Introduction
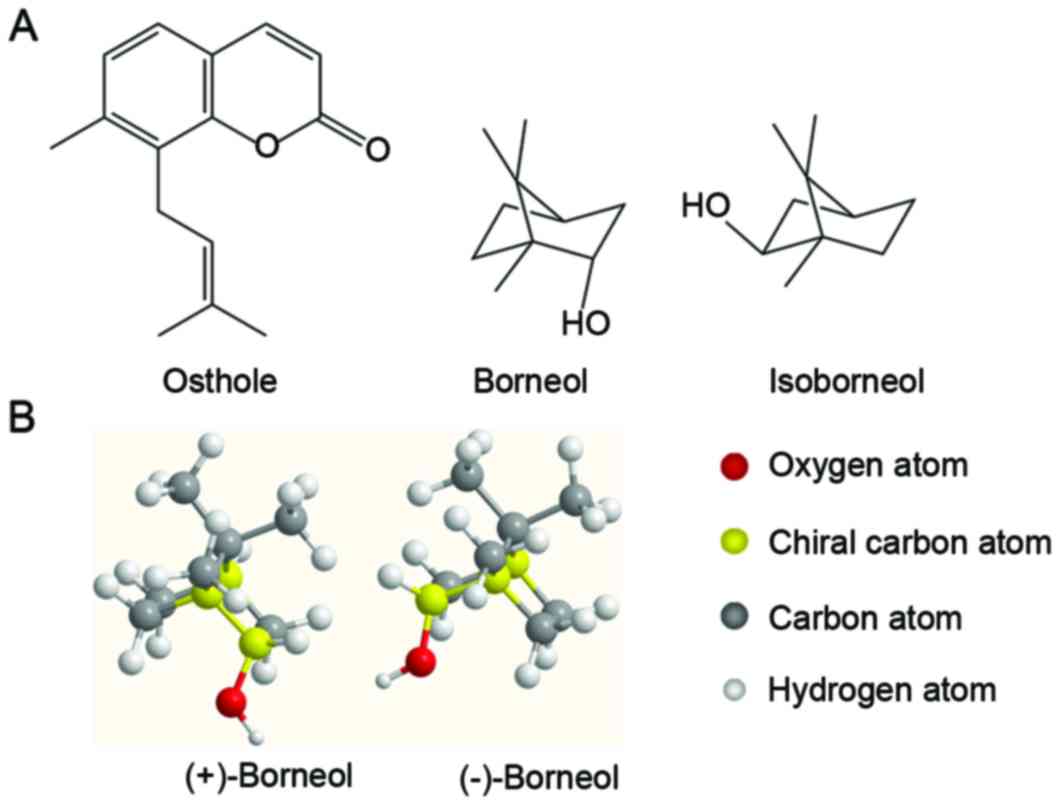
Osthole [7-methoxy-8-(3-methyplent-2-enyl) coumarin;
Fig. 1A], a poorly water-soluble
natural coumarin, is the main active component of the Fructus
Cnidii and Heracleum moellendorffii Hance plant species.
Osthole has been confirmed to possess numerous beneficial
bioactivities including anti-inflammatory (1), neuroprotection (2) and antiosteoporotic activities
(3). In addition, osthole has
beneficial effects in glioblastoma multiforme (4), diabetes and acute ischemic stroke
(5,6). Although osthole possesses many
biological and pharmacological activities, the application of
osthole in the clinic has been limited due to its poor
bioavailability and low plasma concentration (7,8),
which results from rapid elimination by the CYP3A4 enzyme in the
liver (9). In addition, osthole is
excreted in the kidneys and bile, which rely on the excretory
function of P-glycoprotein (10).
Borneol, which is widely used in herbal medicine, is
a component in the essential oils of numerous spice berries
including Lavandula, Thymus vulgaris and
Rosmarinus officinalis Linnaeus (11–13).
In the molecular structure of borneol there is one chiral carbon
atom, which produces two optical isomers: (+)-Borneol and
(−)-borneol (Fig. 1B). Synthetic
borneol [a racemate composed of (+)- and (−)-borneol, isoborneol
(Fig. 1A)] is increasingly being
applied to replace (+)- and (−)-borneol as there is an unlimited
source and it is relatively inexpensive. Borneol has additionally
been used as a Chinese medicine exhibiting various bioactivities
including sedation, anti-inflammation and antioxidant activity
(14–16). Notably, according to Chinese
medicine, borneol was considered as a ‘guide’ drug, regulating and
mediating the delivery of other prescription drugs (17). Previous studies have confirmed that
borneol enhances the bioavailability of other drugs through
pharmacokinetic interactions, including the intestinal absorption
of salvianolic acid B and Akebia saponin (18,19),
the distribution of danshensu to the eye (20) and nasal absorption of geniposide
(21). Previous studies have
identified the differences between (+)- and (−)-borneol in their
interactions with cytochrome P450 enzyme and p-glycoprotein, which
serve an important role in the absorption and elimination of drugs
(22–24). These studies indicated that there
may be differences between the pharmacokinetic interactions of (+)-
and (−)-borneol. In traditional Chinese medicine, co-administration
of borneol with herbal drugs containing osthole, including
Angelica pubescens, Fructus cnidii and Libanotis
buchtormensis (Fisch.) DC, was commonly used (25). In addition, borneol inhibits
cytochrome P450 enzyme, which participates in the elimination of
osthole (26). Collectively the
evidence suggests that borneol may promote the bioavailability of
osthole. However, the pharmacokinetic interactions between osthole
and borneol have not been reported. In addition, to the best of our
knowledge, there is no evidence demonstrating that (+)-borneol and
(−)-borneol possess different effects in the pharmacokinetics of
osthole. Therefore, the aim of the present study was to verify the
effect of borneol on the pharmacokinetics of osthole and to
investigate the differences between treatments with (+)- and
(−)-borneol when co-administration with osthole.
Materials and methods
Chemicals and reagents
(+)-Borneol (98% purity) was purchased from Shenzhen
Oupeng Technology Co., Ltd. (Shenzhen, China). (−)-Borneol (98%
purity) was obtained from Guizhou Miaoyao Biotechnology Co., Ltd.
(Tongren, Guizhou, China). Synthetic borneol (97% purity) was
purchased from Jian Shengda Fragrance Oils Co., Ltd. (Jian, China).
Standard osthole and paeonol were obtained from Chengdu
Purechem-Standard Co., Ltd. (Chengdu, Sichuan, China).
Chromatographic pure methanol, ethyl acetate and other chemicals
and reagents were obtained from Guangzhou Lubex Biological
Technology Co., Ltd. (Guangzhou, Guangdong, China) and were of
analytical grade.
Preparation of standard solutions and
quality control (QC) samples
The stock solution of osthole was prepared by
accurately weighing standard osthole, which was then dissolved and
diluted with chromatographic pure methanol to obtain a series
standard solutions with the following concentrations: 1, 5, 10, 20,
40 and 80 µg/ml. Paeonol standard was weighed precisely to prepare
the internal solution (IS) at a concentration of 2 µg/ml. Quality
control (QC) samples were prepared daily using three concentrations
of osthole standard solutions (10, 40 and 80 µg/ml). A total of 100
µl standard solution was dried prior to the addition of 200 µl
blank blood plasma, followed by vortex-mixing for 3 min. All of the
solutions were maintained at 4°C prior to use.
Sample preparation
An aliquot of blood plasma (100 µl) was added to 50
µl IS solution and 500 µl chromatographic pure methanol, then
vortex-mixed for 3 min. Following centrifugation for 10 min at
12,000 × g and 4°C, the supernatant was transferred into a
centrifuge tube and dried using nitrogen gas. The residue was
re-dissolved with 100 µl chromatographic pure methanol. Following
vortex-mixing for 3 min and centrifugation for 10 min at 12,000 × g
and 4°C, the supernatant was filtered using a 0.22-µm nylon
membrane prior to analysis using high-performance liquid
chromatography (HPLC).
HPLC-ultraviolet (UV) method
HPLC analysis was performed using a Shimadzu HPLC
system (Shimadzu Corporation, Kyoto, Japan). The HPLC system
consisted of a LC solution chromatographic workstation, two pumps
and a UV detector (model no. SPD-20A). Separation was executed
using a Diamonsil C18 column (particle size, 5 µm;
250×4.6 mm; Dikma Co., Beijing, China). The mobile phase consisted
of (A) water and (B) acetonitrile [25/75 (v/v)], with a constant
rate of 1 ml/min and the column temperature was maintained at 25°C
during the whole analysis process. An aliquot (10 µl) of plasma
sample was analyzed by HPLC, and the content of osthole and IS were
detected at a wavelength of 320 nm.
Method validation
Specificity: The specificity study was completed by
comparing chromatograms of blank plasma, blank plasma spiked with
osthole and IS, and plasma samples obtained from rats following
oral administration.
Linearity and sensitivity: A total of 6
concentrations of standard solutions (1, 5, 10, 20, 40 and 80
µg/ml) were used for the calibration curve. The calibration curve
was structured by the peak area ratio (Y) of osthole to IS vs. the
spiked concentrations (X) of the analysis with a 1/X2
weighted least square linear regressions.
Accuracy and precision: Three quality control (QC)
samples were used to test the accuracy and precision, with five
replicates of each concentration. The measured concentrations were
calculated using the calibration curves obtained daily. Intra-day
precision and accuracy were determined by repeated analysis (n=3)
of the QC samples in the same day. Inter-day precision and accuracy
were evaluated by repeated analysis of the QC samples over 3
consecutive days. The precision was determined by the relative
standard deviation (RSD %) and the accuracy as the relative error
(RE %).
Extraction recovery: Extraction recovery was
assessed by comparing the measured concentration vs. the spiked
concentration in three QC samples (n=5).
Stability: The stability test was composed of a
short-term, the freeze-thaw cycle and the long-term stability
tests. Each test was conducted with three QCs (n=3). The short-term
stability test was performed by analysis of QC samples following
storage at room temperature for 24 h. Freeze-thaw cycle stability
was assessed following three freeze-thaw cycles within 3
consecutive days. In each cycle, QC samples were reserved at −80°C
for 24 h and subsequently thawed at room temperature. After
complete thawing, the samples were refrozen at −80°C for 24 h. The
long-term stability test was evaluated by assaying samples
following a period of 2 weeks of storage at −80°C.
Pharmacokinetic study
Male Sprague-Dawley (SD) rats (n=24; weight, 290–310
g; age, 11–14 weeks) were purchased from the Animal Center of
Guangzhou University of Chinese Medicine (Guangzhou, Guangdong,
China). All SD rats were specifically pathogen-free and fed under
standard conditions (a stable temperature at 24±1°C and a 12/12-h
light/dark cycle) for at least 7 days prior to the pharmacokinetics
experiment. All animals were fasted, with access to water only, for
12 h prior to drug administration. Animal experiments were
performed in accordance with procedures approved by the Animal
Experimental Ethics Committee of Guangzhou University of Chinese
Medicine (dSPF 2014 021), and the experimental protocols followed
the ‘Guide for the Care and Use of Laboratory Animals’. All drugs
were dissolved in 5% Tween-80 for the pharmacokinetic studies.
In the pharmacokinetics experiments, the SD rats
were randomly divided into four groups (n=6), each group received
oral administration of osthole (300 mg/kg), and were then given the
following treatments: The control group was given an oral dose with
extra 5% Tween-80 (400 mg/kg) and the (+)-borneol, (−)-borneol and
synthetic borneol groups were given oral doses with an extra 400
mg/kg borneol. The dosage of borneol and osthole applied was based
on that of a previous study (27).
Blood samples were collected at 5, 15, 30, 45, 60, 90, 120, 240,
360, 480 and 720 min following oral administration from the
suborbital venous plexus of the rat eye socket vein. Following
blood collection, the animals were sacrificed following anesthesia.
The blood samples were centrifuged at 12,000 × g and 4°C for 10
min. The supernatant (the blood plasma) was then transferred into a
clean polypropylene tube and maintained in a refrigerator at −20°C
for subsequent analysis.
Data analysis
Pharmacokinetic analysis of osthole was performed
based on a non-compartmental description of the data observed. All
data were expressed as the mean ± standard deviation. The primary
kinetic parameters (AUC0-t, AUC0-∞,
Cmax, Tmax, Vd/F, CL/F and
t1/2) were calculated using The Drug and Statistics
software (version 2.11; Mathematical Pharmacology Professional
Committee of China, Shanghai, China). The area under the plasma
concentration-time curve (AUC) was calculated using the linear
trapezoidal method. In addition, the maximum plasma concentration
(Cmax) and the time to reach the maximum plasma
concentration (Tmax) were obtained from the plasma
concentration-time data. The differences between any two respective
treatment groups were analyzed for significance by one-way analysis
of variance followed by Duncan's multiple range test with SPSS
software (version 19; IBM SPSS, Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significantly
difference.
Results
Method validation
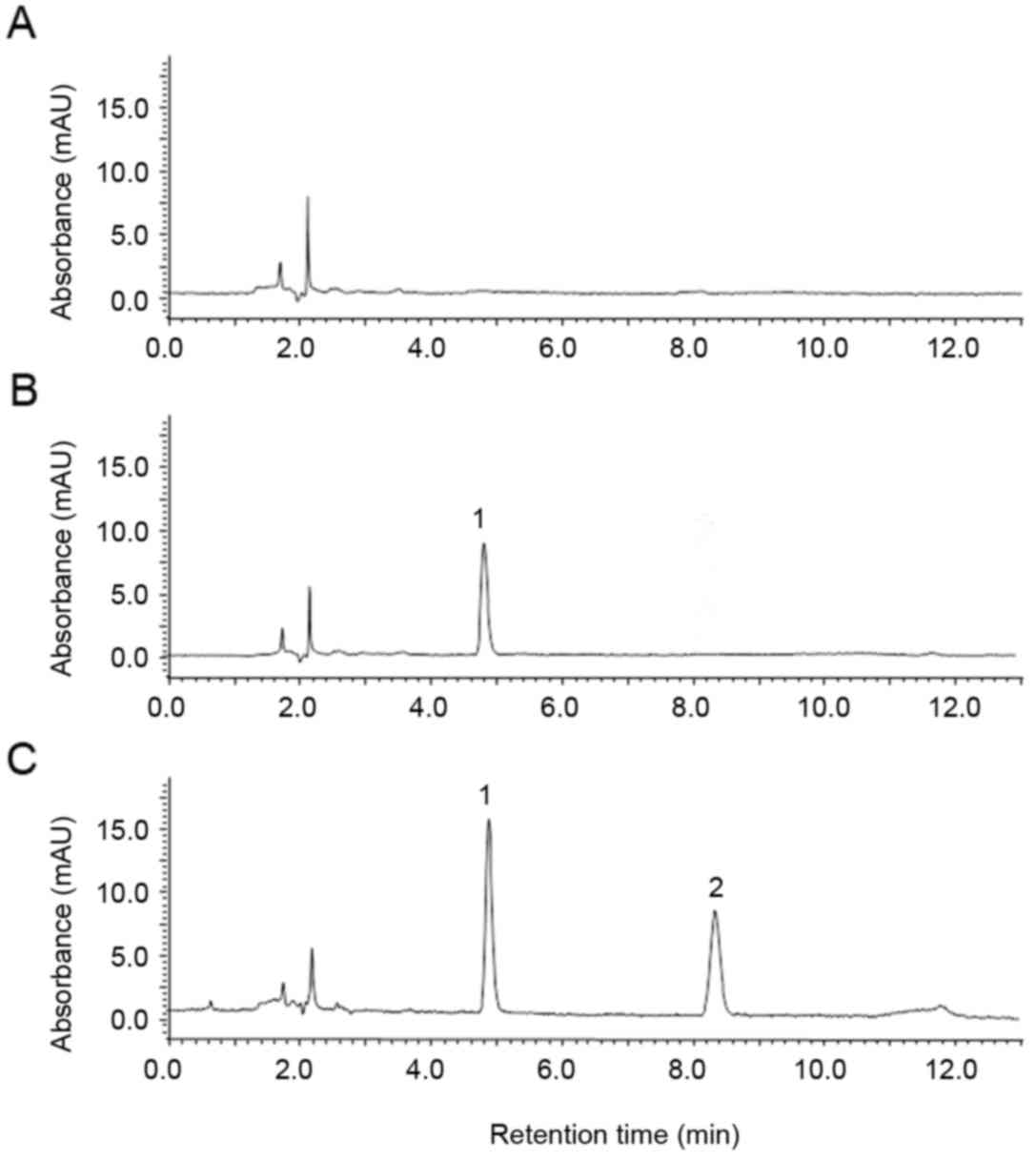
Specificity: The typical chromatograms observed are
depicted in Fig. 2. There were no
interference peaks near the retention time peaks of osthole (8.488
min) and paeonol (4.885 min), with favorable resolution (R>1.5),
which demonstrated that the selectivity of osthole and IS was
favored in the HPLC method.
Linearity and sensitivity: The calibration curves
calculated in the range, 1–80 µg/ml, were linear for the analysis
of osthole from rat plasma. A good linear relation was obtained for
osthole [Y=0.0101X+0.0321 (R2=0.997)].
Accuracy and precision: As presented in Table I, in the 3 QC samples, the
intra-day precision ranged from 1.334–3.373% (RSD) and the
inter-day precision ranged from 1.316–3.702% (RSD). Analytical
accuracy varied from 97.144–101.926%.
 | Table I.The intra- and inter-day accuracy and
precision scores of osthole in rat plasma. |
Table I.
The intra- and inter-day accuracy and
precision scores of osthole in rat plasma.
| Day type | Spiked
concentration (µg/ml) | Osthole, mean ± SD
(µg/ml) | Accuracy (RE,
%) | Precision (RSD,
%) |
|---|
| Intra-day | 5 |
4.857±0.019 | 3.438 | 5.836 |
|
| 20 | 20.308±0.192 | 1.796 | 1.501 |
|
| 80 | 81.541±0.419 | 2.172 | 1.440 |
| Inter-day | 5 |
4.857±0.065 | 3.438 | 3.702 |
|
| 20 | 20.308±0.144 | 1.796 | 1.469 |
|
| 80 | 81.541±0.741 | 2.172 | 1.316 |
Extraction recovery: As presented in Table II, in the 3 QC samples, the
recoveries were all between 94.447 and 101.185%, and the RSDs were
within 0.964 and 3.866%. All of the results indicated that there
was good repeatability of osthole following use as the sample
pretreatment method.
 | Table II.Recovery of osthole in rat plasma
(n=5). |
Table II.
Recovery of osthole in rat plasma
(n=5).
| Spiked
concentration (µg/ml) | Osthole, mean ± SD
(µg/ml) | RSD (%) |
|---|
| 5 |
94.447±3.671 | 3.886 |
| 20 | 100.975±0.974 | 0.964 |
| 80 | 101.185±2.265 | 2.238 |
Stability: As presented in Table III, the percentage of remaining
osthole in the three stability tests was between 97.800 and
103.130%, which indicated that the plasma samples were stable at
20°C for 24 h, −20°C for 7 days and following three freeze-thaw
cycles.
 | Table III.Results of short-term stability,
freeze-thaw cycles and long-term stability of osthole analysis in
rat plasma. |
Table III.
Results of short-term stability,
freeze-thaw cycles and long-term stability of osthole analysis in
rat plasma.
| Stability test | Spiked
concentration (µg/ml) | Osthole, mean ± SD
(µg/ml) | Remaining (%) |
|---|
| Short-term
stability | 5 |
4.890±0.230 |
97.800±4.608 |
|
| 20 | 20.297±0.043 | 101.483±0.213 |
|
| 80 | 82.279±0.626 | 102.849±0.782 |
| Freeze-thaw
cycle | 5 |
5.008±0.057 | 100.168±1.137 |
|
| 20 | 20.434±0.213 | 102.168±1.067 |
|
| 80 | 80.591±1.461 | 100.739±1.826 |
| Long-term
stability | 5 |
4.922±0.113 |
98.441±2.265 |
|
| 20 | 20.310±0.069 | 101.551±0.343 |
|
| 80 | 82.509±0.100 | 103.136±0.124 |
Pharmacokinetic study
The developed HPLC-UV method was applied to
determine the plasma concentration of osthole following oral
administration of (+)-borneol, (−)-borneol and synthetic borneol.
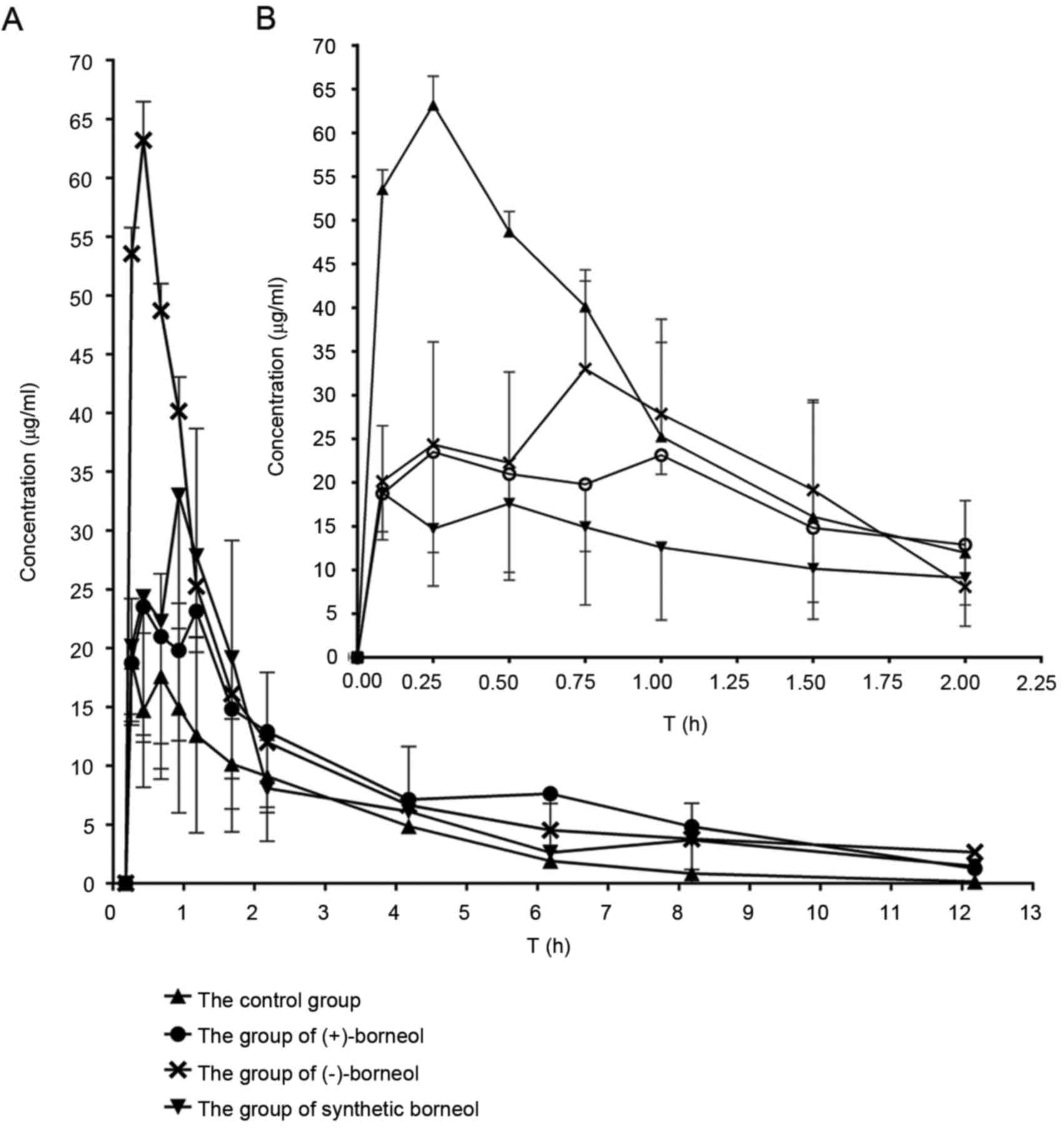
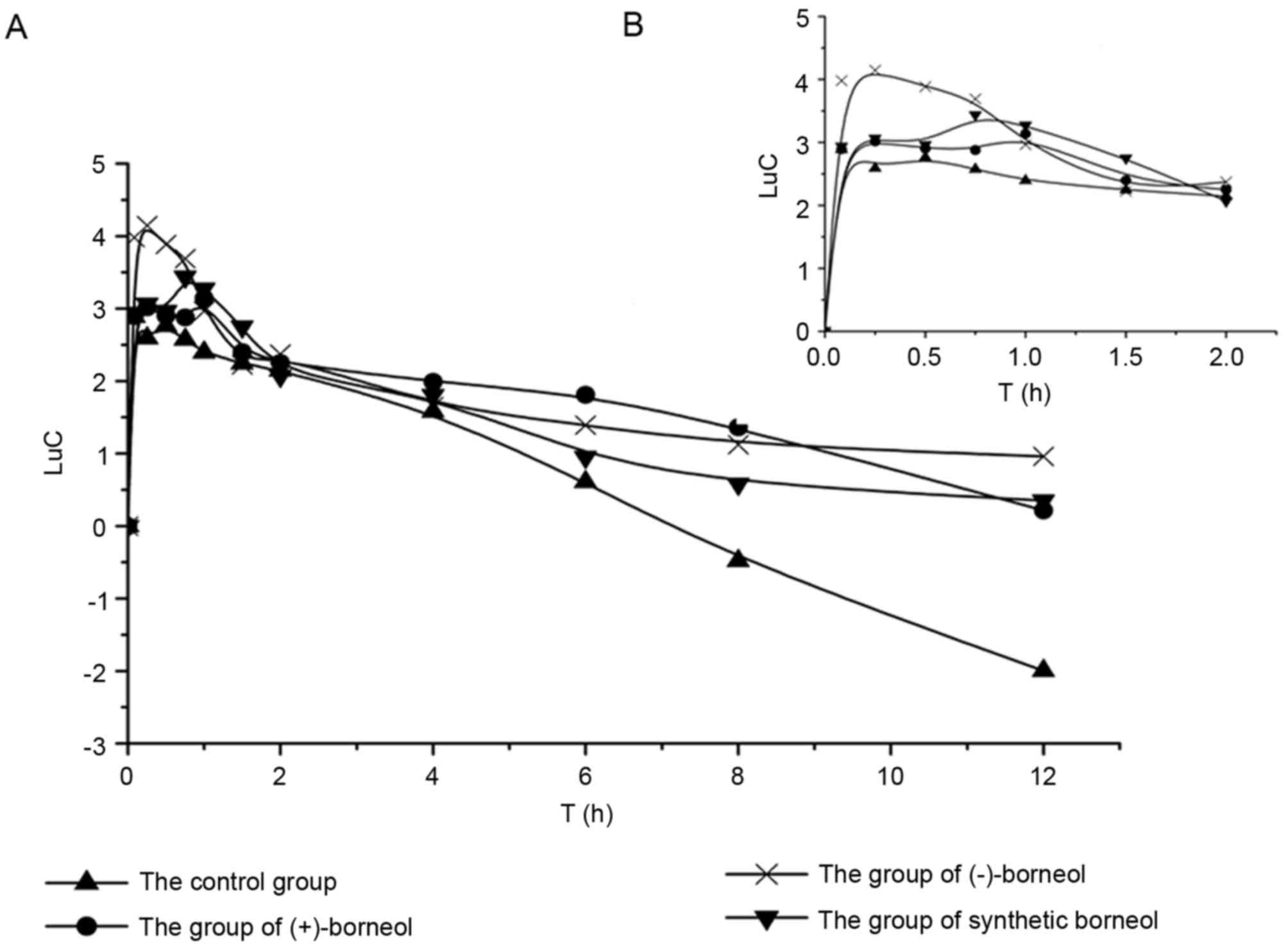
The kinetics curves of osthole in rats are displayed in Fig. 3 and the semi-log plot for the
concentration-time profiles of osthole is presented in Fig. 4. The main pharmacokinetic
parameters of osthole following oral administration are shown in
Table IV, which were then
compared (Fig. 5). The results
demonstrated that the blood concentration and bioavailability of
osthole were markedly enhanced following co-administration with
borneol, however, the differences were significant between borneol
enantiomers.
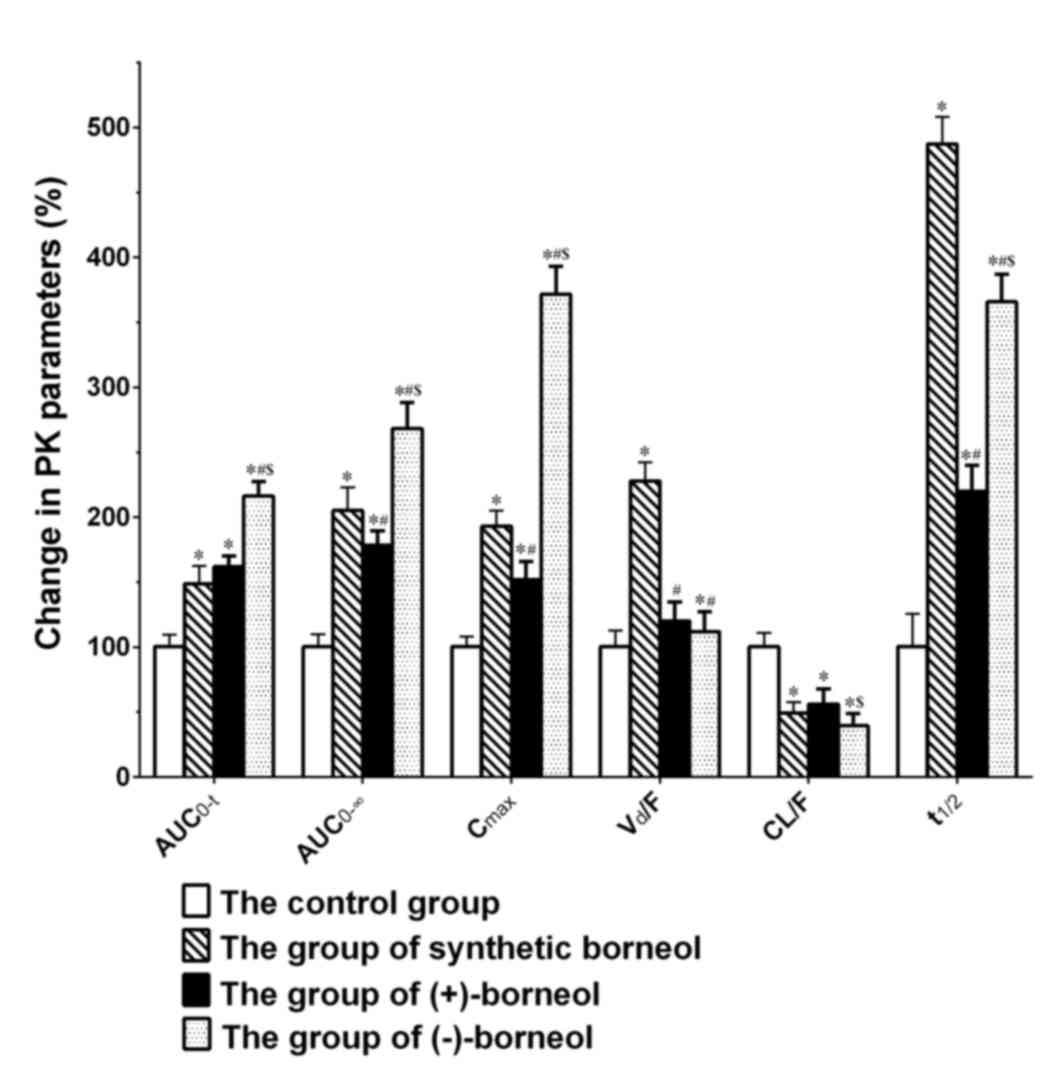 | Figure 5.Percentage change in the main
pharmacokinetic parameters of osthole, including the
AUC0-t, AUC0–∞, Cmax,
Vd/F, CL/F and t1/2 compared with the control
group. *P<0.05 vs. control group; #P<0.05 (+)- and
(−)-borneol vs. synthetic borneol; $P<0.05
(−)-borneol vs. (+)-borneol. AUC0-t, area under the
plasma concentration-time curve from time zero to time t;
AUC0–∞, area under the plasma concentration-time curve
from time zero to infinity; Cmax, maximum (peak) plasma
drug concentration; Vd/F, apparent volume of
distribution after non-intravenous administration; CL/F, apparent
total clearance of the drug from plasma after oral administration;
t1/2, elimination half-life. |
 | Table IV.Main pharmacokinetic parameters of
osthole following oral administration of osthole alone or combined
with borneol. |
Table IV.
Main pharmacokinetic parameters of
osthole following oral administration of osthole alone or combined
with borneol.
| Parameters | Control | Synthetic
borneol | (+)-Borneol | (−)-Borneol |
|---|
| AUC0-t
(mg*h/l,) | 60.208±5.522 |
89.200±2.394a |
97.272±5.017a |
129.961±6.679a |
| AUC0-∞
(mg*h/l,) | 61.617±5.959 |
126.134±10.999a |
109.780±6.761a,b |
165.001±2.268a–c |
| Cmax (mg/l) | 17.161±1.345 |
33.059±2.048a |
26.046±2.351a,b |
63.718±3.638a–c |
| Tmax (h) |
0.500±0.000 |
0.750±0.000 |
0.542±0.368b |
0.250±0.000a,b |
| Vd/F (l/kg) | 13.943±3.089 |
31.714±4.784a |
16.710±5.398a |
15.547±0.738a,c |
| CL/F (l/h/kg) |
4.911±0.524 |
2.394±0.215a |
2.742±0.171a |
1.931±0.444a,c |
| t1/2 (h) |
1.950±0.083 |
9.494±0.410a |
4.280±1.591a,b |
7.124±0.814a–c |
As presented in Table
IV and Fig. 5, when compared
with oral administration of osthole alone, there were significant
differences in the primary kinetic parameters (AUC0-∞,
Cmax, CL/F and t1/2) of osthole following
co-administration with extra borneol. Firstly, when osthole was
co-administered with (+)-borneol, (−)-borneol or synthetic borneol,
the AUC0-∞ of osthole was significantly enhanced by
78.167, 167.786 and 104.708%, respectively, when compared with
those in the osthole group alone. Secondly, the Cmax
value of osthole was significantly promoted by 51.769, 271.289 and
92.635%, respectively. Thirdly, the CL/F of osthole reduced by
44.174, 60.686 and 51.251%, respectively. Finally, the
t1/2 of osthole increased by 115.754, 259.125 and
378.592%, respectively. In addition, when osthole was
co-administrated with (−)-borneol or synthetic borneol, the time to
reach the Cmax was different to that of osthole
alone.
Synthetic borneol was used as a reference substance
in the present study. As presented in Table IV and Fig. 5, the key kinetic parameters
(AUC0-∞, Cmax, Vd/F and
t1/2) of osthole in the (+)- and (−)-borneol groups were
significantly different when compared with those in the synthetic
borneol group. When compared with the synthetic borneol group, the
AUC0-∞, Cmax, Vd/F and
t1/2 values of osthole in the (+)-borneol group
significantly reduced by 12.965, 21.214, 47.310 and 54.919%,
respectively. However, when compared with synthetic borneol, the
AUC0-∞ and Cmax of osthole in the (−)-borneol
group were promoted by 30.814 and 92.743%, respectively. In
addition, the Vd/F and t1/2 values decreased
by 50.977 and 24.962%, respectively and the time to reach
Cmax was also reduced.
(+)- and (−)-borneol had different effects on the
bioavailability of osthole, as osthole co-administered with
(−)-borneol was assimilated more rapidly. As presented in Table IV and Fig. 5, when compared with the (+)-borneol
group, the Cmax of osthole in the (−)-borneol group was
markedly enhanced by 144.640%, with a significantly shorter
Tmax. In addition, the CL/F of osthole in the
(−)-borneol group was greatly decreased with a rate of 29.576%,
while the AUC0-∞ value of osthole in the (−)-borneol
group enhanced by 50.301%.
Discussion
When focusing on the total pharmacokinetics trend of
osthole treatment alone or co-administration with extra borneol,
the kinetic curve could be interpreted as the fast absorption of
osthole with a rapid post-absorption phase. The post-absorption
phase can be subdivided into distribution and elimination phases,
which are depicted in Fig. 4. The
rapid absorption phase was observed from starting time to
Tmax. During this time, osthole was rapidly absorbed,
which was in agreement with the results of a previous study
(28). However, the absorption
rate increased when an oral dose was given with extra borneol; the
absorption rate was the fastest in the (−)-borneol group. The
effect of borneol on promoting the absorption of oral drugs was
widespread without a clear mechanism (29). During the distribution phase, blood
concentrations reduced slowly due to the distribution and
re-release of osthole into the tissues including the heart, liver,
spleen, lungs and kidneys, which may lead to a higher plasma
concentrations (7). In the
elimination phase, osthole was eliminated quickly by the CYP3A4
enzyme in liver (9). However, the
elimination rate decreased when osthole treatment was combined with
borneol. The inhibitory effect of borneol on the CYP3A4 enzyme may
induce this effect (23,24).
In the control group treated with osthole only, the
pharmacokinetic parameters of osthole were partially consistent
with the results observed by Zheng et al (30), in which osthole also exhibited a
low Cmax, high CL and short t1/2 following
oral administration. However, the primary kinetic parameters,
AUC0-∞ and Cmax, of osthole were enhanced
following co-administration with extra borneol, while the CL/F of
osthole decreased. This result indicated that borneol may enhance
gastrointestinal absorption of osthole and inhibit its metabolism.
The increased absorption rate led to a higher Cmax, this
phenomenon was also observed in tetramethylpyrazine phosphate
treatment with borneol (31).
Previous studies have demonstrated that osthole was rapidly
metabolized through ten phase I and three phase II metabolites in
the hepatocyte by CYP3A4 enzyme (9,32).
(+)-Borneol, (−)-borneol and isoborneol all inhibit the CYP3A4
enzyme (24). The inhibition of
CYP3A4 enzyme by borneol decreased CL/F (Fig. 4). This impaired elimination has
also been observed in salvanic acid B and tetramethylpyrazine
phosphate treatments when co-administrated with borneol (18,31).
Notably, the promoted Cmax and inhibited CL/F values all
contributed to the significantly enhanced AUC0-∞ of
osthole when combined with extra borneol.
Synthetic borneol was used as a reference substance
in the present study. Synthetic borneol was composed of
(+)-borneol, (−)-borneol and isoborneol. The proportion of (+)- and
(−)-borneol in synthetic borneol should not be <55%. According
to previous studies, (+)-borneol, (−)-borneol and isoborneol have
different effects on the CYP3A4 enzyme (33,34).
When compared with osthole combined with synthetic borneol, the
AUC0-∞, Cmax, Vd/F and
t1/2 of the (+)-borneol group were significantly
decreased, which may be attributable to the weaker inhibitory
effect on CYP3A4 enzyme and P-glycoprotein of (+)-borneol (22–24).
In addition, in the (−)-borneol group, the AUC0-t,
AUC0-∞, Cmax and t1/2 were
significantly enhanced, while CL/F was diminished, compared with
the synthetic borneol group. The enhanced Cmax may be a
result of the strong promotional effect of (−)-borneol on
absorption and the diminished CL/F may occur as a result of the
greater inhibitory effect on CYP3A4 enzyme. All of these
contributed to a higher AUC0-t and AUC0-∞ in
the (−)-borneol group when compared with that of the synthetic
borneol, which has additionally been noted in the permeation of
gardenia extract (35).
The influence of (−)-borneol on AUC0-t,
AUC0-∞, Cmax and CL/F of osthole was stronger
than that of (+)-borneol, which may be due to the faster absorption
and stronger inhibition of the CYP3A4 enzyme by (−)-borneol
(23,36). The different effects on the
absorption rate and CYP3A4 enzyme of the two borneol enantiomers
may be as a result of their different optical activities. As
presented in Fig. 1B, (+)-borneol
possesses the same chemical structure as (−)-borneol except for a
hydroxyl oriented in the opposite direction to the geminal dimethyl
bridge in chiral carbon atom. The biological activity of drugs is
greatly associated with the optical activity. Enantiomers are
considerably different in potency, pharmacological activity and
pharmacokinetic profile, as the molecules with which they interact
in biological systems are additionally optically active (37,38).
The different pharmacological activity between (+)-borneol and
(−)-borneol are also observed when interacting with the
γ-aminobutyric acid receptor (15).
In conclusion, to the best of our knowledge, this is
the first study to demonstrate the enhanced effect of borneol on
the blood concentration and bioavailability of osthole following
oral administration in rats. In addition, there were significant
differences between the borneol enantiomers when interacting with
osthole, with (−)-borneol having a stronger promotional effect on
the pharmacokinetic parameters of osthole.
Acknowledgements
The present study was supported by grants from the
Hong Kong, Macao and Taiwan Science & Technology Cooperation
Program of China (grant no. 2014DFH30010), the Guangdong
International Cooperation Project (grant no. 2013508102016), the
Science and Technology Planning Project of Guangdong Province,
China (grant nos. 2014A020221050, 2013B090600007, 2013B090600026
and 2012B090600007), the National Natural Science Foundation of
China (grant no. 81503318), Guangzhou University of Chinese
Medicine Youth Elite Project (QNYC20170106) and Guangdong Province
Universities and Colleges Pearl River Scholar Funded Scheme
(2011).
References
|
1
|
Liao PC, Chien SC, Ho CL, Wang EI, Lee SC,
Kuo YH, Jeyashoke N, Chen J, Dong WC, Chao LK and Hua KF: Osthole
regulates inflammatory mediator expression through modulating
NF-κB, mitogen-activated protein kinases, protein kinase C, and
reactive oxygen species. J Agr Food Chem. 58:10445–10451. 2010.
View Article : Google Scholar
|
|
2
|
Chen T, Liu W, Chao X, Qu Y, Zhang L, Luo
P, Xie K, Huo J and Fei Z: Neuroprotective effect of osthole
against oxygen and glucose deprivation in rat cortical neurons:
Involvement of mitogen-activated protein kinase pathway.
Neuroscience. 183:203–211. 2011. View Article : Google Scholar
|
|
3
|
Zhang Q, Qin L, He W, Van Puyvelde L, Maes
D, Adams A, Zheng H and De Kimpe N: Coumarins from Cnidium monnieri
and their antiosteoporotic activity. Planta Med. 73:13–19. 2007.
View Article : Google Scholar
|
|
4
|
Lin YC, Lin JC, Hung CM, Chen Y, Liu LC,
Chang TC, Kao JY, Ho CT and Way TD: Osthole inhibits insulin-like
growth factor-1-induced epithelial to mesenchymal transition via
the inhibition of PI3K/Akt signaling pathway in human brain cancer
cells. J Agr Food Chem. 62:5061–5071. 2014. View Article : Google Scholar
|
|
5
|
Lee WH, Lin RJ, Lin SY, Chen YC, Lin HM
and Liang YC: Osthole enhances glucose uptake through activation of
AMP-activated protein kinase in skeletal muscle cells. J Agr Food
Chem. 59:12874–12881. 2011. View Article : Google Scholar
|
|
6
|
Chao XD, Zhou J, Chen T, Liu W, Dong W, Qu
Y, Jiang X, Ji X, Zhen H and Fei Z: Neuroprotective effect of
osthole against acute ischemic stroke on middle cerebral ischemia
occlusion in rats. Brain Res. 1363:206–211. 2010. View Article : Google Scholar
|
|
7
|
Shi JF, Chen Q, Yang W, Yang HP, Liu J,
Wang XM and He X: Comparative study of pharmacokinetics and tissue
distribution of osthole in rats after oral administration of pure
osthole and Libanotis buchtormensis supercritical extract. J
Ethnopharmacol. 145:25–31. 2013. View Article : Google Scholar
|
|
8
|
Zhao G, Peng C, Du W and Wang S:
Pharmacokinetic study of eight coumarins of Radix Angelicae
Dahuricae in rats by gas chromatography-mass spectrometry.
Fitoterapia. 89:250–256. 2013. View Article : Google Scholar
|
|
9
|
Zhang LF, Hu X, Wang P and Zhang L:
Metabolism of osthol in isolated hepatocytes of rat. Yao Xue Xue
Bao. 44:1131–1135. 2009.(In Chinese).
|
|
10
|
Ambudkar SV, Sarfaty C Kimchi, Sauna ZE
and Gottesman MM: P-glycoprotein: From genomics to mechanism.
Oncogene. 22:7468–7485. 2003. View Article : Google Scholar
|
|
11
|
Hassanpouraghdam MB, Hassani A, Vojodi L,
Asl B Hajisamadi and Rostami A: Essential oil constituents of
Lavandula officinalisChaix. from Northwest Iran. J Agr Food Chem.
22:167–171. 2011.
|
|
12
|
Angioni A, Barra A, Cereti E, Barile D,
Coïsson JD, Arlorio M, Dessi S, Coroneo V and Cabras P: Chemical
composition, plant genetic differences, antimicrobial and
antifungal activity investigation of the essential oil of
Rosmarinus officinalis L. J Agr Food Chem. 52:3530–3535. 2004.
View Article : Google Scholar
|
|
13
|
Nezhadali A, Navavi M and Rajabian M:
Chemical composition of the essential oil of Thymus vulgaris L.
from Iran. J Essent Oil Bear Pl. 15:368–372. 2012. View Article : Google Scholar
|
|
14
|
Harish R, Divakar S, Srivastava A and
Shivanandappa T: Isolation of antioxidant compounds from the
methanolic extract of the roots of Decalepis hamiltonii (Wight and
Arn.). J Agric Food Chem. 53:7709–7714. 2005. View Article : Google Scholar
|
|
15
|
Granger RE, Campbell EL and Johnston GA:
(+)- And (−)-borneol: Efficacious positive modulators of GABA
action at human recombinant alpha1beta2gamma2L GABA(A) receptors.
Biochem pharmacol. 69:1101–1111. 2005. View Article : Google Scholar
|
|
16
|
Mihara S and Shibamoto T: The role of
flavor and fragrance chemicals in TRPA1 (transient receptor
potential cation channel, member A1) activity associated with
allergies. Allergy Asthma Clin Immunol. 11:112015. View Article : Google Scholar :
|
|
17
|
Lai XJ, Zhang L, Li JS, Liu HQ, Liu XH, Di
LQ, Cai BC and Chen LH: Comparative pharmacokinetic and
bioavailability studies of three salvianolic acids after the
administration of Salviae miltiorrhizae alone or with synthetical
borneol in rats. Fitoterapia. 82:883–888. 2011. View Article : Google Scholar
|
|
18
|
Ren-Zhong W, Yan-Yan X, Yan-Ping L,
Mao-Jin Z and Chang-Xiao L: Enhancing effects of different dosages
of borneol on pharmacokinetics of salvanic acid B after oral
administration to rats. J Asian Nat Prod Res. 14:538–544. 2012.
View Article : Google Scholar
|
|
19
|
Zhou Y, Li W, Chen L, Ma S, Ping L and
Yang Z: Enhancement of intestinal absorption of akebia saponin D by
borneol and probenecid in situ and in vitro. Environ Toxicol
Pharmacol. 29:229–234. 2010. View Article : Google Scholar
|
|
20
|
Li Z, Sun D, Yang H, Liu X, Luan L, Bai J
and Cui H: Effect of borneol on the distribution of danshensu to
the eye in rabbit via oral administration. Curr Eye Res.
35:565–572. 2010. View Article : Google Scholar
|
|
21
|
Lu Y, Chen X, Du S, Wu Q, Yao Z and Zhai
Y: The in situ and in vivo study on enhancing effect of borneol in
nasal absorption of geniposide in rats. Arch Pharm Res. 33:691–696.
2010. View Article : Google Scholar
|
|
22
|
He H, Shen Q and Li J: Effects of borneol
on the intestinal transport and absorption of two P-glycoprotein
substrates in rats. Arch Pharm Res. 34:1161–1170. 2011. View Article : Google Scholar
|
|
23
|
Jinno N, Tagashira M, Tsurui K and Yamada
S: Contribution of cytochrome P450 and UDT-glucuronosyltransferase
to the metabolism of drugs containing carboxylic acid groups: Risk
assessment of acylglucuronides using human hepatocytes.
Xenobiotica. 44:677–686. 2014. View Article : Google Scholar
|
|
24
|
Seo KA, Kim H, Ku HY, Ahn HJ, Park SJ, Bae
SK, Shin JG and Liu KH: The monoterpenoids citral and geraniol are
moderate inhibitors of CYP2B6 hydroxylase activity. Chem Biol
Interact. 174:141–146. 2008. View Article : Google Scholar
|
|
25
|
Dai DZ, Du GM, Wang J, Jiang JJ, Chen ZM
and Zhang ZC: Comparison of penetration enhancer on skin of azone
and borneolum to osthole. J Jinling Institute Technol. 24:90–93.
2008.
|
|
26
|
Hu DH, Wang YG, Chen ZW, MA ZC, Liang QD,
Xiao CY, Tan HL, Tang XL, Li H, Shen GL, et al: Effect of compound
Danshen Dripping Pills on rat hepatic cytochrome P450. Chin J
Pharmacol Toxicol. 27:678–684. 2013.
|
|
27
|
Cai Z, Hou S, Li Y, Zhao B, Yang Z, Xu S
and Pu J: Effect of borneol on the distribution of gastrodin to the
brain in mice via oral administration. J Drug Target. 16:178–184.
2008. View Article : Google Scholar
|
|
28
|
Yun F, Kang A, Shan J, Zhao X, Bi X and Di
L: A rapid and sensitive LC-MS/MS method for the determination of
osthole in rat plasma: Application to pharmacokinetic study. Biomed
Chromatogr. 27:676–680. 2013. View Article : Google Scholar
|
|
29
|
Zhang HY, W KW, Qian L, et al: Effect of
borneol on promoting absorption of oral drugs. Chin J Exp Tradit
Med Formulae. 18:294–297. 2012.(In Chinese).
|
|
30
|
Zheng LQ, Z DS and Liu JH: The study on
the distribution in tissue of osthole in rats by the RP-HPLC. Chin
Pharm J. 11:1666–1668. 2006.(In Chinese).
|
|
31
|
Yan-Yu X, Qi-Neng P and Zhi-Peng C: The
enhancing effect of synthetical borneol on the absorption of
tetramethylpyrazine phosphate in mouse. Int J Pharm. 337:74–79.
2007. View Article : Google Scholar
|
|
32
|
Lv X, Wang CY, Hou J, Zhang BJ, Deng S,
Tian Y, Huang SS, Zhang HL, Shu XH, Zhen YH, et al: Isolation and
identification of metabolites of osthole in rats. Xenobiotica.
42:1120–1127. 2012. View Article : Google Scholar
|
|
33
|
Kim H, Kim KB, Ku HY, Park SJ, Choi H,
Moon JK, Park BS, Kim JH, Yea SS, Lee CH, et al: Identification and
characterization of potent CYP2B6 inhibitors in
woohwangcheongsimwon suspension, an herbal preparation used in the
treatment and prevention of apoplexy in Korea and China. Drug Metab
Dispos. 36:1010–1015. 2008. View Article : Google Scholar
|
|
34
|
Yu B, Ruan M, Dong X, Yu Y and Cheng H:
The mechanism of the opening of the blood-brain barrier by borneol:
A pharmacodynamics and pharmacokinetics combination study. J
Ethnopharmacol. 150:1096–1108. 2013. View Article : Google Scholar
|
|
35
|
Lu Y, Du S, Yao Z, Zhao P and Zhai Y:
Study on natural borneol and synthetic borneol affecting mucosal
permeability of gardenia extract. Zhongguo Zhong Yao Za Zhi.
34:1207–1210. 2009.(In Chinese).
|
|
36
|
Zhou JW: The influence and mechanism of
borneol, synthetic borneol and menthol on the P-glycoprotein. PhD
dissertationNanjing Normal University. China Knowledge Resource
Integrated Database 6:2011.(In Chinese).
|
|
37
|
Caldwell J: Do single enantiomers have
something special to offer? Hum Psychopharm Clin. 16:S67–S71. 2001.
View Article : Google Scholar
|
|
38
|
Islam MR, Mahdi JG and Bowen ID:
Pharmacological importance of stereochemical resolution of
enantiomeric drugs. Drug Saf. 17:149–165. 1997. View Article : Google Scholar
|