Introduction
Obesity is a major public health problem worldwide.
It is associated with increased risks of various diseases,
including type 2 diabetes, hyperlipidemia, hypertension,
arteriosclerosis, fatty liver and cardiovascular diseases (1,2).
Obesity triggers the transformation of pre-adipocytes into
adipocytes, and is characterized an increase in number and size of
mature adipocytes (3). Adipose
tissue is primarily composed of adipocytes, and is important for
energy homeostasis by regulating lipid metabolism (4). Therefore, adipocyte differentiation
and the amount of lipid accumulation are involved in the expansion
of adipose tissue (2).
The 3T3-L1 cell line is a well-established model for
studying the conversion of pre-adipocytes into mature adipocytes
and investigating the molecular mechanisms of lipogenesis and
adipogenesis (5). Lipogenesis and
adipogenesis are controlled by several transcription factors such
as sterol regulatory element-binding protein 1 (SREBP1), peroxisome
proliferator activated receptor γ (PPARγ) and CCAAT/enhancer
binding protein-α (C/EBPα), which are primarily expressed in
adipose tissue (6). These
transcription factors regulate the lipid homeostasis by modulating
the expression of target genes, including fatty acid synthase
(FAS), stearoyl-CoA desaturase-1 (SCD1), glycerol-3-phosphate
O-acyltransferase (GPAT), adipocyte protein 2 (aP2), associated
with fat accumulation. Furthermore, AMP-activated protein kinase
(AMPK) activation can inhibit pre-adipocyte differentiation and is
accompanied by inhibition of transcription factors, including
SREBP1, PPARγ and C/EBPα (7). AMPK
activation inhibits acetyl-CoA carboxylase (ACC) activity directly
via phosphorylation, and thus increases expression of carnitine
palmitoyltransferase I (CPT1) (8).
Therefore, AMPK signaling pathway is considered to be an important
anti-obesity mechanism.
Yanhusuo (Corydalis yanhusuo W.T. Wang
extract), is a well-known traditional Chinese herbal medicine, and
has been used in China for treating pain, including chest
impediment, heart pain, dysmenorrhea and amenorrhea (9). The pharmacological effects of
yanhusuo are attributable mainly to the alkaloids in the plant, and
studies have documented the hepatoprotective effects (10), anti-tumor (11), anti-inflammatory (12) and anti-hypertensive (13) activities of the extract.
Tetrahydropalmatine (THP) is the main active component of yanhusuo.
Recently, THP has received increasing attention because various
pharmacological actions, including anti-coagulant,
anti-nociceptive, anti-hyperalgesic, anti-oxidant, anti-viral and
anti-inflammatory effects, were demonstrated to be induced by this
compound (14). However, there are
no reports documenting the anti-obesity effect of THP. The present
study investigated the inhibitory effect of TPH on differentiation
of 3T3-L1 pre-adipocytes and the mechanisms of action.
Materials and methods
Materials
THP was purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). 3T3-L1 mouse embryonic fibroblast cells were
purchased from the American Type Culture Collection (Manassas, VA,
USA). Dulbecco's Modified Eagle's medium (DMEM), fetal bovine serum
(FBS), and bovine calf serum (BCS), were purchased from Gibco;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). All chemicals
were purchased from Sigma-Aldrich; Merck KGaA. Rabbit anti-mouse
polyclonal FAS (3180; 1:2,000), rabbit anti-mouse monoclonal SCD1
(2794; 1:2,000), rabbit anti-mouse polyclonal PPARγ (2435;
1:2,000), rabbit anti-mouse polyclonal C/EBPα (2295; 1:2,000),
rabbit anti-mouse polyclonal phospho (p)-AMPKα (2531L; 1:2,000),
rabbit anti-mouse polyclonal AMPKα (2532S; 1:2,000), rabbit
anti-mouse polyclonal p-ACC (3661L; 1:2,000) and rabbit anti-mouse
polyclonal ACC (3662; 1:2,000) antibodies were from Cell Signaling
Technology, Inc. (Danvers, MA, USA). SREBP1 (sc-366; 1:1,000),
actin (sc-1616; 1:1,000) and horseradish peroxidase
(HRP)-conjugated goat anti-mouse polyclonal IgG antibody (sc-1616;
1:1,000) were obtained from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA). Reverse transcriptase was supplied by Promega Corporation
(Madison, WI, USA). QuantiTect SYBR-Green PCR kit was purchased
from Qiagen GmbH (Hilden, Germany).
Cell culture and adipocyte
differentiation
Mouse 3T3-L1 pre-adipocytes were cultured at 37°C
with 5% CO2 atmosphere in DMEM medium, 10% BCS, 100 U/ml
penicillin, 100 µg/ml streptomycin. For standard adipocyte
differentiation, 3T3-L1 cells were plated at a density of
5×105 cell/ml and incubated for 2 days until they
reached confluence. Pre-adipocytes (designated day 0) were cultured
in differentiation medium (DMEM, 10% FBS, 0.5 mM
3-isobutyl-1-methylxanthine, 1 µM dexamethasone and 10 µg/ml
insulin) for 4 days, switched to post- differentiation medium
containing 10% FBS and 10 µg/ml insulin, and then changed to 10%
FBS medium for an additional 2 days. During adipocyte
differentiation, 3T3-L1 cells were treated with THP at a
concentration of 0, 10, 20 or 40 µM from day 0–4. The positive
control was treated 10 µM of pioglitazone (PIO; Sigma-Aldrich;
Merck KGaA) and the same concentration of differentiation standard
adipogenic medium.
Cell viability assay
Cells were seeded at 5×103 cells/well in
96-well plate and incubated in the presence or absence of THP (0,
10, 20 or 40 µM). Following 96 h of culturing, 20 µl of Cell Titer
96® AQueous One Solution Cell Proliferation Assay kit
reagent (Promega Corporation, Madison, WI, USA) was added to each
well, incubated for 1 h, and absorbance at 490 nm was measured
using a microplate reader.
Measurement of triglyceride (TG)
content
For TG determination, cells were collected and lysed
in lysis buffer (25 mM sucrose, 20 mM Tris-HCl, 1 mM EDTA and 1 mM
EGTA). The cellular contents of TG were measured using Infinity™
triacyglycerol reagents (Asan Pharmaceuticals Co., Ltd., Seoul,
Korea) according to the manufacturer's instructions. The protein
concentration was determined by using a Bio-Rad protein assay
reagent (Bio-Rad Laboratories, Inc., Hercules, CA, USA) according
to the manufacturer's instructions.
Measurement of glycerol-3-phosphate
dehydrogenase (GPDH) activity
For GPDH determination, cells were harvested on day
8, washed twice with PBS, and collected with a scraper into 25 mM
Tris buffer containing 1 mM EDTA and 1 mM dithiothreitol (pH 7.5).
The harvested cells were sonicated for 10 sec, and then centrifuged
at 6,950 × g for 5 min at 4°C. The supernatants were analyzed using
GPDH activity assay kit (Takara Bio, Inc., Otsu, Japan) according
to the manufacturer's protocols.
Oil-red O staining
Following differentiation, culture medium was
removed and cells were gently rinsed with PBS once. Cells were
fixed with 10% formalin for 1 h at room temperature, and then
stained with filtered Oil Red O solution (6:4 ratio of stock
solution and water) for 2 h at room temperature. Finally, cells
were washed with distilled water and dried. The images were
captured using an Olympus IX71 inverted microscope (Olympus
Corporation, Tokyo, Japan). The stained lipid droplets were
dissolved in isopropanol and quantified by spectrophotometrical
analysis (540 nm).
Western blot analysis
Cells were harvested, and total protein extracts
were prepared using a PRO-PREP™ protein extraction solution (Intron
Biotechnology, Inc., Seongnam, Korea) and insoluble protein was
removed by centrifugation at 11,750 × g for 15 min at 4°C. The
supernatant was collected from the lysates and protein
concentrations were determined using a Bio-Rad protein assay
reagent (Bio-Rad Laboratories, Inc.) according to the
manufacturer's instructions. For western blotting, 40 µg protein
per lane was separated on an 8% SDS-PAGE and then transferred to
polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA). The membranes were incubated with blocking solution
(tris-bufferd saline/Tween 20, TBST) containing 5% skim milk (w/v)
for 1 h at room temperature, followed by incubation overnight at
4°C with the indicated primary antibodies and further incubated
with the HRP-conjugated secondary antibodies for 1 h at room
temperature. Immunoreactive proteins were visualized by enhanced
chemiluminescence solution (GE Healthcare Life Sciences, Chalfont,
UK) Image J software version 1.50 (http://rsb.info.nih.gov/ij/download.html; National
Institutes of Health, Bethesda, MD, USA) was used for the
quantification of the results of western blotting.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total mRNA was isolated using an Easy-Blue kit
(Intron Biotechnology, Inc., Seongnam, Korea) according to the
manufacturer's instructions. RNA was quantified using a Nanodrop
ND-1000 UV-Vis spectrophotometer (Nanodrop; Thermo Fisher
Scientific, Inc., Wilmington, DE, USA). SREBP1c, FAS, SCD1, GPAT,
PPAR-γ, C/EBPα, aP2, CPT1 and β-actin mRNA levels were measured by
a LightCycler Real-Time PCR system (Roche Applied Science,
Penzberg, Germany) using the QuantiTect SYBR-Green PCR kit. mRNA
levels were normalized to the housekeeping gene, β-actin. The
primer sequences used were as follows; SREBP1c, sense
5′-GCGCTACCGGTCTTCTATCA-3′, anti-sense 5′-TGCTGCCAAAAGACAAGGG-3′;
FAS, sense 5′-GATCCTGGAACGAGAACAC-3′, anti-sense
5′AGACTGTGGAACACGGTGGT-3′; SCD1, sense 5′-CGAGGGTTGTTGTTGATCTG-3′,
anti-sense 5′-ATAGCACTGTTGGCCCTGGA-3′; GPAT, sense
5′-GGTAGTGGATACTCTGTCGTCCA-3′, anti-sense 5′-CAGCAACATCATTCGGT-3′;
PPARγ, sense 5′-AGGCCGAGAAGGAGAAGCTGTTC-3′, anti-sense
5′-TGGCCACCTCTTTGCTCTTGCTC-3′; C/EBPα, sense
5′-GGGTGAGTTCATGGAGAATGG-3′, anti-sense
5′-CAGTTTGGCAAGAATCAGAGCA-3′; aP2, sense
5′-TCTCACCTGGCCTCTTCCTTTGGCTC-3′, anti-sense
5′-TTCCATCCAGGCCTCTTCCTTTGGCTC-3′; CPT1, sense
5′-TGTCCAAGTATCTGGCAGTCG-3′, anti-sense 5′-CATAGCCGTCAGCAACC-3′;
β-actin sense 5′-GGACTCCTATGGTGGGTGACGAGG-3′, anti-sense
5′-GGGAGAGCATAGCCCTCGTAGAT-3′. RT-qPCR was performed in a 25 µl
reaction mixture containing 1 µl cDNA and primers using the ABI
PRISM 7000 (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The double-stranded DNA-specific dye, SYBR-Green I, was
incorporated into the PCR buffer provided by the QuantiTect
SYBR-Green PCR kit (Qiagen GmbH) to quantitate the PCR products by
using the ∆∆Cq method (15). PCR was performed at 95°C for 30
sec, followed by 60°C for 30 sec, and 72°C for 1 min. The last
cycle was followed by a final extension step of 72°C for 10
min.
Statistical analysis
Results were represented as mean ± standard error of
the mean and differences between groups were analyzed by one-way
analysis of variance followed by Student Newman Keuls. P<0.05
was considered to indicate a statistically significant difference.
Calculations were performed using the SigmaStat software version
3.5 (Systat Software, Inc., Chicago, IL, USA).
Results
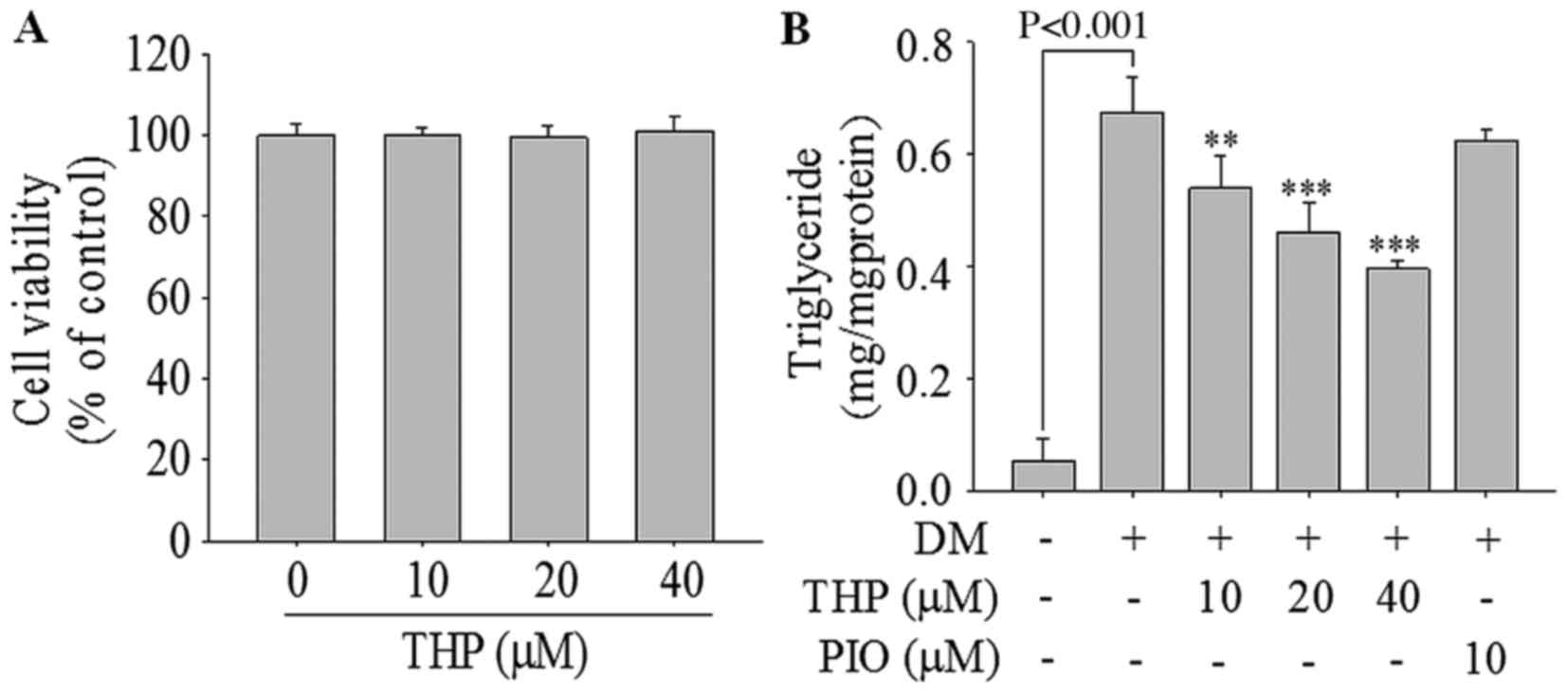
Effects of THP on cell viability
To examine the cell viability following THP
treatment, 3T3-L1 cells were treated with various concentrations of
THP (0–40 µM) for 96 h. THP did not exhibit any cellular toxicity
up to 40 µM (Fig. 1A), and thus
concentrations from 0 to 40 µM of THP were employed in the
subsequent studies. To explore the effect of THP on differentiation
of pre-adipocytes into adipocytes, 3T3-L1 pre-adipocytes were
treated with various concentrations of THP (0, 10, 20 or 40 µM) for
4 days.
Effects of THP on adipocyte
differentiation
On the day 8 of incubation, the change in the
contents of TG in THP-treated adipocytes was examined. As
demonstrated in Fig. 1B, THP
significantly reduced TG accumulation in a concentration-dependent
manner. Compared with the differentiation medium control level, TG
contents were significantly reduced by 13.1% at 10 µM (P<0.01),
34.4% at 20 µM (P<0.001) and 44.7% at 40 µM (P<0.001) of THP.
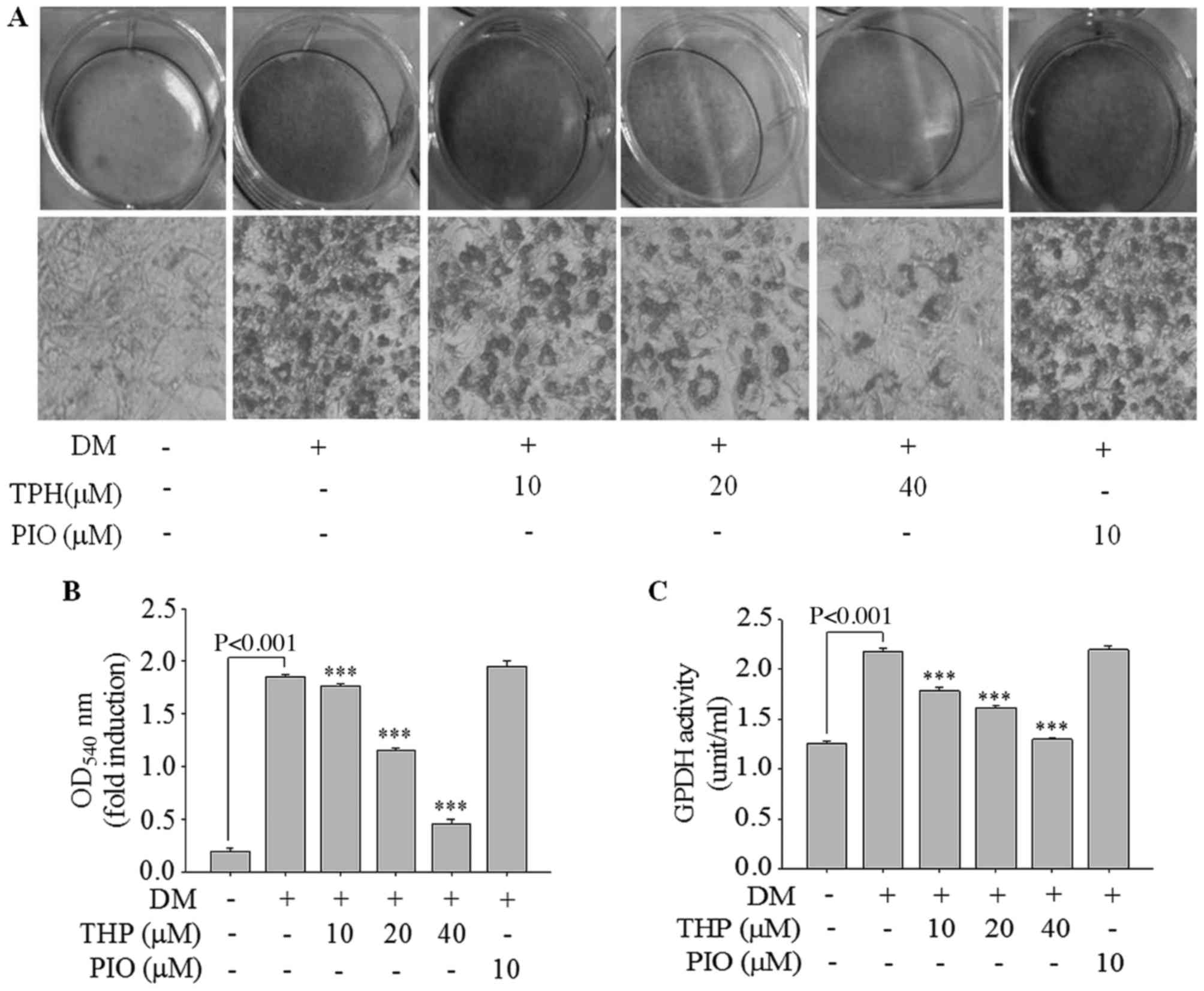
Additionally, lipid accumulation was quantified by Oil Red O
staining. As presented in Fig. 2A,
THP markedly inhibited lipid droplet accumulation in a
concentration-dependent manner. Consistent with results of lipid
droplet accumulation, absorbance value of the eluted dyes measured
at 540 nm were concentration-dependently decreased (Fig. 2B). Additionally, the effect of THP
on the activity of GPDH was examined, as cytosolic GPDH is
important role for the synthesis of TG (16). As demonstrated in Fig. 2C, THP treatment of 3 T3-L1
adipocytes resulted in a marked decrease in GPDH activity in a
dose-dependent manner. These results suggested that THP efficiently
inhibited adipocyte differentiation in 3T3-L1 adipocytes and may
have potential anti-obesity effects.
Effects of THP on SREBP1 target
protein and gene expressions
To investigate the THP on the expression of genes
involved in lipid metabolism, the expressions SREBP1, FAS and SCD1
genes responsible for adipogenesis were examined using western blot
analysis and RT-qPCR. THP significantly inhibited the expressions
of proteins (Fig. 3A) and genes
(Fig. 3B) SREBP1c, FAS and SCD1 in
a concentration-dependent manner, which are all associated with
adipogenesis. In addition, the gene expression of GPAT was
inhibited in a concentration-dependent manner compared with the
differentiation medium group (P<0.01; Fig. 3B). These results suggested that THP
suppresses the adipogenesis gene expression of the SREBP1c target
genes, such as FAS, SCD1 and GPAT, resulting in the inhibition of
3T3-L1 differentiation.
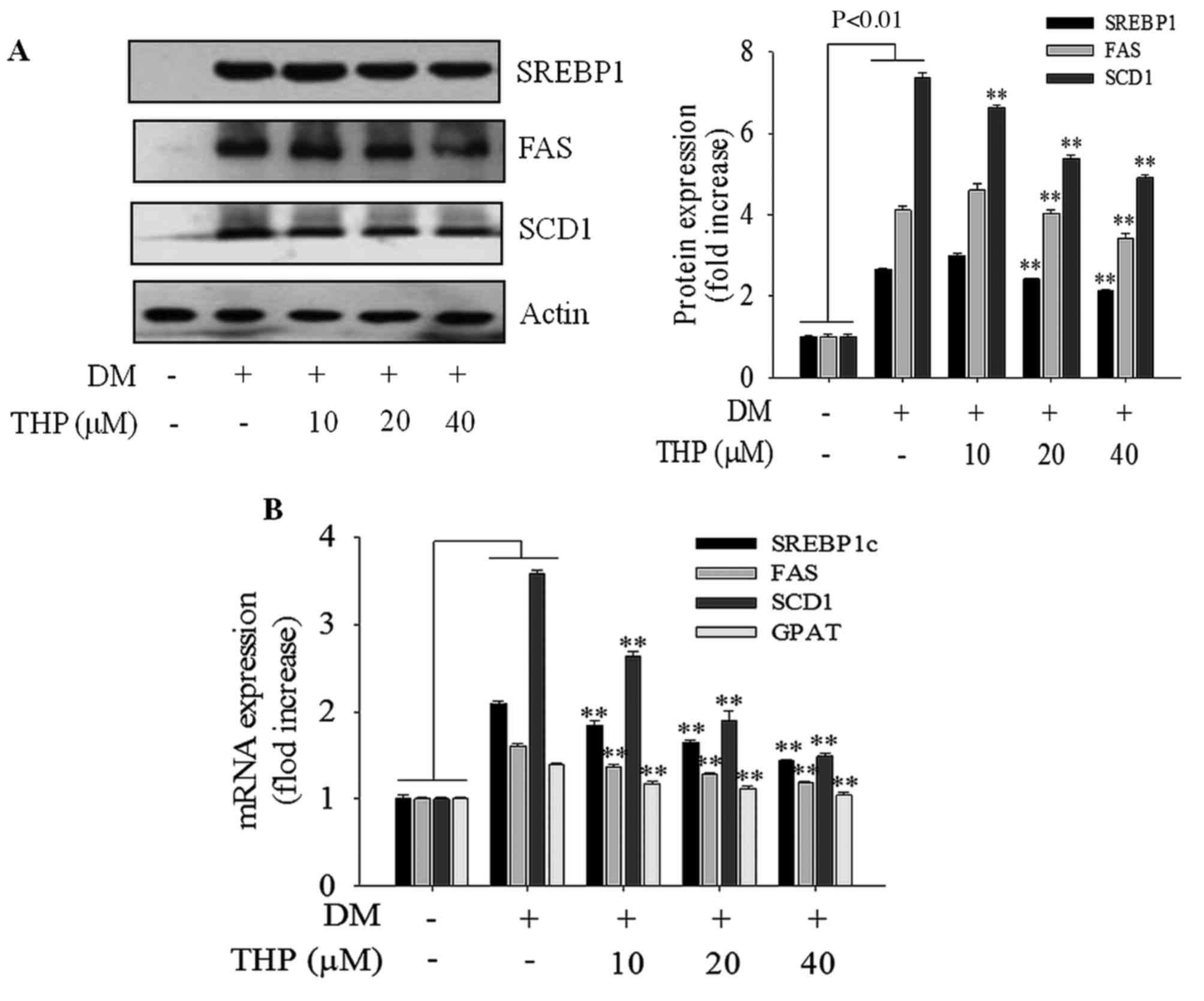 | Figure 3.Effects of THP on SREBP1 target
protein and gene expressions. Confluent cells were treated with
various concentrations (0, 10, 20 or 40 µM) of THP from day 0 to 4.
On day 8, completely differentiated cells were lysed to extract
total protein. (A) Protein extracts were prepared and subjected to
western blot analysis using SREBP1c, FAS and SCD1, (B) On day 8,
completely differentiated cells were used to extract total mRNA.
The expression of adipogenesis-associated genes SREBP1c, FAS, SCD1
and GPAT were measured by reverse transcription-quantitative
polymerase chain reaction. Data are represented as the mean ±
standard error of the mean (n=3). **P<0.01 vs. DM control. THP,
tetrahydropalmatine; DM, differentiation medium; SREBP1c, sterol
regulatory element-binding protein 1; FAS, fatty acid synthase;
SCD1, stearoyl-CoA desaturase-1; GPAT, glycerol-3-phosphate
O-acyltransferase. |
Effects of THP on PPARγ and C/EBPα
expression
PPARγ and C/EBPα are the primary transcription
factors involved in adipogenesis and lipogenesis (4). To further investigate the mechanism
on 3T3-L1 differentiation, expression of PPARγ and C/EBP-α at the
protein and mRNA level were monitored by western blot and RT-qPCR
analyses, respectively. THP markedly suppressed expression of PPARγ
and C/EBP-α protein (Fig. 4A) and
mRNA (Fig. 4B) levels in a
concentration-dependent manner. PPARγ and C/EBP-α promote terminal
differentiation by trans-activating the expression of downstream
adipocyte-specific genes, including aP2 (17). In the current results, aP2 gene
expression was reduced in a concentration-dependent manner compared
with the differentiation medium group (P<0.01; Fig. 4B). These results strongly indicated
that THP suppresses the expression of PPARγ, C/EBP-α and aP2,
resulting in the inhibition of 3T3-L1 differentiation.
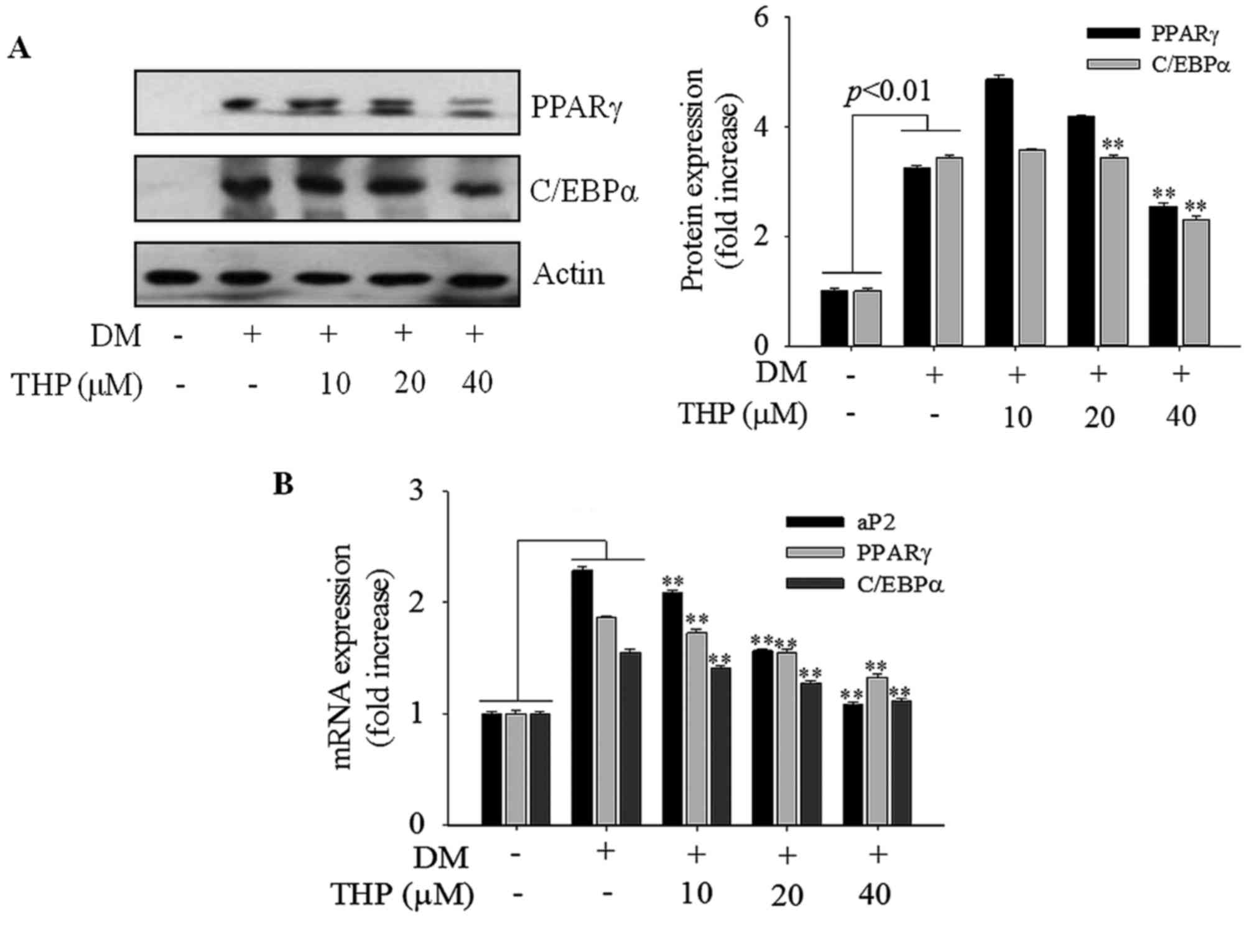 | Figure 4.Effects of THP on aP2, PPARγ and
C/EBPα expression. Confluent cells were treated with various
concentrations (0, 10, 20 or 40 µM) of THP from day 0 to 4. On day
8, completely differentiated cells were lysed to extract total
protein. (A) Protein extracts were prepared and subjected to
western blot analysis for PPARγ and CEBPα. (B) PPARγ, C/EBPα and
aP2 mRNA levels were measured by reverse transcription-quantitative
polymerase chain reaction. Data are represented as the mean ±
standard error of the mean (n=3). **P<0.01 vs. DM control. THP,
tetrahydropalmatine; DM, differentiation medium; PPARγ, peroxisome
proliferator activated receptor γ; C/EBPα, CCAAT/enhancer binding
protein-α; aP2, adipocyte protein 2. |
Effects of THP on AMPK activation
To investigate whether the activation of AMPK is
involved in the adipocyte TG lowering effect of THP, the
phosphorylated forms of AMPKα (Thr172), and its
immediate substrate ACC (Ser79) were detected using
western blotting. As demonstrated in Fig. 5A and B, THP treatment markedly
enhanced the level of p-AMPKα Thr172 (P<0.01) and
p-ACC Ser79 (P<0.01), indicating that AMPK/ACC
signaling is involved in the suppression of adipogenesis caused by
THP (Fig. 5A and B). AMPK leads to
fatty acid β-oxidation through inactivation of ACC and increasing
the expression of CPT-1 (8). The
present results indicated that THP increased gene expression of
CPT1 in a concentration-dependent manner (P<0.01; Fig. 5C) compared with the control group.
These results indicated that THP may inhibit adipogenesis via AMPK
activation.
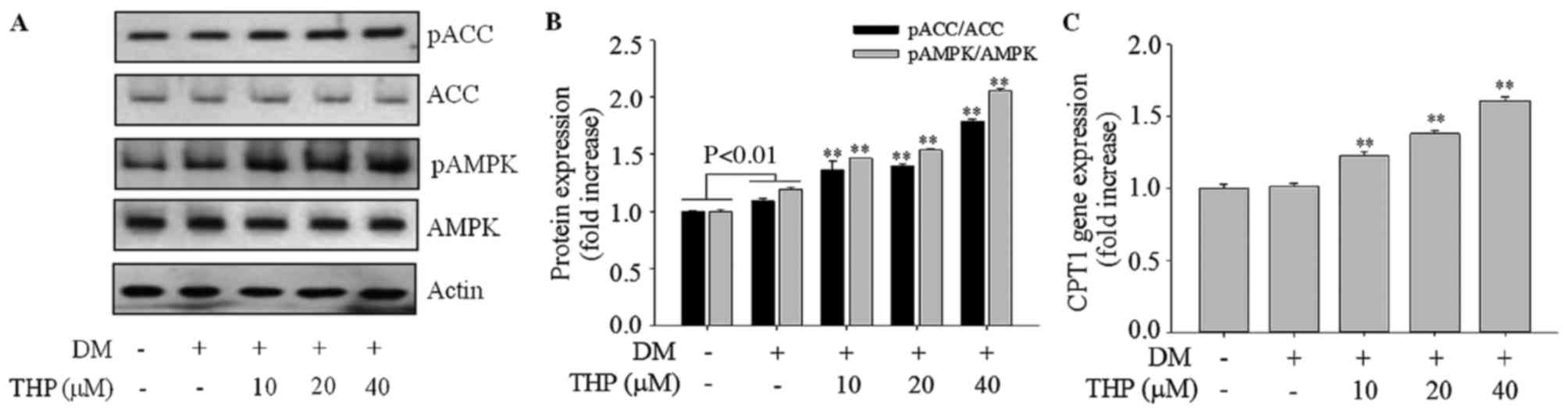 | Figure 5.Effects of THP on AMPK and ACC
phosphorylation and CPT1 gene expression in 3T3-L1 cells. Confluent
cells were treated with various concentrations (0, 10, 20 or 40 µM)
THP from day 0 to 4. On day 8, completely differentiated cells were
lysed to extract total protein or mRNA. (A and B) Protein extracts
were prepared and subjected to western blot analysis using p-AMPK,
AMPK, p-ACC, ACC, and β-actin antibodies. β-actin protein levels
were used as an internal control to elevate relative expression of
the protein. (C) CPT1 gene expression was measured by reverse
transcription-quantitative polymerase chain reaction. Data are
represented as the mean ± standard deviation (n=3). **P<0.01 vs.
DM control. THP, tetrahydropalmatine; DM, differentiation medium;
p, phospho; AMPK, AMP-activated protein kinase; ACC, acetyl-CoA
carboxylase; CPT1, carnitine palmitoyltransferase 1. |
Discussion
Yanhusuo (Corydalis yanhusuo W.T. Wang
extract) has been used as a traditional herbal medicine for
centuries. Tertiary and quaternary alkaloids have been isolated
from yanhusuo and identified, which are responsible for the
biological activities of the crude drug (18). Among the primary constituents, THP
is of particular interest. Although THP has been demonstrated to
exert several pharmacological effects, including anti-coagulant,
anti-nociceptive, anti-hyperalgesic, anti-oxidant, anti-viral and
anti-inflammatory activities (14), its anti-obesity effect has not yet
been reported. To the best of our knowledge, the present study is
the first to demonstrate that THP suppressed 3T3-L1 adipocyte
differentiation and lipid accumulation, and may act via the AMPK
signaling pathway.
Previous studies suggest that lipid accumulation and
adipocyte differentiation are associated with the occurrence and
development of obesity (19–21).
In the present study, THP effectively inhibited adipocyte
differentiation and lipid droplet formation in a
concentration-dependent manner. Additionally, THP significantly
reduced TG accumulation and GPDH activity in a
concentration-dependent manner, which indicates that THP inhibits
adipogenesis during adipocyte differentiation. Adipocyte
differentiation is the result of a complex process regulated by a
number of lipogenic and adipogenic factors. SREBP1c preferentially
regulates the lipogenic process by activating genes involved in
fatty acid and TG (20). It is a
major transcription factor involved in lipogenesis, and it induces
the expression of lipogenic genes, including FAS, SCD1 and GPAT
(1). Furthermore, Farmer (21) reported that SREBP1c acts to induce
the expression of PPARγ and C/EBPα. PPARγ and C/EBPα are the most
important transcriptional regulators known to have central role in
the adipogenesis (6,22). The results of the present study
demonstrated that THP downregulated the expression of SREBP1c and
its target genes, FAS, SCD1 and GPAT, in a concentration-dependent
manner. In addition, THP concentration-dependently downregulated
the expression of PPARγ and C/EBPα, master transcription factors
involved in regulation of adipogenic genes expression, and aP2, a
marker of adipocyte differentiation. These results suggested that
THP exerted a lipid-lowering effect by suppressing the expression
of lipogenic and adipogenic regulators that are crucial for
adipocyte differentiation and lipid accumulation.
AMPK is a pivotal enzyme with a crucial role in
fatty acid synthesis and oxidation via regulation of the expression
and activation of enzymes and genes involved in lipid metabolism
(23). In addition, AMPK regulates
pre-adipocyte differentiation and adipogenesis (24). AMPK activation directly inhibits
ACC activity via phosphorylation, and indirectly inhibits ACC
expression by suppressing SREBP1 (19,25).
Furthermore, inhibition of ACC by AMPK through phosphorylation
leads to the transportation of CPT1 long chain fatty acids into the
mitochondria for β-oxidation, which functions as a major regulatory
enzyme in fatty acid oxidation (26).
The current results indicated that THP stimulated
AMPK and ACC phosphorylation, and increased gene expression of CPT1
in a concentration-dependent manner. These results suggest that THP
suppressed adipocyte differentiation through AMPK activation and
ACC inactivation, and thus, inhibited the gene expression of
SREBP1c, FAS, SCD1, GPAT, PPARγ, C/EBPα and aP2 resulting in lipid
accumulation.
In conclusion, these results suggest that THP may
inhibit adipocyte differentiation and adipogenesis through the AMPK
signal pathway in 3T3-L1 adipocytes. These results provide
molecular information for further investigation of the mechanisms
by which THP alters lipid metabolism.
References
|
1
|
Huang B, Yuan HD, Kim DY, Quan HY and
Chung SH: Cinnamaldehyde prevents adipocyte differentiation and
adipogenesis via regulation of peroxisome proliferator-activated
receptor-γ (PPARγ) and AMP-activated protein kinase (AMPK)
pathways. J Agric Food Chem. 59:3666–3673. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yuan HD and Piao GC: An active part of
Artemisia sacrorum Ledeb. Inhibits adipogenesis via the AMPK
signaling pathway in 3T3-L1 adipocytes. Int J Mol Med. 27:531–536.
2011.PubMed/NCBI
|
|
3
|
Kowalska K, Olejnik A, Rychlik J and
Grajek W: Cranberries (Oxycoccus quadripetalus) inhibit lipid
metabolism and modulate leptin and adiponectin secretion in 3T3-L1
adipocytes. Food Chem. 185:383–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim BH, Han S, Lee H, Park CH, Chung YM,
Shin K, Lee HG and Ye SK: Metformin enhances the anti-adipogenic
effects of atorvastatin via modulation of STAT3 and TGF-β/Smad3
signaling. Biochem Biophys Res Commun. 456:173–178. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kang SI, Shin HS and Kim SJ: Sinensetin
enhances adipogenesis and lipolysis by increasing cyclic adenosine
monophosphate levels in 3T3-L1 adipocytes. Biol Pharm Bull.
38:552–558. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ji S, Doumit ME and Hill RA: Regulation of
Adipogenesis and key adipogenic gene expression by 1,
25-Dihydroxyvitamin D in 3T3-L1 Cells. PLoS One. 10:e01261422015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kang SW, Kang SI, Shin HS, Yoon SA, Kim
JH, Ko HC and Kim SJ: Sasa quelpaertensis Nakai extract and its
constituent p-coumaric acid inhibit adipogenesis in 3T3-L1 cells
through activation of the AMPK pathway. Food Chem Toxicol.
59:380–385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ceddia RB: The role of AMP-activated
protein kinase in regulating white adipose tissue metabolism. Mol
Cell Endocrinol. 366:194–203. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu H, Waldbauer K, Tang L, Xie L, McKinnon
R, Zehl M, Yang H, Xu H and Kopp B: Influence of vinegar and wine
processing on the alkaloid content and composition of the
traditional Chinese medicine Corydalis Rhizoma (Yanhusuo).
Molecules. 19:11487–11504. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan J, He X, Feng S, Zhai Y, Ma Y, Liang S
and Jin C: Up-regulation on cytochromes P450 in rat mediated by
total alkaloid extract from Corydalis yanhusuo. BMC Complement
Altern Med. 14:3062014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao JL, He TC, Li YB and Wang YT: A
traditional Chinese medicine formulation consisting of Rhizoma
Corydalis and Rhizoma Curcumae exerts synergistic anti-tumor
activity. Oncol Rep. 22:1077–1083. 2009.PubMed/NCBI
|
|
12
|
Yun KJ, Shin JS, Choi JH, Back NI, Chung
HG and Lee KT: Quaternary alkaloid, pseudocoptisine isolated from
tubers of Corydalis turtschaninovi inhibits LPS-induced nitric
oxide, PGE(2), and pro-inflammatory cytokines production via the
down-regulation of NF-kappaB in RAW 264.7 murine macrophage cells.
Int Immunopharmacol. 9:1323–1331. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chueh FY, Hsieh MT, Chen CF and Lin MT:
DL-tetrahydropalmatine-produced hypotension and bradycardia in rats
through the inhibition of central nervous dopaminergic mechanisms.
Pharmacology. 51:237–244. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee B, Sur B, Yeom M, Shim I, Lee H and
Hahm DH: L-tetrahydropalmatine ameliorates development of anxiety
and depression-related symptoms induced by single prolonged stress
in rats. Biomol Ther (Seoul). 22:213–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang M, Liu L, Xiao T and Guo W:
Detection of the expression level of miR-140 using realtime
fluorescent quantitative PCR in knee synovial fluid of
osteoarthritis patients. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
37:1210–1214. 2012.(In Chinese). PubMed/NCBI
|
|
16
|
Kim YS, Lee YM, Kim JH and Kim JS:
Polygonum cuspidatum inhibits pancreatic lipase activity and
adipogenesis via attenuation of lipid accumulation. BMC Complement
Altern Med. 13:2822013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi SS, Cha BY, Lee YS, Yonezawa T,
Teruya T, Nagai K and Woo JT: Magnolol enhances adipocyte
differentiation and glucose uptake in 3T3-L1 cells. Life Sci.
84:908–914. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang XL, Zheng Z, Hong Z and Fan G:
Advancements on chemical components and quality control of rhizoma
corydalis. Lishizhen Med Mater Med Res. 22:227–229. 2011.
|
|
19
|
Mcilroy GD, Tammireddy SR, Maskrey BH,
Grant L, Doherty MK, Watson DG, Delibegović M, Whitfield PD and
Mody N: Fenretinide mediated retinoic acid receptor signalling and
inhibition of ceramide biosynthesis regulates adipogenesis, lipid
accumulation, mitochondrial function and nutrient stress signalling
in adipocytes and adipose tissue. Biochem Pharmacol. 100:86–97.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rao Y, Liu H, Gao L, Yu H, Tan JH, Ou TM,
Huang SL, Gu LQ, Ye JM and Huang ZS: Discovery of natural alkaloid
bouchardatine as a novel inhibitor of adipogenesis/lipogenesis in
3T3-L1 adipocytes. Bioorg Med Chem. 23:4719–4727. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Farmer SR: Transcriptional control of
adipocyte formation. Cell Metab. 4:263–273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang X, Ji J, Yan G, Wu J, Sun X, Shen J,
Jiang H and Wang H: Sildenafil promotes adipogenesis through a PKG
pathway. Biochem Biophys Res Commun. 396:1054–1059. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fryer LG and Carling D: AMP-activated
protein kinase and the metabolic syndrome. Biochem Soc Trans.
33:362–366. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim ED, Kim E, Lee JH and Hyun CK:
Gly-Ala-Gly-Val-Gly-Tyr, a novel synthetic peptide, improves
glucose transport and exerts beneficial lipid metabolic effects in
3T3-L1 adipoctyes. Eur J Pharmacol. 650:479–485. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang BB, Zhou G and Li C: AMPK: An
emerging drug target for diabetes and the metabolic syndrome. Cell
Metab. 9:407–416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He Y, Li Y, Zhao T, Wang Y and Sun C:
Ursolic acid inhibits adipogenesis in 3T3-L1 adipocytes through
LKB1/AMPK pathway. PLoS One. 8:e701352013. View Article : Google Scholar : PubMed/NCBI
|