Introduction
Stroke is a major cause of death and disability
worldwide, however treatment options remain limited (1,2). A
small fraction of patients benefit from administration of
recombinant tissue-type plasminogen activator; however, this has a
short time window, as well as additional limitations (3) In a previous study, brain remodelling
and plasticity, including neurogenesis and angiogenesis, has
emerged as a novel promising therapeutic target for stroke
(4). Persistent neurogenesis in
the subventricular zone (SVZ) of the lateral ventricle and the
subgranular zone (SGZ) of the dentate gyrus may be stimulated by
cerebral ischemia and other injuries as part of the endogenous
repair response (5). Angiogenesis,
the formation of new microvessels from the existing vasculature,
improves tissue microperfusion in the peri-infarction region
following a stroke (6).
Guanosine (GUO), a guanine-based purine, serves
several important roles in the central nervous system (7,8).
Endogenous GUO levels increase within 2 h of focal stroke and
remain elevated for 7 days (9). An
accumulating body of evidence indicates that exogenous
administration of GUO prior to or immediately following
experimental stroke confers acute neuroprotection following
cerebral ischemia (10–14). However, any benefits to an ischemic
stroke from delayed treatment of GUO remain unknown. Notably, GUO
has been reported to promote neurite outgrowth from PC12 cell
cultures (15) and proliferation
of neural stem cells (16). In
addition, GUO has been indicated to promote myelination in a murine
model of spinal cord injury (17)
and induce synaptogenesis in the brain of healthy animals (18). These results suggest that GUO may
serve as a restorative target.
Therefore, the present study involved investigation
of whether delayed treatment with GUO (24 h following stroke)
improves the long-term functional outcome following a stroke, as
well as exploring the possible mechanisms underlying GUO
restorative effects.
Materials and methods
Animals and experimental model of
photothrombotic stroke
The experiments were approved by the Institutional
Animal Care and Use Committee of Tongji Medical College, Huazhong
University of Science and Technology (Wuhan, China), and were in
accordance with the guidelines of the Institute of Laboratory
Animal Resources (Washington, DC, USA). A total of 120 male
C57BL/6J wild-type mice (weight, 20–25 g; age, 8–10 weeks old) were
purchased from the Tongji Medical College Experimental Animal
Centre (Wuhan, China). Animals were housed in a temperature- and
humidity-controlled environment with a 12 h light/dark cycle and
free access to food and H2O. Focal stroke was induced by
photothrombosis (PT) as described previously (19). Mice were anesthetized using 10%
chloral hydrate (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) (35
mg/kg) intraperitoneally and placed in a stereotactic apparatus. A
midline incision of the skin exposed the skull. A dose of 0.1 ml
rose bengal solution (10 mg/ml in normal saline; Sigma-Aldrich;
Merck KGaA) was injected intraperitoneally 5 min prior to
illumination. For illumination, a cold light source (KL1500 LCD;
Zeiss AG, Oberkochen, Germany) with a 4-mm aperture was centred 2
mm lateral from the bregma. The brain was illuminated through the
intact skull for 15 min. All mice survived the procedure and
exhibited behavioural deficits.
Drug administration
All animals subjected to PT-induced stroke were
randomly assigned to receive GUO or vehicle following stroke
induction. Researchers were blinded to experimental groups. GUO
(0.5 mg/ml in sterile saline; Sigma-Aldrich; Merck KGaA) was
administered intraperitoneally (i.p.; 8 mg/kg) beginning 24 h
following the stroke and then daily for 7 days. The vehicle group
received an equal volume of saline. Bromo-deoxyuridine (BrdU; 10
mg/ml in sterile saline; Sigma-Aldrich; Merck KGaA) was injected
i.p. (100 mg/kg) beginning 24 h following the stroke and then twice
daily until the animals were sacrificed.
Measurement of infarct volume
The animals were sacrificed 7 days post-stroke.
Anesthetized mice were transcardially perfused with cold PBS
followed by 4% paraformaldehyde. Brains were subsequently removed,
fixed in fresh 4% formaldehyde solution at 4°C overnight and
immersed in 30% sucrose until they sank. Then the brains were
frozen at −80°C. The frozen brains were cut into 10-µm coronal
sections on a cryostat (CM3050S; Leica Microsystems GmbH, Wetzlar,
Germany). For each brain, 15 sequential sections were taken at
100-µm intervals and processed for Nissl staining. Sections were
stained with 0.1% crystal violet solution for 10 min at room
temperature. Infarct volumes were measured using an image analysis
program (ImageJ version 1.46r; National Institutes of Health,
Bethesda, MD, USA).
Behavioural testing
Behavioural tests were carried out prior to the PT
procedure and 1, 7, 14 and 28 days following PT using the modified
neurological severity scale (mNSS), grid walking test and cylinder
test.
mNSS
mNSS is a composite of motor, sensory, balance and
reflex tests. Neurological function is graded on a scale of 0 to 18
(normal score, 0; maximal deficit score, 18). A total of 1 score
point is awarded for the inability to perform the test or for the
lack of a tested reflex; therefore, a more severe injury has an
increased score (20).
Grid walking test
Animals were placed on an elevated wire grid and
video-recorded when they walked. The number of contra- and
ipsilateral faults for each limb and the total number of steps
taken were counted, the ratio between foot faults and total steps
was calculated (21).
Cylinder test
The animal was placed in a transparent cylinder and
video-recorded. Forelimb preference during vertical exploration of
the cylinder was evaluated by recording the forelimb contacts.
Animals were subjected to one trial on day 1 prior to PT. The
asymmetry score for each animal was calculated by the formula
previously described (22).
Immunohistochemistry
Frozen sections were incubated with a blocking
buffer (1X PBS/5% normal goat serum/0.3% Triton X-100, Goodbio,
Wuhan, China) for 1 h at room temperature. The sections were
subsequently incubated at 4°C overnight with the following primary
antibodies: mouse monoclonal anti-BrdU (catalogue no. sc-32323,
dilution, 1:100, Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
rabbit polyclonal anti-BrdU (catalogue no. ab19944, dilution,
1:500, Abcam, Cambridge, MA, USA), goat polyclonal
anti-doublecortin (DCX; catalogue no. sc-8066, dilution, 1:200;
Santa Cruz Biotechnology, Inc.), mouse monoclonal neuronal nuclei
(NeuN; Catalogue no. MAB377, dilution, 1:500; EMD Millipore,
Billerica, MA, USA) and von Willebrand factor (vWF; catalogue no.
ab7356, dilution, 1:1,000; EMD Millipore). Following washing with
PBS, the sections were incubated for 1 h at room temperature with
two secondary antibodies: Alexa Fluor 488 (catalogue no. CA11001s,
dilution, 1:200; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and Alexa Fluor 594 (catalogue no. CA11012s,
dilution, 1:200; Invitrogen; Thermo Fisher Scientific, Inc.).
Incubation was conducted in the dark and then washed with PBS three
times. For BrdU immunofluorescence, brain sections were pre-treated
with 2 M HCl at 37°C for 30 min and then washed with PBS six times
at room temperature before being incubated with blocking solution.
The images were captured using a fluorescence microscope (BX51;
Olympus Corporation, Tokyo, Japan).
Western blot
Mice were anesthetized and decapitated at 14 days
post-stroke. The ipsilateral peri-infarct cortices and cognate
regions from the contralateral hemisphere were sampled. The total
protein of each tissue was extracted according to the instructions
of the protein reagent kit (Goodbio, Wuhan, China). Protein
concentration of each sample was determined using the bicinchoninic
acid assay (Sigma-Aldrich; EMD Millipore). Protein samples (20 µg
per lane) were subsequently separated by 10% SDS-PAGE and
transferred to polyvinylidene difluoride membranes. Two primary
antibodies were incubated with the membranes overnight at 4°C:
anti-brain-derived neurotrophic factor (BDNF; catalogue no. AB1779,
dilution, 1:200; EMD Millipore), anti-vascular endothelial growth
factor (VEGF; catalogue no. sc-152, dilution, 1:200; Santa Cruz
Biotechnology, Inc.) and β-actin (catalogue no. 4970, dilution,
1:1,000; Cell Signalling Technology, Danvers, MA, USA).
Subsequently, membranes were incubated with goat anti-rabbit
horseradish peroxidase-conjugated IgG secondary antibody (catalogue
no. AB501, dilution, 1:1,000; Novoprotein, Shanghai, China) for 2 h
at room temperature. was used as a loading control for all
experiments. Densitometry analysis was performed using the Image J
software with normalization to β-actin.
Statistical analysis
All data are represented as mean ± standard error of
mean and were analysed using SPSS software (version 21.0; IBM SPSS,
Armonk, NY, USA). The treatment effects on the behaviour score at
different time points were analysed using repeated measures one-way
analysis of variance, followed by Tukey-Kramer post hoc tests. When
comparing two groups, statistical analysis of data was performed
using Student's t-test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Delayed administration of GUO does not
reduce infarct volume but improved the post-stroke long-term
functional outcome
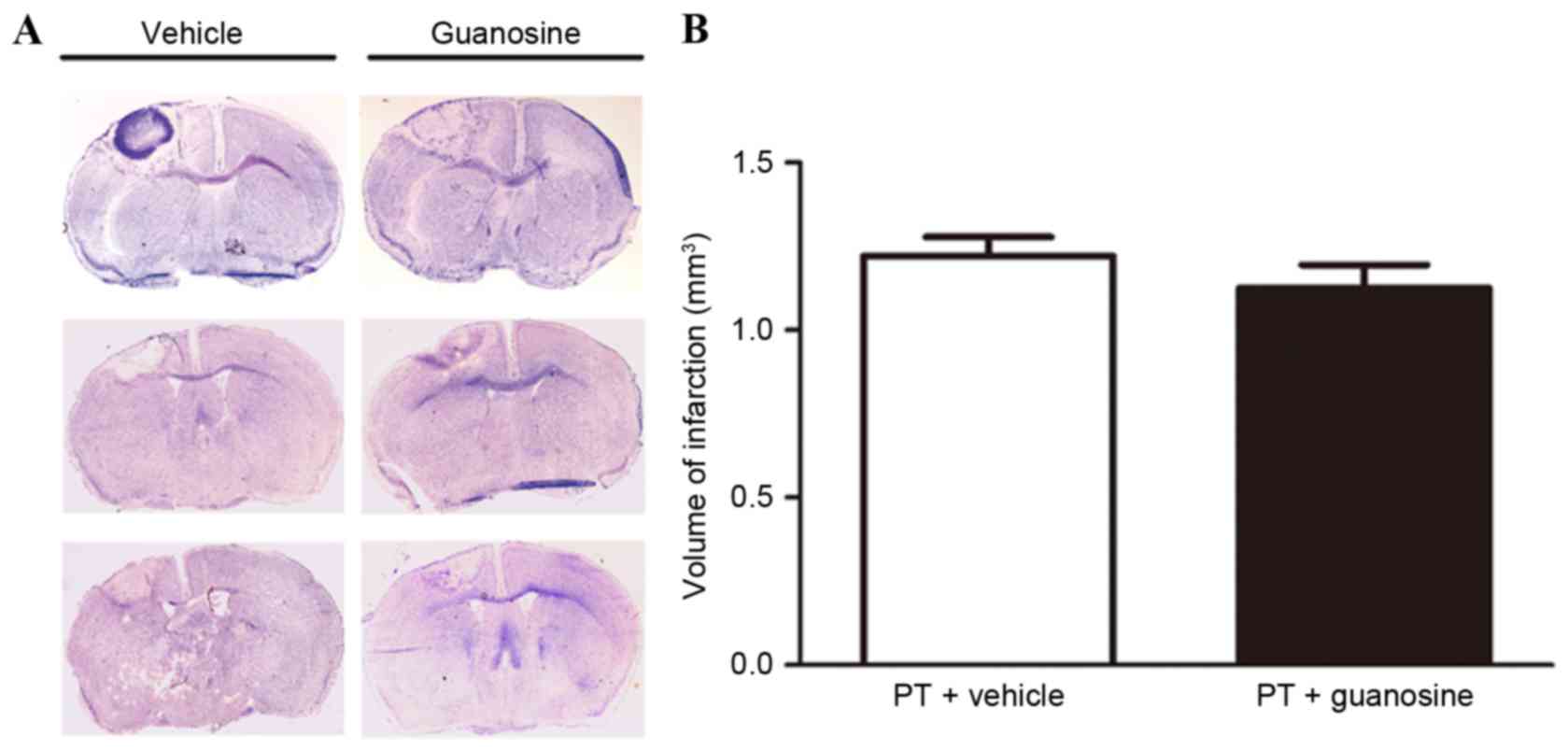
There was no statistical difference in infarct
volume on day 7 post-stroke between the two groups (1.220±0.110 vs.
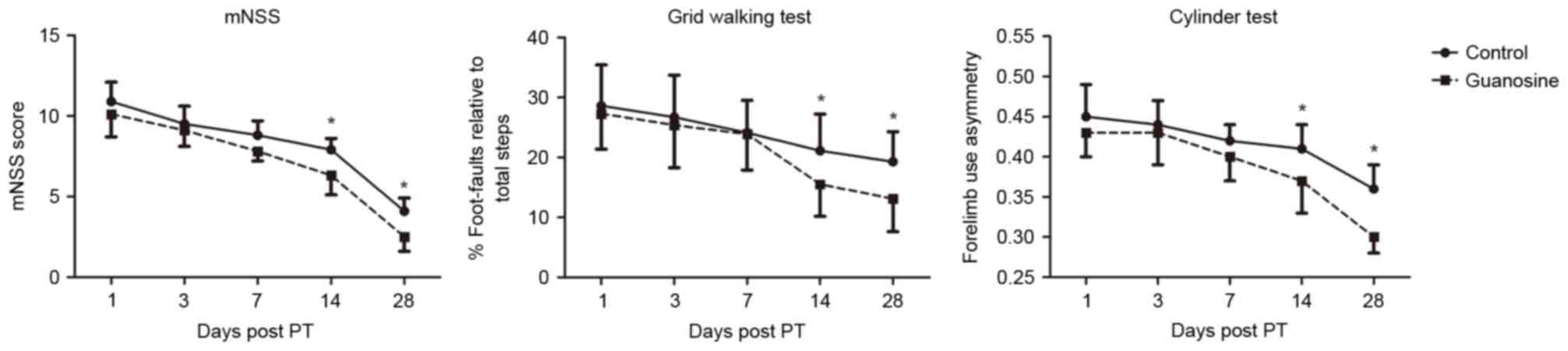
1.125±0.120 mm3, n=8 for each group, P>0.05; Fig. 1). In addition, no difference was
detected on days 1 and 7 post-stroke, in mNSS score, foot fault or
forelimb asymmetry between the vehicle group and GUO group
(Fig. 2A-C). However, a
significant reduction in the mNSS score and foot fault was observed
on days 14 and 28 post-stroke following GUO treatment (P<0.05;
Fig. 2A and B). Similarly,
treatment with GUO significantly improved the function of the
impaired forelimb on day 14, and the effect was maintained up to 28
days post-stroke. The results indicated that delayed administration
of GUO was able to promote long-term functional recovery following
ischemic stroke (Fig. 2C).
Delayed administration of GUO enhances
neurogenesis in the ischemic brain
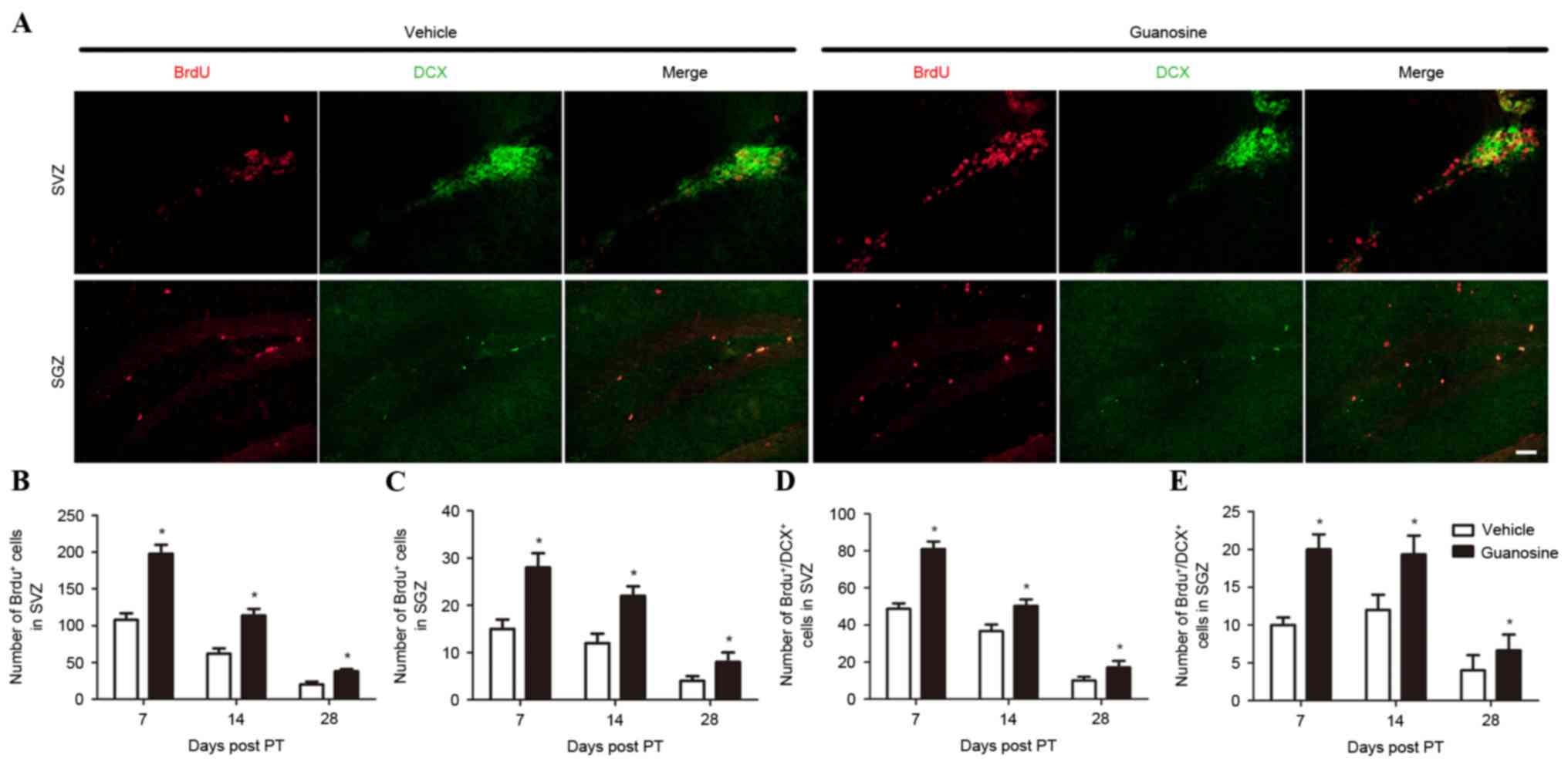
GUO significantly increased the number of
BrdU+ cells in the ipsilateral SVZ and SGZ when compared
with the vehicle group (P<0.05; Fig. 3A-C). These data indicated that
administration of GUO enhances cell proliferation following
stroke.
In addition, GUO significantly increased the number
of BrdU+ cells co-labelled with DCX, a marker of neural
progenitor cells, in the ipsilateral SVZ and SGZ compared with the
vehicle group (P<0.05; Fig. 3D and
E) at all time points following stroke induction, suggesting
that delayed administration of GUO promotes proliferation of neural
progenitor cells in the SVZ and SGZ following stroke.
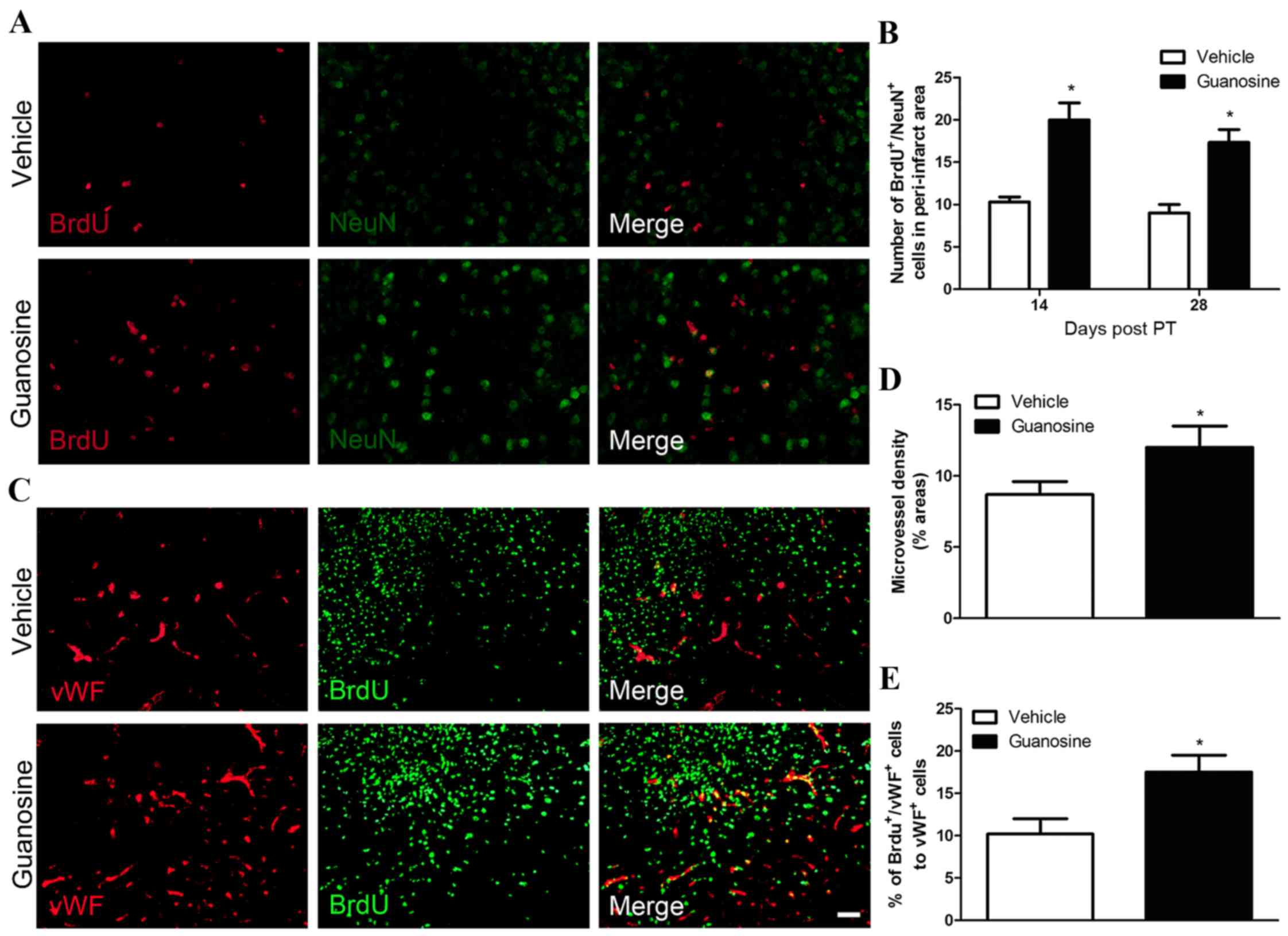
To further investigate whether the proliferative
neural progenitor cells is able to differentiate into functional
neurons, double immunostaining was performed with NeuN (a marker of
mature neurons) and BrdU. GUO significantly increased the number of
BrdU+/NeuN+ cells in the peri-infarction
cortex following stroke, when compared with the vehicle group
(P<0.05; Fig. 4A and B). These
data indicated that GUO enhances the differentiation of new neural
progenitor cells into mature neurons within the peri-infarction
region following stroke.
Delayed administration of GUO enhances
angiogenesis in the ischemic brain
To examine whether GUO influences the formation of
new blood vessels in the ischemic brain, all blood vessels in the
peri-infarction cortex were counted using vWF immunostaining. vWF
is a vascular endothelial cell marker. GUO significantly increased
vascular density in the peri-infarction cortex compared with the
vehicle group (P<0.05; Fig. 4C and
D). Furthermore, these results presented a significant increase
in the percentage of BrdU+/vWF+ cells in mice
treated with GUO, when compared with the vehicle group (P<0.05;
Fig. 4E). These data suggested
that GUO enhances angiogenesis following stroke.
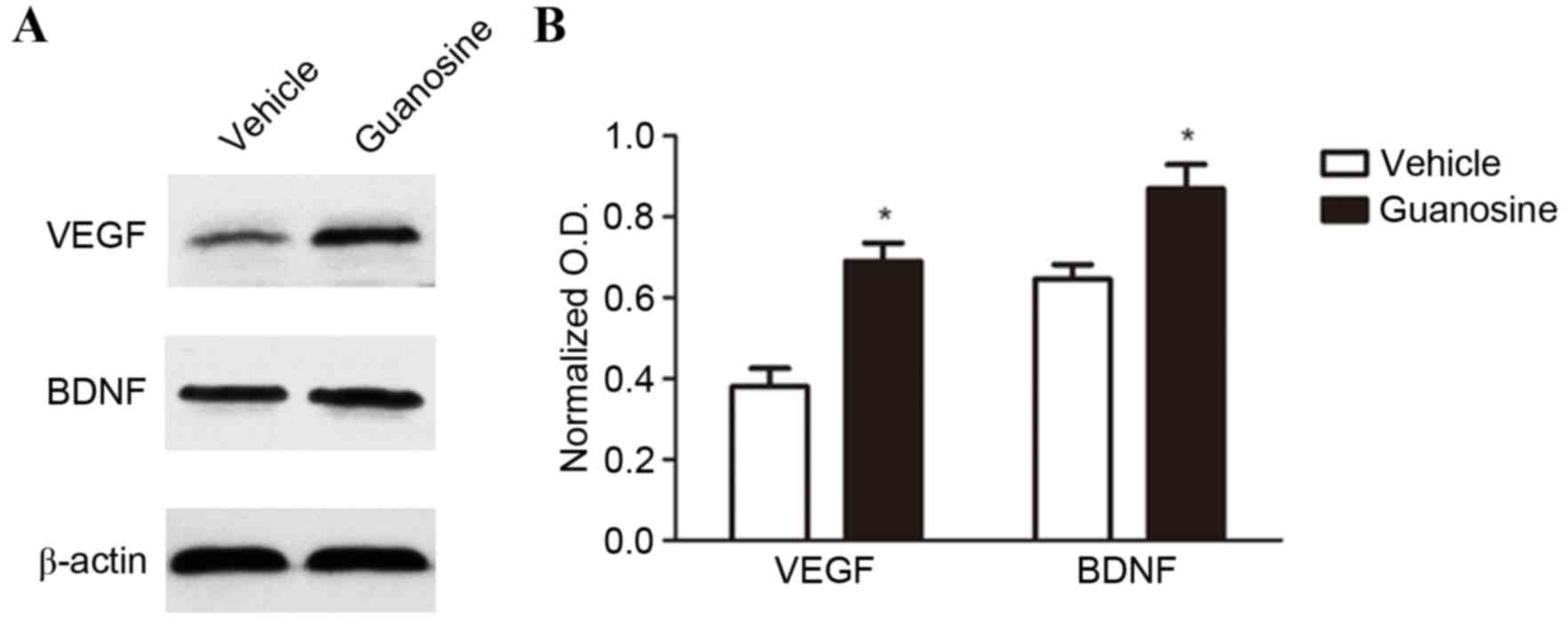
Delayed administration of GUO promotes
the expression of neurotrophic factors
Western blot analysis demonstrated that GUO
significantly increased the expression of VEGF and BDNF in the
ischemic brain at day 14 post-stroke compared to the vehicle
(P<0.05; Fig. 5).
Discussion
The primary result of the present study was that
delayed administration of GUO improved long-term functional outcome
following a PT-induced stroke; however, did not reduce infarct
volume. In addition, GUO enhanced post-ischemic neurogenesis and
angiogenesis, which likely contributed to the restorative effects
of GUO. Furthermore, GUO increased the expression of two key
neurotrophins, BDNF and VEGF, suggesting that neurotrophic effects
may contribute to the enhancing effects of GUO on post-ischemic
neurogenesis and angiogenesis.
Treatment with GUO prior to or immediately following
experimental cerebral ischemia confers acute neuroprotection in
multiple in vitro and in vivo stroke models (10–14).
The mechanisms responsible for the neuroprotective effects may be
associated with the anti-oxidative stress, anti-excitatory toxicity
and anti-apoptosis activities of GUO (9,10,13,23).
In the present study, delayed administration of GUO was
investigated, to identify whether it improved long-term functional
outcome following a stroke. The results indicated that GUO
administered 24 h following PT accelerated long-term recovery. In
particular, delayed GUO treatment only improved neurological
functions from 14 days following the stroke and did not improve
functions during the acute phase, which suggested that delayed GUO
treatment may promote functional recovery through restorative
rather than acute neuroprotective mechanisms.
In addition, the infarct volume at 7 days following
stroke was not reduced. This result is consistent with previous
studies in which infarct volume was only reduced by GUO when it was
administered within a tight administration schedule (11,12).
These results suggest that delayed treatment with GUO did not exert
an acute neuroprotective effect on cerebral ischemia, resulting in
an unchanged infarct size.
GUO has been indicated to induce neurogenesis in SVZ
in a mouse Parkinsonism model (24) and synaptogenesis in the healthy rat
brain (18). However, whether GUO
increases neurogenesis or angiogenesis post-stroke has never been
studied, to the best of the authors' knowledge. GUO significantly
increased the number of BrdU+ cells in the SVZ and the
SGZ, indicating that GUO promotes cell proliferation following
stroke. As the number of BrdU+/DCX+ cells
increased in the SVZ in GUO-treated mice, GUO enhanced
proliferation of endogenous neural progenitor cells. At 14 and 28
days post-stroke, treatment with GUO significantly increased the
number of BrdU+/NeuN+ cells in the
peri-infarct region, when compared with the vehicle-treated group,
suggesting that GUO promoted cell proliferation and the
differentiation of new neural progenitor cells into mature neurons
within the peri-infarction region. GUO was demonstrated to increase
the microvessel density and Brdu+/vwF+ cells
in the peri-infarct region, when compared with the vehicle group,
indicating angiogenesis post-stroke was enhanced and may contribute
to neurological recovery.
Growth and neurotrophic factors have been
demonstrated to promote neurogenesis and angiogenesis and improve
neurological function following cerebral ischemia (25,26).
Previous in vitro studies have repeatedly demonstrated the
neurotrophic effects of GUO (27).
The present results further suggested that GUO significantly
increased BDNF and VEGF levels in ipsilateral brain post-stroke.
BDNF and VEGF are two important neurotrophic factors that have
multiple effects on neurogenesis and angiogenesis, for example,
they stimulate adult neurogenesis and promote migration of new
neurons in the SVZ and dentate gyrus (28,29).
In addition, the expression of VEGF is associated with an increase
in vascular density in the ischemic penumbra (30). Elevated BDNF and VEGF levels may
contribute to the enhanced neurogenesis and angiogenesis by GUO.
However, the causative link between them has not been investigated,
therefore further studies are warranted.
In conclusion, delayed administration of GUO
enhances neurogenesis and angiogenesis post-ischemic stroke and
increases the expression of BDNF and VEGF. This contributes to
improved long-term functional recovery.
Acknowledgments
The authors of the present study would like to thank
Professor Di Song (Tongji Hospital, Tongji Medical College,
Huazhong University of Science and Technology, Wuhan, China) for
her comments on the manuscript.
References
|
1
|
Lozano R, Naghavi M, Foreman K, Lim S,
Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et
al: Global and regional mortality from 235 causes of death for 20
age groups in 1990 and 2010: A systematic analysis for the Global
Burden of Disease Study 2010. Lancet. 380:2095–2128. 2012.
View Article : Google Scholar
|
|
2
|
Lackland DT, Roccella EJ, Deutsch AF,
Fornage M, George MG, Howard G, Kissela BM, Kittner SJ, Lichtman
JH, Lisabeth LD, et al: Factors influencing the decline in stroke
mortality: A statement from the American Heart Association/American
Stroke Association. Stroke. 45:315–353. 2014. View Article : Google Scholar
|
|
3
|
Adams HP Jr, Adams RJ, Brott T, del Zoppo
GJ, Furlan A, Goldstein LB, Grubb RL, Higashida R, Kidwell C,
Kwiatkowski TG, et al: Guidelines for the early management of
patients with ischemic stroke: A scientific statement from the
Stroke Council of the American Stroke Association. Stroke.
34:1056–1083. 2003. View Article : Google Scholar
|
|
4
|
Hermann DM and Chopp M: Promoting brain
remodelling and plasticity for stroke recovery: Therapeutic promise
and potential pitfalls of clinical translation. Lancet Neurol.
11:369–380. 2012. View Article : Google Scholar :
|
|
5
|
Ming GL and Song H: Adult neurogenesis in
the mammalian central nervous system. Annu Rev Neurosci.
28:223–250. 2005. View Article : Google Scholar
|
|
6
|
Liu J, Wang Y, Akamatsu Y, Lee CC, Stetler
RA, Lawton MT and Yang GY: Vascular remodeling after ischemic
stroke: Mechanisms and therapeutic potentials. Prog Neurobiol.
115:138–156. 2014. View Article : Google Scholar
|
|
7
|
Schmidt AP, Lara DR and Souza DO: Proposal
of a guanine-based purinergic system in the mammalian central
nervous system. Pharmacol Ther. 116:401–416. 2007. View Article : Google Scholar
|
|
8
|
Lanznaster D, Dal-Cim T, Piermartiri TC
and Tasca CI: Guanosine: A neuromodulator with therapeutic
potential in brain disorders. Aging Dis. 7:657–679. 2016.
View Article : Google Scholar :
|
|
9
|
Uemura Y, Miller JM, Matson WR and Beal
MF: Neurochemical analysis of focal ischemia in rats. Stroke.
22:1548–1553. 1991. View Article : Google Scholar
|
|
10
|
Chang R, Algird A, Bau C, Rathbone MP and
Jiang S: Neuroprotective effects of guanosine on stroke models in
vitro and in vivo. Neurosci Lett. 431:101–105. 2008. View Article : Google Scholar
|
|
11
|
Rathbone MP, Saleh TM, Connell BJ, Chang
R, Su C, Worley B, Kim M and Jiang S: Systemic administration of
guanosine promotes functional and histological improvement
following an ischemic stroke in rats. Brain Res. 1407:79–89. 2011.
View Article : Google Scholar
|
|
12
|
Connell BJ, Di Iorio P, Sayeed I,
Ballerini P, Saleh MC, Giuliani P, Saleh TM, Rathbone MP, Su C and
Jiang S: Guanosine protects against reperfusion injury in rat
brains after ischemic stroke. J Neurosci Res. 91:262–272. 2013.
View Article : Google Scholar
|
|
13
|
Hansel G, Ramos DB, Delgado CA, Souza DG,
Almeida RF, Portela LV, Quincozes-Santos A and Souza DO: The
potential therapeutic effect of guanosine after cortical focal
ischemia in rats. PLoS One. 9:e906932014. View Article : Google Scholar :
|
|
14
|
Hansel G, Tonon AC, Guella FL, Pettenuzzo
LF, Duarte T, Duarte MM, Oses JP, Achaval M and Souza DO: Guanosine
protects against cortical focal ischemia. Involvement of
inflammatory response. Mol Neurobiol. 52:1791–1803. 2015.
View Article : Google Scholar
|
|
15
|
Gysbers JW and Rathbone MP: GTP and
guanosine synergistically enhance NGF-induced neurite outgrowth
from PC12 cells. Int J Dev Neurosci. 14:19–34. 1996. View Article : Google Scholar
|
|
16
|
Su C, Wang P, Jiang C, Ballerini P,
Caciagli F, Rathbone MP and Jiang S: Guanosine promotes
proliferation of neural stem cells through cAMP-CREB pathway. J
Biol Regul Homeost Agents. 27:673–680. 2013.
|
|
17
|
Jiang S, Khan MI, Lu Y, Wang J, Buttigieg
J, Werstiuk ES, Ciccarelli R, Caciagli F and Rathbone MP: Guanosine
promotes myelination and functional recovery in chronic spinal
injury. Neuroreport. 14:2463–2467. 2003. View Article : Google Scholar
|
|
18
|
Gerrikagoitia I and Martínez-Millán L:
Guanosine-induced synaptogenesis in the adult brain in vivo. Anat
Rec (Hoboken). 292:1968–1975. 2009. View
Article : Google Scholar
|
|
19
|
Jang JY, Choi YW, Kim HN, Kim YR, Hong JW,
Bae DW, Park SJ, Shin HK and Choi BT: Neuroprotective effects of a
novel single compound 1-methoxyoctadecan-1-ol isolated from Uncaria
sinensis in primary cortical neurons and a photothrombotic ischemia
model. PLoS One. 9:e853222014. View Article : Google Scholar :
|
|
20
|
Chen J, Sanberg PR, Li Y, Wang L, Lu M,
Willing AE, Sanchez-Ramos J and Chopp M: Intravenous administration
of human umbilical cord blood reduces behavioral deficits after
stroke in rats. Stroke. 32:2682–2688. 2001. View Article : Google Scholar
|
|
21
|
Clarkson AN, Overman JJ, Zhong S, Mueller
R, Lynch G and Carmichael ST: AMPA receptor-induced local
brain-derived neurotrophic factor signaling mediates motor recovery
after stroke. J Neurosci. 31:3766–3775. 2011. View Article : Google Scholar :
|
|
22
|
de Vasconcelos Dos Santos A, da Costa Reis
J, Paredes B Diaz, Moraes L, Jasmin, Giraldi-Guimarães A and
Mendez-Otero R: Therapeutic window for treatment of cortical
ischemia with bone marrow-derived cells in rats. Brain Res.
1306:149–158. 2010. View Article : Google Scholar
|
|
23
|
Thomazi AP, Boff B, Pires TD, Godinho G,
Battú CE, Gottfried C, Souza DO, Salbego C and Wofchuk ST: Profile
of glutamate uptake and cellular viability in hippocampal slices
exposed to oxygen and glucose deprivation: Developmental aspects
and protection by guanosine. Brain Res. 1188:233–240. 2008.
View Article : Google Scholar
|
|
24
|
Su C, Elfeki N, Ballerini P, D'Alimonte I,
Bau C, Ciccarelli R, Caciagli F, Gabriele J and Jiang S: Guanosine
improves motor behavior, reduces apoptosis, and stimulates
neurogenesis in rats with parkinsonism. J Neurosci Res. 87:617–625.
2009. View Article : Google Scholar
|
|
25
|
Lichtenwalner RJ and Parent JM: Adult
neurogenesis and the ischemic forebrain. J Cereb Blood Flow Metab.
26:1–20. 2006. View Article : Google Scholar
|
|
26
|
Zhao C, Deng W and Gage FH: Mechanisms and
functional implications of adult neurogenesis. Cell. 132:645–660.
2008. View Article : Google Scholar
|
|
27
|
Rathbone MP, Middlemiss PJ, Gysbers JW,
Andrew C, Herman MA, Reed JK, Ciccarelli R, Di Iorio P and Caciagli
F: Trophic effects of purines in neurons and glial cells. Prog
Neurobiol. 59:663–690. 1999. View Article : Google Scholar
|
|
28
|
Schabitz WR, Steigleder T, Cooper-Kuhn CM,
Schwab S, Sommer C, Schneider A and Kuhn HG: Intravenous
brain-derived neurotrophic factor enhances poststroke sensorimotor
recovery and stimulates neurogenesis. Stroke. 38:2165–2172. 2007.
View Article : Google Scholar
|
|
29
|
Jin K, Zhu Y, Sun Y, Mao XO, Xie L and
Greenberg DA: Vascular endothelial growth factor (VEGF) stimulates
neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 99:pp.
11946–11950. 2002; View Article : Google Scholar :
|
|
30
|
Hermann DM and Zechariah A: Implications
of vascular endothelial growth factor for postischemic
neurovascular remodeling. J Cereb Blood Flow Metab. 29:1620–1643.
2009. View Article : Google Scholar
|