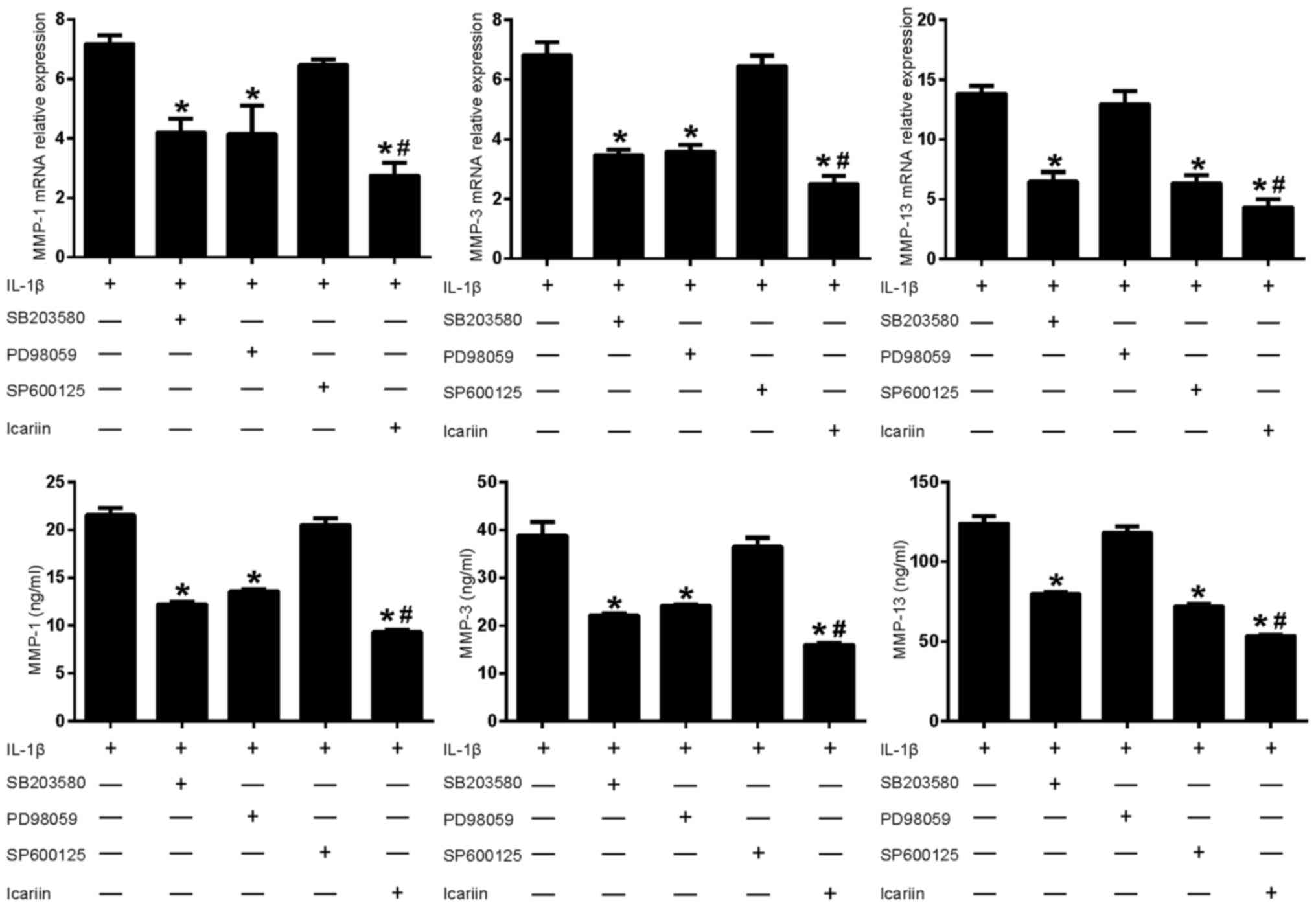 | Figure 3.Effects of mitogen-activated protein
kinase pathway inhibitors and Icariin on MMP-1, MMP-3 and MMP-13
expression. Human SW1353 chondrosarcoma cells were pretreated with
Icariin (20 µM), the p38 inhibitor SB203580 (10 µM), the ERK
inhibitor PD98059 (10 µM) or the JNK inhibitor SP600125 (10 µM) for
1 h. IL-1β (10 ng/ml) was then added to stimulate the cells. After
24 h, mRNA and protein levels of MMP-1, MMP-3 and MMP-13 were
detected using reverse transcription-quantitative polymerase chain
reaction and ELISA, respectively. Data are expressed as the mean ±
standard deviation. For both mRNA and protein expressions
*P<0.05 compared with untreated IL-1β group;
#P<0.05 compared with inhibitor-treated groups. MMP,
matrix metalloproteinase; ERK, extracellular signal-regulated
kinase; JNK, c-Jun N-terminal kinase; IL, interleukin; ELISA,
enzyme-linked immunosorbent assay. |
Effects of MAPK pathway activators on
MMP-1, MMP-3 and MMP-13 in IL-1β-stimulated SW1353 cells
Based on the results of the present study and
previously published reports (14,15),
Icariin exhibited an inhibitory effect on the expression of P-p38,
P-ERK, P-JNK, MMP-1, MMP-3 and MMP-13, and it was speculated that
p38 and ERK were required for MMP-1 and MMP-3 expression, whereas
p38 and JNK were required for MMP-13 expression in IL-1β-stimulated
SW1353 cells. According to this hypothesis, an activator of p38 and
ERK may be able to reverse the inhibitory effects of Icariin on
MMP-1 and MMP-3, and an activator of p38 and JNK may reverse the
inhibitory effect of Icariin on MMP-13. Therefore, cells were
treated with Icariin with or without U-46619 (a p38 and ERK
activator) co-treatment, and changes to the expression levels of
MMP-1 and MMP-3 were detected. The expression of MMP-13 was
detected after the cells were treated with Icariin with or without
co-treatment with Anisomycin (a p38 and JNK activator). Western
blot analysis demonstrated that levels of P-p38, P-ERK and P-JNK
were increased by treatment with the activators U-46619 and
Anisomycin (Fig. 4). Furthermore,
treatment with MAPK pathway activators appeared to produce a
corresponding increase in MMP-1, MMP-3 or MMP-13 expression, which
was not observed when cells were treated with Icariin alone
(Fig. 5).
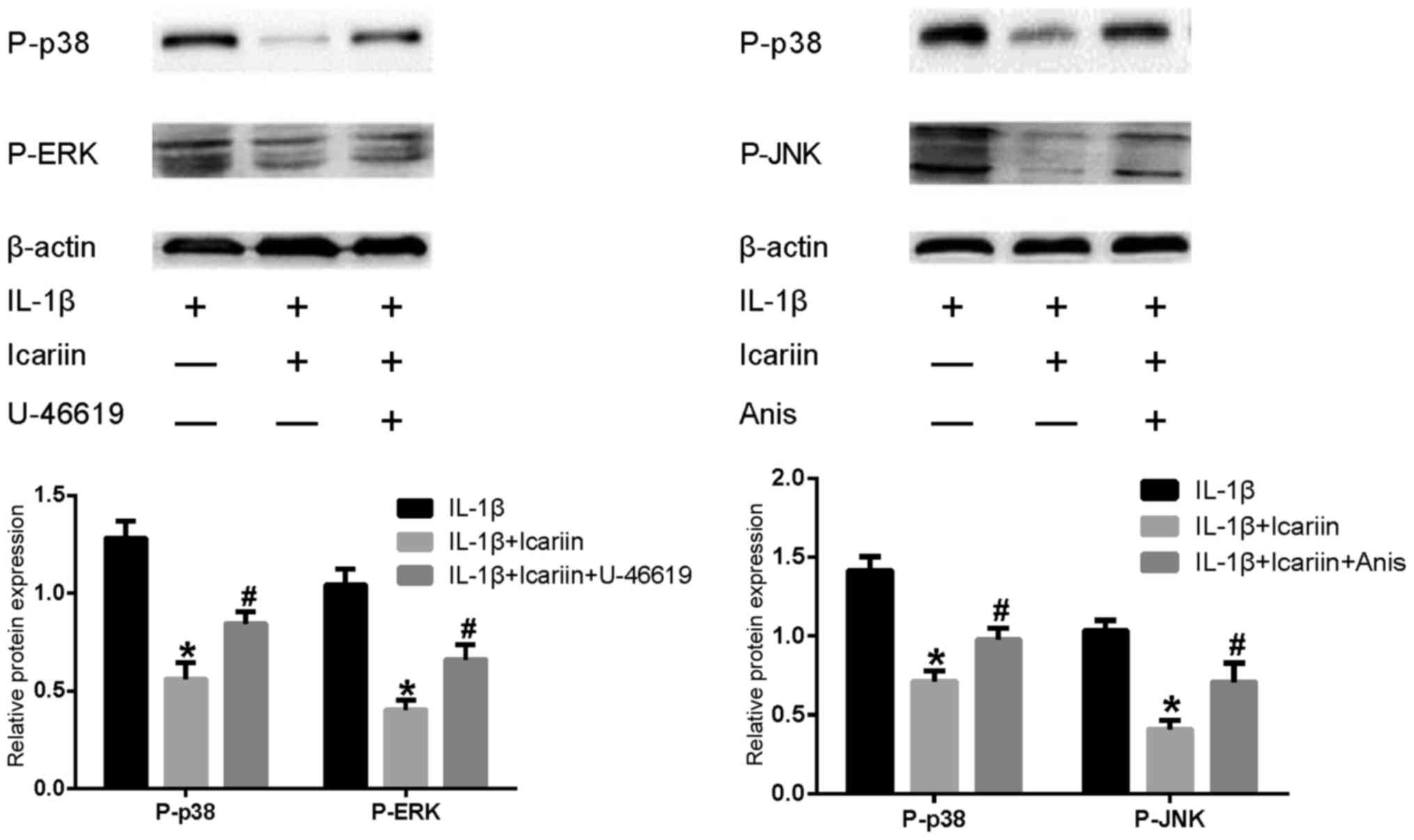 | Figure 4.Effects of MAPK pathway activators and
Icariin on P-p38, P-ERK and P-JNK levels. Human SW1353
chondrosarcoma cells were pretreated with Icariin (20 µM), the p38
and ERK activator U-46619 (50 µM) or the p38 and JNK activator
Anisomycin (10 µg/ml) for 1 h. IL-1β (10 ng/ml) was then added to
stimulate the cells. After 24 h, levels of P-p38, P-ERK, P-JNK were
assessed by western blot analysis. Densitometry of the protein
bands revealed that the MAPK pathway activators significantly
increased P-p38, P-ERK and P-JNK levels compared with
IL-1β-stimulated cells treated with Icariin alone. *P<0.05
compared with combined activator group; #P<0.05
compared with untreated IL-1β group. MAPK, mitogen-activated
protein kinase; ERK, extracellular signal-regulated kinase; JNK,
c-Jun N-terminal kinase; IL, interleukin; Anis, anisomycin. |
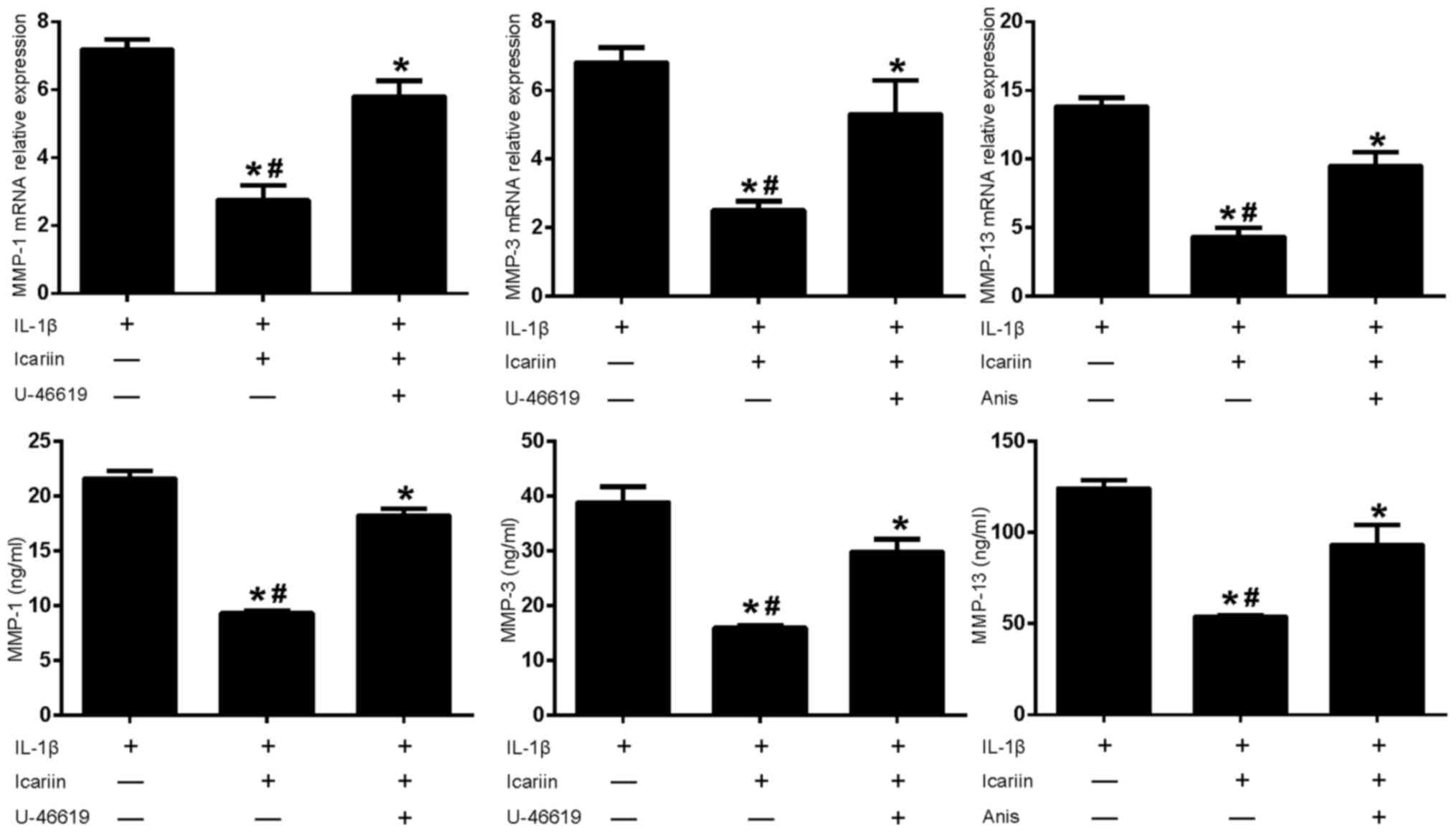 | Figure 5.Effects of MAPK pathway activators and
Icariin on MMP-1, MMP-3 and MMP-13 expression. Human SW1353
chondrosarcoma cells were pretreated with Icariin (20 µM), the p38
and ERK activator U-46619 (50 µM) or the p38 and JNK activator
Anisomycin (10 µg/ml) for 1 h. IL-1β (10 ng/ml) was then added to
stimulate the cells. After 24 h, mRNA and protein expression levels
of MMP-1, MMP-3 and MMP-13 were assessed using reverse
transcription-quantitative polymerase chain reaction and ELISA.
Data are expressed as the mean ± standard deviation. Levels of
MMP-1, MMP-3 and MMP-13 increased when MAPK pathway activators
where used in conjunction with Icariin. For both mRNA and protein
expressions *P<0.05 compared with untreated IL-1β group;
#P<0.05 compared with activator-treated group. MAPK,
mitogen-activated protein kinase; MMP, matrix metalloproteinase;
ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal
kinase; IL, interleukin; Anis, anisomycin; ELISA, enzyme-linked
immunosorbent assay. |
Discussion
OA is a heterogeneous and complex joint pathology,
characterized by the progressive degradation of cartilage,
ultimately resulting in complete loss of articular cartilage
(16). The precise mechanism of OA
pathogenesis has not yet been elucidated, and no effective
treatments to block the progression of the disease are currently
available. Inhibition of the enzymatic degradation of ECM
components and the maintenance of the cellular phenotype are two of
the main therapeutic strategies that are currently under
investigation (17).
MMPs serve a crucial role in the degradation of
articular cartilage. Among the various MMPs, MMP-1, MMP-3 and
MMP-13 can be found primarily in cartilage (18), where they target and degrade
collagen, proteoglycan, osteonectin and perlecan, thus
participating in OA progression (19). Previous research has investigated
the potential of several MMP inhibitors as candidates for the
treatment of OA and other diseases. However, the development of
most of these compounds has been discontinued due to various
reasons, such as toxicity, low specificity, severe off-target
effects, poor bioavailability and efficacy (20). Plant-derived compounds have
received considerable attention as potential therapeutic strategies
for the treatment of OA, due to their beneficial properties
(21,22). The proinflammatory cytokine IL-1β,
which is one of the most critical catabolic factors that
participate in OA pathogenesis, can enhance the production of MMPs.
In the present study, IL-1β was used to develop a cellular OA
model. The present results confirmed that IL-1β represents a potent
inflammatory stimulus that can lead to overexpression of MMP-1,
MMP-3 and MMP-13 in human SW1353 chondrocytes. The inhibitory
effect of Icariin on the IL-1β-induced upregulation of MMP-1, MMP-3
and MMP-13 was also demonstrated.
It has previously been demonstrated that in OA
cartilage, levels of P-MAPKs, including p38, JNK and ERK are
upregulated (7). MAPK pathways can
activate the downstream production of MMPs, including MMP-1, MMP-3
and MMP-13 (23,24). Mengshol et al demonstrated
that the induction of MMPs by IL-1 depends on different signaling
pathways (14). For example, IL-1
induction of MMP-13 requires p38 and JNK activity. The p38
inhibitor SB203580 has been reported to protect the cartilage
against degeneration, via inhibiting the expression of MMP-3 and
MMP-13 in the anterior cruciate ligament transection rat model of
OA, and in IL-1-stimulated cartilage explant culture (25). Results from the present study
demonstrated that the expression levels of MMP-1, MMP-3, MMP-13,
P-p38, P-ERK and P-JNK were downregulated by Icariin in
IL-1β-stimulated SW1353 cells. Icariin exhibited a better
inhibitory effect on the expression of MMP-1, MMP-3 and MMP-13
compared with the single MAPK inhibitor. However, the JNK inhibitor
SP600125 did not significantly affect the expression of MMP-1 and
MMP-3, and the ERK inhibitor PD98059 did not significantly affect
the expression of MMP-13. Based on the present results and
previously published reports (14,15),
it was speculated that p38 and ERK were required for MMP-1 and
MMP-3 expression, whereas p38 and JNK were required for MMP-13
expression in IL-1β-stimulated SW1353 cells. Subsequently,
treatment with U-46619 (a p38 and ERK activator) was demonstrated
to reverse the inhibitory effect of Icariin on MMP-1 and MMP-3. And
treatment with Anisomycin (a p38 and JNK activator) was revealed to
reverse the inhibitory effect of Icariin on MMP-13. Therefore, that
the suppressive effects of Icariin on MMP-1 and MMP-3 were
hypothesized to be partly achieved by inhibiting the activation of
p38 and ERK, whereas its effects on MMP-13 were partly achieved by
inhibiting the activation of p38 and JNK. This was consistent with
a previous study (14). The
present study demonstrated that Icariin inhibited the IL-1β-induced
expression of MMP-1, MMP-3 and MMP-13, and the phosphorylation of
p38, ERK and JNK in SW1353 cells. These results reveal the ability
of Icariin to block numerous pathways participating in degenerative
cartilage damage, and suggest a potential for Icariin as an
alternative strategy for OA treatment.
Acknowledgements
The authors would like to thank Professor Wei-Xue
Tang (Laboratory Research Center, The First Affiliated Hospital of
Chongqing Medical University, Chongqing, China) for her technical
assistance.
References
|
1
|
Robinson WH, Lepus CM, Wang Q, Raghu H,
Mao R, Lindstrom TM and Sokolove J: Low-grade inflammation as a key
mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol.
12:580–592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dai L, Wu H, Yu S, Zhao H, Xue L, Xu M,
Shen Z and Hu M: Effects of OsteoKing on osteoporotic rabbits. Mol
Med Rep. 12:1066–1074. 2015.PubMed/NCBI
|
|
3
|
Krasnokutsky S, Attur M, Palmer G, Samuels
J and Abramson SB: Current concepts in the pathogenesis of
osteoarthritis. Osteoarthritis Cartilage. 16:(Suppl 3). S1–S3.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hwang HS, Park SJ, Cheon EJ, Lee MH and
Kim HA: Fibronectin fragment-induced expression of matrix
metalloproteinases is mediated by MyD88-dependent TLR-2 signaling
pathway in human chondrocytes. Arthritis Res Ther. 17:3202015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kapoor M, Martel-Pelletier J, Lajeunesse
D, Pelletier JP and Fahmi H: Role of proinflammatory cytokines in
the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 7:33–42.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thalhamer T, McGrath MA and Harnett MM:
MAPKs and their relevance to arthritis and inflammation.
Rheumatology (Oxford). 47:409–414. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boileau C, Martel-Pelletier J, Brunet J,
Schrier D, Flory C, Boily M and Pelletier JP: PD-0200347, an α2δ
ligand of the voltage gated calcium channel, inhibits in vivo
activation of the Erk1/2 pathway in osteoarthritic chondrocytes: A
PKCalpha dependent effect. Ann Rheum Dis. 65:573–580. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gebauer M, Saas J, Sohler F, Haag J, Söder
S, Pieper M, Bartnik E, Beninga J, Zimmer R and Aigner T:
Comparison of the chondrosarcoma cell line SW1353 with primary
human adult articular chondrocytes with regard to their gene
expression profile and reactivity to IL-1beta. Osteoarthritis
Cartilage. 13:697–708. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sze SC, Tong Y, Ng TB, Cheng CL and Cheung
HP: Herba Epimedii: Anti-oxidative properties and its medical
implications. Molecules. 15:7861–7870. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen SR, Xu XZ, Wang YH, Chen JW, Xu SW,
Gu LQ and Liu PQ: Icariin derivative inhibits inflammation through
suppression of p38 mitogen-activated protein kinase and nuclear
factor-kappaB pathways. Biol Pharm Bull. 33:1307–1313. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma HP, Ming LG, Ge BF, Zhai YK, Song P,
Xian CJ and Chen KM: Icariin is more potent than genistein in
promoting osteoblast differentiation and mineralization in vitro. J
Cell Biochem. 112:916–923. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsieh TP, Sheu SY, Sun JS and Chen MH:
Icariin inhibits osteoclast differentiation and bone resorption by
suppression of MAPKs/NF-κB regulated HIF-1α and PGE(2) synthesis.
Phytomedicine. 18:176–185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mengshol JA, Vincenti MP, Coon CI,
Barchowsky A and Brinckerhoff CE: Interleukin-1 induction of
collagenase 3 (matrix metalloproteinase 13) gene expression in
chondrocytes requires p38, c-Jun N-terminal kinase and nuclear
factor kappaB: Differential regulation of collagenase 1 and
collagenase 3. Arthritis Rheum. 43:801–811. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X, Li F, Fan C, Wang C and Ruan H:
Effects and relationship of ERK1 and ERK2 in interleukin-1β-induced
alterations in MMP3, MMP13, type II collagen and aggrecan
expression in human chondrocytes. Int J Mol Med. 27:583–589.
2011.PubMed/NCBI
|
|
16
|
Alcaraz MJ, Megías J, García-Arnandis I,
Clérigues V and Guillén MI: New molecular targets for the treatment
of osteoarthritis. Biochem Pharmacol. 80:13–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsumoto T, Cooper GM, Gharaibeh B,
Meszaros LB, Li G, Usas A, Fu FH and Huard J: Cartilage repair in a
rat model of osteoarthritis through intraarticular transplantation
of muscle-derived stem cells expressing bone morphogenetic protein
4 and soluble Flt-1. Arthritis Rheum. 60:1390–1405. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amălinei C, Căruntu ID, Giuşcă SE and
Bălan RA: Matrix metalloproteinases involvement in pathologic
conditions. Rom J Morphol Embryol. 51:215–228. 2010.PubMed/NCBI
|
|
19
|
Shiomi T, Lemaître V, D'Armiento J and
Okada Y: Matrix metalloproteinases, a disintegrin and
metalloproteinases, and a disintegrin and metalloproteinases with
thrombospondin motifs in non-neoplastic diseases. Pathol Int.
60:477–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang M, Sampson ER, Jin H, Li J, Ke QH, Im
HJ and Chen D: MMP13 is a critical target gene during the
progression of osteoarthritis. Arthritis Res Ther. 15:R52013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Henrotin Y, Lambert C, Couchourel D,
Ripoll C and Chiotelli E: Nutraceuticals: Do they represent a new
era in the management of osteoarthritis?-A narrative review from
the lessons taken with five products. Osteoarthritis Cartilage.
19:1–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeong JW, Lee HH, Lee KW, Kim KY, Kim SG,
Hong SH, Kim GY, Park C and Kim HK: Mori folium inhibits
interleukin-1β-induced expression of matrix metalloproteinases and
inflammatory mediators by suppressing the activation of NF-κB and
p38 MAPK in SW1353 human chondrocytes. Int J Mol Med. 37:452–460.
2016.PubMed/NCBI
|
|
23
|
Yoon HY, Lee EG, Lee H, Cho IJ, Choi YJ,
Sung MS, Yoo HG and Yoo WH: Kaempferol inhibits IL-1β-induced
proliferation of rheumatoid arthritis synovial fibroblasts and the
production of COX-2, PGE2 and MMPs. Int J Mol Med. 32:971–977.
2013.PubMed/NCBI
|
|
24
|
Liacini A, Sylvester J, Li WQ, Huang W,
Dehnade F, Ahmad M and Zafarullah M: Induction of matrix
metalloproteinase-13 gene expression by TNF-alpha is mediated by
MAP kinases, AP-1, and NF-kappaB transcription factors in articular
chondrocytes. Exp Cell Res. 288:208–217. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen WD, Jiang Q, Chen DY, Xu H and Zhang
YF: Effects of intra-articular injection of p38 mitogen-activated
protein kinase inhibitor on matrix metalloproteinase in articular
cartilage of a rat model of osteoarthritis. Zhongguo Yi Xue Ke Xue
Yuan Xue Bao. 29:777–781. 2007.(In Chinese). PubMed/NCBI
|