Introduction
Alcoholism is a global social and medical problem,
and the accompanying alcoholic liver disease (ALD) is one of the
most common causes of chronic liver disease, and contributor to
morbidity and mortality rates worldwide. ALD encompasses a series
of conditions ranging from simple steatosis through to
steatohepatitis and fibrosis, cirrhosis and hepatocellular
carcinoma (1).
Bilirubin, a major end-product of the breakdown of
heme, is widely used for the evaluation of ALD severity (2). Increased levels of bilirubin in the
plasma have been reported in ALD rats and in patients with
different stages of ALD without bile duct obstruction (3–5).
Accumulated bilirubin further aggravates liver injury via the
inhibition of various enzyme systems, RNA synthesis and protein
synthesis, and via the uncoupling of oxidative phosphorylation in
liver mitochondria (6). The
dysfunction of bilirubin transportation contributes to the
development of hyperbilirubinemia or cholestatic liver diseases
(7–9). Under normal conditions, unconjugated
bilirubin enters hepatocytes via facilitated diffusion, which is
mediated by the organic anion transporting polypeptide (OATP)
family of proteins expressed on the basolateral membrane, and is
extensively glucuronidated by UDP-glucuronosyltransferase (UGT1A1).
Finally, the glucuronide metabolite is excreted into the bile via
multidrug resistance-associated protein 2 (MRP2) localized on the
canalicular plasma membrane (7–9).
OATP1B2, OATP1A1 and OATP1A4 in the mouse liver mediate uptake
(10). Mutations of these
transporter genes cause hereditary hyperbilirubinemia in humans,
further suggesting that altered transporter expression or function
is pivotal in the pathogenesis of cholestasis, including Rotor
syndrome and Dubin-Johnson syndrome (8,9).
Transport regulatory mechanisms have been investigated in bile duct
ligation (11),
lipopolysaccharide-induced cholestasis and in a
rhesus-rotavirus-induced biliary atresia mouse model (12). However, few studies have
systematically investigated these mechanisms in a murine model of
ALD.
The constitutive androstane receptor (CAR; NR1I3),
known as a xenobiotic receptor, has been implicated in the
detoxification and transportation of bilirubin or bile acid, the
regulation of drug metabolism, lipid metabolism, cholesterol
biosynthesis, cytokine and insulin signaling, the modulation of
apoptosis, and tumor development via the transcriptional regulation
of downstream target genes (11,13–15).
CAR target genes comprise those encoding phase II enzymes,
including UGT isoforms (UGT1A1, UGT1A6 and UGT1A9), uptake/efflux
transporters, including OATPs (OATP1, OATP2 and OATP4) and MRPs
(MRP2, MRP3 multidrug resistance protein 1 (MDR1), cytochrome P450
(CYP) 2B6, CYP2C9, CYP3A4, glutathione S-transferase (GST) A1 and
GSTA2 (14,16–18).
Among these target genes, UGT1A1, OATPs and MRPs are key components
of the bilirubin clearance pathway, thus CAR has a regulatory role
in bilirubin clearance.
The elevated bilirubin levels in ALD and the role of
CAR in bilirubin clearance raise the question of whether ethanol
alters the expression of components of the bilirubin transport
systems or detoxifying enzymes via CAR. The present study
investigated the expression of different components of bilirubin
clearance and of the xenobiotic receptor, CAR. In addition, the
role of CAR activation in the hepatic and renal bilirubin clearance
pathway of a murine model of ALD was investigated using the direct
ligand, 1,4-bis-[2-(3,5-dichlorpyridyloxy)] benzene (TCPOBOP), and
the indirect ligand, phenobarbital (PB), which does not bind to the
receptor but translocates CAR to the nucleus (18,19).
Materials and methods
Chemicals
TCPOBOP was purchased from Sigma-Aldrich; Merck
Millipore (Darmstadt, Germany), PB was purchased from Spectrum
(Scottsdale, NJ, USA) and dimethyl sulfoxide (DMSO) was purchased
from Sigma-Aldrich; Merck Millipore.
Animals and treatment
A total of 48 male mice (8–10 weeks old; 20–25 g) on
a C57BL/6 J background were purchased from Shanghai Laboratory
Animal Center, Chinese Academy of Sciences (Shanghai, China) and
housed in a specific pathogen-free animal facility under constant
temperature (20–22°C), controlled humidity (45–55%) and a standard
12-h light/dark cycle. All experimental procedures were approved by
the Institutional Animal Committee of Wenzhou Medical University
(Wenzhou, China) and performed according to the Guide for the Care
and Use of Laboratory Animals (Wenzhou Medical University, Wenzhou,
China).
The regular Lieber-DeCarli ethanol liquid diet (ETOH
group) or an isocaloric control diet (pair-fed group) were
purchased from Trophic Animal Feed High-tech Co., Ltd. (Nantong,
China), and were fed to the male mice ad libitum, as
previously described (20). The
percentages of caloric intake from ethanol (maltose dextrin),
proteins, carbohydrates and fats were 35.5, 18, 11.5 and 35%,
respectively. In the first week, mice were gradually introduced to
the ethanol liquid diet containing 0.75% ethanol (w/v) for 2 days,
1.50% ethanol (w/v) for 2 days and 3.75% ethanol (w/v) for 3 days
to acclimatize the mice to the liquid diet, and were then
introduced to a 5% ethanol (w/v) diet for 4 weeks. No differences
in body weight or the quantity of food consumed were found among
the treated groups. In the following 3 days, the clinically used
CAR ligand, PB (100 mg/kg/d; dissolved in DMSO) and the specific
rodent CAR ligand, TCPOBOP (3 mg/kg/d; dissolved in corn oil),
which has a higher potency, were administered intraperitoneally
(i.p) to the mice in the ETOH group (11). As a vehicle, mice were injected i.p
with 100 µl of DMSO (ETOH/DS group) or corn oil (ETOH/CO group) for
comparison with the ETOH/PB and ETOH/TCP groups, respectively. A
total of four animals were investigated in each treatment group. A
total of 3 days following injection, the animals were anesthetized
with 5% chloral hydrate (40 mg/kg, i.p) and sacrificed using the
cervical dislocation method following blood collection. Blood was
collected from the orbital sinus of the animals by traumatic
avulsion of the globe from the orbit using a pair of tissue
forceps. Sections of liver tissue were fixed in 4% paraformaldehyde
solution and paraffin-embedded for light microscopy. In addition,
liver tissues were cut into sections measuring <5 mm in
thickness, embedded in OCT compound and frozen in liquid nitrogen.
The remaining liver samples and the serum samples were stored at
−80°C for further analysis.
Liver sectioning and histological
staining
The paraformaldehyde-fixed, paraffin-embedded liver
samples were cut into 5-µm-thick sections and the OCT
compound-embedded liver samples were cut into 8-µm-thick frozen
sections, respectively. A light microscope was used to visualize
hematoxylin and eosin staining, as well as Oil Red O
(Sigma-Aldrich; Merck Millipore) staining to quantify steatosis and
inflammation in each group.
Serum biochemistry
Serum was prepared from the blood by centrifugation
at 1,200 g at 4°C for 10 min. The levels of total serum bilirubin
(TB), conjugated bilirubin (CB), alanine aminotransferase (ALT),
aspartate aminotransferase (AST) and alkaline phosphatase (ALP)
were determined in the pair-fed, ETOH, ETOH/PB, ETOH/TCP, ETOH/CO
and ETOH/DS mice using a total/direct bilirubin kit (Sigma-Aldrich;
Merck Millipore) and an ALT/AST assay kit (Jiancheng Bioengineering
Institute, Nanjing, China) according to the respective
manufacturers' protocols.
Western blot analysis
For isolation of total liver and kidney membranes,
tissue was homogenized in 1 mM NaHCO3 (pH 7.4),
containing complete protease inhibitor (Beyotime Institute of
Biotechnology, Haimen, China). Homogenates were gauze filtered, and
total membranes were isolated by centrifugation at 100,000 ×
g for 1 h at 4°C. as previously described (12,21).
Nuclear protein was extracted using the CelLytic™ NuCLEAR™
Extraction kit (Sigma-Aldrich; Merck Millipore). In addition,
protein concentrations were determined using a BCA protein Assay
kit (Beyotime Institute of Biotechnology). A total of 20 µg protein
was loaded to each well of 10% polyacrylamide gels for
electrophoresis. Proteins were blotted onto a polyvinylidene
fluoride (PVDF) membrane (Merck Millipore). PVDF membranes were
blocked using TBS containing 0.1% Tween 20 and 4% skim milk powder.
Blots were incubated with polyclonal rabbit anti-mouse lamin-B
(dilution, 1:5,000; cat. no. ab-65986; Abcam, Cambridge, UK),
OATP1A1 (dilution, 1:1,000; cat. no. sc-47265; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), OATP1A4 (dilution, 1:1,000;
cat. no. sc-33610; Santa Cruz Biotechnology, Inc.), OATP1B2
(dilution, 1:1,000; cat. no. sc-134460, Santa Cruz Biotechnology.
Inc.), MRP2 (dilution, 1:2,000; cat. no. sc-20766, Santa Cruz
Biotechnology, Inc.), CAR (dilution, 1:1,000; cat. no. ab-186869;
Abcam), GAPDH (dilution, 1:1,000; cat. no. AG019; Beyotime
Institute of Biotechnology) primary antibodies or monoclonal goat
anti-mouse UGT1A1 primary antibody (dilution, 1:1,000; cat. no.
sc-27419; Santa Cruz Biotechnology, Inc.) at 4°C overnight,
respectively. Following washing with 1X TBS containing 0.1% Tween
20 for 3 times, there was incubation conducted using horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody
(dilution, 1:10,000; cat. no. A0208; Beyotime Institute of
Biotechnology) and enhanced chemiluminescence substrate reagent
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). The time for
secondary antibody incubation was <1 h at 37°C. The
quantification of protein expression was determined via image
processing and analysis using Image Lab 3.0 software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Total RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
RNA was extracted from the frozen liver and kidney
samples using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and complementary DNA (cDNA) synthesis for qPCR analysis was
performed using a commercial kit (Toyobo Co., Ltd., Osaka, Japan)
with subsequent melting curve analysis, according to the
manufacturer's protocol. The PCR reaction mixture (forward primer,
0.4 µl; reverse primer, 0.4 µl; cDNA, 1 µl; diethylpyrocarbonate,
3.2 µl) was prepared using SYBR Green Real-time PCR Master Mix-Plus
(Toyobo Co., Ltd.). The thermocycling parameters were as follows:
pre-denaturation at 94°C for 1 min, denaturation at 95°C for 15
sec, annealing at 60°C for 15 sec, extension at 72°C for 15 sec for
40 cycles of amplification. PCR amplification was performed using
specific primers (Table I). Data
were analyzed using Bio-Rad CFX manager (Bio-Rad Laboratories,
Inc.). The levels of gene expression were calculated as a ratio to
the expression of the housekeeping gene, β-actin.
 | Table I.Mouse primers. |
Table I.
Mouse primers.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| Oatp1a1 |
GTCTTACGAGTGTGCTCCAGAT |
GGAATACTGCCTCTGAAGTGGATT |
| Oatp1a4 |
GACGGCTCAGTGTTCATTC |
CTTCTAGCTGGTCCCTCTT |
| Oatp1b2 |
GATCCTTCACTTACCTGTTCAA |
CCTAAAAACATTCCACTTGCCATA |
| Mrp2 |
GCTTCCCATGGTGATCTCTT |
ATCATCGCTTCCCAGGTACT |
| Ugt1a1 |
TCTGAGCCCTGCATCTATCTG |
CCCCAGAGGCGTTGACATA |
| Car |
AAAGCAGGGTCAGCGAGGAG |
AGTCAGGGCGTGGAAATGATAGC |
| β-actin |
CTGGCACCACACCTCCTACA | AGTACTTGCGCACAG
GAGGA |
Immunofluorescence analysis
The NCTC1469 normal mouse liver cell line was
purchased from Zhongqiao (Shanghai, China). The cells were
maintained at 37°C in a humidified atmosphere of 5% CO2
in RPMI 1640 supplemented with penicillin/streptomycin (100 IU/ml
and 100 µg/ml, respectively) and 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.). Prior to the experiment, cell
viability was measured via Trypan blue exclusion. The hepatocytes
were plated on glass coverslips at a density of 2.0×105
cells/ml in 6 wells and incubated in a CO2 incubator for
3 h at 37°C. The cells were pretreated with or without 2 mM
phenobarbital (PB) for 6 h followed by treatment with or without
100 µM alcohol for 12 h at 37°C. The cells were fixed with 4%
formaldehyde/PBS for 15 min. 10% Goat serum (Gibco; Thermo Fisher
Scientific, Inc.) in TBS containing 0.1% Triton X-100 was used to
block nonspecific binding, as previously described (22). Membranes were incubated with CAR
primary antibody at a dilution of 1:100 overnight and with
corresponding secondary antibodies conjugated to Delight 594
(Bioworld Technology, Inc., Minneapolis, MN, USA) at a dilution of
1:200 for another 2 h at room temperature. The coverslips were
sealed with antifade mounting medium (Beyotime Institute of
Biotechnology) following staining with DAPI (Beyotime, Haimen;
China) for 10 min, and were visualized using a fluorescence
microscope (Olympus, Tokyo, Japan).
Statistical analysis
Data are shown as the mean ± standard deviation.
Statistical analysis was performed using two-tailed Student's
t-test or one-way analysis of variance followed by post
hoc tests (LSD-t or Dunnett's T3) for multigroup comparisons.
SPSS 12.0 program (SPSS, Inc., Chicago, IL, USA) was used for
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Biochemical and histopathological
changes in the experimental ALD mouse model
To investigate the role of CAR in bilirubin
clearance in ALD, wild-type C57BL6 mice were fed with a
Lieber-DeCarli liquid diet or a pair-fed control diet for 4 weeks,
followed by i.p. injections with different CAR ligands for 3 days.
The levels of ALT, AST and ALP in the EtOH group were higher,
compared with those in the pair-fed controls (105±30.66, vs.
26.75±2.99 U/l; 187.8±55.34, vs. 111.3±19.52 U/l and 155±12.83, vs.
65.25±37.35 U/l, respectively; P<0.05) and were also elevated in
the EtOH/TCP group, compared with those in its vehicle (EtOH/CO)
group (510.3±151.2, vs. 103.8±25.63 U/l; 287.8±91.09, vs.
174.5±42.15 U/l and 284.5±46.65, vs. 148.8±13.74 U/l, respectively;
P<0.05), as shown in Table II.
The levels of AST and ALP in the EtOH/DS group (EtOH/PB vehicle)
were lower, compared with those in the EtOH group (115±25.59, vs.
187.8±55.34 U/l and 106±18.94, vs. 155±12.83 U/l, respectively;
P<0.05). Compared with the pair-fed controls, the levels of
hepatic total bilirubin and direct bilirubin were significantly
elevated following alcohol ingestion (0.33±0.10, vs. 0.75±0.17
µmol/l and 0.25±0.10, vs. 0.45±0.13 µmol/l, respectively;
P<0.05), and were markedly decreased following administration of
the high affinity CAR agonist, TCPOBOP (0.55±0.13, vs. 0.15±0.06
µmol/l and 0.43±0.10, vs. 0.13±0.05 µmol/l, respectively;
P<0.05). The level of total bilirubin was reduced following
indirect ligand PB injection whereas only a marginal alteration was
observed in the level of direct bilirubin (Table II). Furthermore, the levels of
total and direct bilirubin in the EtOH/DS group (EtOH/PB vehicle)
were downregulated, compared with those in the EtOH group
(0.45±0.06, vs. 0.75±0.17 µmol/l and 0.2±0.08, vs. 0.45±0.13
µmol/l, respectively; P<0.05). Taken together, chronic alcohol
consumption led to ALD, and increased the levels of total and
direct bilirubin. In addition, the CAR agonists, TCPOBOP and PB,
decreased total and direct bilirubin levels.
 | Table II.Serum biochemistry. |
Table II.
Serum biochemistry.
| Characteristic | Pair-fed | EtOH | EtOH/TCP | EtOH/CO | EtOH/PB | EtOH/DS |
|---|
| Total bilirubin
(µmol/l) |
0.33+0.10a | 0.75+0.17 |
0.15+0.06b | 0.55+0.13 |
0.23+0.05b |
0.45+0.06a |
| Direct bilirubin
(µmol/l) |
0.25+0.10a | 0.45+0.13 |
0.13+0.05b | 0.43+0.10 | 0.10+0.08 |
0.2+0.08a |
| ALT (U/l) |
26.75+2.99a | 105+30.66 |
510.3+151.2b | 103.8+25.63 | 51.5+12.92 | 79.3+19.41 |
| AST (U/l) |
111.3+19.52a | 187.8+55.34 |
287.8+91.09b | 174.5+42.15 | 125.3+29.3 |
115+25.59a |
| ALP (U/l) |
65.25+37.35a | 155+12.83 |
284.5+46.65b | 148.8+13.74 | 86+27.06 |
106+18.94a |
H&E staining and oil red O staining revealed
that chronic alcohol ingestion caused liver macrovesicular and
microvesicular steatosis, cellular edema and ballooning
degeneration, and nuclear pycnosis. These liver injuries were
ameliorated by the CAR ligands, TCPOBOP and PB (Fig. 1A and B). In addition, TCPOBOP
treatment resulted in hepatocyte enlargement, based on an elevated
liver/body weight ratio (P<0.001; Fig. 1C). Chronic alcohol intake also
significantly dysregulated the liver/body weight ratio, as shown in
Fig. 1C (P<0.01).
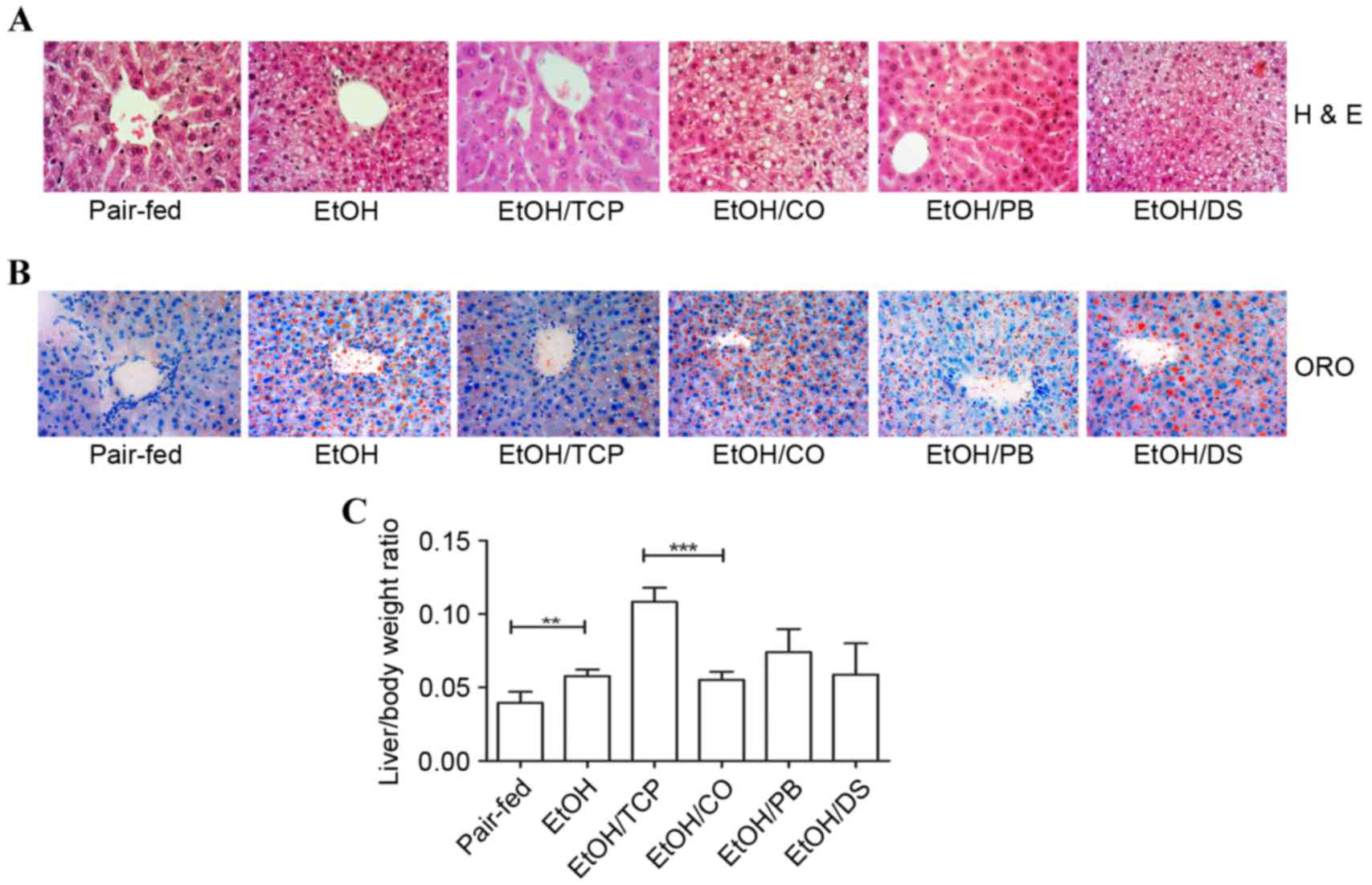 | Figure 1.Effect of CAR agonists, TCP and PB,
and their vehicles, corn oil and dimethyl sulfoxide, on
alcohol-induced fatty liver in mice. (A) Liver sections were
stained with H&E (original magnification, ×400). (B) ORO
staining of the liver sections showing the number of oil drops in
the sections from each group (original magnification, ×400). (C)
Liver/body weight ratio in each group. Scale bar=50 µm. (n=4,
**P<0.01 and ***P<0.001). CAR, constitutive androstane
receptor; PB, pentobarbital; TCP,
1,4-bis-[2-(3,5-dichlorpyridyloxy)]benzene; CO, corn oil; DS,
dimethyl sulfoxide; H&E, hematoxylin and eosin; ORO, oil red
O. |
Effect of chronic alcohol consumption
on hepatic and extrahepatic bilirubin clearance
The mRNA and protein levels of the primary mouse
hepatic bilirubin uptake transporters, OATP1A4 and OATP1B2,
remained unaltered following 4 weeks of chronic alcohol consumption
(Fig. 2A and C). However, the mRNA
level of hepatic OATP1A1 in the EtOH group was markedly reduced by
~60-fold (Fig. 2A). The protein
levels of OATP1A1 were decreased by 60%, compared with those of the
pair-fed group (Fig. 2C), in
accordance with the downregulated mRNA levels (Fig. 2A). Furthermore, alcohol intake
induced the mRNA and protein expression of
bilirubin-glucuronidating UGT1A1 4.9- and 1.5-fold (Figs. 2B and C), respectively, as
previously observed in a rat model of ALD (5). The mRNA levels of the excretion pump,
MRP2, were significantly decreased 2.2-fold (Fig. 2B), whereas the protein level of
MRP2 was deregulated (P<0.05; Fig.
2C), compared with that of the pair-fed group.
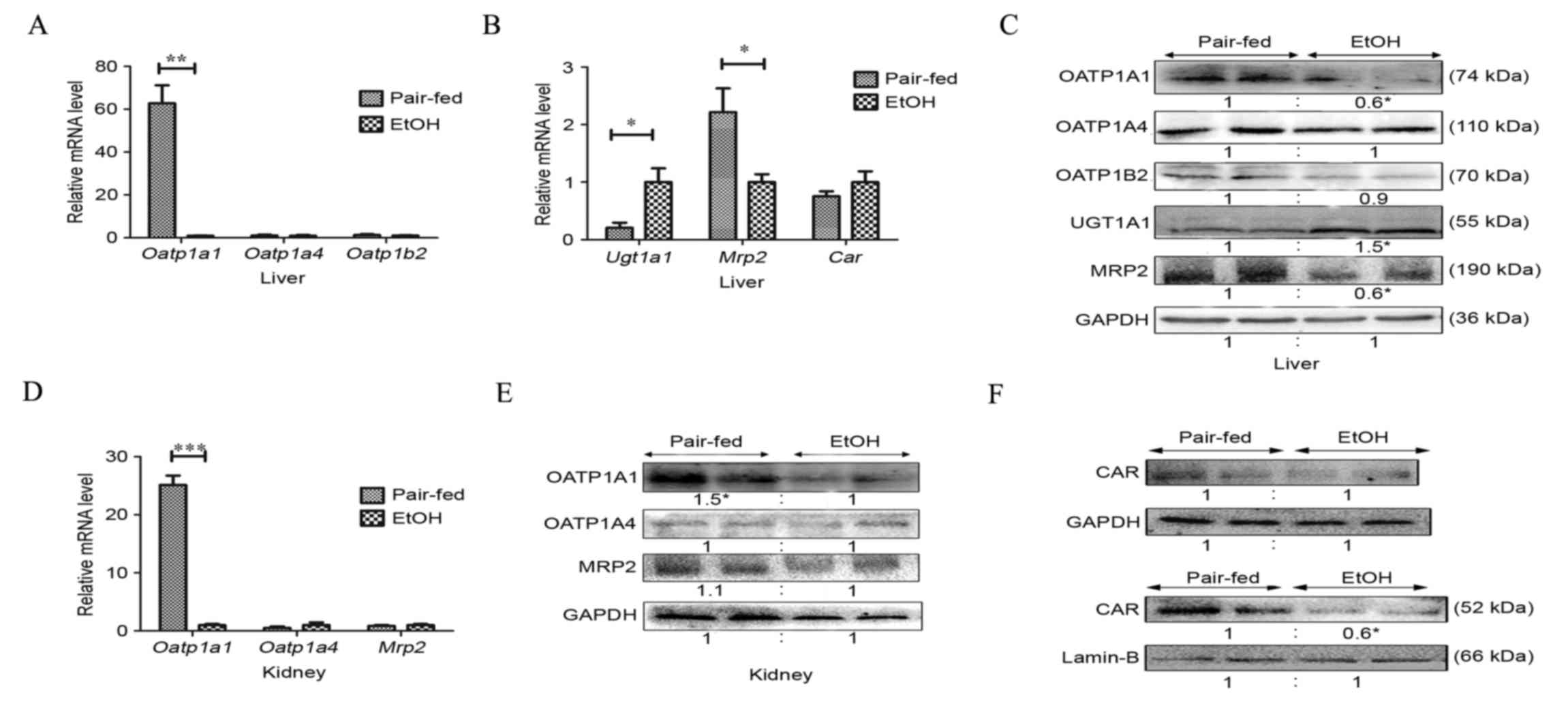 | Figure 2.Effects of chronic alcohol consumption
on bilirubin uptake, detoxification and excretion. (A) mRNA levels
of OATP1a1, OATP1a4 and OATP1b2 uptake transporters in mouse livers
were assayed following 4 weeks on an alcoholic diet. (B) Western
blotting analysis of protein expression of liver OATP1a1, OATP1a4,
OATP1b2, UGT1A1 and MRP2. (C) mRNA levels of liver detoxification
and excretion enzymes, UGT1A1 and MRP2, in mice. (D) Nuclear
extracts from liver and liver membrane were subjected to western
blot analysis to determine the protein level of CAR. (E) Kidney
tissue was subjected to reverse transcription-quantitative
polymerase chain reaction analysis for determination of mRNA levels
of OATP1a1, OATP1a4 and MRP2. (F) Western blot analysis of OATP1a1,
OATP1a4 and MRP2 in the kidney (n=4 per group; *P<0.05,
**P<0.01 and ***P<0.001). CAR, constitutive androstane
receptor; OATP, organic anion transporting polypeptide; UGT,
UDP-glucuronosyltransferase; MRP, multidrug resistance-associated
protein. |
OATP1A1, OATP1A4 and MRP2 also have been detected in
the apical membrane of renal proximal tubular cells (10,17,23,24).
Under pathological conditions, including cholestasis, bilirubin
glucuronides are secreted into the plasma and then eliminated by
the kidney (10). Adaptive renal
transporter induction has been confirmed in mouse cholestatic
models of common bile duct ligation (23). To investigate the extrahepatic
pathway of bilirubin clearance in the ALD mouse model, the present
study examined the renal expression of these transporters. The mRNA
and protein expression levels of OATP1A1 in the kidney were
decreased by 25- and 1.6-fold, respectively, compared with those in
the pair-fed group (Figs. 2D and
E). The expression levels of renal OATP1A4 and MRP2 remained
unaffected (Figs. 2D and E).
Previous reports on renal bilirubin transport in an ALD mouse model
are limited.
Effect of chronic alcohol consumption
on the expression of nuclear receptor CAR
The present study also examined whether the
expression of CAR was affected by chronic alcohol ingestion. The
alcohol-fed mice showed limited variation in mRNA and protein
levels of CAR in livers of the alcohol-fed mice, whereas the
protein level of CAR in nuclear extracts was negatively regulated
by 60%, compared with that of the pair-fed group (Fig. 2B and F), suggesting that alcohol
inhibited CAR translocation to the nucleus.
Effect of the TCPOBOP and PB on
hepatic and extrahepatic bilirubin clearance in a murine model of
ALD
To determine the role of the TCPOBOP and PB CAR
ligands in the regulation of bilirubin clearance in ALD mice,
TCPOBOP, PB and their vehicles were administered (i.p.). A marked
increase was observed in the mRNA expression levels of liver
Oatp1a1, Oatp1a4 and Ugt1a1 in the EtOH/PB group, compared with
that of the vehicle EtOH/DS group (2.5-, 1.3- and 4.6-fold,
respectively) whereas no significant change in the levels of
Oatp1b2 or Mrp2 were observed (Fig. 3A
and B). An increase in the mRNA expression of Mrp2 of
>2-fold was observed in the EtOH/TCP group, compared with its
vehicle EtOH/CO group, whereas the mRNA levels of Oatp1a1, Oatp1a4,
Oatp1b2 and Ugt1a1 were not altered significantly (Fig. 3A and B). Accordingly, the protein
levels of UGT1A1 in the liver were induced following treatment with
TCPOBOP and PB, compared with the levels in their vehicles (1.5-
and 2-fold, respectively, P<0.05; Fig. 3C). MRP2 was also increased
significantly in the EtOH/TCP group (1.4-fold; P<0.05) and
marginally induced in the EtOH/PB group (1.3-fold; P>0.05;
Fig. 3C). No significant
enhancement in the relative protein levels of OATP1A1, OATP1A4 or
OATP1B2 were found in the EtOH/TCP or EtOH/PB groups (Fig. 3C).
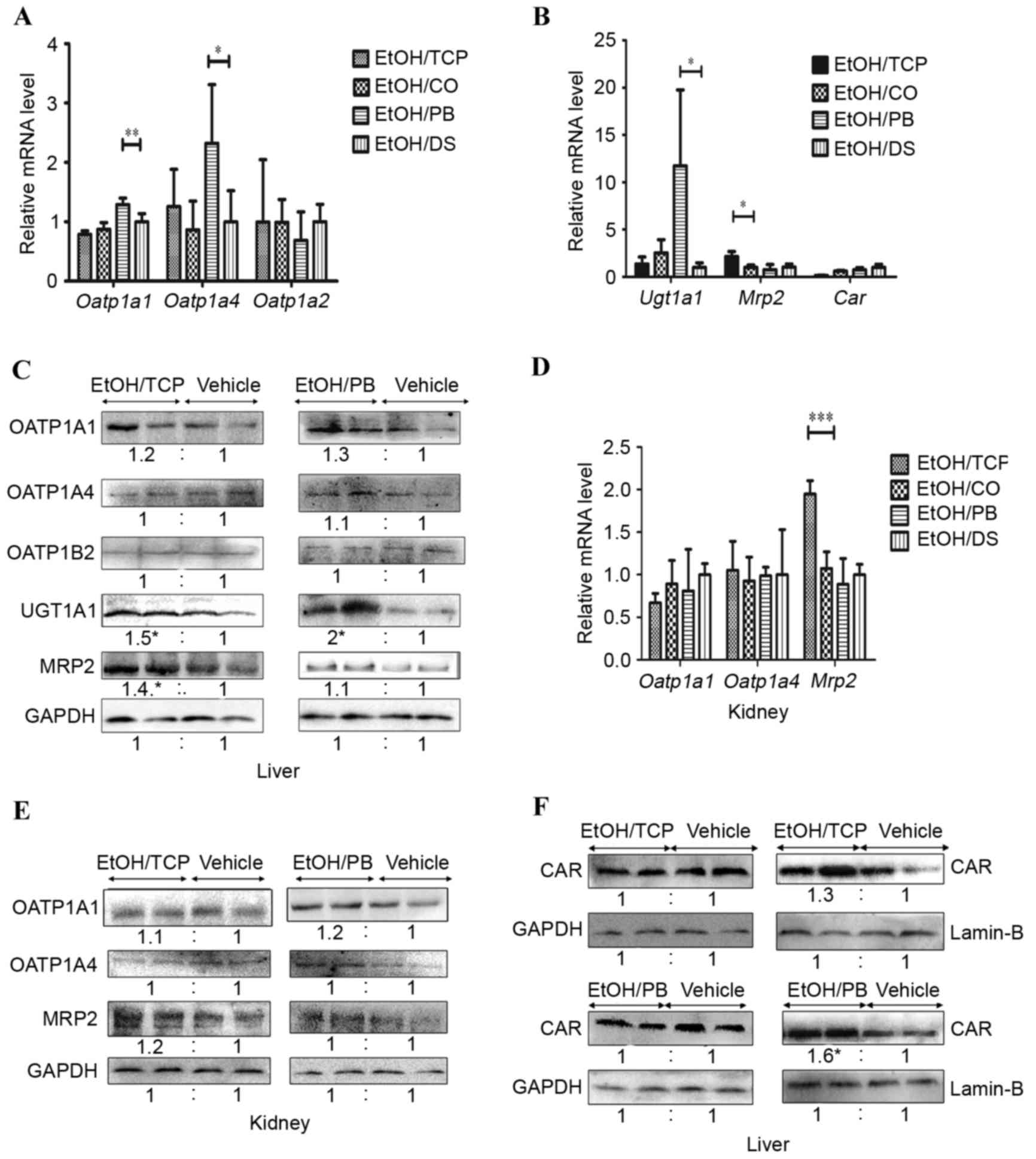 | Figure 3.TCP and PB selectively increase the
expression of bilirubin-detoxifying enzymes, transporters and CAR
in the liver and kidney. (A) RT-qPCR analysis of total liver mRNA
expression of Oatp1a1, Oatp1a4 and Oatp1b2. (B) RT-qPCR analysis of
total liver mRNA expression of Ugt1A1, Mrp2 and Car. (C) Western
blot analysis of liver protein expression levels of OATP1A1,
OATP1A4, OATP1B2, UGT1A1 and MRP2. (D) RT-qPCR analysis of renal
mRNA levels of Oatp1a1, Oatp1a4, Mrp2. (E) Western blot analysis of
renal protein expression levels of OATP1A1, OATP1A4 and MRP2. (F)
Western blot analysis of nuclear extracts from liver and liver
membranes for CAR determination. n=4 in each group (*P<0.05,
**P<0.01 and ***P<0.001). CAR, constitutive androstane
receptor; OATP, organic anion transporting polypeptide; UGT,
UDP-glucuronosyltransferase; MRP, multidrug resistance-associated
protein; TCP, 1,4-bis-[2-(3,5-dichlorpyridyloxy)]benzene; PB,
pentobarbital; CO, corn oil; DS, dimethyl sulfoxide; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction. |
To further elucidate the effect of CAR ligands on
the extrahepatic bilirubin clearance pathway in ALD mice, the
present study detected the renal expression of OATP1A1, OATP1A4 and
MRP2 transporters. The mRNA levels of Oatp1a1 and Oatp1a4 remained
unchanged following treatment with the two agonists, whereas the
mRNA level of Mrp2 was induced 2-fold by TCP (Fig. 3D), with no clear difference in
protein levels (Fig. 3E).
Effect of CAR ligands on the
expression of CAR in a murine model of ALD
The present study also determined whether the
expression of CAR was affected by CAR ligands in the ALD mice. The
total liver mRNA and protein expression levels of CAR were not
induced by TCPOBOP or PB treatment (Fig. 3B and F), as described previously
(17). However, it was found that
the protein expression of CAR in the nuclear extract was increased
1.6-fold following PB administration (Fig. 3F), as previously described
(18). However, no significant
alteration in the protein level of CAR was observed in the nuclear
extract following TCP administration, compared with that of its
vehicle.
In vitro subcellular localization of
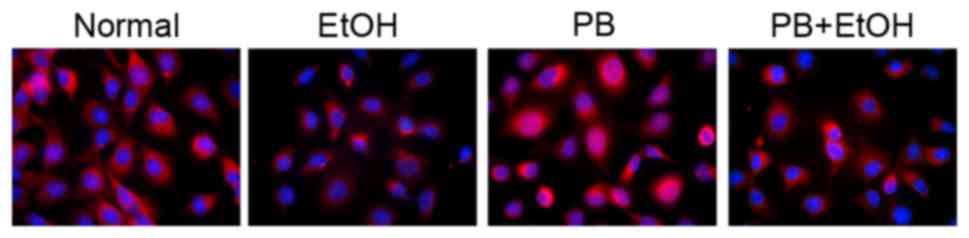
CAR
To further determine whether alcohol suppressed CAR
translocation to the nucleus, the present study measured the
expression of CAR using immunofluorescence analysis in normal mouse
hepatocytes. As shown in Fig. 4,
fluorescence was observed in the alcohol-treated cells, although
few fluorescence dots were identified in the nuclei of the
alcohol-treated cells. These results indicated that alcohol
impaired the PB-induced translocation of CAR from the cytoplasm to
the nucleus.
Discussion
In the present study, plasma levels of bilirubin and
the hepatic and extrahepatic bilirubin clearance pathways were
examined in a murine model of ALD. It was found that chronic
alcohol intake elevated plasma levels of bilirubin and induced the
mRNA and protein levels of UGT1A1, which mediated the
detoxification of bilirubin to less toxic substrates for biliary
and urinary excretion, and induced alternative elimination
pathways, including the MRP3 and MRP4 pathways. Despite the
increased levels of UGT1A1, chronic alcohol consumption reduced the
expression of hepatic MRP2, as reported by Zinchuk et al
(22). Alcohol significantly
reduced the transcriptional and post-transcriptional levels of
OATP1A1 in the liver and kidney, which may have been a response to
limit the cellular accumulation of bilirubin in the liver and the
renal reuptake of bilirubin. Few reports have described the effects
of ethanol on the reduction of OATP1A1. The altered levels of
OATP1A1 and MRP2 may account for the pathological elevation of
bilirubin in ALD, and the induction of UGT1A1 may be an adaptive
response to the increased levels of bilirubin.
The present study also found that the protein levels
of CAR in the nuclear extracts decreased with alcohol intake and
increased with exposure to the CAR ligand, PB, which translocates
CAR to the nucleus and causes the total liver protein to remain
constant. This indicated that alcohol impaired CAR translocation.
Furthermore, the distribution analysis of fluorescence in
vitro confirmed that alcohol impaired the translocation of CAR
and the PB-induced nuclear translocation of CAR. Hepatic OATPs,
UGT1A1 and MRP2 represent CAR target genes, as previously described
(11,18,25).
Therefore, alterations in transporters may be associated with the
dysfunctional translocation of CAR and requires further
investigation.
The nuclear translocation of CAR is a key step in
inducer transactivation. Cytoplasmic CAR (NR1I3) forms heterodimers
with the retinoid X receptor α (NR2B1) upon activation by its
ligand to mediate binding with nuclear DNA response elements in the
promoter regions, which is defined by different canonical hexamer
sequences in the target genes (25). The phenobarbital-responsive
enhancer module (PBREM) and the xenobiotic-responsive enhancer
module have been identified as the CAR binding sites in response to
CAR activation (13).
In order to further elucidate the effect of CAR on
serum levels of bilirubin and the bilirubin clearance pathway in
the murine model of ALD, the ALD mice were injected with the
rodent-specific CAR agonist, TCPOBOP, and indirect CAR ligand, PB,
both of which promoted bilirubin clearance by decreasing plasma
bilirubin levels (Table II). PB
also induced the hepatic transcription of OATP1A1 and OATP1A4. The
stimulatory effects of TCPOBOP on the expression levels of these
two uptake transporters were moderate (P>0.05), whereas PB and
TCPOBOP had no significant effect on OATP1B2. In the absence of
mouse OATP1A and 1B transporters, the biliary excretion of
conjugated bilirubin was reduced by 50%. This finding indicated
that, under physical conditions, at least half of the conjugated
bilirubin was initially transported into the plasma, only to be
taken up by hepatocytes downstream via OATP1A and 1B (26). Thus, the induction of OATP1A1 and
OATP1A4 following the administration of CAR agonists explains the
reduction in serum-conjugated bilirubin via the reuptake of
bilirubin glucuronides (Table
II). In addition, PB enhanced the transcriptional and
post-transcriptional levels of UGT1A1, whereas TCPBOTOP stimulated
the protein overexpression of UGT1A1 in the ALD murine model.
UGT1A1, which is pivotal in the clearance of bilirubin, is the
first glucuronidation enzyme targeted by CAR by binding to a
distinct PBREM of UGT1A1 (27,28).
TCPOBOP induced the expression of MRP2, consistent with a study by
Petrick et al (17),
however, MRP2 was less affected by PB. The induction of
detoxification enzymes and the biliary excretion pump may also
account for the accelerated serum bilirubin clearance following
treatment with CAR agonists. The present study also detected renal
transporters by administering these two ligands and found that
OATP1A1 and OATP1A4 remained unaltered by the CAR ligands, and the
mRNA expression of Mrp2 was induced by TCPOBOP, but not PB. This
limited effect may be attributed to the lower renal expression of
CAR, as previously described (11,17).
Similarly, previous studies have demonstrated that CAR agonists
upregulate the expression levels of OATP2, UGT1A1 and MRP2 to
inhibit cholestasis, with subsequent reduced serum levels of
bilirubin, in a model of common bile duct ligation (11). Huang et al (29,30)
observed that TCPOBOP increased clearance under an acute dose of
bilirubin, with induction of UGT1A1, MRP2, GSTA1 and GSTA2 in the
bilirubin clearance pathway in the livers of wild-type, but not
CAR-deficient, mice. Despite increased induction of its target
gene, the expression of CAR was not induced by activators of CAR,
as reported previously (17).
In the present study, in addition to protective
effects as a bilirubin cleaner, CAR agonists also alleviated the
degree of steatosis induced by alcohol, as shown using H&E and
oil red O staining. The CAR agonist, PB, has been shown to improve
lipid metabolism and enhance insulin sensitivity in diabetic rats
(31). Furthermore, CAR agonists
have been reported to inhibit the expression of lipogenic genes,
including acetyl-CoA carboxylase (Acc-2) and stearoyl-CoA
desaturase-1 (Scd-1), and rats deficient in Acc-2 or Scd-1 are
protected from hepatic steatosis induced by a high-fat diet
(32). Similarly, CAR agonists
alleviate steatosis of the liver by improving lipid metabolism,
which requires further investigation. However, the CAR agonist,
TCPOBOP, elevated the levels of ALT and AST, and enlarged
hepatocytes by increasing the liver/body weight ratio as a side
effect. Therefore, the selection of appropriate CAR activators is
essential for bilirubin clearance in hepatocytes and for improved
lipid metabolism.
In conclusion, the results of the present study
revealed that chronic alcohol consumption impaired the
translocation of CAR, leading to defective bilirubin clearance
accompanied by the reduction of hepatic and renal OATP1A1, the
reduction of hepatic MRP2 and the induction of UGT1A1. The results
also demonstrated that the activation of CAR by different ligands
promotes bilirubin clearance by selectively inducing OATP1A1,
OATP1A4, UGT1A1 and MRP2 in a murine model of ALD.
Acknowledgements
This study was supported by the Zhejiang Provincial
Natural Science Foundation of China (grant nos. LY12H03003 and
Y2110768).
References
|
1
|
Orman ES, Odena G and Bataller R:
Alcoholic liver disease: Pathogenesis, management, and novel
targets for therapy. J Gastroenterol Hepatol. 28 Suppl 1:S77–S84.
2013. View Article : Google Scholar
|
|
2
|
Chalmers DM, Rinsler MG, MacDermott S,
Spicer CC and Levi AJ: Biochemical and haematological indicators of
excessive alcohol consumption. Gut. 22:992–996. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torkadi PP, Apte IC and Bhute AK:
Biochemical evaluation of patients of alcoholic liver disease and
non-alcoholic liver disease. Indian J Clin Biochem. 29:79–83. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zollner G, Fickert P, Zenz R, Fuchsbichler
A, Stumptner C, Kenner L, Ferenci P, Stauber RE, Krejs GJ, Denk H,
et al: Hepatobiliary transporter expression in percutaneous liver
biopsies of patients with cholestatic liver diseases. Hepatology.
33:633–646. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kardon T, Coffey MJ, Bánhegyi G, Conley
AA, Burchell B, Mandl J and Braun L: Transcriptional induction of
bilirubin UDP-glucuronosyltransrase by ethanol in rat liver.
Alcohol. 21:251–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Flitman R and Worth MH Jr: Inhibition of
hepatic alcohol dehydrogenase by bilirubin. J Biol Chem.
241:669–672. 1966.PubMed/NCBI
|
|
7
|
Bodeman CE, Dzierlenga AL, Tally CM,
Mulligan RM, Lake AD, Cherrington NJ and McKarns SC: Differential
regulation of hepatic organic cation transporter 1, organic
anion-transporting polypeptide 1a4, bile-salt export pump and
multidrug resistance-associated protein 2 transporter expression in
lymphocyte-deficient mice associates with interleukin-6 production.
J Pharmacol Exp Ther. 347:136–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Keppler D: The roles of MRP2, MRP3,
OATP1B1, and OATP1B3 in conjugated hyperbilirubinemia. Drug Metab
Dispos. 42:561–565. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Erlinger S, Arias IM and Dhumeaux D:
Inherited disorders of bilirubin transport and conjugation: New
insights into molecular mechanisms and consequences.
Gastroenterology. 146:1625–1638. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iusuf D, van de Steeg E and Schinkel AH:
Functions of OATP1A and 1B transporters in vivo: Insights from
mouse models. Trends Pharmacol Sci. 33:100–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wagner M, Halilbasic E, Marschall HU,
Zollner G, Fickert P, Langner C, Zatloukal K, Denk H and Trauner M:
CAR and PXR agonists stimulate hepatic bile acid and bilirubin
detoxification and elimination pathways in mice. Hepatology.
42:420–430. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang H, Plösch T, Lisman T, Gouw AS, Porte
RJ, Verkade HJ and Hulscher JB: Inflammation mediated
down-regulation of hepatobiliary transporters contributes to
intrahepatic cholestasis and liver damage in murine biliary
atresia. Pediatr Res. 66:380–385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang H and Wang H: Signaling control of
the constitutive androstane receptor (CAR). Protein Cell.
5:113–123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tojima H, Kakizaki S, Yamazaki Y, Takizawa
D, Horiguchi N, Sato K and Mori M: Ligand dependent hepatic gene
expression profiles of nuclear receptors CAR and PXR. Toxicol Lett.
212:288–297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luisier R, Lempiäinen H, Scherbichler N,
Braeuning A, Geissler M, Dubost V, Müller A, Scheer N, Chibout SD,
Hara H, et al: Phenobarbital Induces cell cycle transcriptional
responses in mouse liver humanized for constitutive androstane and
pregnane X receptors. Toxicol Sci. 139:501–511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Assenat E, Gerbal-Chaloin S, Larrey D,
Saric J, Fabre JM, Maurel P, Vilarem MJ and Pascussi JM:
Interleukin 1beta inhibits CAR-induced expression of hepatic genes
involved in drug and bilirubin clearance. Hepatology. 40:951–960.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Petrick JS and Klaassen CD: Importance of
hepatic induction of constitutive androstane receptor and other
transcription factors that regulate xenobiotic metabolism and
transport. Drug Metab Dispos. 35:1806–1815. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saini SP, Mu Y, Gong H, Toma D, Uppal H,
Ren S, Li S, Poloyac SM and Xie W: Dual role of orphan nuclear
receptor pregnane X receptor in bilirubin detoxification in mice.
Hepatology. 41:497–505. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen X, Meng Z, Wang X, Zeng S and Huang
W: The nuclear receptor CAR modulates alcohol-induced liver injury.
Lab Invest. 91:1136–1145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu W, Zhu B, Peng X, Zhou M, Jia D and Gu
J: Activation of farnesoid X receptor attenuates hepatic injury in
a murine model of alcoholic liver disease. Biochem Biophys Res
Commun. 443:68–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fisher CD, Lickteig AJ, Augustine LM,
Elferink RP Oude, Besselsen DG, Erickson RP and Cherrington NJ:
Experimental non-alcoholic fatty liver disease results in decreased
hepatic uptake transporter expression and function in rats. Eur J
Pharmacol. 613:119–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zinchuk V, Zinchuk O, Akimaru K, Moriya F
and Okada T: Ethanol consumption alters expression and
colocalization of bile salt export pump and multidrug resistance
protein 2 in the rat. Histochem Cell Biol. 127:503–512. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee J, Azzaroli F, Wang L, Soroka CJ,
Gigliozzi A, Setchell KD, Kramer W and Boyer JL: Adaptive
regulation of bile salt transporters in kidney and liver in
obstructive cholestasis in the rat. Gastroenterology.
121:1473–1484. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Masereeuw R and Russel FG: Regulatory
pathways for ATP-binding cassette transport proteins in kidney
proximal tubules. AAPS J. 14:883–894. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Geier A, Wagner M, Dietrich CG and Trauner
M: Principles of hepatic organic anion transporter regulation
during cholestasis, inflammation and liver regeneration. Biochim
Biophys Acta. 1773:283–308. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van de Steeg E, Wagenaar E, van der
Kruijssen CM, Burggraaff JE, de Waart DR, Elferink RP, Kenworthy KE
and Schinkel AH: Organic anion transporting polypeptide
1a/1b-knockout mice provide insights into hepatic handling of
bilirubin, bile acids, and drugs. J Clin Invest. 120:2942–2952.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sugatani J, Kojima H, Ueda A, Kakizaki S,
Yoshinari K, Gong QH, Owens IS, Negishi M and Sueyoshi T: The
phenobarbital response enhancer module in the human bilirubin
UDP-glucuronosyltransferase UGT1A1 gene and regulation by the
nuclear receptor CAR. Hepatology. 33:1232–1238. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tolson AH and Wang H: Regulation of
drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR. Adv
Drug Deliv Rev. 62:1238–1249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang W, Zhang J, Chua SS, Qatanani M, Han
Y, Granata R and Moore DD: Induction of bilirubin clearance by the
constitutive androstane receptor. Proc Natl Acad Sci USA. 100:pp.
4156–4161. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang W, Zhang J, Chua SS, Qatanani M, Han
Y, Granata R and Moore DD: Induction of bilirubin clearance by the
constitutive androstane receptor (CAR). Proc Natl Acad Sci USA.
100:pp. 4156–4161. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Venkatesan N, Davidson MB, Simsolo RB and
Kern PA: Phenobarbital treatment enhances insulin-mediated glucose
metabolism and improves lipid metabolism in the diabetic rat.
Metabolism. 43:348–356. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao J, He J, Zhai Y, Wada T and Xie W: The
constitutive androstane receptor is an anti-obesity nuclear
receptor that improves insulin sensitivity. J Biol Chem.
284:25984–25992. 2009. View Article : Google Scholar : PubMed/NCBI
|