Introduction
Esophageal cancer is one of the most frequently
diagnosed cancers and is responsible for numerous cancer-associated
mortalities worldwide (1), with an
increasing incidence annually (2).
Esophageal cancer has two predominant histological types:
esophageal squamous cell carcinoma (ESCC), which accounts for 95%
of all esophageal cancers in China, and esophageal adenocarcinoma,
which frequently occurs in developed countries (1).
Radiotherapy is an essential therapeutic method in
the treatment of patients with inoperable and locally advanced
ESCC. However, the response of esophageal cancer to radiotherapy is
variable, and the majority of ESCC patients do not benefit from
radiotherapy due to radioresistance (3–6).
Therefore, searching for molecular markers which may enhance the
radiosensitivity of esophageal cancer is of primary concern, in
order to improve clinical outcomes.
Liver kinase B (LKB) 1, additionally termed,
serine/threonine kinase 11, is important in various biological
processes, including cell growth, apoptosis and DNA damage
response, cell motility, energy metabolism and cell polarity
(7,8). Mutations in the LKB1 gene have been
associated with a broad spectrum of human cancers, and LKB1 has
been indicated to be a critical tumor suppressor (9,10).
Recently, various studies have suggested that LKB1 is involved in
the regulation of radiosensitivity of cancer cells, and may be
important in regulating the radiosensitivity of different tumor
types (11,12). However, the potential function of
LKB1 in esophageal cancer radiotherapy remains to be
elucidated.
The present study investigated the role of LKB1 in
the radiosensitivity of esophageal cancer and its molecular
mechanism. The results may aid in providing a novel mechanism to
improve the efficacy of radiotherapy in the treatment of esophageal
cancer patients in the future.
Materials and methods
Animal experiments
All animal experiments were approved by the Ethics
Committee of The First Affiliated Hospital of Fujian Medical
University (Fujian, China). A total of 156 male BALB/C nude mice,
(age, 5–6 weeks; weight, 16–20 g) were provided by the Shanghai
Laboratory Animal Research Center (Shanghai, China). The mice were
maintained in individual cages at a controlled temperature (22±2°C)
and humidity (55%), under 12/12 h light/dark cycles, with free
access to food and water. To develop xenograft tumors, Eca-109
cells transfected with LKB1 overexpression plasmid or empty vector
were harvested, washed with PBS and implanted into the hind limb of
the BALB/C nude mice (2×106 cells/0.1 ml). When xenograft tumors
reached a mean diameter of 5 mm, the ‘radiation group’ animals were
irradiated every 4 days with a dose of 12 Gy radiation in three
fractions. Tumor growth was measured every 2 days by a caliper
until day 25, and the tumor volume (mm3) was calculated according
to the formula: Tumor volume (mm3) = length (mm) × width
(mm2)/2.
Cell culture
Eca-109 cells were obtained from the Cell Bank of
the Chinese Academy of Sciences (Shanghai, China). The cells were
maintained in Dulbecco's modified Eagle's medium (Hyclone; GE
Healthcare, Logan, UT, USA) supplemented with 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
100 µg/ml penicillin at 37°C in a humidified atmosphere containing
5% CO2. Compound C was purchased from EMD Millipore
(Billerica, MA, USA) and used at a concentration of 20 µM.
Cell transfection
The LKB1-pcDNA3.1 plasmid was synthesized by
Shenzhen Zhonghong Boyuan Biological Technology Co., Ltd.
(Shenzhen, China). A total of 1 µg plasmid DNA was transfected into
the Eca-109 cells using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
protocol.
MTT assay
Cell viability was determined via an MTT assay. The
cells were plated in 96-well plates at a density of 5×104
cells/well. Following incubation for 1, 2, 3 and 4 days, 5 µl of
MTT solution (1 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was added to each well, and the plates were incubated for
an additional 4 h. Finally, dimethyl sulfoxide (Sigma-Aldrich;
Merck KGaA) was added to dissolve the formazan crystals and the
absorbance was measured at a wavelength of 570 nm.
Colony formation assay
Eca-109 cells were seeded into six-wells plates, at
a density of 500 cells/well. Following overnight culture at 37°C,
the cells were exposed to radiation at 0, 2, 4, 6 and 8 Gy with an
average dose rate of 100 cGy/min. Following incubation at 37°C in
an environment containing 5% CO2 for 10 days, the
colonies were fixed in 4% formaldehyde at room temperature for 30
min, and stained with crystal violet at room temperature for 2 h.
Stained cells were observed under a microscope (TS100; Nikon
Corporation, Tokyo, Japan) and colonies containing >50 cells
were counted as clonogenic survivors.
Flow cytometry
The Annexin V-FITC and Propidium Iodide (PI)
Apoptosis assay kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China) was used to determine the percentage of cells undergoing
apoptosis, according to the manufacturer's protocol. Briefly, cells
were harvested 8 h post-irradiation at a dose of 8 Gy. Following
washing with PBS, 1×106 cells were dual-stained with 10 µl PI and 5
µl Alexa Fluor 488-Annexin V at room temperature for 15 min, and
subsequently analyzed by flow cytometry (BD FACSCanto II flow
cytometer; BD Biosciences, Franklin Lakes, NJ, USA) using the BD
FACSCanto™ Clinical software version 2.1 (BD Biosciences).
Western blot analysis
Cells were lysed in a radioimmunoprecipitation assay
lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China)
at room temperature for 5 min. Cell lysates were centrifuged at
13,000 × g for 5 min at room temperature, and the supernatants were
harvested. Protein concentrations were quantified using a BCA kit
(Pierce; Thermo Fisher Scientific, Inc.). Equal amounts of
extracted protein samples (5 µg) were separated by 12% SDS-PAGE and
transferred onto polyvinylidence difluoride membranes (EMD
Millipore). The membranes were blocked with 3% bovine serum albumin
(Sangon Biotech Co., Ltd., Shanghai, China) at 4°C overnight, and
the blots were probed with rabbit polyclonal antibodies against
LKB1 (catalog no. ab79355; 1:500), rabbit polyclonal to active
caspase-3 (catalog no. ab2302; 1:200), mouse monoclonal to cleaved
poly(ADP-ribose) polymerase (PARP; catalog no. ab13907; 1:200),
rabbit polyclonal to microtubule-associated protein 1 light chain 3
α/β (LC3 α/β; catalog no. ab128025; 1:800) (all from Abcam,
Cambridge, MA, USA), rabbit monoclonal to phosphorylated
(p)-AMP-activated protein kinase (AMPK) α (Thr172; catalog no.
4188; 1:400), and mouse monoclonal to beclin-1 (catalog no. 4122;
1:400) (both from Cell Signaling Technology, Inc., Danvers, MA,
USA) and mouse monoclonal to GAPDH (catalog no. ab8245; 1:2,000;
Abcam) at 37°C for 2 h. Following washing with Tris buffered saline
Tween-20, the membranes were incubated with goat anti-mouse
horseradish peroxidase-conjugated immunoglobulin (Ig) G (catalog
no. sc-2005; 1:5,000) or goat anti-rabbit horseradish
peroxidase-conjugated IgG (catalog no. sc-2004; 1:5,000) (both from
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) secondary
antibodies at 37°C for 1 h, and protein bands were visualized by a
SuperSignal West Femto kit (Pierce; Thermo Fisher Scientific,
Inc.). Blots were semi-quantified by densitometric analysis using
ImageJ software version 1.46 (National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
Statistical analysis was performed using SPSS
statistical software, version 19.0 (IBM SPSS, Armonk, NY, USA).
Data are presented as the mean ± standard deviation of 3
independent experiments. The statistical significance of the
differences between groups was assessed using unpaired Student's
t-test for pair-wise comparisons or one-way analysis of variance
followed by a post hoc least significant difference test for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
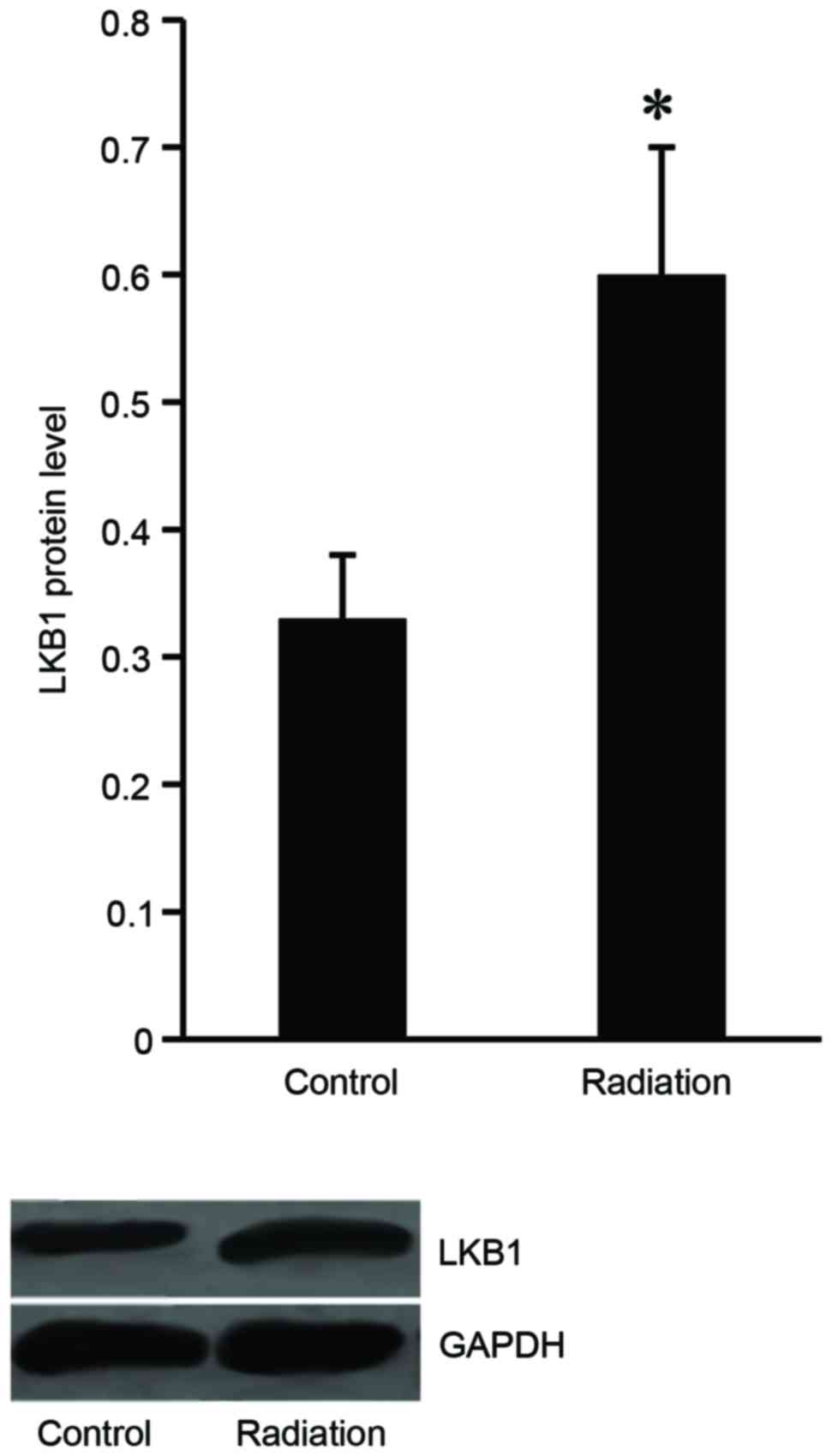
LKB1 expression is induced in Eca-109
cells following radiation treatment
To investigate the effect of radiation treatment on
LKB1 expression, the Eca-109 cells were subjected to irradiation
from 1–8 Gy 10 times, and then western blot analysis was performed
to examine LKB1 expression level in the Eca-109 cells. The results
demonstrated that LKB1 protein expression increased in the
irradiated cells compared with the untreated cells (Fig. 1).
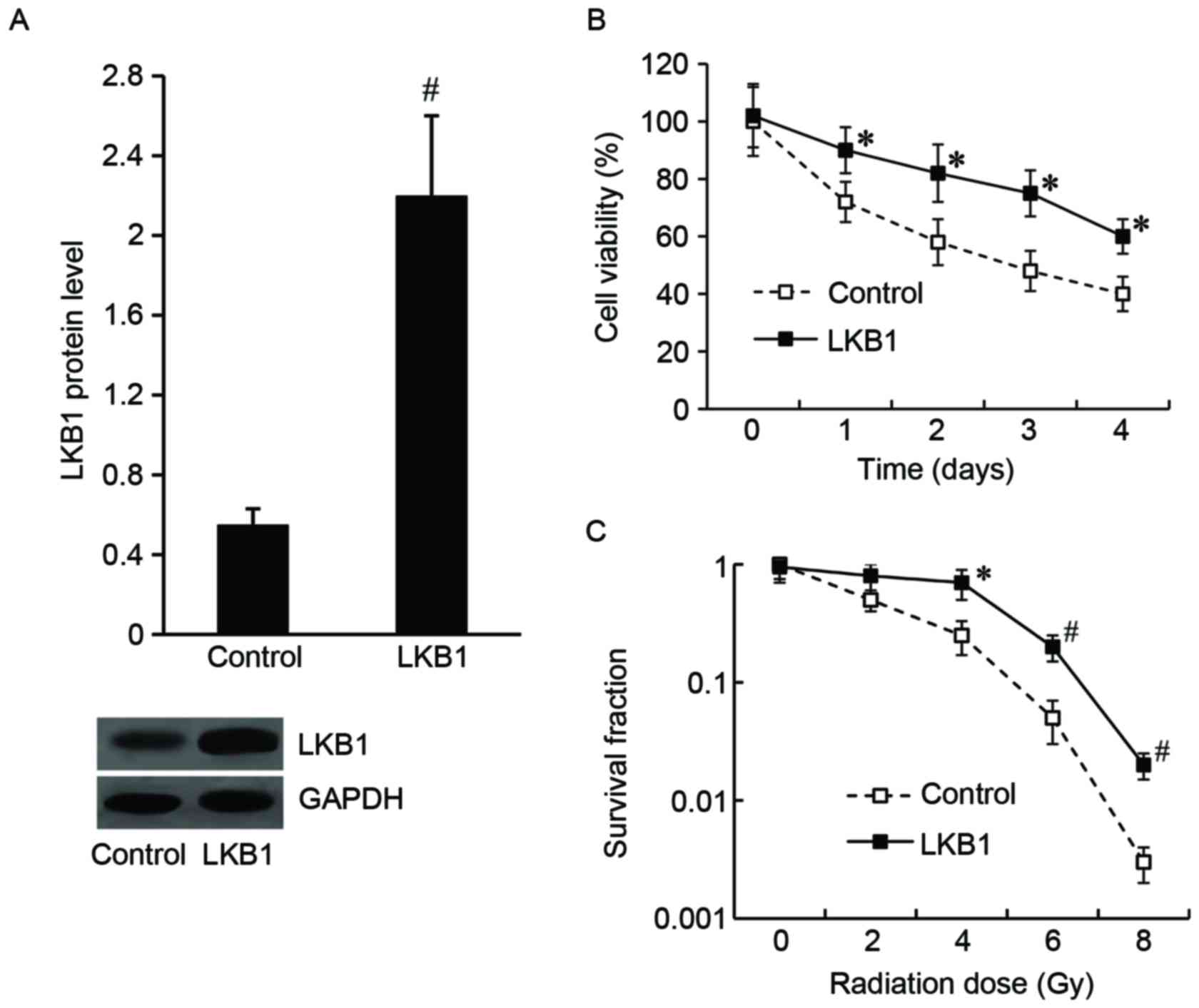
LKB1 modulates the radiosensitivity of
Eca-109 cells
To determine the effect of LKB1 on the modulation of
Eca-109 cell radiosensitivity, Eca-109 cells were transfected with
the LKB1-pcDNA3.1 plasmid to overexpress LKB1, and the pcDNA3.1
vector was transfected as the control. As demonstrated in Fig. 2A, the LKB1 protein level was
significantly increased in LKB1-pcDNA3.1-transfected cells compared
with the control.
Following irradiation at a dose of 8 Gy for 24 h, an
MTT assay was performed and the results demonstrated that the cell
viability was significantly increased in the LKB1-pcDNA3.1 group
compared with the control group (Fig.
2B). The results of the colony formation assay demonstrated
that the LKB1-pcDNA3.1-transfected cells exhibited an increased
survival fraction compared with control cells (Fig. 2C).
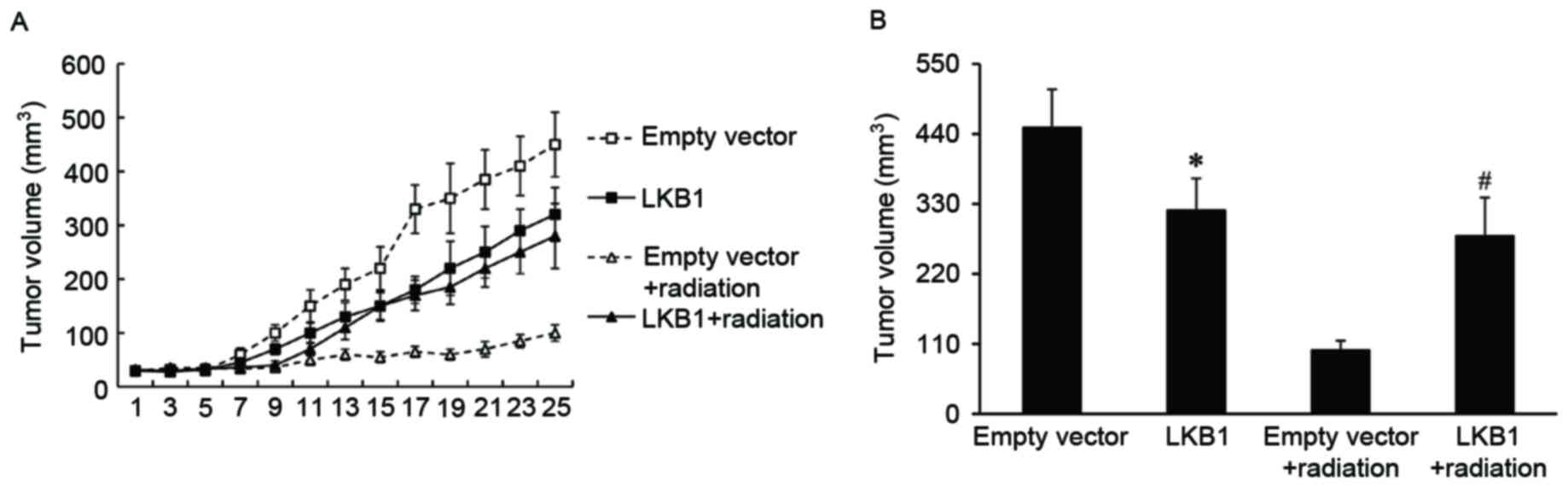
LKB1 induces radioresistance in
xenograft tumor models
To investigate if LKB1 affected the radiosensitivity
of esophageal cancer in vivo, xenograft tumors with a high
level of LKB1 expression were established in BALB/c nude mice and
exposed to irradiation. Xenograft tumors transduced with empty
vector were used as a control. It was observed that the tumor
volume was significantly increased in LKB1-overexpressed tumors
compared with that of the control tumors, when treated with
radiation (Fig. 3).
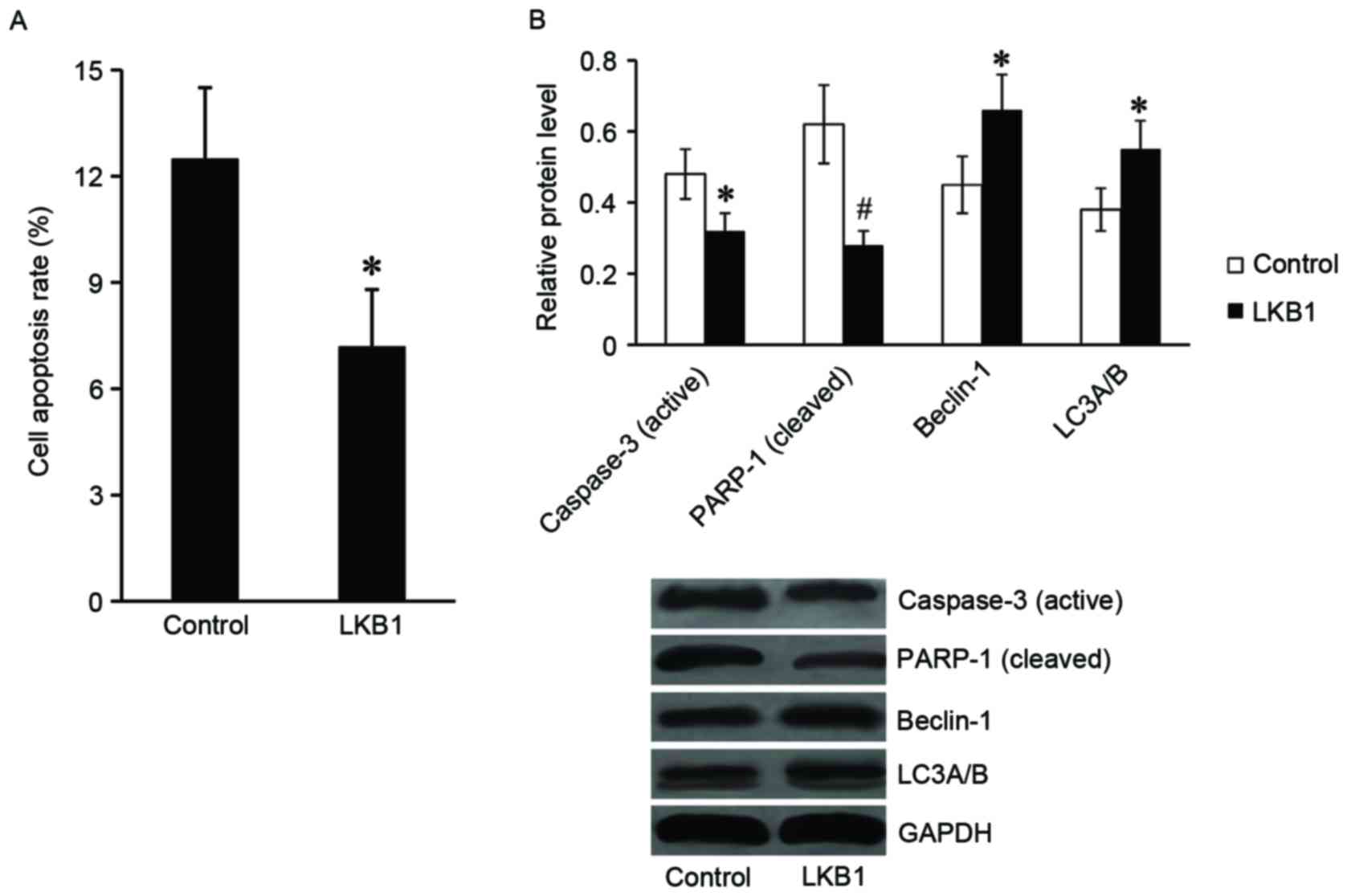
LKB1 inhibits apoptosis and activates
autophagy of Eca-109 cells following radiation treatment
Subsequently, the present study explored the role of
LKB1 in apoptosis and autophagy of Eca-109 cells following
radiation. Following exposure to radiation at 8 Gy for 24 h, the
cells were harvested for flow cytometry and western blot assays.
The results of the flow cytometry assay demonstrated that the cell
apoptosis rate was significantly downregulated in
LKB1-pcDNA3.1-transfected cells compared with the control cells
(Fig. 4A). In addition, the
expression of apoptotic proteins, including the active caspase-3
and cleaved PARP-1 were markedly reduced in
LKB1-pcDNA3.1-transfected cells compared with control cells. The
expression levels of autophagy-associated proteins (beclin-1 and
LC3α/β) were revealed to be significantly increased in
LKB1-pcDNA3.1-transfected cells compared with control cells
(Fig. 4B).
Effect of LKB1 on radiosensitivity of
Eca-109 cells is mediated by AMPK
To further examine the molecular mechanism
underlying the effect of LKB1 on radiosensitivity of Eca-109 cells,
the cells were transfected with the LKB1-pcDNA3.1 plasmid and/or
treated with compound C, an AMPK inhibitor, and then subjected to
irradiation. The activation of AMPK was measured by AMPKα
phosphorylation on Thr172. The western blot analysis demonstrated
that the expression level of p-AMPKα (Thr172) in
LKB1-pcDNA3.1-transfected cells was significantly upregulated
compared with pcDNA3.1-transfected cells, following radiation
treatment. Compound C effectively inhibited p-AMPKα (Thr172)
protein expression (Fig. 5A). As
presented in e. 5B and C, following irradiation treatment, the cell
viability and survival fraction were significantly increased in
LKB1-pcDNA3.1-transfected cells compared with control cells.
However, following inhibition of AMPK, the increased cell viability
and survival fraction induced by LKB1 was inhibited.
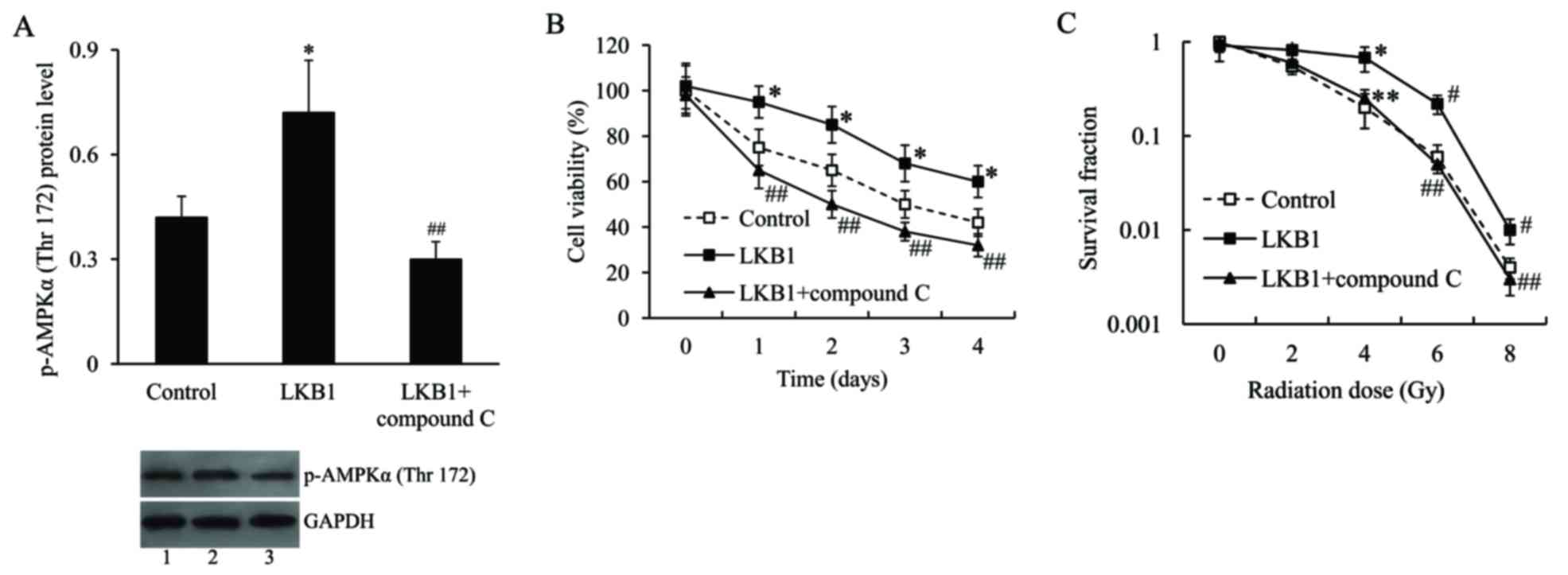 | Figure 5.The effect of LKB1 on radiosensitivity
of Eca-109 cells was mediated by AMPK. (A) Representative image and
quantification of expression level of p-AMPKα (Thr172) in Eca-109
cells transfected with LKB1-pcDNA3.1 plasmid and then treated with
compound C, following radiation exposure. Lane 1, control; lane 2,
LKB1; lane 3, LKB1 + compound C. (B) Cell viability of Eca-109
cells transfected with LKB1-pcDNA3.1 plasmid and treated with
compound C, following radiation exposure. (C) Survival fraction of
Eca-109 cells transfected with LKB1-pcDNA3.1 plasmid and treated
with compound C, following radiation exposure. *P<0.05,
#P<0.01 vs. control group; **P<0.05 and
##P<0.01 vs. LKB1 group. LKB1, liver kinase B1; p,
phosphorylated; AMPK, AMP-activated protein kinase. |
Discussion
To the best of our knowledge, LKB1 appears to
exhibit dual characteristics in human cancers: LKB1 acts as a
well-known tumor suppressor by suppressing cell growth and
metastasis, however it additionally enhances chemo and
radioresistance of various tumor cells. Using genetically
engineered mouse models of primary lung adenocarcinoma, it was
previously demonstrated that loss of the LKB1 gene impairs the
response of Kras-mutant lung cancers to standard chemotherapy
(13) and renders the tumors less
responsive to radiotherapy (11).
Saigusa et al (12)
revealed that in patients with locally advanced rectal cancer
treated with pre-operative chemoradiotherapy, LKB1 gene expression
levels are increased in patients with a poor pathological response
and tumor recurrence, suggesting that LKB1 expression may be
involved in resistance to chemoradiotherapy. In addition, Xia et
al (14) demonstrated that
LKB1 enhances chemoresistance in breast cancer. These reports
indicate that LKB1 may serve different roles in the
radiosensitivity of various types of tumor. Wang et al
(15) revealed that downregulation
of LKB1 is associated with esophageal cancer progression and LKB1
may inhibit esophageal cancer cell proliferation. However, few
studies have reported the association between LKB1 and
radiosensitivity of esophageal cancer. The present study
demonstrated that LKB1 expression was significantly upregulated in
Eca-109 cells in response to radiation, and it was hypothesized
that LKB1 may modulate the radiation response of esophageal cancer.
Following this, the in vivo and in vitro studies
revealed that LKB1 overexpression suppressed the esophageal cancer
cell response to irradiation, which confirmed that LKB1 induced
radioresistance of esophageal cancer.
Radiation induces an apoptotic response which
destroys the cells following radiotherapy (16). The intrinsic and extrinsic
apoptotic pathways lead to the activation of caspases, which
proteolytically cleave the substrate PARP. Autophagy is ‘the second
apoptosis’ which is important in the programmed cell apoptotic
response (17). Autophagy may be
activated as a response of ionizing radiation and targeting
autophagy is an antitumor strategy which is currently of interest
(18–20). The present study revealed that LKB1
suppressed apoptosis and activated autophagy of esophageal cancer
cells with radiotherapy. These data suggested that apoptosis and
authophagy were the potential mechanisms underlying the effect of
LKB1 on the induction of radioresistance in the esophageal cancer
cells.
LKB1 has previously been demonstrated to regulate
the activities of various signal transduction pathways, including
bone morphogenetic protein receptor signaling (21) and the Notch (22) and Wnt (23) signaling pathways. LKB1 is a primary
upstream kinase of AMPK, an enzyme that regulates a wide variety of
cellular functions, including growth, metabolism, stress, autophagy
and polarity (24,25). Ionizing radiation may activate AMPK
in various human cancer cells (26). It has been suggested that AMPK may
be important in the cellular response to radiotherapy (27–29).
To explore the potential molecular mechanism underlying the
function of LKB1 in esophageal cancer radiosensitivity, the present
study investigated AMPK signaling. LKB1 increases AMPK activity by
phosphorylating its Thr172 residue (30,31).
Compound C, an AMPK inhibitor, was used to suppress AMPK activity.
It was demonstrated that the promotive effect of radioresistance
induced by LKB1 on Eca-109 cells, was attenuated by AMPK
inhibition. These results suggested that AMPK mediates the
radioresistance of Eca-109 cells via LKB1.
In conclusion, LKB1 acts as a tumor suppressor in
various cancers, however it was revealed to exhibit a differing
function with exposure to radiotherapy. To the best of our
knowledge, the present study demonstrated for the first time, that
LKB1 induced radioresistance of esophageal cancer cells to
irradiation via suppression of apoptosis and activation of
autophagy, and this effect was mediated by AMPK. Further studies
are required to investigate the association between increased
autophagy and increased radioresistance of esophageal cancer cells
to further clarify the molecular mechanisms underlying these
events. The findings will aid in the understanding of the
occurrence of radioresistance in esophageal cancer treatment, and
may provide a novel target to maximize the efficiency of esophageal
cancer radiotherapy in the future.
References
|
1
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schuchert MJ, Luketich JD and Landreneau
RJ: Management of esophageal cancer. Curr Probl Surg. 47:845–946.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fareed KR, Kaye P, Soomro IN, Ilyas M,
Martin S, Parsons SL and Madhusudan S: Biomarkers of response to
therapy in oesophago-gastric cancer. Gut. 58:127–143. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gillies RS, Middleton MR and Blesing C: A
reply to evidence-based radiation oncology: Oesophagus. Radiother
Oncol. 94:387–388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Berger B and Belka C: Evidence-based
radiation oncology: Oesophagus. Radiother Oncol. 92:276–290. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Borghesi S, Hawkins MA and Tait D:
Oesophagectomy after definitive chemoradiation in patients with
locally advanced oesophageal cancer. Clin Oncol (R Coll Radiol).
20:221–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li N, Huang D, Lu N and Luo L: Role of the
LKB1/AMPK pathway in tumor invasion and metastasis of cancer cells
(Review). Oncol Rep. 34:2821–2826. 2015.PubMed/NCBI
|
|
8
|
Gan RY and Li HB: Recent progress on liver
kinase B1 (LKB1): Expression, regulation, downstream signaling and
cancer suppressive function. Int J Mol Sci. 15:16698–16718. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou W, Zhang J and Marcus AI: LKB1 Tumor
suppressor: Therapeutic opportunities knock when LKB1 is
inactivated. Genes Dis. 1:64–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Momcilovic M and Shackelford DB: Targeting
LKB1 in cancer-exposing and exploiting vulnerabilities. Br J
Cancer. 113:574–584. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Herter-Sprie GS, Korideck H, Christensen
CL, Herter JM, Rhee K, Berbeco RI, Bennett DG, Akbay EA, Kozono D,
Mak RH, et al: Image-guided radiotherapy platform using single
nodule conditional lung cancer mouse models. Nat Commun.
5:58702014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saigusa S, Inoue Y, Tanaka K, Toiyama Y,
Kawamura M, Okugawa Y, Okigami M, Hiro J, Uchida K, Mohri Y and
Kusunoki M: Significant correlation between LKB1 and LGR5 gene
expression and the association with poor recurrence-free survival
in rectal cancer after preoperative chemoradiotherapy. J Cancer Res
Clin Oncol. 139:131–138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Z, Cheng K, Walton Z, Wang Y, Ebi H,
Shimamura T, Liu Y, Tupper T, Ouyang J, Li J, et al: A murine lung
cancer co-clinical trial identifies genetic modifiers of
therapeutic response. Nature. 483:613–617. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xia C, Ye F, Hu X, Li Z, Jiang B, Fu Y,
Cheng X, Shao Z and Zhuang Z: Liver kinase B1 enhances
chemoresistance to gemcitabine in breast cancer MDA-MB-231 cells.
Oncol Lett. 8:2086–2092. 2014.PubMed/NCBI
|
|
15
|
Wang YQ, Dai WM, Chu XY, Yang B, Zhao M
and Sun Y: Downregulation of LKB1 suppresses Stat3 activity to
promote the proliferation of esophageal carcinoma cells. Mol Med
Rep. 9:2400–2404. 2014.PubMed/NCBI
|
|
16
|
Piao LS, Hur W, Kim TK, Hong SW, Kim SW,
Choi JE, Sung PS, Song MJ, Lee BC, Hwang D and Yoon SK:
CD133+ liver cancer stem cells modulate radioresistance
in human hepatocellular carcinoma. Cancer Lett. 315:129–137. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paglin S, Hollister T, Delohery T, Hackett
N, McMahill M, Sphicas E, Domingo D and Yahalom J: A novel response
of cancer cells to radiation involves autophagy and formation of
acidic vesicles. Cancer Res. 61:439–444. 2001.PubMed/NCBI
|
|
18
|
Gewirtz DA, Hilliker ML and Wilson EN:
Promotion of autophagy as a mechanism for radiation sensitization
of breast tumor cells. Radiother Oncol. 92:323–328. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zois CE and Koukourakis MI:
Radiation-induced autophagy in normal and cancer cells: Towards
novel cytoprotection and radio-sensitization policies? Autophagy.
5:442–450. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moretti L, Cha YI, Niermann KJ and Lu B:
Switch between apoptosis and autophagy: Radiation-induced
endoplasmic reticulum stress? Cell Cycle. 6:793–798. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Raja E, Tzavlaki K, Vuilleumier R, Edlund
K, Kahata K, Zieba A, Morén A, Watanabe Y, Voytyuk I, Botling J, et
al: The protein kinase LKB1 negatively regulates bone morphogenetic
protein receptor signaling. Oncotarget. 7:1120–1143. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Just PA, Poncy A, Charawi S, Dahmani R,
Traore M, Dumontet T, Drouet V, Dumont F, Gilgenkrantz H, Colnot S,
et al: LKB1 and notch pathways interact and control biliary
morphogenesis. PLoS One. 10:e01454002015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Zhang K, Wang J, Wu X, Liu X, Li
B, Zhu Y, Yu Y, Cheng Q, Hu Z, et al: Underexpression of LKB1 tumor
suppressor is associated with enhanced Wnt signaling and malignant
characteristics of human intrahepatic cholangiocarcinoma.
Oncotarget. 6:18905–18920. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lipovka Y and Konhilas JP: AMP-activated
protein kinase signalling in cancer and cardiac hypertrophy.
Cardiovasc Pharm Open Access. 4:pii: 1542015.
|
|
25
|
Dasgupta B and Chhipa RR: Evolving lessons
on the complex role of AMPK in normal physiology and cancer. Trends
Pharmacol Sci. 37:192–206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sanli T, Rashid A, Liu C, Harding S,
Bristow RG, Cutz JC, Singh G, Wright J and Tsakiridis T: Ionizing
radiation activates AMP-activated kinase (AMPK): A target for
radiosensitization of human cancer cells. Int J Radiat Oncol Biol
Phys. 78:221–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Storozhuk Y, Hopmans SN, Sanli T, Barron
C, Tsiani E, Cutz JC, Pond G, Wright J, Singh G and Tsakiridis T:
Metformin inhibits growth and enhances radiation response of
non-small cell lung cancer (NSCLC) through ATM and AMPK. Br J
Cancer. 108:2021–2032. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Muaddi H, Chowdhury S, Vellanki R, Zamiara
P and Koritzinsky M: Contributions of AMPK and p53 dependent
signaling to radiation response in the presence of metformin.
Radiother Oncol. 108:446–450. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fasih A, Elbaz HA, Hüttemann M, Konski AA
and Zielske SP: Radiosensitization of pancreatic cancer cells by
metformin through the AMPK pathway. Radiat Res. 182:50–59. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shaw RJ, Kosmatka M, Bardeesy N, Hurley
RL, Witters LA, DePinho RA and Cantley LC: The tumor suppressor
LKB1 kinase directly activates AMP-activated kinase and regulates
apoptosis in response to energy stress. Proc Natl Acad Sci USA.
101:3329–3335. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hardie DG and Hawley SA: AMP-activated
protein kinase: The energy charge hypothesis revisited. Bioessays.
23:1112–1119. 2001. View Article : Google Scholar : PubMed/NCBI
|