Introduction
Diabetes and its associated complications, such as
diabetic nephropathy (DN) have become a serious health problem.
Approximately one-third of all diabetic patients suffer from DN
(1), which has significant social
and economic burdens (2) and may
be the leading cause of end-stage renal disease (ESRD). In the
United States ~200,000 patients receive ESRD care due to diabetic
kidney disease, with 50,000 new patients starting dialysis yearly
(3,4). The primary indicators of DN are
continuous albuminuria, high blood pressure and progressive renal
damage. However, the specific pathogenic mechanisms remain to be
fully elucidated. Hyperglycemia has an important part in the
development of DN; however, additional factors, such as
inflammation due to fibrosis, are considered to be important for
the initiation and progression of diabetic nephropathy.
The major pathological alterations of DN include
mesangial expansion, extracellular matrix (ECM) alterations,
tubulointerstitial fibrosis and glomerular sclerosis. Transforming
growth factor-β (TGF-β) has been identified to be a key regulator
of fibrosis in DN (5).
Overexpression of TGF-β may promote epithelial-mesenchymal
transition (EMT) and renal sclerosis, ultimately leading to organ
failure (6). Chen et al
(7) demonstrated that suppression
of the TGF-β/mothers against decapentaplegic signaling pathway may
greatly ameliorate streptozotocin (STZ)-induced fibrosis and
albumin levels in the urine of rats (7). Therefore, therapeutic agents that may
inhibit TGF-β and its signaling pathways may also reduce the
progress of DN.
At present, despite the wide use of therapeutic
approaches focused on managing hyperglycemia and high blood
pressure, numerous patients continue to suffer from progressive and
severe renal injury. Therefore, it is important to develop novel
renal protective drugs for the treatment of DN. Berberine (BBR) is
a type of isoquinolone alkaloid that is extracted from the widely
used Chinese herb, Rhizoma coptidis. Recent studies have
indicated that BBR has multiple pharmacological activities,
including hypolipidemic, antioxidant and glucose-lowering,
suggesting that it may have clinical potential as an alternative
therapeutic drug for diabetic complications (8,9). Liu
et al (10) reported that
BBR effectively lowered blood glucose and lipid levels through
suppression of oxidative stress. However, it remains to be
elucidated if BBR exerts its beneficial effects in DN via
regulation of TGF-β. The present study used a STZ-induced DN rat
model to investigate the effect of BBR on the activation of TGF-β
and its associated pathways in DN.
Materials and methods
Materials
Male Wistar rats (n=50; weight, 150±10 g) were
obtained from Laboratory Animal Center of Henan Province
(Zhengzhou, China). The rats were raised at an ambient temperature
of 24±1°C with 12-h light/dark cycle and 45±5% humidity, and free
access to a standard chow diet and water for 1 week prior to the
experiment. The present study received ethical approval from the
Nanyang Institute of Technology (Nanyang, China).
Rats were randomly assigned into control (n=10) and
diabetic (n=40) groups. The rats in the diabetic group received a
single intraperitoneal injection of STZ (60 mg/kg, Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany). The control group were
injected with citrate buffer solution (0.1 M, pH 4.4). The
development of hyperglycemia was confirmed by measuring fasting
blood glucose (FBG) at 72 h following injection. Those with FBG
over 11.1 mM/l were considered diabetic. A total of 30 diabetic
rats were selected and randomly divided into DN, BBR and metformin
groups (n=10/group).
The control and DN groups were orally treated with
double distilled water (0.5 ml/100 g) per day (vehicle) for 12
weeks. The BBR group received 400 mg/kg BBR (dissolved in sodium
carboxymethyl cellulose and mixed by magnetic stirring apparatus
for at least half an hour) orally every day, and the metformin
group were treated with 500 mg/kg metformin solution. The control
group rats were fed standard rodent chow and the remaining groups
were fed high-fat food (78.8% normal diet, 10% lard, 10% yolk
powder, 1% cholesterol and 0.2% cholate). All rats had free access
to water, and the urine was collected at the end of 83 days from
the metabolic cages 24 h prior to the end of the experiment. At the
end of the 12th week, all the animals were anesthetized with 10%
chloralhydrate (Lot 30037574; Sinopharm Chemical Reagent Beijing
Co., Ltd.) and sacrificed. Kidney samples were dissected and
rapidly excised, weighed and frozen in liquid nitrogen and stored
at −80°C or fixed in 10% neutral-buffered formalin.
Quantification of body weight and
urinary albumin
All rats were fasted for 24 h; however, water was
placed in the metabolic cages and urine collected prior to
sacrifice. Urinary protein excretion was determined by the Bradford
method (P0006; Beyotime Institute of Biotechnology, Beijing,
China).
Quantification of creatinine (Scr) and
blood urea nitrogen (BUN)
Serum levels of Scr and BUN were quantified using a
Hitachi 7080 Chemistry Analyzer (Hitachi, Ltd., Tokyo, Japan).
Histopathological analysis
Kidney tissues were fixed in 10% phosphate buffered
formalin solution and embedded in paraffin. Paraffin sections (2–3
µm) were stained with periodic acid-Schiff (PAS) and Masson's
trichrome. The degree of glomerulosclerosis, defined as ECM
deposition and mesangial expansion, was randomly selected and
evaluated at a magnification of ×40 for 20 cortical fields using a
previously described scoring system (0–4 grades) (11).
Immunohistochemistry analysis
Immunohistochemistry was performed on paraffin
sections (3-µm) using a microwave-based antigen retrieval technique
as previously described (12). The
slides were incubated overnight at 4°C in a humidified chamber with
anti-TGF-β (sc-101574; 1:500) or anti-α-smooth muscle actin (α-SMA;
sc-53142; 1:500) (both from Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) antibodies. The next day, the sections were incubated with
a biotinylated goat-anti-rat secondary antibody (115–035-003;
1:10,000; Jackson ImmunoResearch Laboratories, Inc., West Grove,
USA) and subsequently in an avidin-horseradish peroxidase solution.
Immunostaining was visualized with 0.05% diaminobenzidine. Sections
were examined using an Olympus DP72 microscope (Olympus
Corporation, Tokyo, Japan) and imaged with a high-resolution camera
at a magnification of ×400. Areas with positive staining were
quantified by using ImageJ version 1.6.0_24 (National Institutes of
Health, Bethesda, MD, USA; https://imagej.nih.gov/ij/download.html) and expressed
as a percentage of the entire glomerulus or selected
tubulointerstitial area.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The renal cortex was collected by carefully
dissecting the renal pelvis and medullar tissues, and was
subsequently frozen at −80°C for analysis of the gene of interest
(13). TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was
used to isolate total RNA from kidney tissues following the
manufacturer's protocol. A standard reverse transcriptase reaction
kit (Takara Biotechnology, Co., Ltd., Dalian, China) was used to
synthesize cDNA. qPCR was performed using the UltraSYBR Mixture kit
with ROX I (CW2602M; CW Biotech, Beijing, Beijing, China;) on an
ABI 7500 Sequence Detection system (Thermo Fisher Scientific,
Inc.). The primers used are presented in Table I. Each sample was analyzed in
triplicate. The relative mRNA expression levels were calculated
using the 2−ΔΔCq method (14). The expression of GAPDH was used as
the endogenous reference control.
 | Table I.Primers used for quantitative
polymerase chain reaction. |
Table I.
Primers used for quantitative
polymerase chain reaction.
| Gene | Forward (5′-3′) | Reverse (5′-3′) | Product length
(bp) |
|---|
| TGF-β |
GATACGCCTGAGTGGCTGTCT |
GGAAGGGTCGGTTCATGTCAT | 202 |
| α-SMA |
TGTACCCAGGCATTGCTGACA |
TCTGCTGGAAGGTAGATAAG | 150 |
| Vimentin |
GAGATCGCCACCTACAGGAG |
TCATCGTGGTGCTGAGAATC | 206 |
| NF-κB |
TGCGACAGATGGGCTACACAG |
TTTGCGGAAGGATGTCTCCAC | 200 |
| GAPDH |
GGTTGTCTCCTGTGACTTCAA |
TGCTGTAGCCATATTCATTGT | 125 |
Western blotting
Renal cortices tissues were lysed in
radioimmunoprecipitation (RIPA) buffer (Sanbio, Beijing, China).
Proteins from kidney tissues and cultured cells were extracted with
RIPA lysis buffer, and western blot analysis was performed as
previously described (15).
Following centrifugation at 15,000 × g for 15 min at 4°C,
supernatant protein concentration was quantified using a Bradford
assay kit (P0006; Beyotime Institute of Biotechnology). With 50 µg
proteins were separated by 12% SDS-PAGE and transferred onto a
polyvinylidene difluoride membrane, which was then blocked for 1 h
with 5% non-fat dry milk in PBS containing 0.1% Tween-20. The
membranes were then incubated with the TGF-β (sc-101574; 1:1,000),
α-SMA (sc-53142; 1:1,000), Vimentin (sc-73259; 1:1,000) (all from
Santa Cruz Biotechnology, Inc.), NF-κB (48,862; 1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA) overnight at 4°C. The
membranes were washed with Tris-buffered saline and incubated for 1
h with secondary antibodies (111–035-003 or 115-035-003; 1:10,000;
Jackson ImmunoResearch Laboratories, Inc.). Following washing,
protein bands were detected by a chemiluminescence reagent
(Applygen Technologies, Inc., Beijing, China).
Statistical analysis
Data were analyzed using SPSS version 17.0 (SPSS,
Inc., Chicago, IL, USA) and are presented as the mean ± standard
deviation. Significance between groups was evaluated by one way
analysis of variance followed by a Newman-Keuls post hoc test
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of BBR on body weight, blood
glucose, and 24 h urinary protein
At the end of experiments, rats in DN group
experienced significant weight loss (P<0.05) when compared with
the control group (data not shown). No significant difference was
identified in terms of body weight in the BBR and metformin groups
when compared with the control group. Following induction of
diabetes, the blood glucose level was increased in the DN, BBR and
metformin groups. However, treatment with BBR and metformin did not
significantly decrease the blood level when compared with the DN
group (Table II).
 | Table II.Characteristics of rats in the
different treatment groups. |
Table II.
Characteristics of rats in the
different treatment groups.
| Group | Urine albumin | Glucose | Scr (µM/l) | BUN (mM/l) |
|---|
| Control | 14.92±4.23 | 6.1±0.6 | 28.9±5.2 | 5.24±0.36 |
| DN |
110.3±12.04a | 21.5±4.3a | 78.4±8.9a |
9.71±0.82b |
| DN+BBR |
87.6±11.42c | 22.9±3.7 |
49.6±11.1c |
7.33±1.01c |
| DN+Metformin | 94.51±16.21 | 19.1±6.8 | 53.4±7.8c |
6.67±0.99c |
During the experimental period, diabetic rats had a
significantly greater urine protein excretion. However, after
12-week treatment with BBR, the rats had significantly reduced 24 h
urine albumin when compared with the DN group (P<0.05). In
addition, High fat food plus STZ injection caused a significant
increase of Scr and BUN levels in diabetic rats. However, 12 weeks
treatment with BBR can reduced the levels of Scr and BUN compared
with the DN group (P<0.05; Table
II).
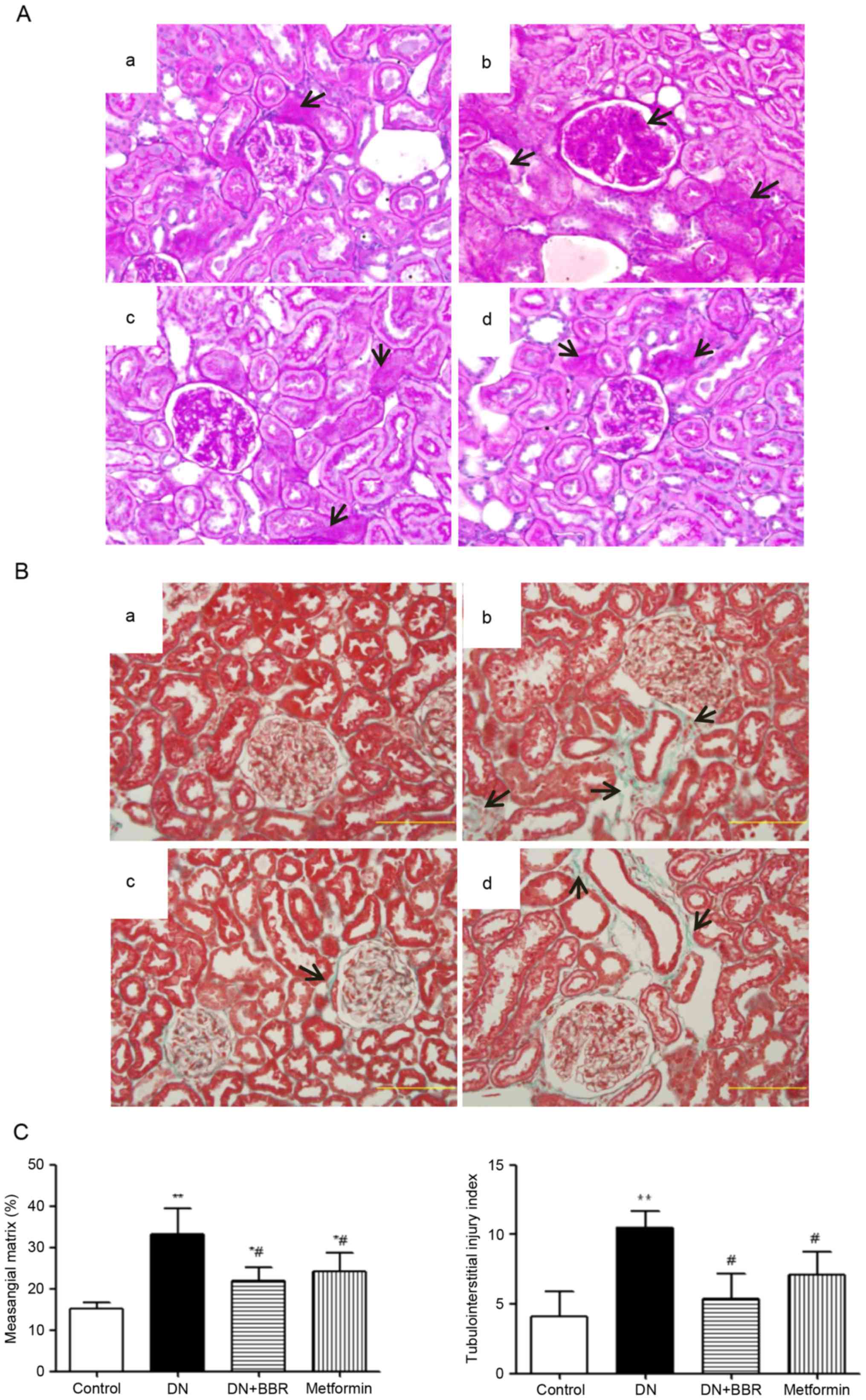
PAS and Masson staining
Both PAS and Masson staining demonstrated
significant histological alterations in the DN rats. The DN rats
had notable glomerular hypertrophy and mesangial matrix expansion
compared to the control group (Fig.
1). However, treatment with BBR or metformin significantly
ameliorated these pathological alterations (P<0.01; Fig. 1C).
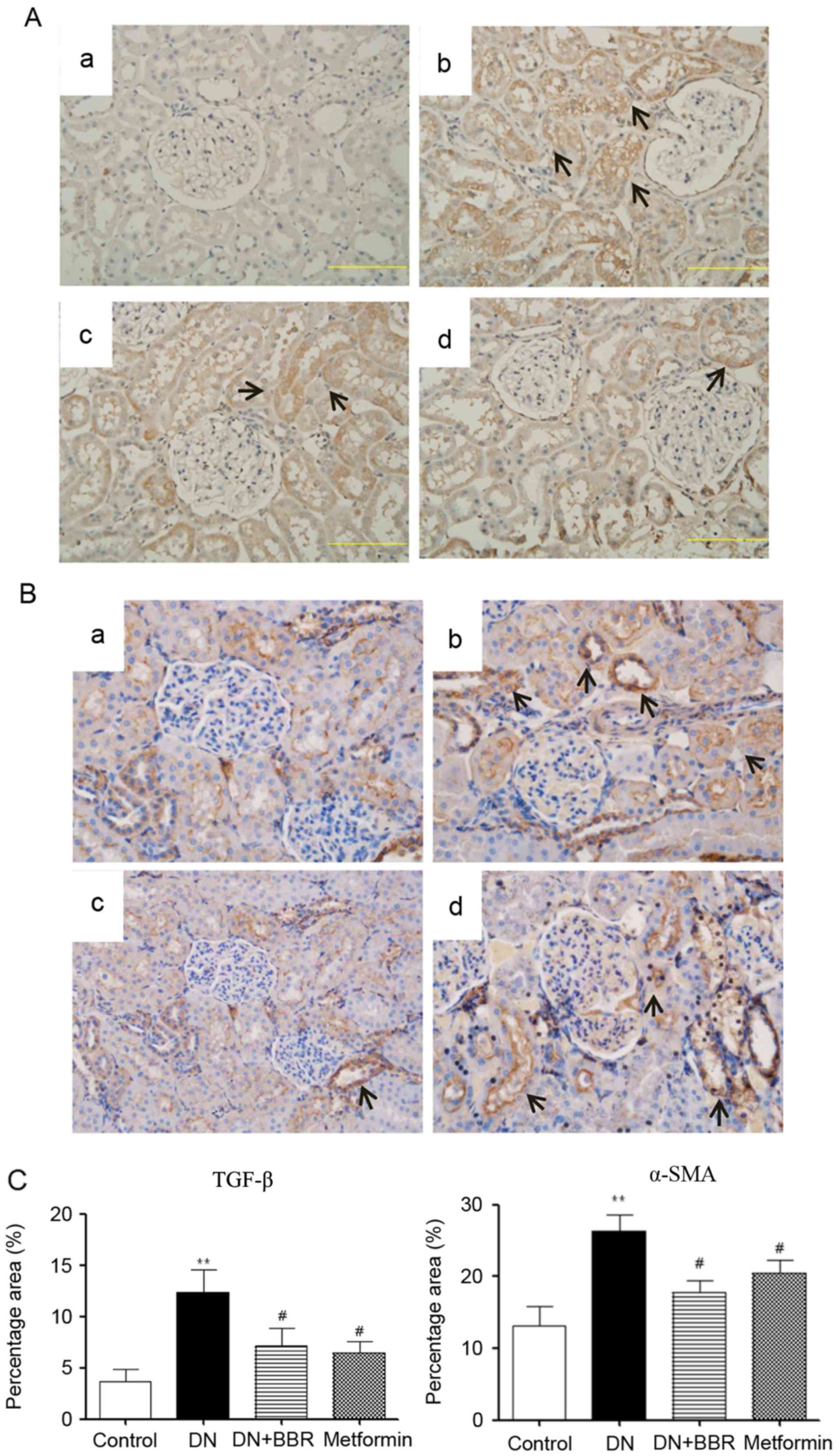
Immunohistochemical staining of TGF-β
and α-SMA
TGF-β and α-SMA expression levels in renal tissues
were observed by immunohistochemical staining (Fig. 2A and B). The expression levels of
TGF-β and α-SMA in the DN group were increased compared with the
normal group. However, BBR treatment ameliorated this effect. In
addition, metformin also reduced the expression of TGF-β and α-SMA;
however, there no significant difference was identified between the
BBR and metformin groups (Fig.
2C).
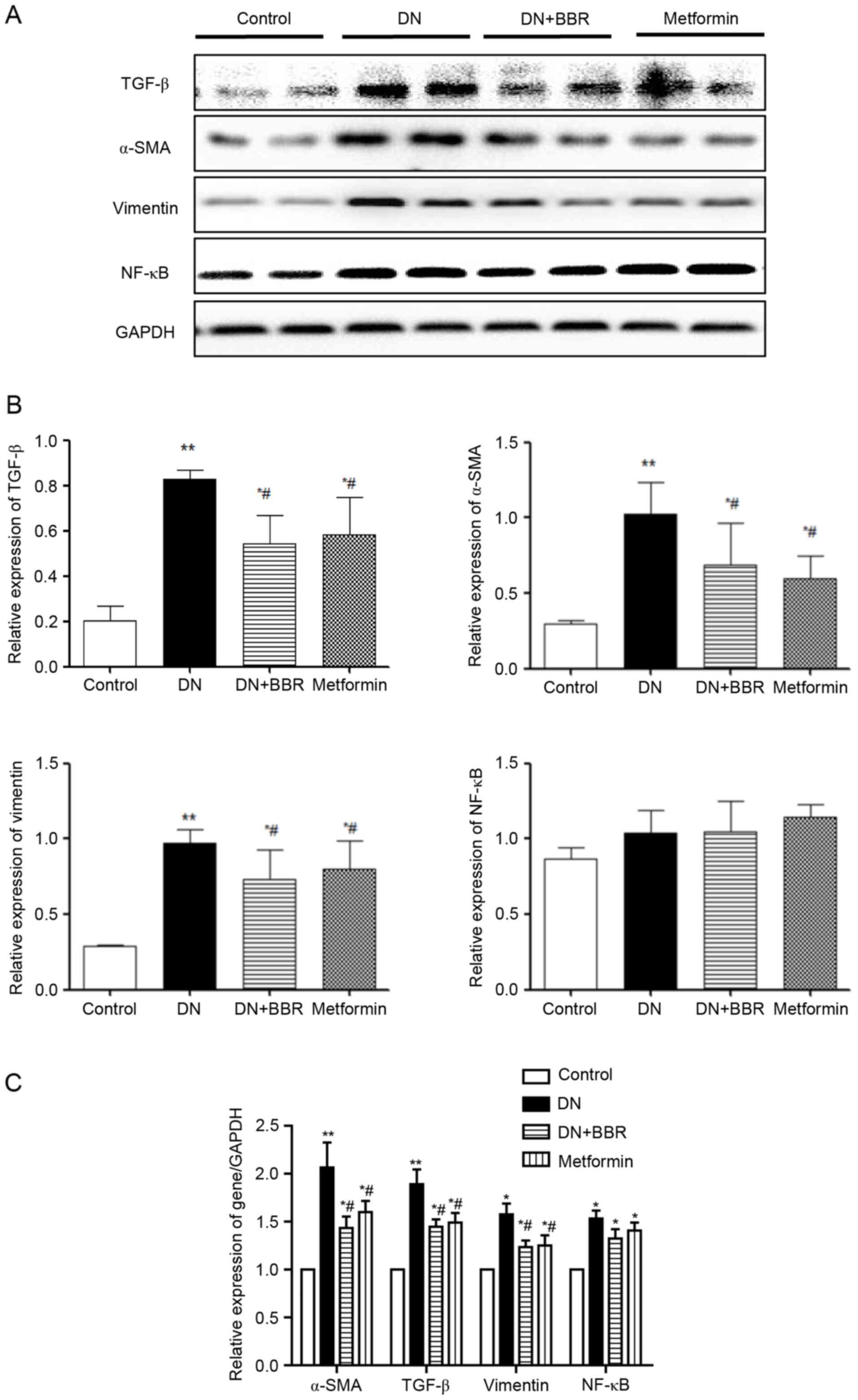
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and western blotting of TGF-β,
vimentin, NF-κB and α-SMA
In order to determine the underlying mechanisms by
which BBR inhibits renal fibrosis, the mRNA and protein expression
levels of TGF-β, vimentin, nuclear factor-κB (NF-κB) and α-SMA in
diabetic kidney tissues were assessed using RT-qPCR and western
blotting, respectively. The mRNA and protein expression levels of
TGF-β, vimentin, and α-SMA were significantly increased in the DN
group compared with the control group (Fig. 3). However, treatment with BBR may
significantly reduce the expression level of those genes
(P<0.05; Fig. 3C) when compared
with the DN group. Additionally, no significant difference was
identified between the mRNA or protein expression levels of NF-κB
in the control group compared with the DN group.
Discussion
DN is characterized by glomerular hypertrophy,
thickness of the basement, tubular and glomerular membranes and
accumulation of ECM, which leads to tubulointerstitial and
glomerular fibrosis and sclerosis. TGF-β has a key role in the
development of matrix production and the replacement of the normal
renal tubulointerstitium with fibrous scarring; however, effective
methods for alleviation of fibrosis during DN remain to be
elucidated.
High fat food combined with STZ injection may
exacerbate kidney injury. Previous studies determined that
seriously injured rat kidneys also exhibit increased levels of Scr
12 weeks after induction of the diabetic model (16,17).
The present study determined that BBR effectively reduced the serum
Scr and BUN levels, which was in accordance with previous studies
(18,19). In addition, BBR treatment also
reduced the expression of TGF-β and α-SMA, which have been
identified to have an important role in the ECM-synthesizing
process. These findings have demonstrated that BBR may have a
significant protective effect against diabetic renal injury.
The earliest detectable alteration in the
pathogenesis of diabetic nephropathy is an expansion of the
glomerular mesangium, which occurs due to excessive accumulation of
ECM proteins. During this process, TGF-β and α-SMA have been
identified to have an important role in the ECM. TGF-β contributes
to a variety of biological processes, including cell proliferation,
differentiation, apoptosis, autophagy and production of the ECM. A
previous study revealed that TGF-β was upregulated in injured
kidneys in both patients and animal disease models (20). The downregulation of TGF-β
signaling pathways may alleviate kidney fibrosis (21,22).
During DN, fibroblasts are a critical component of the repair
mechanism, and increased TGF-β may lead to an increased
transformation of these cells into activated myofibroblasts,
indicated by increased expression of α-SMA. Hinz et al
(23) reported that increased
α-SMA expression enhances fibroblast contractile activity (23). Recent studies have identified a
limited number of drugs that may reverse fibrosis (24,25).
However, in present study, Chinese herbs have been identified to
have a favorable curative effect in fibrosis.
Traditional Chinese herbs have been widely used in
the treatment of diabetes in China for thousands of years.
Currently, various nutraceutical ingredients, frequently of
botanical origin, have been investigated in terms of their ability
to treat fibrosis. Meng et al (26) determined that Astragali
radix may alleviate renal tubulointerstitial fibrosis by
inhibition of tubular EMT and fibroblast activation (26). BBR, extracted from Coptis
chinensis, was first proved to have anti-hyperglycemic activity in
1986 (27,28). However, its effect on DN,
particularly in fibrosis, remains to be elucidated. The present
study observed that BBR may inhibit the expression of TGF-β and
α-SMA in kidney tissues. This may contribute to its ability to
ameliorate the symptoms of DN.
In conclusion, the present study demonstrated that
BBR, extracted from Rhizoma coptidis, had a beneficial
effect on TGF-β and α-SMA expression levels. Furthermore, BBR
treatment improved renal fibrosis in DN. However, BBR was not
observed to regulate blood glucose in the present study. Future
studies should focus on the underlying molecular mechanism in order
to allow for the clinical use of BBR.
Acknowledgements
The current study was supported by a grant from the
Henan Province Science and Technology Key Project (grant no.
162102310256).
References
|
1
|
Atkins RC and Zimmet P: Diabetic kidney
disease: Act now or pay later. Kidney Int. 77:375–377. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cooper ME: Diabetes: Treating diabetic
nephropathy-still an unresolved issue. Nat Rev Endocrinol.
8:515–516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reidy K, Kang HM, Hostetter T and Susztak
K: Molecular mechanisms of diabetic kidney disease. J Clin Invest.
124:2333–2340. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Badal SS and Danesh FR: New insights into
molecular mechanisms of diabetic kidney disease. Am J Kidney Dis.
63 2 Suppl 2:S63–S83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fan Y, Li X, Xiao W, Fu J, Harris RC,
Lindenmeyer M, Cohen CD, Guillot N, Baron MH, Wang N, et al: BAMBI
elimination enhances alternative TGF-β signaling and glomerular
dysfunction in diabetic mice. Diabetes. 64:2220–2233. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Du J, Hong S, Dong L, Cheng B, Lin L, Zhao
B, Chen YG and Chen X: Dynamic sialylation in transforming growth
factor-β (TGF-β)-induced epithelial to mesenchymal transition. J
Biol Chem. 290:12000–12013. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen KH, Hung CC, Hsu HH, Jing YH, Yang CW
and Chen JK: Resveratrol ameliorates early diabetic nephropathy
associated with suppression of augmented TGF-β/smad and ERK1/2
signaling in streptozotocin-induced diabetic rats. Chem Biol
Interact. 190:45–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen X, Zhang Y, Zhu Z, Liu H, Guo H,
Xiong C, Xie K, Zhang X and Su S: Protective effect of berberine on
doxorubicin-induced acute hepatorenal toxicity in rats. Mol Med
Rep. 13:3953–3960. 2016.PubMed/NCBI
|
|
9
|
Adil M, Kandhare AD, Dalvi G, Ghosh P,
Venkata S, Raygude KS and Bodhankar SL: Ameliorative effect of
berberine against gentamicin-induced nephrotoxicity in rats via
attenuation of oxidative stress, inflammation, apoptosis and
mitochondrial dysfunction. Ren Fail. 38:996–1006. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu C, Wang Z, Song Y, Wu D, Zheng X, Li
P, Jin J, Xu N and Li L: Effects of berberine on amelioration of
hyperglycemia and oxidative stress in high glucose and high fat
diet-induced diabetic hamsters in vivo. Biomed Res Int.
2015:3138082015.PubMed/NCBI
|
|
11
|
Zhang H, Li P, Burczynski FJ, Gong Y, Choy
P, Sha H and Li J: Attenuation of diabetic nephropathy in otsuka
long-evans tokushima fatty (OLETF) rats with a combination of
Chinese herbs (Tangshen Formula). Evid Based Complement Alternat
Med. 2011:6137372011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao TT, Zhang HJ, Lu XG, Huang XR, Zhang
WK, Wang H, Lan HY and Li P: Chaihuang-Yishen granule inhibits
diabetic kidney disease in rats through blocking TGF-β/Smad3
signaling. PLoS One. 9:e908072014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hou CC, Wang W, Huang XR, Fu P, Chen TH,
Sheikh-Hamad D and Lan HY: Ultrasound-microbubble-mediated gene
transfer of inducible Smad7 blocks transforming growth factor-beta
signaling and fibrosis in rat remnant kidney. Am J Pathol.
166:761–771. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu SF, Chang SY, Lee TC, Chuang LY, Guh
JY, Hung CY, Hung TJ, Hung YJ, Chen PY, Hsieh PF and Yang YL:
Dioscorea alata attenuates renal interstitial cellular fibrosis by
regulating Smad- and epithelial-mesenchymal transition signaling
pathways. PLoS One. 7:e474822012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Renno WM, Abdeen S, Alkhalaf M and Asfar
S: Effect of green tea on kidney tubules of diabetic rats. Br J
Nutr. 100:652–659. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuo CW, Shen CJ, Tung YT, Chen HL, Chen
YH, Chang WH, Cheng KC, Yang SH and Chen CM: Extracellular
superoxide dismutase ameliorates streptozotocin-induced rat
diabetic nephropathy via inhibiting the ROS/ERK1/2 signaling. Life
Sci. 135:77–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Liu H, Li N, Zhang Q and Zhang H:
The protective effect of fucoidan in rats with
streptozotocin-induced diabetic nephropathy. Mar Drugs.
12:3292–3306. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mapanga RF, Tufts MA, Shode FO and
Musabayane CT: Renal effects of plant-derived oleanolic acid in
streptozotocin-induced diabetic rats. Ren Fail. 31:481–491. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rodell CB, Rai R, Faubel S, Burdick JA and
Soranno DE: Local immunotherapy via delivery of interleukin-10 and
transforming growth factor β antagonist for treatment of chronic
kidney disease. J Control Release. 206:131–139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang Y, Kim B, Park YK, Koo SI and Lee JY:
Astaxanthin prevents TGFβ1-induced pro-fibrogenic gene expression
by inhibiting Smad3 activation in hepatic stellate cells. Biochim
Biophys Acta. 1850:178–185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu J, Shi J, Li M, Gui B, Fu R, Yao G,
Duan Z, Lv Z, Yang Y, Chen Z, et al: Activation of AMPK by
metformin inhibits TGF-β-induced collagen production in mouse renal
fibroblasts. Life Sci. 127:59–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hinz B, Celetta G, Tomasek JJ, Gabbiani G
and Chaponnier C: Alpha-smooth muscle actin expression upregulates
fibroblast contractile activity. Mol Biol Cell. 12:2730–2741. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramos AM, González-Guerrero C, Sanz A,
Sanchez-Niño MD, Rodríguez-Osorio L, Martín-Cleary C,
Fernández-Fernández B, Ruiz-Ortega M and Ortiz A: Designing drugs
that combat kidney damage. Expert Opin Drug Discov. 10:541–556.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meng XM, Tang PM, Li J and Lan HY:
TGF-β/Smad signaling in renal fibrosis. Front Physiol. 6:822015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meng LQ, Tang JW, Wang Y, Zhao JR, Shang
MY, Zhang M, Liu SY, Qu L, Cai SQ and Li XM: Astragaloside IV
synergizes with ferulic acid to inhibit renal tubulointerstitial
fibrosis in rats with obstructive nephropathy. Br J Pharmacol.
162:1805–1818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yao J, Kong W and Jiang J: Learning from
berberine: Treating chronic diseases through multiple targets. Sci
China Life Sci. 58:854–859. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singh IP and Mahajan S: Berberine and its
derivatives: A patent review (2009–2012). Expert Opin Ther Pat.
23:215–231. 2013. View Article : Google Scholar : PubMed/NCBI
|