Introduction
Recent advances in immunology and molecular biology
have demonstrated the important role served by the immune system in
cancer development (1). Patients
with colorectal cancer (CRC), which is the fourth most frequently
diagnosed cancer and the second leading cause of cancer-associated
mortality in the United States, exhibit weakened immune responses
(2). Immune infiltrates in CRC are
of clinical importance; they may aid the prediction of metastatic
invasion and possibly clinical outcome (3,4).
Furthermore, certain studies have suggested that aberrations of
local immune infiltrations and circulating lymphocyte subsets may
have clinical significance (5,6).
Therefore, several studies are currently investigating the value of
specific complementary and non-invasive biomarkers for use in CRC
diagnosis, which may also improve cost-benefit ratio (7). Peripheral blood mononuclear cells
(PBMCs) represent a reservoir of inflammatory cells that contribute
to the progression of various diseases (8,9), and
the characterization of CRC patients based on lymphocyte
imbalances, such as a reduced cluster of differentiation
(CD)4+/CD8+ ratio (10) and an enrichment of regulatory T
cells (11), has previously been
reported.
Previous research has demonstrated that T cell
expression of programmed death-1 (PD-1) and T cell immunoglobulin
and mucin protein-3 (Tim-3) can induce T cell exhaustion in CRC
patients (12,13). Tim-3 has been reported to have a
critical role in regulating the immune response against viral
infection and carcinoma. Furthermore, there is growing evidence
that Tim-3 may function as a regulator of the anti-tumor immune
response and the development of cancer (14). Previous research has suggested that
PD-1hi Tim-3+ T cells are strongly associated
with post-transplantation leukemia relapse in patients with acute
myelocytic leukemia (15).
Furthermore, levels of Tim-3 were significantly increased on
CD4+ T cells and CD8+ T cells, and were
associated with higher cancer stages in renal cell carcinoma
(16). Unregulated Tim-3
expression in natural killer (NK) cells is associated with disease
stage, and predicts a poorer prognosis, in melanoma (17) and lung adenocarcinoma (18). However, there is limited data on
Tim-3 expression in the peripheral lymphocytes of patients with
CRC.
The present study determined the frequency of
CD3+CD56− T cells,
CD3−CD56+ NK cells and
CD3+CD56+ natural killer T (NKT) cells
expressing Tim-3 in the peripheral blood of preoperative CRC
patients. The expression of Tim-3 in lymphocyte subsets from
postoperative blood samples of patients with CRC was also
investigated. The results were correlated with the
clinicopathological parameters of the patients. Decreased Tim-3
expression on NK cells significantly correlated with tumor node
metastasis (TNM) stage. Furthermore, Tim-3 expression rapidly
recovered to baseline levels post-surgery. These results may be
useful in targeting specific cell types, and may provide valuable
information in the prediction of tumor progression.
Materials and methods
Participants
Chinese individuals (n=127) including 89 CRC
patients and 38 age- and gender-matched healthy volunteers [healthy
control group (HC)] were recruited from Qilu Hospital of Shandong
University (Jinan, China), during May-October 2013. Basic patient
information, clinical data and laboratory results, including serum
concentration of three CRC biomarkers [carcinoembryonic antigen
(CEA), cancer antigen 199 (CA199) and cancer antigen 724 (CA724)]
were retrieved from the medical records for each patient. An
experienced pathologist reviewed the histopathological criteria,
including the tumor differentiation and TNM stage. HC individuals
had no abnormal laboratory results and no family history of
autoimmune diseases. The demographic and clinicopathological
characteristics of the patients with CRC and HCs are presented in
Table I. The Qilu Hospital of
Shandong University ethics committee approved the study, and
written informed consent was acquired from each participant.
Peripheral blood samples were obtained from the participants and
processed within 6 h.
 | Table I.Demographic and clinicopathological
parameters of CRC patients and HC volunteers participating in the
study. |
Table I.
Demographic and clinicopathological
parameters of CRC patients and HC volunteers participating in the
study.
| A, CRC patients
(n=89) |
|---|
|
|---|
| Clinical
parameter | Value |
|---|
| Gender |
|
|
Male | 56 |
|
Female | 33 |
| Age (years) | 59 (24–80) |
| Lesion
location |
|
|
Rectum | 47 |
|
Colon | 42 |
| Surgery |
|
| No | 36 |
|
Yes | 53 |
| Pathological
type |
|
|
Adenocarcinoma | 80 |
| Other
or unclear | 9 |
| Tumor
differentiation |
|
|
Well | 15 |
|
Median | 48 |
|
Poor | 19 |
|
Unclear | 7 |
| Lymph node
metastasis |
|
|
Absent | 52 |
|
Present | 30 |
|
Unclear | 7 |
| TNM stage |
|
| I | 5 |
| II | 42 |
|
III | 27 |
| IV | 15 |
|
| B, HC volunteers
(n=38) |
|
| Clinical
parameter | Value |
|
| Gender |
|
|
Male | 22 |
|
Female | 16 |
| Age (years) | 52.5 (22–85) |
Flow cytometry
PBMCs were obtained by centrifugation with
Ficoll-Paque Plus (GE Healthcare Life Sciences, Chalfont, UK). PBMC
staining for flow cytometry analysis was performed using the
following fluorochrome-conjugated monoclonal antibodies:
CD3-peridinin chlorophyll protein-Cy5 (555334), CD56-flourescein
isothiocyanate (340410) and Tim-3-phycoerythrin (565570) (BD
Biosciences, San Jose, CA, USA), according to the manufacturer's
protocol. The peripheral blood was incubated with anti-CD3,
anti-CD56 and anti-Tim-3 for 20 min at 4°C in the dark. An isotype
control was performed alongside the test antibodies. Three-color
FACS Calibur (BD Biosciences, San Jose, CA, USA) analysis was used
to investigate Tim-3 expression and calculate mean fluorescence
intensity (MFI) on CD3+CD56− T cells,
CD3− CD56+ NK cells and
CD3+CD56+ NKT cells.
Statistical analysis
All data were analyzed using GraphPad Prism 5.0
software (GraphPad Software, Inc. La Jolla, CA, USA). Paired data
within donors were analyzed using a paired Student's t-test.
Unpaired data, between healthy and CRC patients, were analyzed
using an unpaired Student's t-test. Comparison of Tim-3 expression
based on demographic and clinical parameters was performed using an
unpaired t-test to compare two groups, and one-way analysis of
variance to compare more than two groups, followed by Tukey's test.
Spearman's rank correlation analysis was used to calculate the
correlation coefficient. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of the study
population
The distribution of selected clinicopathological
features of the CRC patients and HC volunteers are presented in
Table I. Amongst 89 CRC cancer
patients, 47 (52.8%) cases were classified as rectal cancer and 42
(47.2%) as colon cancer. Most CRC patients (89.9%) were
histologically confirmed as adenocarcinoma, with the remaining 9
(10.1%) patients classified as ‘other carcinoma’. A total of 53
(59.6%) patients had previously undergone surgery. TNM staging,
which was performed according to guidelines set out by The Union
for International Cancer Control/American Joint Committee on Cancer
TNM Classification (7th edition) (19), classified 49 CRC patients (52.8%)
as stage I–II, and 42 CRC patients (47.2%) as stage III–IV.
Levels of lymphocyte subsets in CRC
patients and HC
The percentage of T cells, NK cells and NKT cells
were investigated in peripheral blood from preoperative CRC
patients and HC volunteers. The gating strategy used to distinguish
the various lymphocyte populations is depicted in Fig. 1A. Compared with healthy
individuals, the percentage of circulating NK cells in CRC patients
was significantly decreased (12.34±6.77 vs. 17.98±8.02%, P=0.0027;
Fig. 1B). The levels of T cells
and NKT cells from preoperative CRC patients and healthy controls
demonstrated no significant difference (68.24±11.27 vs.
65.14±10.10%, and 5.42±5.05 vs. 5.28±4.35%, P>0.05; Fig. 1C and D). These results demonstrated
that the level of NK cells was reduced in CRC patients.
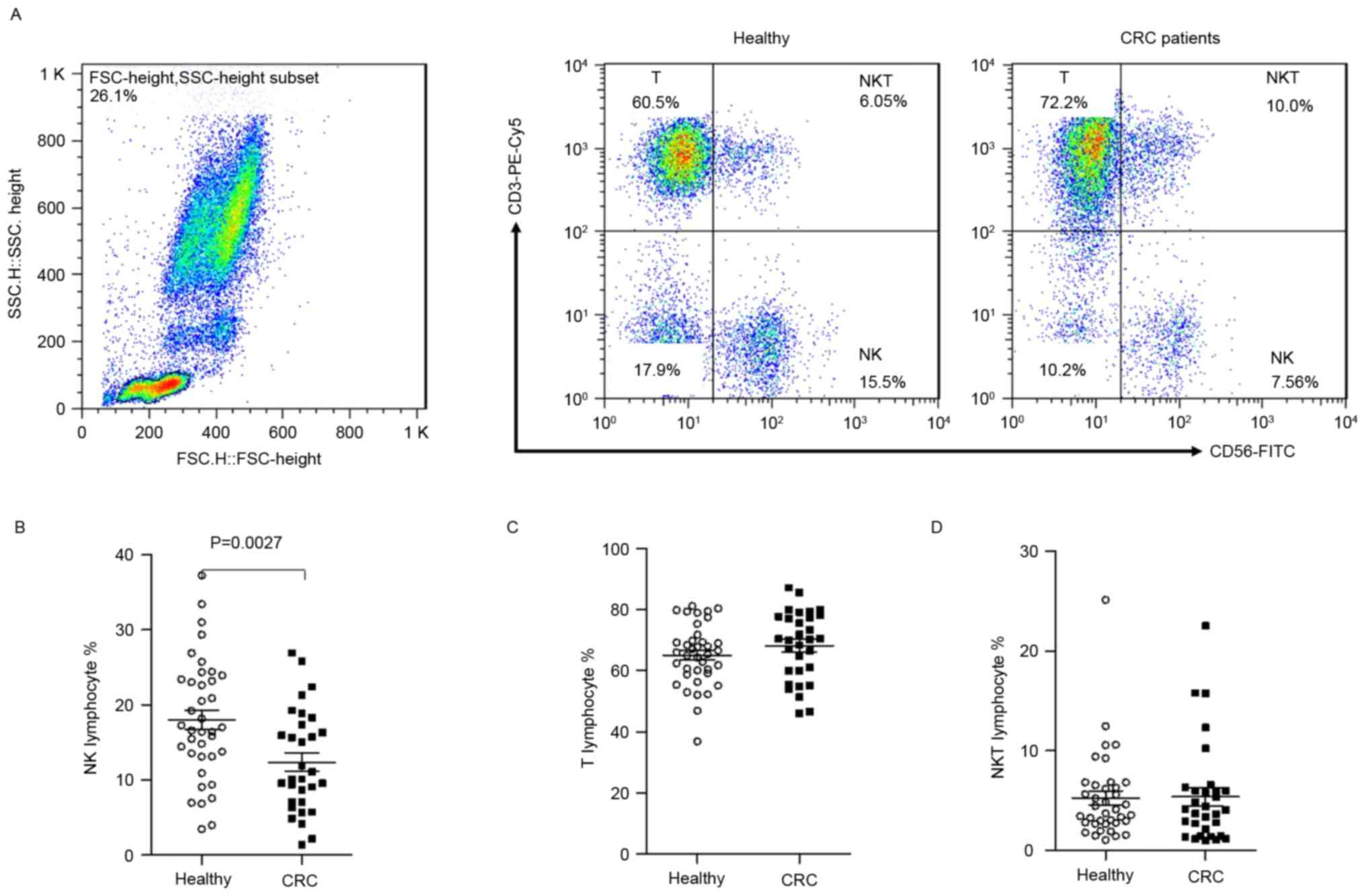 | Figure 1.Decreased numbers of NK cells in
lymphocytes from untreated CRC patients. Flow cytometry was used to
identify CD3+CD56− T cells,
CD3−CD56+ NK cells and
CD3+CD56+ NKT cells from peripheral blood
mononuclear cells isolated from healthy controls and untreated CRC
patients. (A) Harvested cells were initially gated using live
lymphocytes and subsequently on CD3+CD56−,
CD3−CD56+ and CD3+CD56+
cells with ≥30,000 events analyzed for each sample. (B) NK, (C) T
and (D) NKT cells in CRC patients and HCs. Data points represent
the T cell, NK cell and NKT cell percentages. NK, natural killer;
CRC, colorectal cancer; CD, cluster of differentiation; NKT, NK T
cells; FITC, fluorescein isothiocyanate; PE, phycoerythrin; FSC,
forward scatter; SSC, side scatter. |
Tim-3 expression is decreased on
peripheral NK cells in CRC patients
Abnormal expression of Tim-3 on innate immune cells
has been associated with the progression of several clinical
diseases, such as infection, immune diseases and tumors (20,21).
To further define the Tim-3 expression profile on various
lymphocyte subtypes between the two patient groups, flow cytometric
analysis of the Tim-3 expression profile was performed on
peripheral blood from preoperative CRC patients (n=36) and HCs
(Fig. 2A). Overall, the median
expression of Tim-3 was higher on NK cells compared with NKT cells
and T cells. Furthermore, Tim-3+ NK cells were
significantly decreased in CRC cases compared with HCs (71.51±17.39
vs. 81.14±17.07%, P=0.0239; Fig.
2B), however the percentage of Tim-3 expressing T cells and NKT
cells from CRC patients and healthy controls demonstrated no
significant difference. These results indicated that Tim-3 may be
involved in the immune dysfunction of CRC, via an NK cell-mediated
role.
 | Figure 2.Tim-3+ lymphocyte
frequencies. The number of Tim-3+ NK cells was reduced
in CRC patients. (A) Harvested cells were initially gated for
CD3+CD56−, CD3−CD56+,
CD3+CD56+ lymphocytes, and then for
Tim-3+ cells with ≥30,000 events analyzed in each
sample. Data are representative results from different groups of
subjects, and the percentage of Tim-3+ lymphocyte
subsets from individual subjects are presented. (B) Summarized data
demonstrate the percentage of Tim-3+ lymphocytes. The
median is represented by a horizontal line. Tim-3, T cell
immunoglobulin and mucin protein-3; NK, natural killer; CRC,
colorectal cancer; CD, cluster of differentiation; NKT, NK T cells;
FSC, forward scatter. |
Reductions in NK cells and
Tim-3+ NK cells are correlated with TNM stage
The percentage of NK and Tim-3+ NK cells
was correlated with various clinicopathological features (Tables II and III). Associations between the
percentage of NK cells or Tim-3+ NK cells with TNM stage
were assessed by Spearman's rank correlation (Fig. 3A and B). Patients with stage I and
II CRC were pooled in this analysis, due to the limited number of
patients assessed with stage I CRC. Overall, NK cells demonstrated
the most significant reduction with disease stage. Furthermore,
significantly decreased numbers of NK cells and Tim-3+
NK cells were observed in CRC patients with stage IV disease,
compared with HCs (P<0.05). Notably, the percentage of
Tim-3+ NK cells was decreased at stage IV, compared with
stages I–II and III.
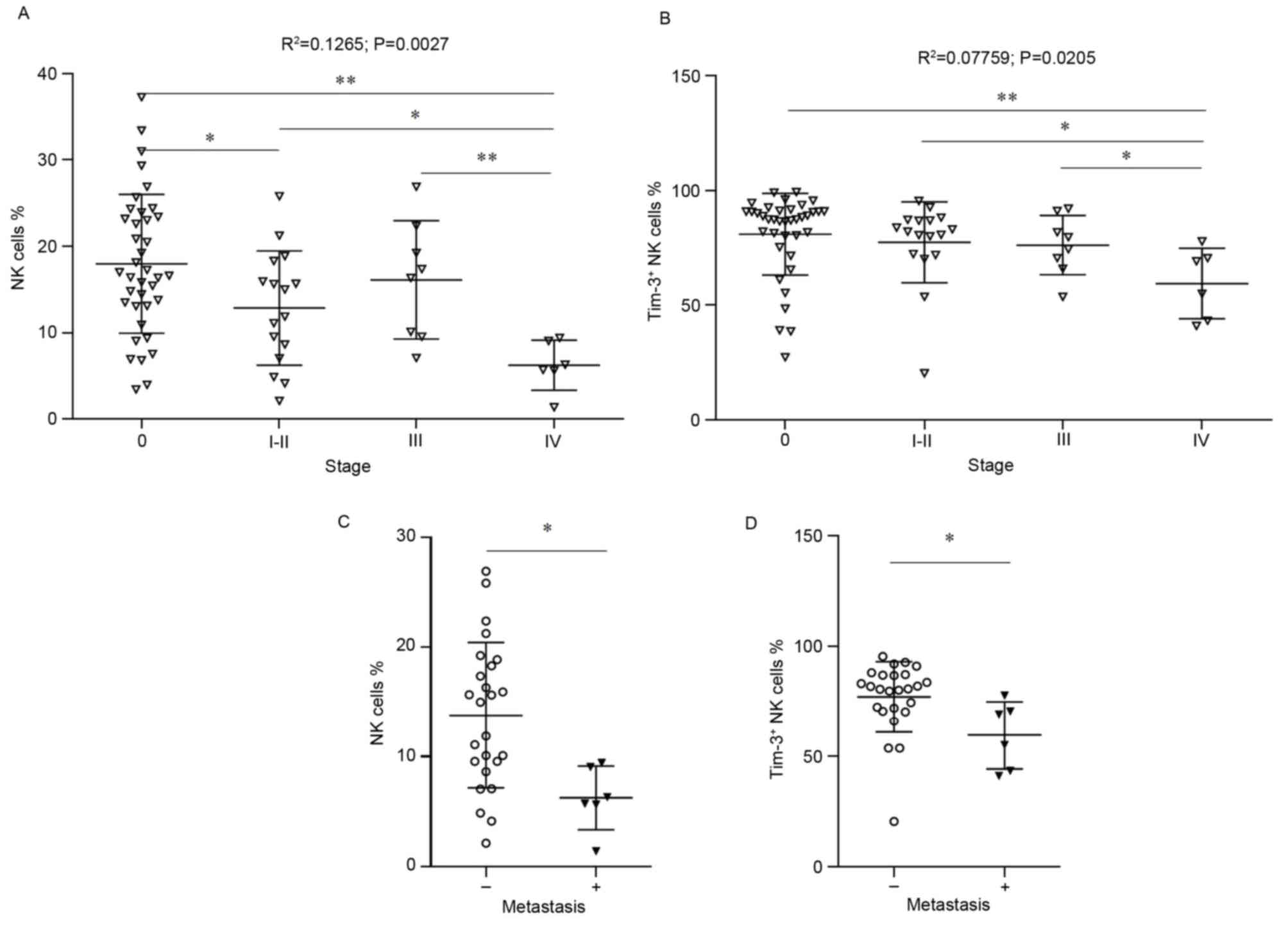 | Figure 3.NK cells and Tim-3+ NK
cells are associated with TNM stage. The association of (A) NK
cells or (B) Tim-3+ NK cell percentage was correlated
with TNM stage. Healthy individuals were designated as stage 0, and
patients were staged (I–IV) post surgery. Probability and
R2 values were calculated for the entire data set by
Spearman's rank correlation. Differences between healthy donors and
patients at each disease stage were calculated by an unpaired
Wilcoxon rank-sum test. *P<0.05 and **P<0.001, vs. stage 0,
I–II or III as indicated in the figure. (C) The frequency of
CD56+CD3− NK cells and (D) Tim-3+
NK cells was higher in CRC patients with metastasis, compared with
CRC patients without metastasis. *P<0.05, vs. non-metastatic
patients. Each symbol represents a single subject; the median value
is indicated by a horizontal line. NK, natural killer; Tim-3, T
cell immunoglobulin and mucin protein-3; TNM, tumor node
metastasis; CRC, colorectal cancer; CD, cluster of differentiation;
NKT, NK T cells. |
 | Table II.Association between percentage of NK
cells and clinicopathological features in patients with colorectal
cancer. |
Table II.
Association between percentage of NK
cells and clinicopathological features in patients with colorectal
cancer.
| Clinical
feature | Median NK cells,
%(range) | P-value |
|---|
| Gender |
| 0.5945 |
|
Male | 9.76
(1.39–26.91) |
|
|
Female | 11.89
(4.14–25.83) |
|
| Age | 10.11
(1.39–26.91) | 0.7732 |
| Lesion
location |
| 0.8359 |
|
Rectum | 11.50
(1.39–26.91) |
|
|
Colon | 9.56
(4.14–25.83) |
|
| Tumor
differentiation |
| 0.6745 |
|
Well | 11.89
(8.64–22.39) |
|
|
Median | 11.10
(1.39–26.91) |
|
|
Poor | 9.56
(4.14–19.25) |
|
| Lymph node
metastasis |
| 0.1802 |
|
Absent | 10.11
(1.39–25.83) |
|
|
Present | 16.87
(5.64–26.91) |
|
 | Table III.Association between the percentage of
Tim-3+ NK cells and clinicopathological features in
patients with colorectal cancer. |
Table III.
Association between the percentage of
Tim-3+ NK cells and clinicopathological features in
patients with colorectal cancer.
| Clinical
feature | Median
Tim-3+ NK cells, % (range) | P-value |
|---|
| Gender |
| 0.3133 |
|
Male | 71.25
(20.44–91.04) |
|
|
Female | 80.57
(43.29–95.40) |
|
| Age | 74.1
(20.44–95.40) | 0.8068 |
| Lesion
location |
| 0.7739 |
|
Rectum | 80.62
(20.44–92.68) |
|
|
Colon | 70.21
(53.65–95.40) |
|
| Tumor
differentiation |
| 0.9197 |
|
Well | 80.57
(20.44–100) |
|
|
Median | 74.49
(41.01–92.68) |
|
|
Poor | 70.60
(53.65–84.20) |
|
| Lymph node
metastasis |
| 0.9889 |
|
Absent | 80.57
(20.44–95.40) |
|
|
Present | 72.54
(53.00–91.04) |
|
Preoperative CRC patients were subsequently
classified as non-metastatic (n=30) or metastatic (n=6). Patients
with stage I/II/III cancer demonstrated the highest percentage of
NK cells, and this was statistically significant when compared with
the metastatic stage IV group (13.81±6.631 vs. 6.252±2.900%,
P=0.0014; Fig. 3C). Furthermore, a
significant difference was observed in the percentage of
Tim-3+ NK cells in non-metastatic patients compared with
metastatic groups (77.01±16.02 vs. 59.48±15.32%, P=0.0218; Fig. 3D). There were no significant
differences in the percentage of Tim-3+ T cells or
Tim-3+ NKT cells (data not shown). Several patients with
non-metastatic disease appear to maintain a higher NK Tim-3
expression; therefore these data suggested that Tim-3 may be a
useful indicator of CRC disease progression, however a larger
cohort of patients are required to further investigate this
hypothesis.
Effects of surgical resection on Tim-3
expression
To investigate the impact of surgical resection on
lymphocyte number and Tim-3 expression, postsurgical blood was
collected from CRC patients that had undergone surgery (n=53).
Blood samples were collected within 1 week of surgery, and these
included 28 cases with stage I/II and 25 cases with stage III/IV.
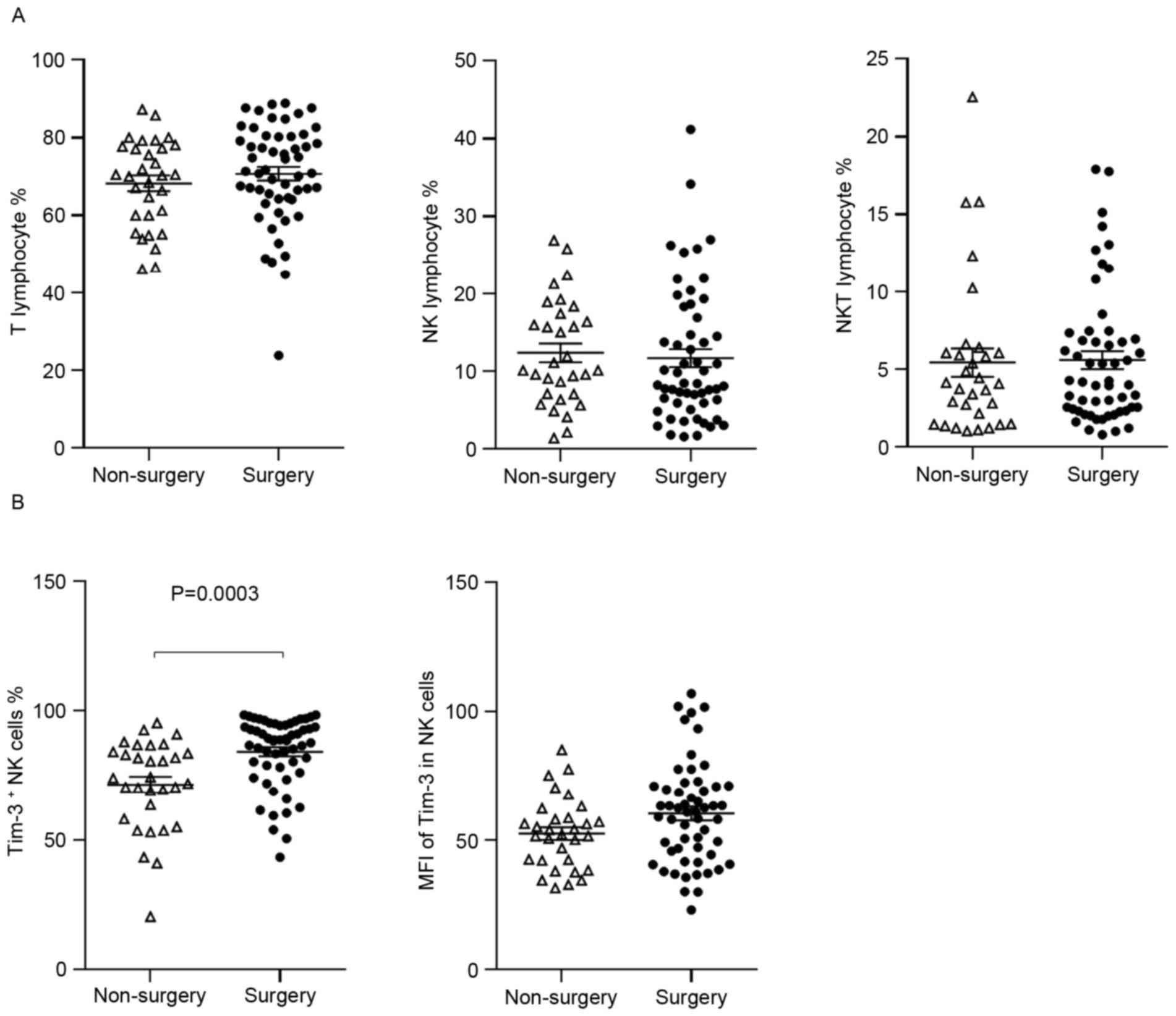
The results indicated no difference in the percentage of lymphocyte
subtypes between the two patient groups (Fig. 4A), however, the percentage of
Tim-3+ NK cells, but not the MFI of Tim-3 on NK cells,
was significantly higher in patients that had received surgery,
compared with preoperative patients (84.31±13.55 vs. 71.51±17.39%,
P=0.0003; Fig. 4B).
To further investigate the impact of surgery on
lymphocyte populations, pre- and post-surgical blood samples were
obtained from 7 patients. No significant difference was observed in
the percentage of lymphocytes (P>0.05; Fig. 5A). However, surgery elevated Tim-3
expression on NK cells to the baseline levels observed in healthy
controls, in the majority of patients with reduced pre-surgical
levels (P=0.0126; Fig. 5B). No
significant difference was observed in the Tim-3 MFI in NK cells,
or in the levels of Tim-3 expression on T cells and NKT cells
(P>0.05; data not shown). These data suggest that
Tim-3-expressing NK cells are more prevalent following resection of
the primary tumor.
NK cells and Tim-3+ NK
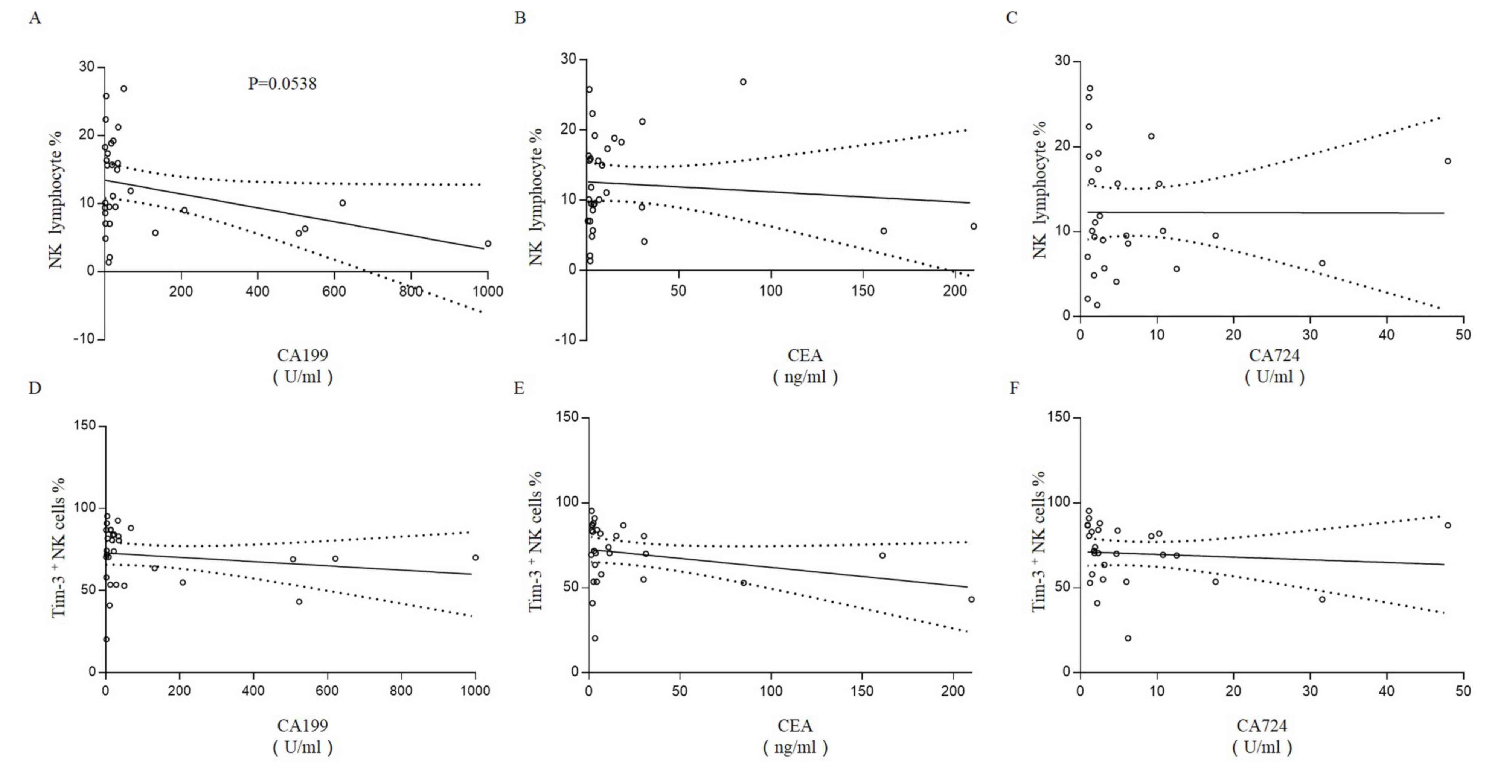
cells are not associated with CA199, CEA or CA724
The association of NK cells and Tim-3+ NK
cells with the preoperative serum concentration of three CRC
biomarkers was assessed. A weak negative association was observed
between the percentage of NK cells and the concentration of CA199,
however this did not reach significance (R2=0.1223,
P=0.0538; Fig. 6A). No correlation
was observed between the percentage of NK cells and the
concentration of CEA (R2=0.0100; Fig. 6B) or CA724 (R2=0.000;
Fig. 6C). Furthermore, there
appeared to be a weak negative correlation between
Tim-3+ NK cells and the serum concentration of CA199
(R2=0.030; Fig. 6D),
CEA (R2=0.088; Fig. 6E)
and CA724 (R2=0.0086; Fig.
6F), however these were not significant (P>0.05 for all
cases).
Discussion
The peripheral blood from cancer patients contains a
reservoir of cells and cell products derived from the primary tumor
and distant metastases, which may contain valuable diagnostic or
prognostic information. The collection of blood samples is also
minimally invasive, and this resource has the potential for
wide-scale use in CRC biomarker screening. Considering the
convenience of peripheral blood biomarker detection and the
influence of Tim-3 on immune cell function, it would be valuable to
investigate the mechanistic role of Tim-3 as a potential biomarker
in the progression of CRC. The present study used peripheral blood
samples to more precisely characterize the expression of Tim-3 on
lymphocytes, in response to CRC. The percentage of total NK and
Tim-3+ NK cells was significantly downregulated in
patients with CRC; these NK cells would normally participate in a
typical immune response. Notably, TNM stage was associated with the
expression of Tim-3 on NK cells; the presence of Tim-3+
NK cells was significantly lower in patients with stage IV tumors,
suggesting a possible association between Tim-3 expression and CRC
metastasis. Furthermore, a previously unreported rebound in Tim-3
expression in NK cells was observed following surgical resection of
the primary tumor. Distant metastasis is a critical event that
impacts on the prognosis of patients with CRC. The present study
indicated that patients with distant metastases had a significantly
lower proportion of NK cells and Tim-3+ NK cells,
compared with patients without metastases. These results therefore
indicated that Tim-3+ NK cells may serve a role in
disease progression.
Poor cytotoxic activity of peripheral NK cells has
been associated with an increased risk of cancer (22). Furthermore, NK cell infiltration in
tumor tissue predicts improved prognosis, particularly in CRC
(23,24). In light of this research, NK cells
have since been targeted as a potential cancer therapeutic
(25). Tim-3 is constitutively
expressed at high levels on resting NK cells (26), however, research regarding the
function of Tim-3 on NK cells is conflicting. One study indicated
that Tim-3 may deliver inhibitory signals and inhibit normal NK
cell-mediated cytotoxicity (26),
whereas another study suggested that Tim-3 may act as an activated
receptor, where exposure to galectin-9 results in enhanced
interferon-γ production by Tim-3+ NK cells (27). Previous research has demonstrated
that the number of NK cells is significantly reduced in CRC
patients compared with healthy controls (28), which is consistent with the
findings of the current study. These results indicate that NK cell
function is damaged in patients with CRC, and an improved
understanding of Tim-3 function, in the context of carcinogenesis
and tumor development, may prove useful in the development of
future therapeutics. Dysregulation of Tim-3 expression on NK cells
is a feature of several diseases, with upregulation observed in
advanced melanoma (17),
hepatitis-C infection (20) and
lung cancer (29), and
downregulation observed in human immunodeficiency virus infection
(30). To our knowledge, this is
the first report of a decreased proportion of Tim-3+ NK
cells in patients with CRC, a scenario that is compatible with
Tim-3's role as an activated receptor on normal NK cells.
Furthermore, the reduction in Tim-3 expression was associated with
a loss of Tim-3-expressing cells, because patients undergoing
surgery demonstrated no significant attenuation in the mean
fluorescence intensity of Tim-3, compared with untreated patients
(data not shown). In CRC patients, serum CEA, CA199 and CA724 may
be useful in the diagnosis of colorectal carcinoma (31,32).
The relationship between the proportion of
CD3−CD56+ NK cells in CRC patients and these
serum biomarkers was investigated. Although there appeared to be an
association between serum CA199 levels and the proportion of
CD3−CD56+ NK cells in CRC patients, this did
not reach statistical significance.
One approach to activating an anti-tumor immune
response, which is at the forefront of current cancer
immunotherapy, has been termed ‘immune checkpoint blockade’. This
strategy is largely driven by the success of therapies targeting
cytotoxic T-lymphocyte associated protein-4 and PD-1, and several
clinical trials focusing on anti-immune checkpoint antibodies and
antagonists are currently underway (12). However, a large number of patients
do not currently benefit from this type of cancer therapy (33), and this has catalyzed interest in
targeting novel immune checkpoint receptors. Tim-3 has therefore
attracted attention as a novel immune checkpoint receptor. Notably,
treatment with anti-Tim-3 and anti-PD-ligand 1 antibodies
significantly limited tumor growth in vivo (34). In CRC, previous research
demonstrated significantly higher levels of circulating
Tim-3+PD-1+CD8+ T cells,
indicating that Tim-3 blockage may be a potential therapeutic
approach for CRC patients (13).
However, given the expression of Tim-3 on other immune cells,
including NK cells, Tim-3 therapy should be cautious, and the
choice of targeted immune checkpoint should be founded on knowledge
of the immune system. The present study also demonstrated that
surgical resection of the primary tumor rapidly reverses Tim-3
expression in NK cell populations, which has significant
implications for the timing of Tim-3 based therapies. However, this
will require large and properly controlled clinical studies to
verify and screen novel therapeutics.
The present study indicated that reduced NK cell
Tim-3 expression is associated with CRC progression and
presentation of poor prognostic clinical parameters. For the first
time, Tim-3 has been demonstrated as a bioactivity marker, which is
expressed in NK cells from patients with CRC. Tim-3 may serve as a
serum biomarker, which could predict disease progression, and
potentially prove useful in identifying patients likely to benefit
from Tim-3-based therapies.
Acknowledgements
This work was supported by grants from the National
Natural Science Foundation of China (grant nos. 31470885, 31300752,
31270971, 81300510, 30901326 and 81072406).
References
|
1
|
Dalgleish AG and O'Byrne KJ: Chronic
immune activation and inflammation in the pathogenesis of AIDS and
cancer. Adv Cancer Res. 84:231–276. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shibata M, Nezu T, Kanou H, Abe H,
Takekawa M and Fukuzawa M: Decreased production of interleukin-12
and type 2 immune responses are marked in cachectic patients with
colorectal and gastric cancer. J Clin Gastroenterol. 34:416–420.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pagès F, Berger A, Camus M, Sanchez-Cabo
F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte
D, et al: Effector memory T cells, early metastasis, and survival
in colorectal cancer. N Engl J Med. 353:2654–2666. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tachibana T, Onodera H, Tsuruyama T, Mori
A, Nagayama S, Hiai H and Imamura M: Increased intratumor
Valpha24-positive natural killer T cells: A prognostic factor for
primary colorectal carcinomas. Clin Cancer Res. 11:7322–7327. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen ZY, Raghav K, Lieu CH, Jiang ZQ, Eng
C, Vauthey JN, Chang GJ, Qiao W, Morris J, Hong D, et al: Cytokine
profile and prognostic significance of high neutrophil-lymphocyte
ratio in colorectal cancer. Br J Cancer. 112:1088–1097. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ling L, Zhao P, Yan G, Chen M, Zhang T,
Wang L and Jiang Y: The frequency of Th17 and Th22 cells in
patients with colorectal cancer at pre-operation and
post-operation. Immunol Invest. 44:56–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pawa N, Arulampalam T and Norton JD:
Screening for colorectal cancer: Established and emerging
modalities. Nat Rev Gastroenterol Hepatol. 8:711–722. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murdoch C, Muthana M, Coffelt SB and Lewis
CE: The role of myeloid cells in the promotion of tumour
angiogenesis. Nat Rev Cancer. 8:618–631. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi C and Pamer EG: Monocyte recruitment
during infection and inflammation. Nat Rev Immunol. 11:762–774.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Diederichsen AC, Hjelmborg Jv, Christensen
PB, Zeuthen J and Fenger C: Prognostic value of the CD4+/CD8+ ratio
of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR
expression on tumour cells. Cancer Immunol Immunother. 52:423–428.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hua W, Yuan A, Zheng W, Li C, Cui J, Pang
Z, Zhang L, Li Z, Goll R and Cui G: Accumulation of FoxP3+ T
regulatory cells in the tumor microenvironment of human colorectal
adenomas. Pathol Res Pract. 212:106–112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singh PP, Sharma PK, Krishnan G and
Lockhart AC: Immune checkpoints and immunotherapy for colorectal
cancer. Gastroenterol Rep(Oxf). 3:289–297. 2015.PubMed/NCBI
|
|
13
|
Xu B, Yuan L, Gao Q, Yuan P, Zhao P, Yuan
H, Fan H, Li T, Qin P, Han L, et al: Circulating and
tumor-infiltrating Tim-3 in patients with colorectal cancer.
Oncotarget. 6:20592–21603. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuchroo VK, Dardalhon V, Xiao S and
Anderson AC: New roles for TIM family members in immune regulation.
Nat Rev Immunol. 8:577–580. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kong Y, Zhang J, Claxton DF, Ehmann WC,
Rybka WB, Zhu L, Zeng H, Schell TD and Zheng H: PD-1(hi)TIM-3(+) T
cells associate with and predict leukemia relapse in AML patients
post allogeneic stem cell transplantation. Blood Cancer J.
5:e3302015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cai C, Xu YF, Wu ZJ, Dong Q, Li MY, Olson
JC, Rabinowitz YM, Wang LH and Sun Y: Tim-3 expression represents
dysfunctional tumor infiltrating T cells in renal cell carcinoma.
World J Urol. 34:561–567. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
da Silva IP, Gallois A, Jimenez-Baranda S,
Khan S, Anderson AC, Kuchroo VK, Osman I and Bhardwaj N: Reversal
of NK-cell exhaustion in advanced melanoma by Tim-3 blockade.
Cancer Immunol Res. 2:410–422. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu L, Huang Y, Tan L, Yu W, Chen D, Lu C,
He J, Wu G, Liu X and Zhang Y: Increased Tim-3 expression in
peripheral NK cells predicts a poorer prognosis and Tim-3 blockade
improves NK cell-mediated cytotoxicity in human lung
adenocarcinoma. Int Immunopharmacol. 29:635–641. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Edge SB, Byrd SR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th.
Springer-Verlag; New York, NY: pp. 143–164. 2010
|
|
20
|
Golden-Mason L, Hurtado CE Waasdorp, Cheng
L and Rosen HR: Hepatitis C viral infection is associated with
activated cytolytic natural killer cells expressing high levels of
T cell immunoglobulin- and mucin-domain-containing molecule-3. Clin
Immunol. 158:114–125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anderson AC: Tim-3, a negative regulator
of anti-tumor immunity. Curr Opin Immunol. 24:213–216. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Imai K, Matsuyama S, Miyake S, Suga K and
Nakachi K: Natural cytotoxic activity of peripheral-blood
lymphocytes and cancer incidence: An 11-year follow-up study of a
general population. Lancet. 356:1795–1799. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koda K, Saito N, Oda K, Seike K, Kondo E,
Ishizuka M, Takiguchi N and Miyazaki M: Natural killer cell
activity and distant metastasis in rectal cancers treated
surgically with and without neoadjuvant chemoradiotherapy. J Am
Coll Surg. 197:254–260. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kondo E, Koda K, Takiguchi N, Oda K, Seike
K, Ishizuka M and Miyazaki M: Preoperative natural killer cell
activity as a prognostic factor for distant metastasis following
surgery for colon cancer. Dig Surg. 20:445–451. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vivier E, Ugolini S, Blaise D, Chabannon C
and Brossay L: Targeting natural killer cells and natural killer T
cells in cancer. Nat Rev Immunol. 12:239–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ndhlovu LC, Lopez-Vergès S, Barbour JD,
Jones RB, Jha AR, Long BR, Schoeffler EC, Fujita T, Nixon DF and
Lanier LL: Tim-3 marks human natural killer cell maturation and
suppresses cell-mediated cytotoxicity. Blood. 119:3734–3743. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gleason MK, Lenvik TR, McCullar V, Felices
M, O'Brien MS, Cooley SA, Verneris MR, Cichocki F, Holman CJ,
Panoskaltsis-Mortari A, et al: Tim-3 is an inducible human natural
killer cell receptor that enhances interferon gamma production in
response to galectin-9. Blood. 119:3064–3072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Halama N, Braun M, Kahlert C, Spille A,
Quack C, Rahbari N, Koch M, Weitz J, Kloor M, Zoernig I, et al:
Natural killer cells are scarce in colorectal carcinoma tissue
despite high levels of chemokines and cytokines. Clin Cancer Res.
17:678–689. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu LY, Chen DD, He JY, Lu CC, Liu XG, Le
HB, Wang CY and Zhang YK: Tim-3 expression by peripheral natural
killer cells and natural killer T cells increases in patients with
lung cancer-reduction after surgical resection. Asian Pac J Cancer
Prev. 15:9945–9948. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jost S, Moreno-Nieves UY, Garcia-Beltran
WF, Rands K, Reardon J, Toth I, Piechocka-Trocha A, Altfeld M and
Addo MM: Dysregulated Tim-3 expression on natural killer cells is
associated with increased Galectin-9 levels in HIV-1 infection.
Retrovirology. 10:742013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang YR, Yan JX and Wang LN: The
diagnostic value of serum carcino-embryonic antigen, alpha
fetoprotein and carbohydrate antigen 19-9 for colorectal cancer. J
Cancer Res Ther. 10 Suppl:S307–S309. 2014. View Article : Google Scholar
|
|
32
|
Zhu Z, Chen Z, Chen C, Yang Z, Xuan W, Hou
Y, Zuo Y and Ren S: Opposite variation tendencies of serum CA724
levels in patients with colon and rectal carcinoma. Mol Clin Oncol.
2:139–145. 2014.PubMed/NCBI
|
|
33
|
Haddad AQ and Margulis V: Tumour and
patient factors in renal cell carcinoma-towards personalized
therapy. Nat Rev Urol. 12:253–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sakuishi K, Apetoh L, Sullivan JM, Blazar
BR, Kuchroo VK and Anderson AC: Targeting Tim-3 and PD-1 pathways
to reverse T cell exhaustion and restore anti-tumor immunity. J Exp
Med. 207:2187–2194. 2010. View Article : Google Scholar : PubMed/NCBI
|