Introduction
The apelin receptor (APJ), a novel G-protein-coupled
receptor, shares homology with the angiotensin II type 1 receptor
and was considered an ‘orphan’ receptor prior to the discovery of
apelin in 1998 (1,2). Apelin is a product of the 77-amino
acid precursor preproapelin, which yields numerous isoforms,
including apelin-12, apelin-13, apelin-17 and apelin-36 (3,4).
Apelin-13 is composed of 13 amino acids at the C-terminus of
preproapelin, and is highly conserved (5); it exhibits the strongest binding to
APJ among all the isoforms (3).
Following the pairing of apelin peptides with cognate ligands, they
have been identified to serve various roles in physiological and
pathophysiological states, including effects on the cardiovascular
system (6–8), fluid equilibrium (9,10),
the adipoinsular signaling pathway (11,12),
the immune system (13) and
neuroprotection (14).
The apelin-APJ system is located in the central and
peripheral nervous systems (3,15).
In the central nervous system (CNS), apelin and its receptors were
detected in pain-associated regions, indicating that apelin may
affect nociception (4,16). Previous studies have indicated that
apelin-13, when administered at the supraspinal level, inhibits
acute or visceral pain (17,18).
Intracerebroventricular apelin-13 administration promotes
antinociception in mice, an effect markedly antagonized by
coagulation factor XIII A chain (an APJ antagonist) and naloxone,
suggesting that apelin is involved in opioid receptor signaling and
corroborating reports that apelin is located in opioid-rich brain
areas, including the arcuate nucleus of the hypothalamus and the
spinal trigeminal nucleus (19).
However, a previous study proposed that an intrathecal injection of
apelin-13 produces hyperalgesia, not antinociception, in the second
phase of a formalin test (20).
The discrepancies between previous studies may result from the
involvement of multiple receptor systems in apelin-13-induced pain
behavior in acute and tonic pain models. Although apelin-13
produces aberrations in acute nociceptive models, it possibly
serves an agonistic role at low concentrations, with these effects
reversed at increased concentrations (20). Alternatively, apelin-13 may
costimulate receptors aside from known opioid binding molecules,
compromising its antinociceptive activity (17). Recent clinical results also
indicated that the ratio of apelin to the endothelin-1 precursor
has a positive correlation with increased vaso-occlusive pain in
pediatric patients with sickle cell disease (21), indicating that the role of the
apelin-APJ system in pain modulation requires further research.
Pain following nerve injury, also termed neuropathic
pain, remains a challenging field within basic and clinical
science. The apelin-APJ system is involved in the modulation of
nociception, and in inflammatory and visceral pain; however, its
involvement in models of neuropathic pain remain unclear. In
addition, the signal transduction pathways of apelin overlap with
those associated with neuropathic pain (22–24).
Therefore, the present study was designed to examine the expression
of the spinal apelin-APJ system and its functional role, and the
mechanisms underlying neuropathic pain caused by chronic
constriction injury (CCI) to the peripheral sciatic nerve.
Materials and methods
Animal preparation
Adult male Sprague-Dawley rats (n=163; 200–220 g;
8–10-weeks-old) were purchased from the Experimental Animal Center
of Guangdong Province (Guangdong, China). Rats were housed in a
temperature-controlled room (24±2°C) with a 12 h light/dark cycle
and food and water available ad libitum. The animals were allowed
to acclimate for ≥3 days, prior to the experiments approved by the
Animal Use and Care Committee for Research and Education of The
First People's Hospital of Foshan (Guangdong, China), in accordance
with the ethical guidelines of the International Association for
the Study of Pain (25). Pain and
suffering were minimized, as was the number of animals used.
CCI model
The CCI neuropathic pain model was established as
previously proposed (26). The
animals were anesthetized by intraperitoneal injection of 10%
chloral hydrate (400 mg/kg). The biceps femoris was dissected to
expose the left sciatic nerve. The area proximal to the sciatic
nerve's trifurcation was freed of adhering tissue, and 4-0 surgical
catgut suture was tied loosely around it with approximately 1 mm
between ligatures. Sham-operated rats underwent the same surgery,
but without ligation. Following the surgery, muscle and skin tissue
was sutured in layers, and treatment with ampicillin (100 mg/kg)
intraperitoneally for the first day was administered. Rats in the
naïve group received neither surgery nor ligation. Each group
contains 6 rats. Rats in the CCI group exhibiting no mechanical and
thermal allodynia at 7 days post-surgery were excluded from the
study.
Intrathecal treatment
In order to determine the effect of single
intrathecal apelin-13 (10 µg; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and ML221 (1, 3, 10 and 30 µg; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) doses on mechanical allodynia and
heat hyperalgesia, the rats were treated with light isoflurane
anesthesia (1–2%). The injection was carried out with a 30-gauge
needle as previously described (27). Apelin-13 or ML221 was slowly
administered in a solution of 10 µl.
A permanent intrathecal catheter (PE-10 polyethylene
tube; BD Biosciences, Franklin Lakes, NJ) was inserted between the
T3 and T4 vertebrae and extended to the
L4 and L5 segments following intraperitoneal
administration of 400 mg/kg chloral hydrate, in order to assess the
effect of continuous injection of apelin-13 or ML221 using
behavioral testing. Cannulated animals were housed individually;
those with surgery-associated neurological ailments were not taken
into account in the final analysis. Drugs were injected through the
catheter in a volume of 10 µl followed by flushing with 10 µl
saline. All injections were performed by the same investigator.
Behavioral tests
Mechanical allodynia was evaluated as previously
described (28). Each rat was
placed individually into a wire mesh-bottomed cage for 30 min of
adaptation prior to the test. Filaments were presented, from
weakest to strongest, at a 90° angle to the plantar surface, with
enough force to slightly bend the paw, and maintained for 6 sec. A
positive response was indicated by the animal quickly withdrawing
or flinching the paw. The stimuli were presented at intervals of 2
min, and the stimuli were increased and decreased sequentially
(‘up-and-down’ technique) to assess paw-withdrawal threshold (PWT).
Interpolation of the 50% threshold (the weakest force causing ≥3
withdrawals in 5 successive applications) was carried out according
to the method of Dixon (29). The
experiments were performed in a blinded manner.
Paw-withdrawal latency (PWL) values following
thermal hyperalgesia were determined using a Plantar Analgesia
Meter (IITC Life Science, Woodland Hills, CA, USA) (30). Individual rats were assessed three
times (left hind paw) and average values were obtained.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was obtained from
L4-L5 spinal cord segments using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's protocol. RNA purity and
concentration were evaluated using the NanoDrop 2000 (Thermo Fisher
Scientific, Inc., Wilmington, DE, USA). RT-qPCR analysis was
carried out to determine the mRNA concentration of apelin and APJ
using the EXPRESS One-Step SYBR Green ER SuperMix kit (Invitrogen;
Thermo Fisher Scientific, Inc.) following the manufacturer's
protocol, on a StepOne Real-Time PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Relative gene expression was
assessed by the 2−ΔΔCq method (31). RT-qPCR amplification was carried
out as previously described (30),
with β-actin as the reference gene for normalization. The
experiments were performed in triplicate. The specific primers used
for sequence detection were as follows: β-actin, forward
5′-GGAGATTACTGCCCTGGCTCCTA-3′ and reverse
5′-GACTCATCGTACTCCTGCTTGCTG-3′; Apelin, forward
5′-CTCTCCTTGACTGCCGTGTGTG-3′ and reverse
5′-GCATGTTGCCTTCTTCTAGCCC-3′; APJ, forward
5′-TGCCTCAACCCCTTCCTCTA-3′ and reverse
5′-GTTCTCCTCCCTTGCACATG-3′.
Western blotting
In order to evaluate the variation in the protein
concentrations of the apelin-APJ system (rats randomly divided into
control group, sham group and CCI groups) and ERK signaling [rats
randomly divided into sham group, CCI group, CCI + dimethylsufoxide
(DMSO) group and CCI + ML221 group], western blot analysis was
performed with L4-L5 spinal cord segment
specimens. Sample homogenization was performed in lysis buffer as
described previously (30).
Protein amounts were determined using the bicinchoninic acid assay.
Equal amounts (20 µg) of total protein were resolved by 10%
SDS-PAGE and electro-transferred onto polyvinylidene fluoride
membranes (Merck KGaA). The membranes were blocked using 5% nonfat
dry milk for 1 h at room temperature (25°C) and incubated overnight
at 4°C with primary antibodies raised against apelin (goat; cat.
no. sc-33469; 1:1,500), APJ (rabbit; cat. no. sc-33823; 1:2,000),
ERK1/2 (mouse; cat. no. sc-514302; 1:1,500), and p-ERK1/2 (rabbit;
cat. no. sc-101760; 1:1,000), all purchased from Santa Cruz
Biotechnology, Inc. For loading control, the blots were probed with
GAPDH antibody (mouse; cat. no. sc-32233; 1:5,000; Santa Cruz
Biotechnology, Inc.). These membranes were further incubated for 2
h with rabbit anti-goat (cat. no. BA1060; 1:2,000; Wuhan Boster
Biological Technology, Ltd., Wuhan, China), goat anti-mouse (cat.
no. BA1051; 1:2,000; Wuhan Boster Biological Technology, Ltd.) and
mouse anti-rabbit (cat. no. L27A9; 1:2,000; Cell Signaling
Technology, Inc., Danvers, MA, USA) IgG horseradish
peroxidase-conjugated secondary antibodies at room temperature.
Band intensities were assessed using Image J software (version
1.48u; National Institutes of Health, Bethesda, MD, USA).
Immunohistochemistry
When neuropathic pain was fully established, the
rats were intraperitoneally administered 400 mg/kg chloral hydrate
for deep anesthesia, and L4-L5 spinal cord
segments were extracted, fixed in 4% paraformaldehyde for 90 min at
room temperature (25°C) and then placed in 30% sucrose solution at
4°C overnight, embedded in optimal cutting temperature (OCT)
compound (Thermo Fisher Scientific, Inc.), and sectioned at 5 µm
for immunofluorescence. Blocking with 2% donkey serum (Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 1 h at
room temperature was followed by overnight incubation (4°C) with
primary antibodies raised against apelin (goat; cat. no. sc-33469;
1:100; Santa Cruz Biotechnology, Inc.) and APJ (rabbit; cat. no.
sc-33823; 1:100; Santa Cruz Biotechnology, Inc.). DyLight 649-
(rabbit anti-goat; cat. no. E032630; 1:200; EarthOx Life Sciences,
Millbrae, CA, USA) or Andy Fluor 488-(goat anti-rabbit; cat. no.
L110B; 1:300; GeneCopoeia, Inc., Rockville, MD, USA) conjugated
secondary antibodies were added for 1 h at room temperature.
Immunoreactivity was captured on a confocal laser scanning
microscope (magnification, ×200) and analyzed with Adobe Photoshop
CS6 (Adobe Systems, Inc., San Jose, CA, USA).
Statistical analysis
All values are expressed as the mean ± standard
error of the mean. The data on mechanical allodynia and thermal
hyperalgesia were analyzed by two-way repeated measures analysis of
variance followed by the Bonferroni post hoc test. Western blotting
and RT-qPCR data were analyzed by one-way analysis of variance
followed by the Newman-Keuls post hoc test. Independent samples
Student's t-test was used to analyze the immunofluorescence data.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Upregulated levels of the spinal
apelin-APJ system are associated with CCI-induced neuropathic
pain
The function of the apelin-APJ system in chronic
neuropathic pain was investigated by examining apelin-APJ system
expression at the mRNA and protein level in CCI rats. CCI caused
rapid and persistent neuropathic pain from the third day following
surgery, which lasted for >2 weeks in the rats. mRNA and protein
levels were assessed at 3, 7 and 14 days post-injury; these time
points corresponded, respectively, to the onset, peak and full
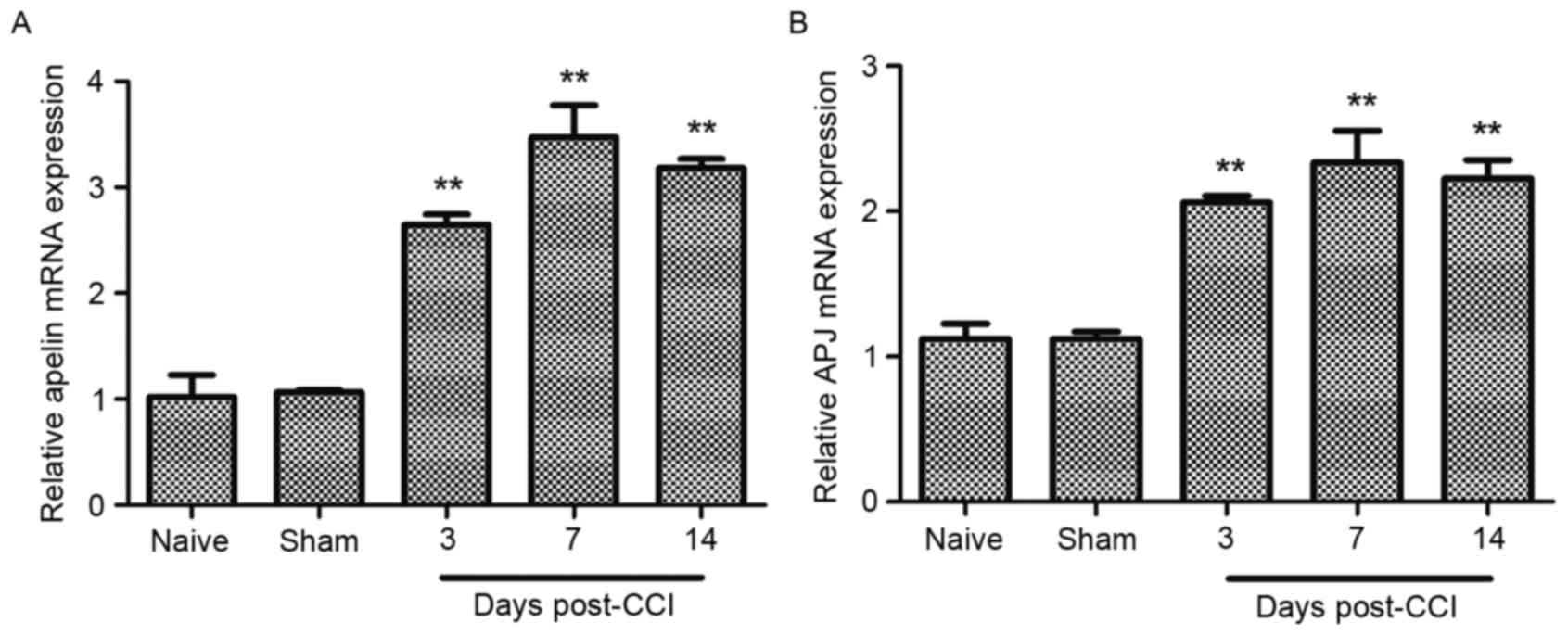
establishment of allodynia post-CCI. RT-qPCR data demonstrated that
apelin mRNA levels continuously increased, peaking at 7 days
post-CCI, with a 3-fold increase in the CCI group compared with the
controls (Fig. 1A). In addition, a
parallel, significant and time-dependent increase in APJ mRNA
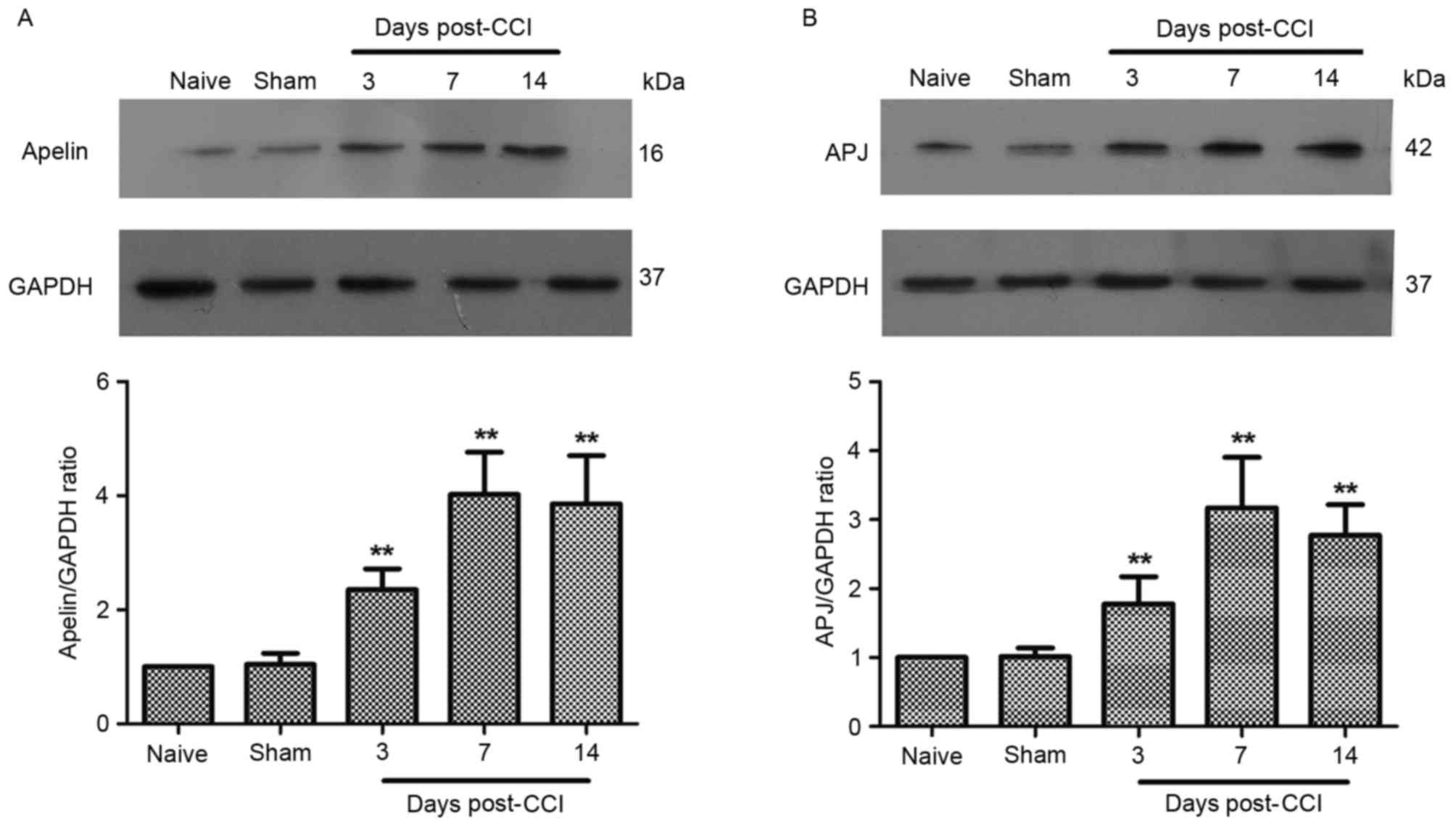
levels, was observed at 3, 7 and 14 days post-CCI (Fig. 1B). Western blot analysis
demonstrated that CCI caused a rapid (within 3 days) and
long-lasting (>14 days) increase in APJ protein amounts in the
spinal cord (Fig. 2B).
Additionally, spinal apelin protein levels were significantly
increased, compared with sham-operated animals, 7 and 14 days
post-surgery (Fig. 2A).
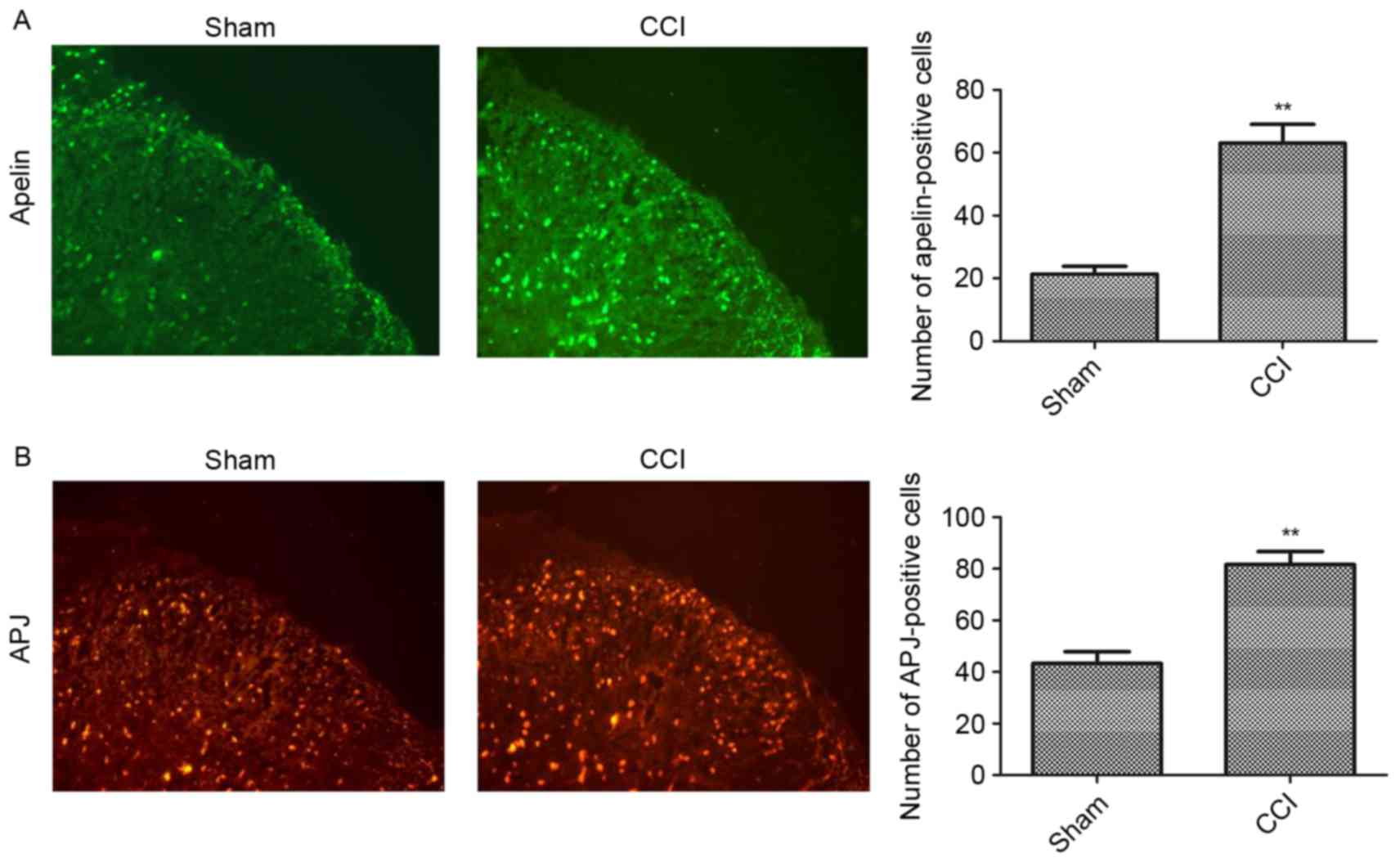
In order to examine spinal cord apelin and APJ
expression levels, immunostaining was carried out in naïve and
CCI-operated mice. As the expression levels of apelin and APJ
peaked at 7 days post-surgery, this time point was selected to
assess the alteration of apelin and APJ expression levels in the
spinal cord. As presented in Fig.
3B, APJ was constitutively expressed in the superficial dorsal
horn. CCI induced a marked increase in APJ expression and an
increased number of APJ-immunoreactive cells was observed in the
CCI group compared with the sham group (Fig. 3B). In addition, low
immunofluorescence intensities were obtained for apelin in sham
animals, while CCI rats exhibited enhanced expression 7 days
post-surgery (Fig. 3A).
The results of the present study suggested that
pain-associated alterations of apelin and APJ levels in the
superficial dorsal horn may be involved in the development and
maintenance of chronic neuropathic pain.
Spinal apelin-APJ system affects
neuropathic pain development and maintenance
Mechanical allodynia and thermal hyperalgesia in the
animals were measured at 1 day before (baseline), and 1, 3, 5 and 7
days following, CCI or sham surgery. It was identified that CCI led
to a gradual reduction in PWT and PWL to a level considered to be
consistent with neuropathic pain, in accordance with a previous
report (26).
In order to investigate the role of increased
expression of the spinal apelin-APJ system in pain sensory
modulation, intrathecal injections of apelin-13 and ML221 were
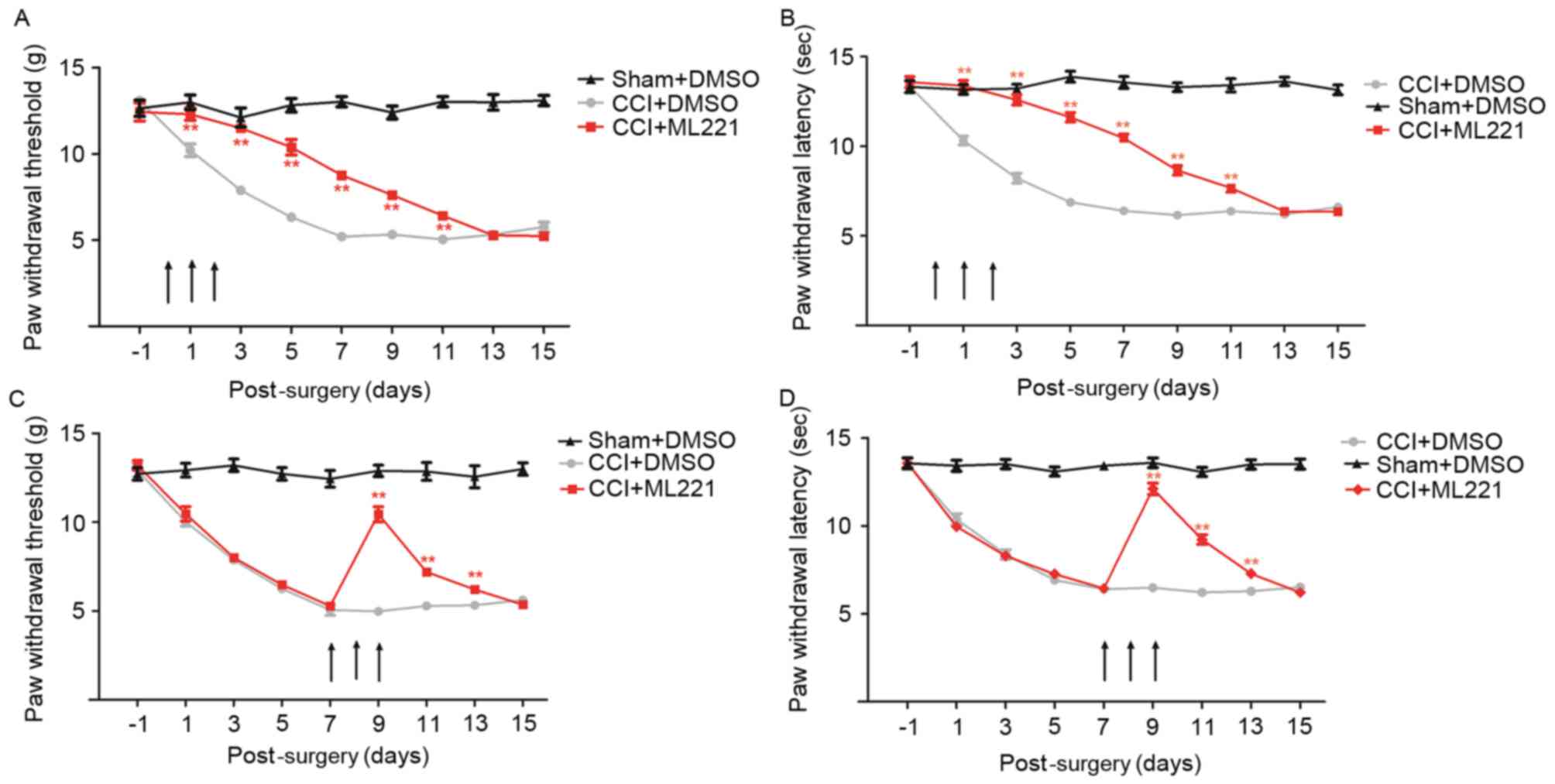
performed in CCI rats. A single intrathecal injection of 10 µg
ML221, 7 days following CCI, caused a transient, marked reduction
of mechanical allodynia and heat hyperalgesia compared with CCI
rats treated with normal saline (Fig.
4A and B). By contrast, an intrathecal injection of apelin-13
did not affect mechanical allodynia or heat hyperalgesia (Fig. 4A and B), suggesting that the spinal
apelin-APJ system contributed to CCI-induced neuropathic pain. Drug
dosages were selected based on a previous study (20).
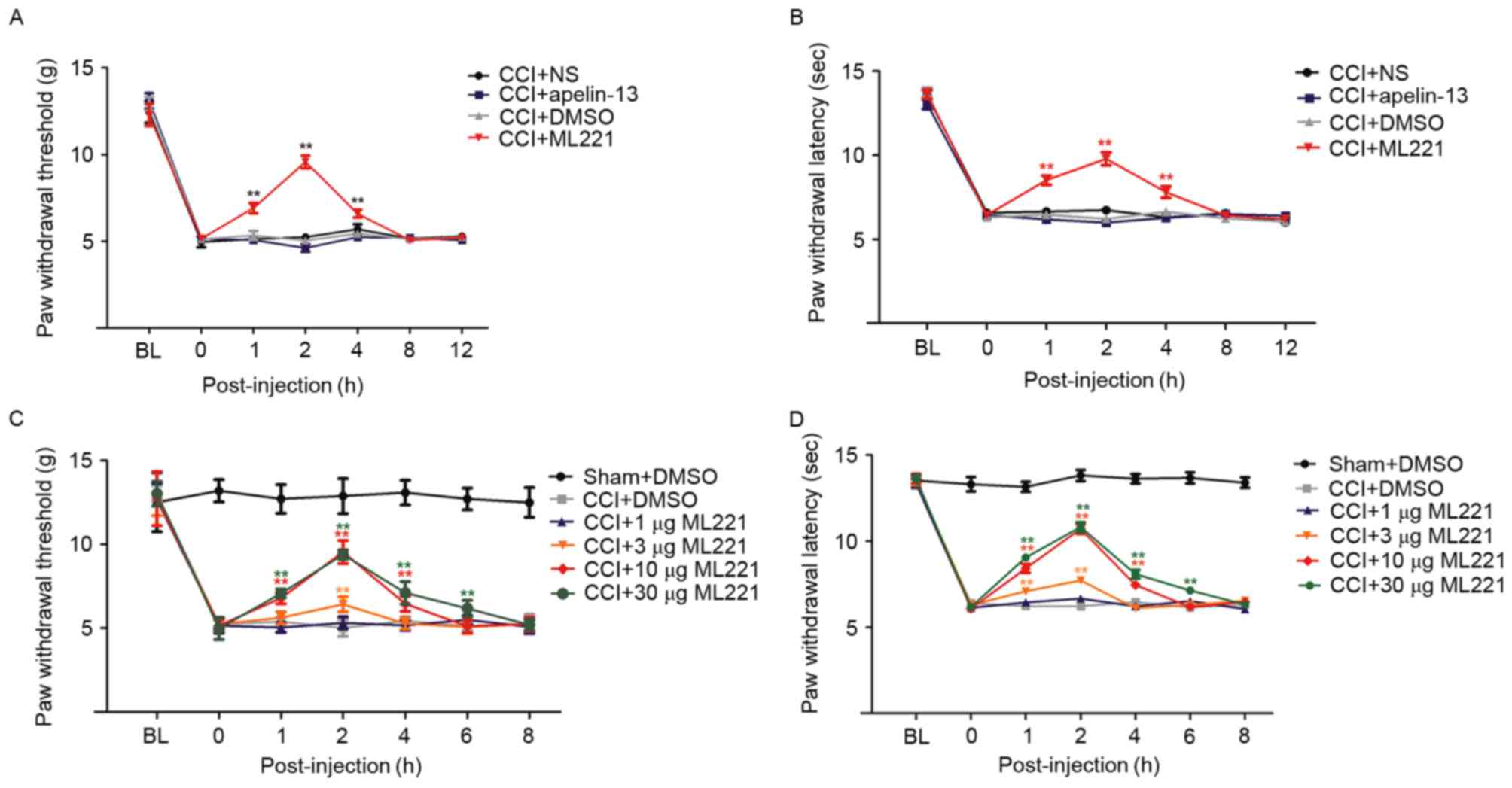 | Figure 4.ML221 alleviates pain
hypersensitivity induced by CCI of the sciatic nerve. The time
course of the effects of intrathecal injection of apelin-13 (10 µg)
and ML221 (10 µg) on (A) mechanical allodynia and (B) thermal
hyperalgesia at 7 days post-CCI. Behavioral tests were performed
before CCI surgery (baseline) and prior to (0 h) and 1, 2, 4, 8 and
12 h following ML221 administration. Increasing doses of ML221 (1,
3, 10 and 30 µg) were administered at 7 days post-CCI. (C) Paw
withdrawal time and (D) paw withdrawal latency were measured prior
to CCI surgery (baseline) and prior to (0 h) and 1, 2, 4, 6 and 8 h
following ML221 administration. A total of eight rats were included
in each group. Data are presented as the mean ± standard error of
the mean. Data were analyzed using two-way analysis of variance.
**P<0.01 vs. CCI + DMSO group. CCI, chronic constriction injury;
DMSO, dimethyl sulfoxide. |
In order to study the dose-effect association of
ML221 in alleviating CCI-induced neuropathic pain, increasing doses
of ML221 (1, 3, 10 and 30 µg) were injected intrathecally 7 days
post-surgery. As presented in Fig. 4C
and D, ML221 inhibited mechanical allodynia and thermal
hyperalgesia in a dose-dependent manner; these effects began 1 h
post-injection, peaked at 2 h and diminished within 4 h. A dose of
3 µg ML221 transiently reduced CCI-induced mechanical allodynia and
heat hyperalgesia, although the reduction was not statistically
significant; this result was not observed with a 1 µg dose. A 10 µg
dose of ML221 significantly increased PWT and PWL between 1 and 4
h. Increased ML221 dosages displayed analgesic effects similar to
those obtained with 10 µg, indicating that a single intrathecal
injection of 10 µg ML221 may be considered the optimal dose for the
reversal of allodynia.
In order to assess the long-term impact of chronic
intrathecal ML221 administration during the initiation of
CCI-induced neuropathic pain, 10 µg ML221 was administered once
daily for 3 days (days 0–2). Pretreatment with ML221 for 3
successive days markedly delayed CCI-induced mechanical allodynia
and heat hyperalgesia for 2–11 days (Fig. 5A and B). In order to determine the
long-term effect of repeated treatment with ML221 in maintaining
CCI-induced neuropathic pain, the same ML221 doses were given at
days 7–9, and produced a somewhat reliable inhibitory effect on
allodynia (Fig. 5C and D).
Analgesia persisted for 4 days following the third treatment with
ML221. The present results suggested that a reduction in the
expression of spinal APJ may inhibit the development and
maintenance of CCI-induced neuropathic pain.
Alleviation of CCI-induced neuropathic
pain by ML221 is associated with phosphorylated ERK downregulation
in the spinal cord
As presented above, increased expression of the
spinal apelin-APJ system contributes to CCI-induced neuropathic
pain. As ERK signaling is implicated in the effects of apelin, the
present study investigated whether apelin-induced nociceptive
behaviors were mediated via the ERK signaling pathway. Rats were
assigned to sham, CCI, CCI + DMSO, and CCI + ML221 groups,
respectively. A single intrathecal injection of ML221 (10 µg) or
vehicle (DMSO) was performed 7 days post-surgery, and
L4-L5 SC segments were harvested 2 h
post-injection for the assessment of ERK and phosphorylated ERK
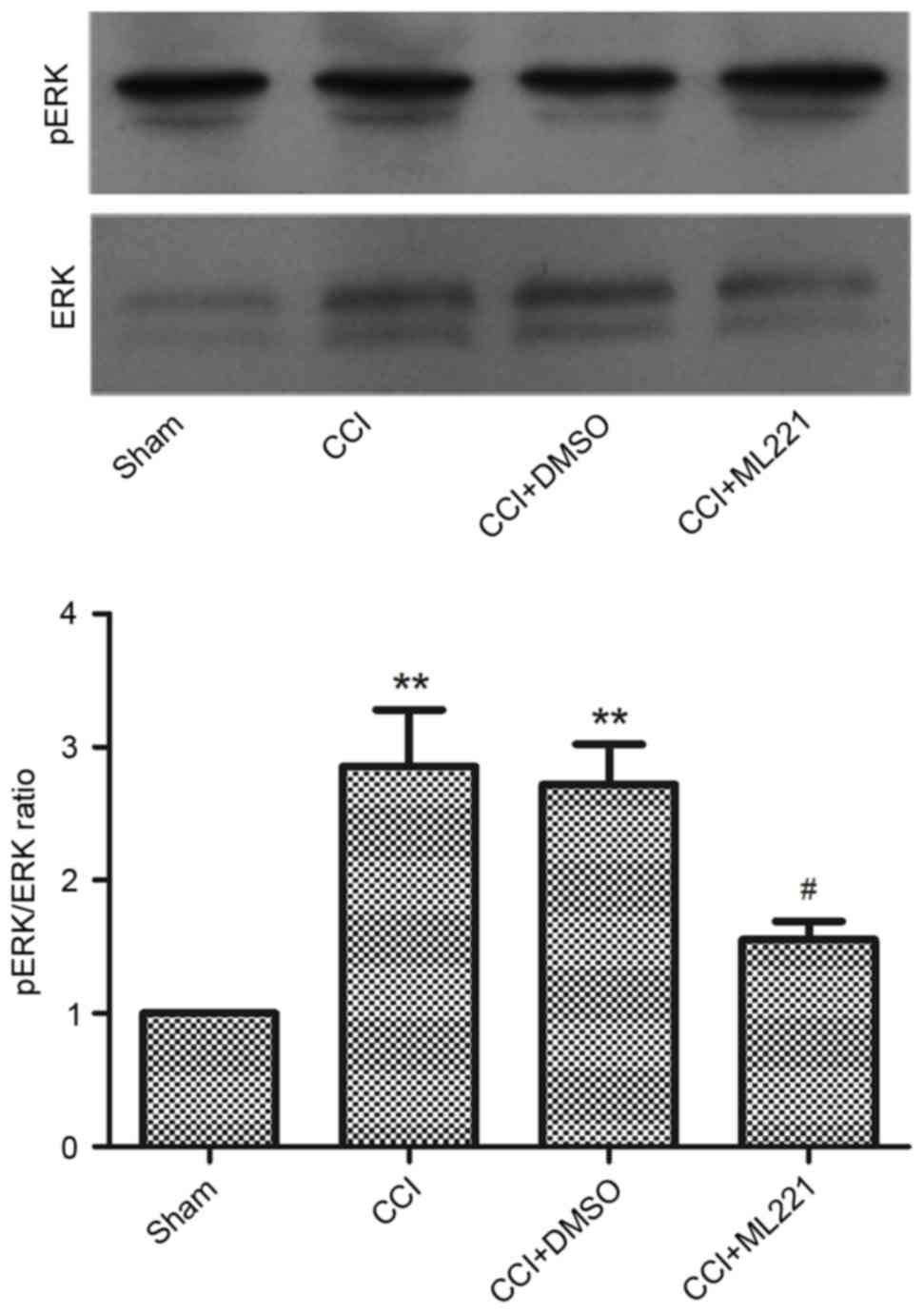
levels. As presented in Fig. 6,
phosphorylated ERK levels were increased in the CCI group compared
with the sham group. Phosphorylated ERK induction was alleviated by
a single intrathecal injection of ML221, corroborating the results
obtained in the behavioral tests as described above. The present
data indicated that the spinal apelin-APJ system may be involved in
neuropathic pain via the ERK signaling pathway.
Discussion
Current evidence suggests that apelin serves a role
in neuronal signaling pathways. In a previous study, apelin was not
detected in glial cells in clinical and rat epilepsy models
(32). In mice with amyotrophic
lateral sclerosis, spinal cord apelin levels are significantly
decreased due to apelin silencing (33). There is increasing evidence to
suggest an association of apelin with inflammatory processes, as
inflammatory mediators, including tumor necrosis factor-α (34), interleukin-6, and interferon-γ
(35) may increase apelin
expression levels, which correlate with markers of inflammation
(36). In addition, apelin was
suggested to be involved in pain modulation.
The present study provides an insight into the
function of apelin in neuropathic pain. It was demonstrated that
the spinal apelin-APJ system serves a role in the modulation of
nerve injury-induced neuropathic pain. Apelin and APJ expression
levels were increased in the ipsilateral spinal dorsal horn
following CCI. Spinal blockade does not activate APJ; rather, it
inhibits CCI-associated neuropathic pain development and
persistence. The apelin-APJ system may contribute to neuropathic
pain via ERK signaling by upregulating phosphorylated ERK in the
spinal dorsal horn. Therefore, the apelin-APJ system may be a novel
mechanism and target in neuropathic pain.
Apelin-13 is the strongest APJ activator expressed
in cells (3,15). As an adipocytokine, apelin is
expressed in and secreted by mature mammalian adipocytes (37). Apelin-13 is also highly expressed
in parts of the CNS, including the hypothalamus, hippocampus,
striatum, pituitary gland, substantia nigra, central gray matter,
dorsal raphe nucleus, amygdala, cerebellum and spinal cord
(19,38–42).
In the present study, a long-lasting increase in apelin mRNA and
protein expression levels in the spinal cord following nerve injury
was observed, as were persistent pain behaviors. Immunofluorescent
staining demonstrated that apelin was expressed in the superficial
spinal dorsal horn. APJ is widely distributed in the CNS and
periphery; the overlap in receptor and peptide expression patterns
demonstrates the variety of neuronal functions of APJ. The present
data indicated that peripheral nerve injury upregulated APJ mRNA
and protein expression from day 3, peaking at day 7, in CCI rats.
Increased APJ immunoreactive signals were observed in the
ipsilateral spinal cord dorsal horn. The present data demonstrate
that the spinal apelin-APJ system is activated during nerve injury,
and therefore may be implicated in pain processes.
Previous studies have reported various effects of
the apelin-APJ system in pain modulation, and suggested that the
differences may be due to apelin-13-induced pain behavior involving
distinct receptor systems in acute and tonic pain models (17,18,20).
In order to further clarify the function of the spinal apelin-APJ
system in neuropathic pain, apelin-13 and ML221 were administered
intrathecally to CCI rats. As presented above, a single intrathecal
injection of 10 µg apelin-13 exhibited no effect on pain behavior;
however, ML221, a newly-synthesized APJ antagonist, significantly
reduced CCI-induced pain hypersensitivity. Nerve injury increased
apelin expression and the APJ antagonist ML221 alleviated
CCI-induced neuropathic pain, indirectly indicating a role for
endogenous apelin in neuropathic pain development, consistent with
previous formalin test data (20).
In the present study, ML221 was used as it inhibits
apelin-13-mediated recruitment of β-arrestin and exhibits 37-fold
increased selectivity for APJ compared with the angiotensin II type
1 receptor (43). The dose-effect
association of ML221 in neuropathic pain was investigated.
Increasing ML221 doses (1, 3, 10 and 30 µg) were administered
intrathecally at day 7 to CCI rats, and 3, 10 and 30 µg partially
reversed mechanical allodynia and heat hyperalgesia. Notably, ML221
at 10 and 30 µg exhibited similar pharmacological effects in
relieving CCI-induced hyperalgesia, indicating that ML221 had
reached peak efficacy. Neuropathic pain involves abnormal
production, distribution and activity of multiple receptors, ion
channels and signaling pathway effectors; therefore, it is
understandable that the inhibition of APJ may not fully reverse
pain behaviors. In a preventive approach, intrathecal
administration of 10 µg ML221 at days 0–2 prevented mechanical
allodynia and markedly reduced the thermal hyperalgesia which
ordinarily occurs 3 days following CCI. Intrathecal administration
of 10 µg ML221 at days 7–9, when thermal and mechanical
hyperalgesia reached full development, attenuated thermal
hyperalgesia and mechanical allodynia at days 7, 9 and 11. The
results of the present study suggested a role for apelin in the
initiation and persistence of neuropathic pain.
The role of apelin in the modulation of pain remains
unclear. Previous evidence indicates that there is functional
cross-talk between APJ and opioid receptors. In a previous study,
apelin mRNA expression was decreased in the lateral hypothalamus of
morphine-dependent mice (44); in
addition, the inhibitory effects of apelin-13 on gastrointestinal
motility involve opioid receptors (45). µ-opioid receptors were suggested to
mediate the antinociceptive activity of apelin-13, which has been
demonstrated to markedly amplify the analgesic effect of morphine
in a tail immersion test and a visceral pain model (17,18).
Spinal apelin-13 has been observed to cause hyperalgesia in a tonic
inflammatory pain model, an effect mediated by APJ and the
γ-aminobutyric acid receptor A, not opioid receptors (20). Similar phenomena involving
interactions between distinct receptor systems have been reported
previously (46). In the present
study, intrathecal injection of 10 µg apelin-13, an increased dose
compared with that which has been demonstrated to induce a
significant antinociceptive effect in a tail-flick test (20), exhibited no impact on pain
sensitivity, while inhibition of APJ significantly reduced
mechanical allodynia and heat hyperalgesia, indicating that
increased apelin and APJ expression levels contribute to
CCI-induced neuropathic pain.
Previous studies in the field of pain research have
focused on the effects of opioid peptides in apelin signaling, and
less research has been focused on the role of apelin, particularly
in chronic pain. APJ possesses a typical 7-transmembrane domain
structure with consensus phosphorylation sites (1). The activation of apelin receptors
inhibits forskolin-stimulated cyclic AMP synthesis (47). In addition to the adenylyl cyclase
inhibition pathway, in vitro studies have demonstrated that
apelin is involved in ERK1/2 induction in a protein kinase
C-dependent pathway (48).
Apelin-13 and apelin-36 have been observed to promote ERK1/2
phosphorylation in Chinese hamster ovary cells and hippocampal
cultures stably producing murine APJ (49,50).
A comparable previous study investigating HEK293 cells transfected
with human APJ also identified ERK1/2 induction by apelin (51). Apelin, acting via APJ, may activate
multiple signaling pathways. The transcription factors or other
cell mediators involved in the action of apelin in CCI-induced
neuropathic pain remain unclear. It is known that ERK induction in
spinal cord neurons through nociceptive activity, involving various
neurotransmitters and relay molecules, is involved in CNS
sensitization and pain hypersensitivity, regulating the activities
of multiple ligands and increasing mRNA synthesis (52). The present study investigated
whether the hyperalgesic effect of the spinal apelin-APJ system on
neuropathic pain was mediated through ERK signaling. As presented
above, intrathecal ML221 administration downregulated
phosphorylated ERK and alleviated pain behaviors. Therefore, ERK
phosphorylation and activation may be a downstream effect of apelin
signaling which drives CCI-induced neuropathic pain. However,
future investigation of the function of apelin, in numerous aspects
of neuropathic pain, is required to clarify the underlying
mechanisms.
Clinical approaches to treating neuropathic pain are
scarce, and the mechanisms behind neuropathic pain are not
completely understood; thus, neurochemical alterations accompanying
neuropathic pain may be considered targets for its treatment. The
present study describes the role of spinal apelin in the processing
of neuropathic pain induced by chronic constriction of the sciatic
nerve, and elucidates receptor and intracellular mechanisms
underlying apelin-mediated pain modulation. The results of the
present study provide a basis for the development of novel
therapeutics for chronic neuropathic pain.
References
|
1
|
O'Dowd BF, Heiber M, Chan A, Heng HH, Tsui
LC, Kennedy JL, Shi X, Petronis A, George SR and Nguyen T: A human
gene that shows identity with the gene encoding the angiotensin
receptor is located on chromosome 11. Gene. 136:355–360. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tatemoto K, Hosoya M, Habata Y, Fujii R,
Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, et
al: Isolation and characterization of a novel endogenous peptide
ligand for the human APJ receptor. Biochem Biophys Res Commun.
251:471–476. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kawamata Y, Habata Y, Fukusumi S, Hosoya
M, Fujii R, Hinuma S, Nishizawa N, Kitada C, Onda H, Nishimura O
and Fujino M: Molecular properties of apelin: Tissue distribution
and receptor binding. Biochim Biophys Acta. 1538:162–171. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hosoya M, Kawamata Y, Fukusumi S, Fujii R,
Habata Y, Hinuma S, Kitada C, Honda S, Kurokawa T, Onda H, et al:
Molecular and functional characteristics of APJ. Tissue
distribution of mRNA and interaction with the endogenous ligand
apelin. J Biol Chem. 275:21061–21067. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee HJ, Tomioka M, Takaki Y, Masumoto H
and Saido TC: Molecular cloning and expression of aminopeptidase A
isoforms from rat hippocampus. Biochim Biophys Acta. 1493:273–278.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Japp AG and Newby DE: The apelin-APJ
system in heart failure: Pathophysiologic relevance and therapeutic
potential. Biochem Pharmacol. 75:1882–1892. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chandrasekaran B, Dar O and McDonagh T:
The role of apelin in cardiovascular function and heart failure.
Eur J Heart Fail. 10:725–732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Quazi R, Palaniswamy C and Frishman WH:
The emerging role of apelin in cardiovascular disease and health.
Cardiol Rev. 17:283–286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brown CH, Ruan M, Scott V, Tobin VA and
Ludwig M: Multi-factorial somato-dendritic regulation of phasic
spike discharge in vasopressin neurons. Prog Brain Res.
170:219–228. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Llorens-Cortes C and Moos F: Opposite
potentiality of hypothalamic coexpressed neuropeptides, apelin and
vasopressin in maintaining body-fluid homeostasis. Prog Brain Res.
170:559–570. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rayalam S, Della-Fera MA, Krieg PA, Cox
CM, Robins A and Baile CA: A putative role for apelin in the
etiology of obesity. Biochem Biophys Res Commun. 368:815–819. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee DK, George SR and O'Dowd BF:
Unravelling the roles of the apelin system: Prospective therapeutic
applications in heart failure and obesity. Trends Pharmacol Sci.
27:190–194. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Horiuchi Y, Fujii T, Kamimura Y and
Kawashima K: The endogenous, immunologically active peptide apelin
inhibits lymphocytic cholinergic activity during immunological
responses. J Neuroimmunol. 144:46–52. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheng B, Chen J, Bai B and Xin Q:
Neuroprotection of apelin and its signaling pathway. Peptides.
37:171–173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Medhurst AD, Jennings CA, Robbins MJ,
Davis RP, Ellis C, Winborn KY, Lawrie KW, Hervieu G, Riley G,
Bolaky JE, et al: Pharmacological and immunohistochemical
characterization of the APJ receptor and its endogenous ligand
apelin. J Neurochem. 84:1162–1172. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reaux A, de Mota N, Skultetyova I, Lenkei
Z, El Messari S, Gallatz K, Corvol P, Palkovits M and
Llorens-Cortes C: Physiological role of a novel neuropeptide,
apelin, and its receptor in the rat brain. J Neurochem.
77:1085–1096. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu N, Wang H, Fan L and Chen Q:
Supraspinal administration of apelin-13 induces antinociception via
the opioid receptor in mice. Peptides. 30:1153–1157. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lv SY, Qin YJ, Wang NB, Yang YJ and Chen
Q: Supraspinal antinociceptive effect of apelin-13 in a mouse
visceral pain model. Peptides. 37:165–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reaux A, Gallatz K, Palkovits M and
Llorens-Cortes C: Distribution of apelin-synthesizing neurons in
the adult rat brain. Neuroscience. 113:653–662. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lv S, Yang YJ, Hong S, Wang N, Qin Y, Li W
and Chen Q: Intrathecal apelin-13 produced different actions in
formalin test and tail-flick test in mice. Protein Pept Lett.
20:926–931. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Smith TP, Schlenz AM, Schatz JC, Maitra R
and Sweitzer SM: Modulation of pain in pediatric sickle cell
disease: Understanding the balance between endothelin mediated
vasoconstriction and apelin mediated vasodilation. Blood Cells Mol
Dis. 54:155–159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cui RR, Mao DA, Yi L, Wang C, Zhang XX,
Xie H, Wu XP, Liao XB, Zhou H, Meng JC, et al: Apelin suppresses
apoptosis of human vascular smooth muscle cells via APJ/PI3-K/Akt
signaling pathways. Amino Acids. 39:1193–1200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Than A, Cheng Y, Foh LC, Leow MK, Lim SC,
Chuah YJ, Kang Y and Chen P: Apelin inhibits adipogenesis and
lipolysis through distinct molecular pathways. Mol Cell Endocrinol.
362:227–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Perjes A, Skoumal R, Tenhunen O, Kónyi A,
Simon M, Horváth IG, Kerkelä R, Ruskoaho H and Szokodi I: Apelin
increases cardiac contractility via protein kinase Cε- and
extracellular signal-regulated kinase-dependent mechanisms. PLoS
One. 9:e934732014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zimmermann M: Ethical guidelines for
investigations of experimental pain in conscious animals. Pain.
16:109–110. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bennett GJ and Xie YK: A peripheral
mononeuropathy in rat that produces disorders of pain sensation
like those seen in man. Pain. 33:87–107. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mestre C, Pélissier T, Fialip J, Wilcox G
and Eschalier A: A method to perform direct transcutaneous
intrathecal injection in rats. J Pharmacol Toxicol Methods.
32:197–200. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dixon WJ: Efficient analysis of
experimental observations. Annu Rev Pharmacol Toxicol. 20:441–462.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang JX, Hua L, Li YQ, Jiang YY, Han D,
Liu H, Tang QQ, Yang XN, Yin C, Hao LY, et al: Caveolin-1 in the
anterior cingulate cortex modulates chronic neuropathic pain via
regulation of NMDA receptor 2B subunit. J Neurosci. 35:36–52. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang X, Peng X, Fang M, Zhou C, Zhao F,
Zhang Y, Xu Y, Zhu Q, Luo J, Chen G and Wang X: Up-regulation of
apelin in brain tissue of patients with epilepsy and an epileptic
rat model. Peptides. 32:1793–1799. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kasai A, Kinjo T, Ishihara R, Sakai I,
Ishimaru Y, Yoshioka Y, Yamamuro A, Ishige K, Ito Y and Maeda S:
Apelin deficiency accelerates the progression of amyotrophic
lateral sclerosis. PloS One. 6:e239682011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Daviaud D, Boucher J, Gesta S, Dray C,
Guigne C, Quilliot D, Ayav A, Ziegler O, Carpene C, Saulnier-Blache
JS, et al: TNFalpha up-regulates apelin expression in human and
mouse adipose tissue. FASEB J. 20:1528–1530. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han S, Wang G, Qi X, Englander EW and
Greeley GH Jr: Involvement of a Stat3 binding site in
inflammation-induced enteric apelin expression. Am J Physiol
Gastrointest Liver Physiol. 295:G1068–G1078. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Malyszko J, Malyszko JS, Pawlak K,
Wolczynski S and Mysliwiec M: Apelin, a novel adipocytokine, in
relation to endothelial function and inflammation in kidney
allograft recipients. Transplant Proc. 40:3466–3469. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Boucher J, Masri B, Daviaud D, Gesta S,
Guigné C, Mazzucotelli A, Castan-Laurell I, Tack I, Knibiehler B,
Carpéné C, et al: Apelin, a newly identified adipokine up-regulated
by insulin and obesity. Endocrinology. 146:1764–1771. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Choe W, Albright A, Sulcove J, Jaffer S,
Hesselgesser J, Lavi E, Crino P and Kolson DL: Functional
expression of the seven-transmembrane HIV-1 co-receptor APJ in
neural cells. J Neurovirol. 6 Suppl 1:S61–S69. 2000.PubMed/NCBI
|
|
39
|
O'Carroll AM, Selby TL, Palkovits M and
Lolait SJ: Distribution of mRNA encoding B78/apj, the rat homologue
of the human APJ receptor, and its endogenous ligand apelin in
brain and peripheral tissues. Biochim Biophys Acta. 1492:72–80.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brailoiu GC, Dun SL, Yang J, Ohsawa M,
Chang JK and Dun NJ: Apelin-immunoreactivity in the rat
hypothalamus and pituitary. Neurosci Lett. 327:193–197. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
de Mota N, Lenkei Z and Llorens-Cortès C:
Cloning, pharmacological characterization and brain distribution of
the rat apelin receptor. Neuroendocrinology. 72:400–407. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee DK, Cheng R, Nguyen T, Fan T,
Kariyawasam AP, Liu Y, Osmond DH, George SR and O'Dowd BF:
Characterization of apelin, the ligand for the APJ receptor. J
Neurochem. 74:34–41. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Maloney PR, Khan P, Hedrick M, Gosalia P,
Milewski M, Li L, Roth GP, Sergienko E, Suyama E, Sugarman E, et
al: Discovery of 4-oxo-6-((pyrimidin-2-ylthio)methyl)-4H-pyran-3-yl
4-nitrobenzoate (ML221) as a functional antagonist of the apelin
(APJ) receptor. Bioorg Med Chem Lett. 22:6656–6660. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Befort K, Filliol D, Darcq E, Ghate A,
Matifas A, Lardenois A, Muller J, Thibault C, Dembele D, Poch O and
Kieffer BL: Gene expression is altered in the lateral hypothalamus
upon activation of the mu opioid receptor. Ann N Y Acad Sci.
1129:175–184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang YJ, Lv SY, Xiu MH, Xu N and Chen Q:
Intracerebroventricular administration of apelin-13 inhibits distal
colonic transit in mice. Peptides. 31:2241–2246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lutfy K, Eitan S, Bryant CD, Yang YC,
Saliminejad N, Walwyn W, Kieffer BL, Takeshima H, Carroll FI,
Maidment NT and Evans CJ: Buprenorphine-induced antinociception is
mediated by mu-opioid receptors and compromised by concomitant
activation of opioid receptor-like receptors. J Neurosci.
23:10331–10337. 2003.PubMed/NCBI
|
|
47
|
Habata Y, Fujii R, Hosoya M, Fukusumi S,
Kawamata Y, Hinuma S, Kitada C, Nishizawa N, Murosaki S, Kurokawa
T, et al: Apelin, the natural ligand of the orphan receptor APJ, is
abundantly secreted in the colostrum. Biochim Biophys Acta.
1452:25–35. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Masri B, Lahlou H, Mazarguil H, Knibiehler
B and Audigier Y: Apelin (65–77) activates extracellular
signal-regulated kinases via a PTX-sensitive G protein. Biochem
Biophys Res Commun. 290:539–545. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Masri B, Morin N, Pedebernade L,
Knibiehler B and Audigier Y: The apelin receptor is coupled to Gi1
or Gi2 protein and is differentially desensitized by apelin
fragments. J Biol Chem. 281:18317–18326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
O'Donnell LA, Agrawal A, Sabnekar P,
Dichter MA, Lynch DR and Kolson DL: Apelin, an endogenous neuronal
peptide, protects hippocampal neurons against excitotoxic injury. J
Neurochem. 102:1905–1917. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bai B, Tang J, Liu H, Chen J, Li Y and
Song W: Apelin-13 induces ERK1/2 but not p38 MAPK activation
through coupling of the human apelin receptor to the Gi2 pathway.
Acta Biochim Biophys Sin (Shanghai). 40:311–318. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ji RR, Gereau RW IV, Malcangio M and
Strichartz GR: MAP kinase and pain. Brain Res Rev. 60:135–148.
2009. View Article : Google Scholar : PubMed/NCBI
|