Introduction
Bone is a basic component of the musculoskeletal
system and is constantly remodeled by the function of osteoblasts
and osteoclasts (1–3). This bone remodeling is maintained in
men throughout life, whereas following menopause, women exhibit
accelerated bone loss due to a decrease in estrogen levels, leading
to osteoporosis (4). Osteoporosis
is a serious social and health problem in a progressively aging
society, and the associated increases in medical expenditure have
become a serious problem in developed and developing countries
(5). Various epidemiological
studies have demonstrated that the intake of various vitamins and
phytoestrogens can act to support the enrichment of bone mineral
density (BMD) (6). Therefore,
utilization of food-based nutrients is considered to be the most
economical and simple way to prevent bone loss. For example,
genistein, which has a similar structure to estrogen, has been
demonstrated to aid in maintaining BMD (6–9).
Furthermore, the simultaneous intake of genistein and menaquinone-7
(MK-7), a major form of vitamin K2, is considered to be
more effective for the maintenance of BMD (10–12).
However, MK-7 is only present in fermented foods. For example, the
traditional Japanese food, Nattō, a type of fermented soybean, is
considered to be beneficial; however, due to its peculiar smell,
its intake is not always common, even in Japan (10), and non-Japanese experience
difficulty with its intake.
In addition to the extremely high concentration of
MK-7 in Nattō (13), another major
form of vitamin K2, menaquinone-4 (MK-4) is found in
meat, eggs and dairy foods; however, its concentration is often low
(14,15). In addition, the bioavailability of
MK-4 is unclear (16).
In the present study, the effects of genistein
and/or MK-4 at dietary obtainable concentrations on the level of
mRNAs and their protein products in MC3T3-E1 cells derived from
neonatal mouse calvaria were evaluated.
Materials and methods
Reagents
Fetal bovine serum (FBS), and phenol red-free
Eagle's minimal essential medium, α-modification (α-MEM), were
obtained from Thermo Fisher Scientific, Inc. (Osaka, Japan).
Genistein, 17-β-estradiol and MK-4 were purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany), and dissolved in
ethanol (Nacalai Tesque, Inc., Kyoto, Japan).
MC3T3-E1 cell culture, treatment and
RNA preparation
Osteoblastic MC3T3-E1 cells derived from the
calvaria of a newborn C57BL/6 mouse were obtained from the Riken
Cell Bank (Tokyo, Japan) and used at passages 3–5. The cells were
maintained in α-MEM containing 10% (v/v) FBS. All cells were plated
at a density of 1×105 cells in 10-cm culture dishes, and
incubated at 37°C in a humidified atmosphere containing 5%
CO2. When subconfluent, the cells were subcultured into
35-mm dishes at a density of 1×105 cells and then
cultured with the media containing 0.1% ethanol (control), 0.1 µM
17-β-estradiol, 1 µM genistein, 1 µM MK-4, 17-β-estradiol +
genistein (0.1 µM + 1 µM), 17-β-estradiol + MK-4 (0.1 µM + 1 µM) or
genistein + MK-4 (1 µM + 1 µM) for 24, 48 or 96 h. The total RNA
was extracted from 24 and 48 h cell cultures using ISOGEN reagent
(Nippon Gene Co., Ltd., Tokyo, Japan). All total RNA samples were
quality checked by RNA Pico Chips using an Agilent 2100 Bioanalyzer
(Agilent Technologies, Inc., Santa Clara, CA, USA), and treated
with RNase-free DNase I recombinant (Roche Diagnostics GmbH,
Mannheim, Germany) to isolate DNA-free RNA.
Identification of mRNA species
upregulated by genistein
From each 24 h culture with or without 1 µM
genistein, 1 mg total RNA was obtained. Oligo-dT (Sigma-Aldrich;
Merck KGaA) purification was performed as previously described
(17). Following this, polyA + RNA
was compared using a mouse GE microarray version 2.0 (Agilent
Technologies, Inc.). Among the mRNAs that were observed to increase
by >3 times following treatment with genistein, 19 mRNAs with
known functions (Table I) were
subjected to reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) in order to confirm the effects of
17-β-estradiol, genistein, MK-4, 17-β-estradiol + MK-4 and
genistein + MK-4 at 24 and 48 h.
 | Table I.mRNAs with expression increased >3
fold in the microarray assay following 24 h treatment with 1 µM
genistein were subjected to reverse transcription-quantitative
polymerase chain reaction to validate the expression level
change. |
Table I.
mRNAs with expression increased >3
fold in the microarray assay following 24 h treatment with 1 µM
genistein were subjected to reverse transcription-quantitative
polymerase chain reaction to validate the expression level
change.
|
|
| Primer sequence |
|---|
|
|
|
|
|---|
| Accession no. | Gene name (genetic
symbol) | Forward | Reverse |
|---|
| NM_007432 | Alkaline phosphatase
3, intestine, not Mn requiring (ALP3) |
agaagctgcaataccacaac |
atttggttgctgttggaact |
|
|
| NM_015804 | ATPase, class VI,
type 11A (ATP11A) |
gacttgtgggtgtgtcgatg |
ggaagagaactgggtggaca |
| NM_007541 | Bone γ
carboxyglutamate protein (BGLAP) |
gggcagagagagaggacagg |
acctgtgctgccctaaagc |
| NM_007557 | Bone morphogenetic
protein 7 (BMP7) |
atggtggtatcgagggtgga |
tctcctacccctacaaggcc |
| NM_013878 | Calcium binding
protein 2 (CABP2) |
ggacccatcagctccacaaa |
ccaggagtttgaccgagacc |
| NM_007689 | Chondroadherin
(CHAD) |
tggataatgggagggaacg |
aaatccccgaccaagaggt |
| NM_010074 | Dipeptidylpeptidase 4
(DPP4) |
gttctggggacaggcatc |
ccagcacatctattcccaca |
| NM_015744 | Ectonucleotide
pyrophosphatase/phosphodiesterase 2 (ENPP2) |
gcaggtatgtcttgagggtca |
ctcgggtgagggacatcg |
|
|
| NM_010258 | GATA binding protein
6 (GATA6) |
gcatttttgctgccatctg |
aaccccgagaacagtgacc |
| NM_008398 | Integrin α 7
(ITGA7) |
cagatcgcatggcactttcg |
atcctcagcacctctgggat |
| M21041 |
Microtubule-associated protein 2
(MAP2) |
accaggatgccagatttggg |
accttcctccatcctccctc |
| NM_144557 | Myosin VIIA and Rab
interacting protein (MYRIP) |
ttcttcaggaccttggcact |
agagctgctgctcctaccag |
| BC059256 | Notch gene homolog 2
(NOTCH2) |
agagagcgagggaagatgga |
gagcacccatacctgacacc |
| NM_172766 | Nuclear factor
related to κ B binding protein (NFRKB) |
cccttggagaccctgctaag |
aaagcctcctggtcccttg |
| NM_008873 | Plasminogen
activator, urokinase (PLAU) |
gtgttggcctttcctcggta |
gtggcagtgtacttggagct |
| NM_173413 | RAB8B, member RAS
oncogene family (RAB8B), mRNA |
ccacaggaatgaacgacca |
gagagcaacgagcatttgtttt |
| NM_009446 | Tubulin, α 3
(TUBA3) |
cgtggtattgctcagcatgc |
agtttgccatctacccagcc |
| NM_009521 | Wingless-related MMTV
integration site 3A (WNT3A) |
ggtgtttctccaccaccatc |
cgctcagctatgaacaagca |
| NM_009524 | Wingless-related MMTV
integration site 5A (WNT5A) |
ccaaacagctgcaacacctc |
cagaaccagccacttagggg |
| NM_007431 | Alkaline
phosphatase, liver/bone/kidney (ALPL) |
ctacgcaccctgttctgagg |
ggcgtccatgagcagaacta |
Quantification of mRNAs using
RT-qPCR
From ~40 ng of total RNA, first-strand cDNA was
synthesized using ReverTra Ace® reverse transcriptase
(Toyobo, Co., Ltd., Osaka, Japan) with 5 pmol oligo-dT primers in a
20 µl reaction mixture, as indicated in the manufacturer's
instructions, at 50°C for 1.5 h. RT-qPCR was performed using the
Stratagene Mx3000P qPCR System (Agilent Technologies, Inc.). The
reaction mixture consisted of a final volume of 20 µl containing 1
µl of cDNA sample, 5 pmol of a set of gene-specific primers
designed using Primer-3-Plus (http://primer3plus.com; Table I), and 10 µl of Brilliant III
Ultra-Fast SYBR Green QPCR Master Mix (Agilent Technologies, Inc.).
The cycling conditions included a denaturing step at 95°C for 2
min, followed by 60 cycles at 95°C for 5 sec and 60°C for 20 sec.
The number of cycles of amplification required to reach the
threshold (quantification cycle; Cq) were obtained using an
amplification plot and the threshold line automatically reported.
To determine the number of copies of the targeted mRNAs in the
samples, the Cq values of the genes were normalized against that of
β-actin using the 2−ΔΔCq method (18). RT-qPCR products were run on 1%
agarose gels and were confirmed as a single band.
Immunocytochemistry of chondroadherin
(CHAD), dipeptidylpeptidase 4 (DPP4/CD26) and alkaline phosphatase
(ALP)
The protein expression levels of CHAD and DPP4 were
immunocytochemically examined, with anti-ALP used as an MC3T3-E1
cell marker. The cells were seeded into BD Bio-Coat Collagen IV
Cellware plates (Cosmo Bio Co., Ltd., Tokyo, Japan) at a density of
1×105 cells/well, and incubated with 17-β-estradiol,
genistein, MK-4, 17-β-estradiol + MK-4 or genistein + MK-4 for 48
or 96 h. The cells were fixed with 4% (w/v) paraformaldehyde in TBS
for 30 min at room temperature. Subsequently, the cells were washed
three times with 200 µl TBS and incubated in blocking solution
containing 1% bovine serum albumin (Sigma-Aldrich; Merck KGaA) and
0.1% Tween-20 in TBS for 1 h at room temperature. Following a
further wash with TBS, the fixed cells were incubated overnight at
37°C with a goat polyclonal anti-ALP antibody (5 µg/ml; cat. no.
AF2910; R&D Systems, Inc., Minneapolis, MN, USA), rabbit
polyclonal anti-CHAD antibody (dilution, 1:500; cat. no.
NBP1-87031, Novus Biologicals, LLC, Littleton, CO, USA) and a
rabbit polyclonal anti-DPP4 antibody (dilution, 1:500; cat. no.
sc-9153, Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The
cells in each well were then washed three times with TBS, and
incubated with fluorescein isothiocyanate-conjugated anti-goat and
tetramethylrhodamine-conjugated anti-rabbit secondary antibodies
(dilution, 1:800; cat. nos. T6778 and F7367, respectively;
Sigma-Aldrich; Merck KGaA) for 1 h at room temperature. The cells
were incubated with DAPI (Cell Signaling Technology, Inc., Danvers,
MA, USA) for nuclear staining and were viewed under a BZ-8000
All-in-One Fluorescence Microscope (Keyence Corporation, Osaka,
Japan).
Measurement of ALP activity
The activity of ALP was measured in 1×105
MC3T3-E1 cells in 12-well culture plates after 96 h of treatment.
The cells were fixed with 4% (w/v) paraformaldehyde in TBS for 30
min at room temperature and washed three times in TBS. The activity
of ALP was measured by adding 0.25 mg/ml naphthol AS-BI phosphate
(Sigma-Aldrich; Merck KGaA) for 1 h. Following a wash with TBS, ALP
activity levels were measured via image capture using a Canon IXY
50S camera (Canon Inc., Tokyo, Japan) and intensity measurement
using Adobe Photoshop version-20160113.r.355 ×64 (Adobe Systems,
Inc., San Jose, CA, USA).
Statistical analysis
All values are presented as the mean ± standard
deviation. In order to compare the differences between the control
and treatment groups, all groups were compared using one-way
analysis of variance with Tukey's honest significant difference
applied as a post hoc test (19).
Statistically significant differences are indicated in each table.
Analyses were performed using SPSS software (version 19; IBM SPSS,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
The half-maximal inhibitory concentration of
genistein against 17-β-estradiol is reportedly 145 nM for estrogen
receptor α and 8.4 nM for estrogen receptor β, and the two
receptors are almost completely occupied by genistein at
concentrations of 1–10 µM (20).
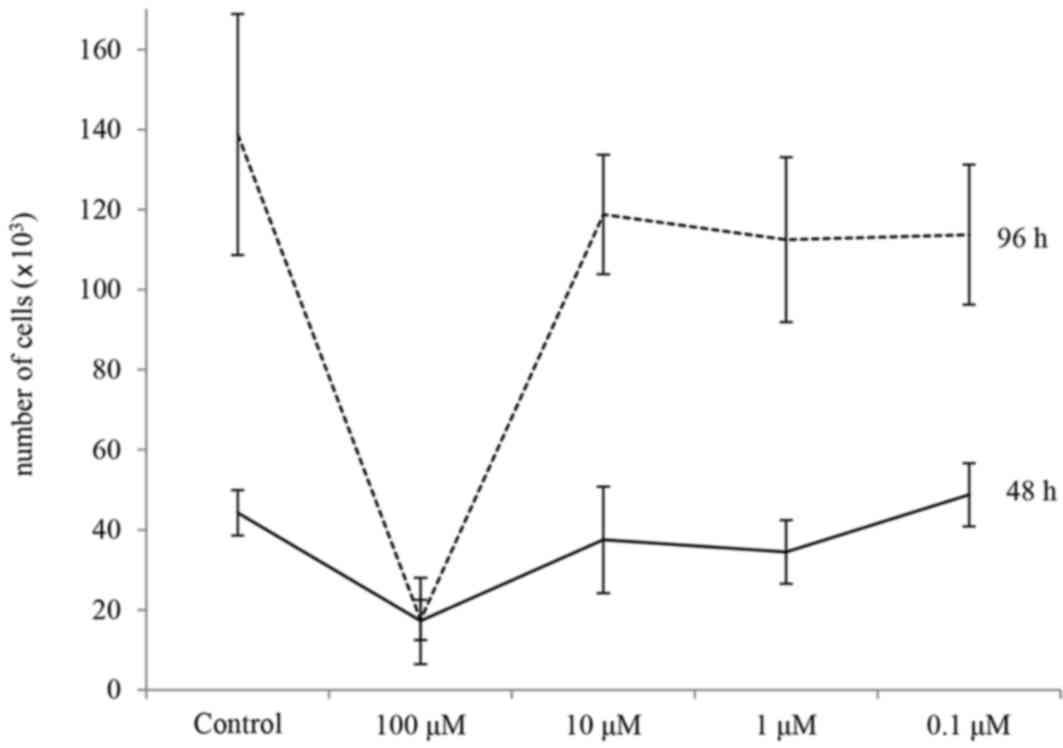
Thus, the effects of 0.1–100 µM of genistein on MC3T3-E1 cell
growth were examined (Fig. 1).
This revealed that 100 µM genistein inhibited cell growth at 24 and
98 h. Thus, in order to select mRNAs that may be involved in
modifying osteoblastic function, mRNAs from cells treated with and
without 1 µM of genistein were compared using microarray analysis
at 24 h. Among the mRNAs from genistein-treated cells, almost
13,000 spots were revealed to exhibit a >3 fold higher signal
compared with mRNAs from cells not treated with genistein. Of
these, the mRNAs without any known function were removed, and those
potentially affecting osteoblastic function (Table I) were selected and subjected to
RT-qPCR in order to confirm their level of expression in cultures
treated with 17-β-estradiol, genistein, MK-4, 17-β-estradiol + MK-4
or genistein + MK-4 for 24 or 48 h.
Among the identified factors, no significant
difference was observed for Wnt family member 3A (WNT3A; Table II). At 24 h, all treated groups
tended to exhibit increased GATA-binding protein 6 (GATA6) mRNA
levels compared with the control; however, these differences were
not significant. This effect was also observed at 48 h, and the
GATA6 level was significantly increased genistein-treated cells
compared with the other treatment groups at 48 h. At 48 h, the
levels of NOTCH2 and WNT5A in the genistein treatment groups tended
to be higher compared with those of other groups, without
statistical significance. NOTCH2 in the MK-4 treatment group was
significantly lower than that in the genistein treatment group
(P=0.0475).
 | Table II.Effects of 17-β-estradiol, genistein
and/or MK-4 on mRNA levels of GATA6, NOTCH2 and WNT5A. |
Table II.
Effects of 17-β-estradiol, genistein
and/or MK-4 on mRNA levels of GATA6, NOTCH2 and WNT5A.
|
| GATA6 | NOTCH2 | WNT5A |
|---|
|
|
|
|
|
|---|
| Treatment | Expression |
P-valuea | Expression |
P-valuea | Expression |
P-valuea |
|---|
| 24 h |
|
|
|
|
|
|
|
Control | 0.01±0.01 | 0.0018 | 0.12±0.14 | 0.049 | 0.33±0.46 | 0.0489 |
|
17-β-estradiol | 10.92±6.05 |
| 2.45±1.40 |
| 3.37±5.10 |
|
|
Genistein | 1.53±2.83 | 0.0018 | 0.54±0.79 |
| 0.60±1.11 |
|
|
MK-4 | 3.01±3.08 | 0.0066 | 0.04±0.10 | 0.0281 | 1.44±2.15 |
|
|
17-β-estradiol+MK-4 | 12.60±29.74 |
| 0.45±0.26 |
| 0.27±0.36 | 0.0469 |
|
Genistein+MK-4 | 11.26±8.15 |
| 0.67±0.84 |
| 3.06±5.49 |
|
| 48 h |
|
|
|
|
|
|
|
Control | 0.75±0.73 | 0.0012 | 1.64±1.32 |
| 2.56±2.38 |
|
|
17-β-estradiol | 5.36±6.67 | 0.0097 | 3.09±4.66 |
| 6.94±9.09 |
|
|
Genistein | 34.20±27.60 |
| 5.58±6.61 |
| 13.16±17.21 |
|
|
MK-4 | 4.18±3.35 | 0.0058 | 0.33±0.38 | 0.0475 | 3.58±3.29 |
|
|
17-β-estradiol+MK-4 | 3.11±2.38 | 0.0037 | 2.01±1.24 |
| 3.71±3.15 |
|
|
Genistein+MK-4 | 2.14±1.82 | 0.0024 | 3.06±3.30 |
| 5.68±4.38 |
The levels of bone γ-carboxyglutamate (BGLAP) mRNA
in all treatment groups, with the exception of genistein at 24 h,
were increased compared with the control group, whether significant
or not; the increase following genistein treatment at 48 h was
statistically significant (Table
III). 17-β-estradiol appeared to increase bone morphogenetic
protein 7 (BMP7) mRNA at 48 h, although this difference was not
significant compared with the 48 h control group. The levels of
CHAD and DPP4 in all of the treatment groups at 48 h were higher
compared with those of the control; however, a significant
difference was observed only in the genistein treatment group.
Similarly, the levels of ectonucleotide
pyrophosphatase/phosphodiesterase 2 (ENPP2) mRNA tended to be
increased following the treatments, however a significant
difference was obtained only in the genistein treatment group at 48
h. A significant increase in ATPase phospholipid-transporting 11A
(ATP11A) mRNA was observed following treatment with genistein +
MK-4 for 48 h compared with the other groups. Differences in the
expression levels of the following mRNAs were not observed between
the treatment and control groups: Calcium-binding protein 2
(CABP2); nuclear factor related to κB-binding protein (NFRKB);
integrin subunit α 7 (ITGA7); microtubule-associated protein 2
(MAP2); plasminogen-activator, urokinase (PLAU); RAB8B, member RAS
oncogene family; and tubulin α 3 (TUBA3).
 | Table III.Effects of 17-β-estradiol, genistein
and/or MK-4 on genes potentially associated with osteoblast
function. |
Table III.
Effects of 17-β-estradiol, genistein
and/or MK-4 on genes potentially associated with osteoblast
function.
|
| BGLAP | BMP7 | CHAD | DPP4 | ENPP2 | ALP3 | ATP11A |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Treatment | Expression |
P-valuea | Expression |
P-valueb | Expression |
P-valuea | Expression |
P-valuea | Expression |
P-valuea | Expression |
P-valuea | Expression |
P-valuec |
|---|
| 24 h |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Control | 6.62±2.50 | 0.0021 | 0.02±0.02 | 0.0623 | 0.22±0.20 | 0.0037 | 0.46±0.37 | 0.0147 | 1.98±0.36 | 0.0066 | 0.26±0.17 | 0.0001 | 0.01±0.01 | <0.0001 |
|
17-β- | 71.34±55.19 |
| 0.25±0.32 |
| 2.98±1.20 |
| 9.23±8.42 |
| 16.26±11.69 | 0.0448 | 3.18±2.34 | 0.026 | 0.02±0.03 | <0.0001 |
|
estradiol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Genistein | 5.67±3.00 | 0.002 | 0.03±0.04 | 0.0454 | 0.20±0.19 | 0.0016 | 0.79±0.91 | 0.009 | 1.96±0.40 | 0.0066 | 0.25±0.18 | 0.0001 | 0.01±0.02 | <0.0001 |
|
MK-4 | 52.57±35.58 | 0.0304 | 0.30±0.51 |
| 2.70±2.41 | 0.1167 | 7.77±8.33 | 0.0385 | 9.58±5.62 | 0.019 | 2.08±1.95 | 0.0028 | 0.01±0.02 | <0.0001 |
|
17-β- | 84.84±190.21 |
| 0.02±0.03 | 0.0379 | 0.19±0.18 | 0.0101 | 0.17±0.13 |
| 45.99±106.03 |
| 0.38±0.31 | 0.0001 | 0.20±0.42 | 0.0003 |
|
estradiol+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MK-4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Genistein+ | 78.00±53.11 |
| 0.14±0.21 |
| 3.15±4.12 | 0.0419 | 10.28±13.17 |
| 25.47±17.67 |
| 3.19±2.59 | 0.0158 | 0.11±0.15 | <0.0001 |
|
MK-4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 48 h |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Control | 29.56±14.22 | 0.0085 | 0.17±0.22 |
| 0.34±0.58 | 0.0118 | 2.56±1.25 | 0.0223 | 7.16±1.70 | 0.0137 | 1.04±0.62 | 0.0005 | 0.16±0.16 | 0.0001 |
|
17-β- | 108.22±66.35 |
| 0.72±0.86 |
| 0.98±0.09 | 0.0232 | 9.64±12.96 |
| 17.18±10.33 |
| 3.93±3.32 | 0.0445 | 0.02±0.02 | <0.0001 |
|
estradiol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Genistein | 243.69±226.79 |
| 0.20±0.27 |
| 10.80±10.09 |
| 56.22±75.94 |
| 91.57±72.08 |
| 10.84±10.40 |
| 0.04±0.07 | <0.0001 |
|
MK-4 | 77.27±38.57 |
| 0.25±0.35 |
| 1.40±1.39 | 0.0045 | 3.73±3.99 | 0.0169 | 19.88±7.95 |
| 2.23±1.39 | 0.0035 | 0.29±0.28 | 0.002 |
|
17-β- | 56.23±26.86 | 0.0368 | 0.09±0.10 |
| 1.64±0.89 | 0.0105 | 3.83±1.41 | 0.0285 | 11.99±6.44 | 0.0261 | 2.26±1.28 | 0.0037 | 0.20±0.09 | 0.0003 |
|
estradiol+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MK-4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Genistein+ | 56.21±30.18 | 0.0368 | 0.09±0.10 |
| 1.24±0.64 | 0.0037 | 6.00±5.57 | 0.027 | 24.42±10.26 |
| 3.61±2.07 | 0.0287 | 0.94±0.66 |
|
MK-4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Alterations in CHAD and DPP4 mRNA levels were
similar to each other (Table
III). As these encode cell membrane-bound proteins that are
suitable for quantitative analysis, the changes in their protein
products were examined immunocytochemically (Fig. 2). At 48 h, an effect of
17-β-estradiol on CHAD or DPP4 was not always apparent. Genistein
treatment induced the appearance of small fluorescent foci for CHAD
and DPP4 along with alterations in cell morphology into oval or
spindle-shape following 48 h treatment; such an effect was not
always observed following MK-4 treatment. However, treatment with
17-β-estradiol + MK-4 induced the appearance of granular and
diffuse CHAD and DPP4 staining, which were also apparent in the
genistein + MK-4 group. After 96 h, alterations in the cell shape
were marked in the MK-4 treatment cultures, which exhibited high
intensities of CHAD and DPP4 staining. The small foci had
disappeared, and larger spots along with diffuse staining appeared.
These alterations were the most marked in Genistein + MK-4-treated
cells.
 | Figure 2.Immunocytochemical analysis of CHAD
and DPP4 proteins in control cells and cells treated with
17-β-estradiol, genistein, MK-4, 17-β-estradiol + MK-4 or genistein
+ MK-4 for 48 and 96 h. Left panel, anti-CHAD or anti-DPP4
staining; center panel, anti-ALP staining; and right panel, merge
with DAPI staining. Scale bar, 100 µm. MK-4, menaquinone-4; CHAD,
chondroadherin; DPP4, pipeptidylpeptidase 4; ALP, alkaline
phosphatase. |
A significant increase in ALP activity was observed
only in the 96 h genistein + MK-4 treatment group compared with the
other groups (Table IV). ALP3
mRNA was significantly increased in the 48 h genistein treatment
compared with all other treatment and control groups (Table III), whereas alkaline phosphatase
liver/bone/kidney mRNA exhibited no differences in expression in
any of the treatment groups (data not shown).
 | Table IV.ALP activities at 96 h. |
Table IV.
ALP activities at 96 h.
| Treatment | ALP activity |
P-valuea |
|---|
| Control | 44367±6992 | 0.0029 |
| 17β-estradiol | 48205±8507 | 0.0175 |
| Genistein | 42629±1646 | 0.0013 |
| MK-4 | 41318±1379 | 0.0007 |
|
17β-estradiol+MK-4 | 43170±2215 | 0.0017 |
| Genistein+MK-4 | 62844±4095 |
|
Discussion
Among the Japanese population, the daily intake of
genistein is reported to be ~12 mg (50 µmol) from 80 g of soy
products (21), and people in
eastern Japan typically consume 50 g of Nattō each day, supplying
3–5 mg of MK-7 (~6 µmol) simultaneously (22). Pre-menopause, Nattō intake is
useful to promote bone formation (11). By contrast, MK-4 is contained in
daily foods like meat, eggs and dairy foods, and can be consumed
daily, but its concentrations are low at 1–10 nmol/100 g, thus,
MK-4 intake is very limited (15).
In the present study, in order to elucidate the utility of MK-4,
mRNAs that exhibited altered levels in response to genistein
treatment were identified initially in MC3T3-E1 cells using a
microarray. However, microarrays are not always useful for
quantitative analysis. Therefore, to validate the effect of
genistein and/or MK-4 on selected mRNAs, in comparison with the
effects of 17-β-estradiol, RT-qPCR was also performed.
Although the mRNAs were selected because genistein
increased their levels by >3 fold compared with the control at
24 h, this effect could not be confirmed in certain mRNAs.
Genistein is a phytoestrogens that exerts similar effects to
17-β-estradiol, although these effects are not always identical.
For example, at 48 h, a significant increase in GATA6 mRNA was
observed following genistein treatment, but not following
17-β-estradiol treatment. However, the 17-β-estradiol + MK-4 and
genistein + MK-4 maintained the expression level of GATA6 at a
level consistent with that of the control group. As GATA6 is
reported to suppress bone differentiation (23), the administration of estrogenic
substances together with MK-4 appears to be beneficial for bone
formation.
Osteoclastogenesis and bone resorption are inhibited
by NOTCH1 and enhanced by NOTCH2 (24). In mouse embryonic stem cells,
recombinant WNT5A has been reported to significantly enhance
osteogenic yield, while recombinant WNT3A or other positive
regulators of β-catenin decrease the expression of osteogenic
markers (25). However, WNT5A is
usually considered to promote osteoclast differentiation and
prevent adipocyte differentiation (26). Genistein alone (27) and MK-4 alone (28,29)
reportedly promote bone formation; however, in the current study,
their co-administration appeared to be more beneficial as it
allowed the maintenance of NOTCH2 and WNT5A mRNAs at the levels
observed in the control cells.
In the current study, genistein and/or MK-4
treatments were shown to increase BGLAP, also known as osteocalcin,
indicating that this promoted an osteoblastic phenotype in the
MC3T3-E1 cells. In fact, cell morphology was altered following 96 h
treatments, also exhibiting a high intensity of ALP. The increase
of BMP7 by 17-β-estradiol also indicated beneficial effect of
estrogenic stimulation on osteoblastic activity. The background of
high activity of ALP by genistein + MK-4 was not always
obvious.
Significant increases in the mRNA level of CHAD by
MK-4 treatment were not consistently observed within 48 h, whereas
strong expression of its protein was clearly visible by
immunocytochemistry. CHAD has been reported to reduce preosteoclast
motility and bone resorption without affecting osteoblast
parameters, including the expression of runt related transcription
factor 2 and BGLAP, the activity of ALP and bone formation
(30). Therefore, the findings of
the current study indicated a beneficial effect on bone formation
in response to genistein + MK-4 treatment. However, the expression
of DPP4 does not always appear to be beneficial for bone formation
(31). Type 2 diabetes is
associated with an increased risk of fracture. Incretins (gastric
inhibitory polypeptide, glucagon like peptide 1, and 2) have an
important role in the regulation of bone turnover and insulin
release, and are digested by DPP4 (31). Therefore, DPP4 inhibitors appear to
be useful for patients with type 2 diabetes, to ameliorate diabetes
and also to prevent bone fracture. DPP4 is potentially induced by
genistein and/or MK-4, of which intake is inevitable in healthy
people via daily food consumption; however the concentrations
obtained from food tend to be low.
ENPP2 encodes a secreted protein (32), which is therefore unsuitable for
immunocytochemical analysis and was not assessed in the current
study. Acidosis increases the mRNA levels of ENPPs and potentially
contributes to decreased mineralization. However, among these ENPP
proteins, the level of ENPP2 is not high in osteoblasts (33). ATP11A likely drives the transport
of ions, such as calcium, across membranes (34); therefore, its increase by genistein
+ MK-4 treatment may be beneficial for the maintenance of bone.
In summary, the results of the present study suggest
that the administration of genistein and/or MK-4 may be beneficial
to maintain and/or improve BMD and bone metabolism. Compared with
MK-7, the dietary intake of MK-4 is more common, despite its low
concentration in foods. The simultaneous administration of MK-4 and
genistein appears to have a substantial beneficial effect; thus,
the consumption of beans with meat, eggs and dairy foods is
advisable to increase osteoblastic activity.
Acknowledgements
This work was in part supported by the Grant-in-Aid,
Ministry of Education, Culture, Sports, Science and Technology,
Japan (grant no. C-165904748124).
References
|
1
|
Milgram JW: Adult bone structure.
Radiologic and Histrogic Pathology of Nontumorous Diseases of Bone
and Joints. 1. Illinoi, USA: Northbrook Publishing Co. Inc.; pp.
1–5. 1990
|
|
2
|
Czernick B: Morphology of normal bone.
Dolfman and Czernick's Bone Tumors. 2nd. Philadelphia, USA:
Elsevier; pp. 3–12. 1998
|
|
3
|
Matsuo K and Irie N: Osteoclast-osteoblast
communication. Arch Biochem Biophys. 473:201–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Turner RT, Riggs BL and Spelsberg TC:
Skeletal effects of estrogen. Endocr Rev. 15:275–300. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ballane G, Cauley JA, Luckey MM and
Fuleihan Gel-H: Secular trends in hip fractures worldwide: Opposing
trends East versus West. J Bone Miner Res. 29:1745–1755. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zaheer K and Akhtar Humayoun MH: An
updated review of dietary isoflavones: Nutrition, processing,
bioavailability and impacts on human health. Crit Rev Food Sci
Nutr. 57:1280–1293. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hertrampf T, Gruca MJ, Seibel J,
Laudenbach U, Fritzemeier KH and Diel P: The bone-protective effect
of the phytoestrogen genistein is mediated via ER alpha-dependent
mechanisms and strongly enhanced by physical activity. Bone.
40:1529–1535. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hur HG, Lay JO Jr, Beger RD, Freeman JP
and Rafii F: Isolation of human intestinal bacteria metabolizing
the natural isoflavone glycosides daidzin and genistin. Arch
Microbiol. 174:422–428. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Katsuyama H, Arii M, Hinenoya H,
Matsushima M, Fushimi S, Tomita M, Okuyama T, Hidaka K, Watanabe Y,
Tamechika Y and Saijoh K: Alterations in bone turnover by
isoflavone aglycone supplementation in relation to estrogen
receptor α polymorphism. Mol Med Rep. 3:531–535. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaneki M, Hodges SJ, Hosoi T, Fujiwara S,
Lyons A, Crean SJ, Ishida N, Nakagawa M, Takechi M, Sano Y, et al:
Japanese fermented soybean food as the major determinant of the
large geographic difference in circulating levels of Vitamin K2:
Possible implications for hip-fracture risk. Nutrition. 17:315–321.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Katsuyama H, Ideguchi S, Fukunaga M,
Fukunaga T, Saijoh K and Sunami S: Promotion of bone formation by
fermented soybean (Natto) intake in premenopausal women. J Nutr Sci
Vitaminol (Tokyo). 50:114–120. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsangalis D, Wilcox G, Shah NP and
Stojanovska L: Bioavailability of isoflavone phytoestrogens in
postmenopausal women consuming soya milk fermented with probiotic
bifidobacteria. Br J Nutr. 93:867–877. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schurgers LJ and Vermeer C: Determination
of phylloquinone and menaquinones in food. Effect of food matrix on
circulating vitamin K concentrations. Haemostasis. 30:298–307.
2000.PubMed/NCBI
|
|
14
|
Tsukamoto Y, Ichise H, Kakuda H and
Yamaguchi M: Intake of fermented soybean (natto) increases
circulating vitamin K2 (menaquinone-7) and gamma-carboxylated
osteocalcin concentration in normal individuals. J Bone Miner
Metab. 18:216–222. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elder SJ, Haytowitz DB, Howe J, Peterson
JW and Booth SL: Vitamin K contents of meat, dairy, and fast food
in the U.S. Diet. J Agric Food Chem. 54:463–467. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sato T, Schurgers LJ and Uenishi K:
Comparison of menaquinone-4 and menaquinone-7 bioavailability in
healthy women. Nutr J. 11:932012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Davis L, Kuehl M and Battey J: Selection
of Poly-A+ RNA on Oligo-dT Cellulose. Basic Methods in
Molecular Biology. 2nd. Connecticut, USA: Appleton & Lange; pp.
344–349. 1994
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dawson-Saunders B and Trapp RG: Comparing
three or more means. IN basic and clinical biostatistics
Connecticut, USA: Appleton & Lange; pp. 124–141. 1990
|
|
20
|
Kuiper GG, Lemmen JG, Carlsson B, Corton
JC, Safe SH, van der Saag PT, van der Burg B and Gustafsson JK:
Interaction of estrogenic chemicals and phytoestrogens with
estrogen receptor β. Endocrinology. 139:4252–4263. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takahashi T, Saito A, Hashimoto S and Sato
C: Isoflavone contents of soybean and soy-based foods produced in
Hokkaido. Rep Hokkaido Inst Pub Hlth. 52:29–36. 2002.
|
|
22
|
Standard tables of food composition in
Japan, . 7th. Ministry of Education Culture, Sports, Science and
Technology. Japan: 2016, (In Japanese).
|
|
23
|
Bhushan R, Grünhagen J, Becker J, Robinson
PN, Ott CE and Knaus P: miR-181a promotes osteoblastic
differentiation through repression of TGF-β signaling molecules.
Int J Biochem Cell Biol. 45:696–705. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zanotti S and Canalis E: Notch signaling
and the skeleton. Endocr Rev. 37:223–253. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Keller KC, Ding H, Tieu R, Sparks NR,
Ehnes DD and Nieden Zur NI: Wnt5a supports osteogenic lineage
decisions in embryonic stem cells. Stem Cells Dev. 25:1020–1032.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kobayashi Y, Uehara S, Udagawa N and
Takahashi N: Regulation of bone metabolism by Wnt signals. J
Biochem. 159:387–392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamaguchi M: Nutritional factors and bone
homeostasis: Synergistic effect with zinc and genistein in
osteogenesis. Mol Cell Biochem. 366:201–221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Katsuyama H, Fushimi S, Yamane K, Watanabe
Y, Shimoya K, Okuyama T, Katsuyama M, Saijoh K and Tomita M: Effect
of vitamin K2 on the development of stress-induced osteopenia in a
growing senescence-accelerated mouse prone 6 strain. Exp Ther Med.
10:843–850. 2015.PubMed/NCBI
|
|
29
|
Shikano K, Kaneko K, Kawazoe M, Kaburaki
M, Hasunuma T and Kawai S: Efficacy of vitamin K2 for
glucocorticoid-induced osteoporosis in patients with systemic
autoimmune diseases. Intern Med. 55:1997–2003. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Capulli M, Olstad OK, Onnerfjord P,
Tillgren V, Muraca M, Gautvik KM, Heinegård D, Rucci N and Teti A:
The C-terminal domain of chondroadherin: A new regulator of
osteoclast motility counteracting bone loss. J Bone Miner Res.
29:1833–1846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Meier C, Schwartz AV, Egger A and
Lecka-Czernik B: Effects of diabetes drugs on the skeleton. Bone.
82:93–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hausmann J, Kamtekar S, Christodoulou E,
Day JE, Wu T, Fulkerson Z, Albers HM, van Meeteren LA, Houben AJ,
van Zeijl L, et al: Structural basis for substrate discrimination
and integrin binding by autotaxin. Nat Struct Mol Biol. 18:198–204.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Orriss IR, Key ML, Hajjawi MO, Millán JL
and Arnett TR: Acidosis is a key regulator of osteoblast
ecto-nucleotidase pyrophosphatase/phosphodiesterase 1 (NPP1)
expression and activity. J Cell Physiol. 230:3049–3056. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
ATP11A ATPase phospholipid transporting
11A. Gene ID: 23250. https://www.ncbi.nlm.nih.gov/gene/23250
|