Introduction
Non-small-cell lung cancer (NSCLC) represents
approximately 80% of lung cancers, and is the leading cause of
cancer death with 5-year survival rates less than 15% (1,2). The
epidermal growth factor receptor (EGFR, also called HER1), a
tumour-promoting factor (3), plays
a considerable role in NSCLC, especially in cells with activating
EGFR mutations, such as L858R and exon-19 deletion (4,5).
EGFR expression was considered as a predictor of survival for
first-line chemotherapy plus cetuximab in patients with advanced
NSCLC (6). EGFR activation can be
inhibited with tyrosine kinase inhibitors (TKIs), including
afatinib, gefitinib, and erlotinib, which disrupt oncogenic cell
signaling pathways and benefit patients with sensitizing mutations.
Approximately 70% of patients respond initially to TKI treatment
but eventually develop resistance with a median time of 10–16
months (7). In at least 50% of
these cases, the mutation T790M in exon 20 of EGFR arises (8). This mutation has been reported to
reverse inactivation of the EGFR induced by TKIs by altering the
structure of the EGFR such that the ability of the EGFR to bind
TKIs is reduced (8). TKIs have
also proved to induce expression of IL-6, which activates the
JAK1/STAT3 signaling pathway through the IL-6 receptor (IL-6R),
leading to increased survival (9–11).
TKI-dependent expression of IL-6 increases expression of the
transforming growth factor β1 (TGF-β1) receptor and enhances TGF-β1
signaling, contributing to the epithelial-mesenchymal transition
(EMT) and tumor progression (12,13).
Probably, it is a new strategy to cure NSCLC with mutation T790M in
EGFR by inhibiting the IL-6/JAK1/STAT3 signaling pathway. It has
been reported that the JAK inhibitor pyridone 6 (P6) combined with
afatinib can inhibit the growth of NSCLC cells in vitro and
in vivo (10). TG101348, a
kind of JAK2 inhibitor, also had the ability to overcome
erlotinib-resistance in NSCLC cells with mutated EGFR (14).
More recently, it has been found that matrine, an
extract of the Chinese traditional medicine radix Sophorae
flavescentis, has antitumor activity in vitro and in
vivo. Multiple studies have confirmed that matrine can inhibit
the proliferation of leukemia cells by suppressing DNA synthesis
and inducing apoptosis, and in combination with commonly used
chemotherapeutics can effectively reverse the drug resistance of
leukemia cells (15,16). Matrine can also decrease the
expression of IL-6 and activation of the IL-6 receptor-mediated
JAK/STAT3 signaling pathway (17–19).
Whether matrine can inhibit activation of the
JAK1/STAT3 signaling pathway in NSCLC is still unknown. In the
present study, we investigated the effects of matrine treatment on
the growth of H1975 cells and activation of the JAK1/STAT3
signaling pathway in the presence or absence of afatinib.
Materials and methods
Reagents
Anti-Bcl-2, anti-p-JAK1, anti-JAK1, anti-p-STAT3,
anti-STAT3, anti-β-actin, anti-cleaved caspase-3, anti-caspase-3
and HRP-conjugated anti-rabbit antibodies and the IL-6 kit were
purchased from Cell Signaling Technology, Inc., (Danvers, MA, USA).
Matrine (≥99% purity by HPLC) was purchased from Sigma-Aldrich (St.
Louis, MO, USA). Afatinib was purchased from SYNkinase (San Diego,
CA, USA).
Cell culture
The T790M EGFR-mutant NCI-H1975 (H1975) cell line
used in this study was obtained from the American Type Culture
Collection (Manassas, VA, USA), and cultured in RPMI-1640,
supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v)
penicillin/streptomycin (all from Invitrogen, Waltham, MA, USA),
and incubated at 37°C in a carbon dioxide incubator.
Cell viability assay
The effect of matrine on the growth of H1975 cells
was detected with a Cell Counting Kit-8 (CCK-8). In the presence or
absence of afatinib, 105 cells per well in a 96-well
plate were treated with or without matrine. Cell viability was
detected after treatment with matrine or afatinib for 24 h.
Solution containing WST-8
[2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium,
monosodium salt] was added to cells according to the manufacturer's
instructions and absorbance was detected at 450 nm. All experiments
were performed in triplicate.
RNA extraction and real-time reverse
transcription PCR (RT-PCR)
Total RNA was extracted from cells with RNAiso plus
reagent (Takara Bio, Dalian, China) according to the manufacturer's
instructions. RNA concentration was determined with a
spectrophotometer. First-strand cDNA was synthesized using a
Transcriptor First Strand cDNA Synthesis kit (Roche Applied
Science, Mannheim, Germany). The amplification conditions were as
follows: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles
of 95°C for 10 sec, 58°C for 10 sec, and 72°C for 10 sec. The PCR
reaction was performed using a 7500 Fast Real-Time quantitative PCR
System (Applied Biosystems; Life Technologies, Foster City, CA,
USA). The primer sequences for real-time PCR of IL-6 were
5′-CACTCACCTCTTCAGAACGAAT-3′ and 5′-TTTGTACTCATCTGCACAGCTC-3′, and
the primer sequences for the internal control were
5′-CCAGAG-CAAGAGAGGCATCCT-3′ and 5′-TAGATGGGCACAGTGTGGGTGA-3′
(20). RNA levels were determined
with the 2ΔΔCt method.
Apoptosis assay
Cell death was detected by flow cytometry with
Annexin V-fluorescein isothiocyanate, according to the
manufacturer's instructions (BD Biosciences, San Jose, CA, USA).
Briefly, 1×106 cells were plated in six-well plates for
24 h followed by treatment with matrine or afatinib for 24 h prior
to Annexin V and propidium iodide (PI) staining (FACSAria; BD
Biosciences). For each dye, appropriate electronic compensation of
the FACSAria sorter was performed to avoid overlapping of the two
emission spectra.
Enzyme-linked immunosorbent asssay
(ELISA)
Supernatant was prepared from H1975 cells treated
with or without matrine in the presence or absence of afatinib for
24 h. The content of IL-6 from the supernatant was detected using
IL-6 ELISA kit (Sigma-Aldrich).
Western blot analysis
Cells were dissolved in lysis buffer and the lysate
was centrifuged at 10,000 × g at 4°C for 30 min after incubation on
ice for 50 min. Lysate of equal concentration measured with the BCA
kit was subjected to 12% (w/v) sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to polyvinylidene fluoride (PVDF) membrane (Amersham
Hybond™ P; GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). After
blocking in 5% (w/v) skimmed milk or bovine serum albumin (BSA),
membranes were incubated with anti-Bcl-2 (1:1,000), anti-p-JAK1
(1:1,000), anti-JAK1 (1:1,000), anti-p-STAT3 (1:1,000), anti-STAT3
(1:1,000), anti-cleaved caspase-3 (1:1,000), anti-caspase-3
(1:1,000), or anti-β-actin (1:2,000) antibodies, followed by
incubation with the appropriate secondary antibody for 1 h at room
temperature. Finally, proteins were detected by chemiluminescence
(PerkinElmer Life and Analytical Sciences, Inc., Massachusetts, MA,
USA).
Small interfering RNA (siRNA)
H1975 cells were transfected with IL-6 siRNA
(sc-39627; Santa Cruz Biotechnology, Inc., Paso Robles, CA, USA) or
negative control siRNA (sc-37007; Santa Cruz Biotechnology, Inc.)
using Lipofectamine 2000 (Invitrogen). Under subconfluent
conditions, transfection reagents with 12 pmol of siRNA and 16 µl
of Lipofectamine 2000 in a final volume of 1.6 ml with Opti-MEM I
(Invitrogen) were added to each flask, and incubated for 48 h prior
to treatment with matrine.
Tumor xenograft studies
H1975 cells were harvested and resuspended in
serum-free media, and 5×106 cells were injected
subcutaneously under the back skin of 5-to-6-week-old male BALB/c
nude mice. The mice were treated with or without matrine (5 g/kg)
in the presence or absence of afatinib (5 mg/kg) formulated in
saline by intraperitoneal injection once daily for 4 weeks. Tumor
volume was estimated once every 3 days for 4 weeks with the
following formula: volume = l × w2 ×
0.536, where l and w are perpendicular measured
diameters. All treatments on animals were in accordance with the
ethical standards.
Statistical analysis
Data are represented as means ± standard error of
the mean (SEM) of three independent experiments and analyzed using
the Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Matrine inhibits the growth of H1975
cells by inducing apoptosis
H1975 cells are resistant to TKIs because of the
T790M mutation in the EGFR. To explore the effects of matrine on
the growth of H1975 cells, cell viability was assessed with the
Cell Counting Kit-8 (CCK-8) assay. Our results indicated that
matrine inhibits the growth of H1975 cells in a dose-dependent
manner with an IC50 value of approximately 2 mM
(Fig. 1B). Cell viability is
dependent on cell proliferation and cell death. Here, matrine
treatment induced apoptosis and cleavage of caspase-3 in H1975
cells (Fig. 2A-C). In addition,
expression of the anti-apoptotic protein B-cell lymphoma 2 (Bcl-2)
decreased in H1975 cells treated with matrine (Fig. 2C). Our results indicate that
matrine inhibits growth of H1975 cells, likely due to the decrease
to Bcl-2 expression and the resulting increase in apoptosis.
Matrine inhibits the IL-6/JAK1/STAT3
signaling pathway in H1975 cells
The JAK1/STAT3 signaling pathway can result in
several biological reactions that prevent apoptosis. To explore
whether matrine treatment impacts the JAK1/STAT3 signaling pathway
in H1975 cells, we measured the phosphorylation of JAK1 and STAT3
by western blot analysis following treatment with different
concentrationsof matrine. Our results demonstrate that matrine
decreased the levels of phosphoryalted JAK1 (p-JAK1) and
phosphoryalted STAT3 (p-STAT3) in a dose-dependent manner, thus
inhibiting the JAK1/STAT3 signaling pathway (Fig. 3A). The binding of IL-6 to its
receptor, IL-6R, contributes to the phosphorylation of JAK1 and
activation of the JAK1/STAT3 signaling pathway. To explore whether
IL-6 is associated with inhibition of JAK1/STAT3 signaling, we
determined IL-6 levels with real-time reverse transcription
(RT-PCR) and an enzyme-linked immunosorbent assay (ELISA) in H1975
cells treated with various concentrations of matrine. We observed
that matrine treatment results in a decrease in the mRNA and
protein levels of IL-6 in a dose-dependent manner (Fig. 3B and C).
Matrine induces apoptosis by
regulating the IL-6/JAK1/STAT3 signaling pathway in H1975
cells
To investigate whether matrine treatment inhibits
the growth of H1975 cells via the IL-6/JAK1/STAT3 signaling
pathway, we targeted IL-6 with siRNA to evaluate the role of
IL-6/JAK1/STAT3 signaling in matrine-induced apoptosis by real-time
RT-PCR. Our results demonstrated that treatment with siRNA
targeting IL-6, which markedly reduces the levles of IL-6 mRNA and
protein (Fig. 4A and B),
influenced the phosphorylation of JAK1 and STAT3 and the expression
of Bcl-2, ultimately inhibiting cell growth (Figs. 4C and 5). To explore whether IL-6 is involved in
matrine-induced apoptosis through the JAK1/STAT3 signaling pathway
in H1975 cells, we also assessed molecular signal, apoptosis and
cell viability in H1975 cells treated with or without IL-6 siRNA in
the presence or absence of matrine. Our data demonstrated that IL-6
siRNA could not enhance inactivation of the JAK1/STAT3 signaling
pathway and apoptosis in H1975 cells treated with matrine (Figs. 4C and 5A and B). Therefore, matrine treatment
inhibits growth of H1975 cells via the IL-6/JAK1/STAT3 signaling
pathway, decreases the expression of Bcl-2, and induces
apoptosis.
Combined treatment with afatinib and
matrine has a greater effect on H1975 cells
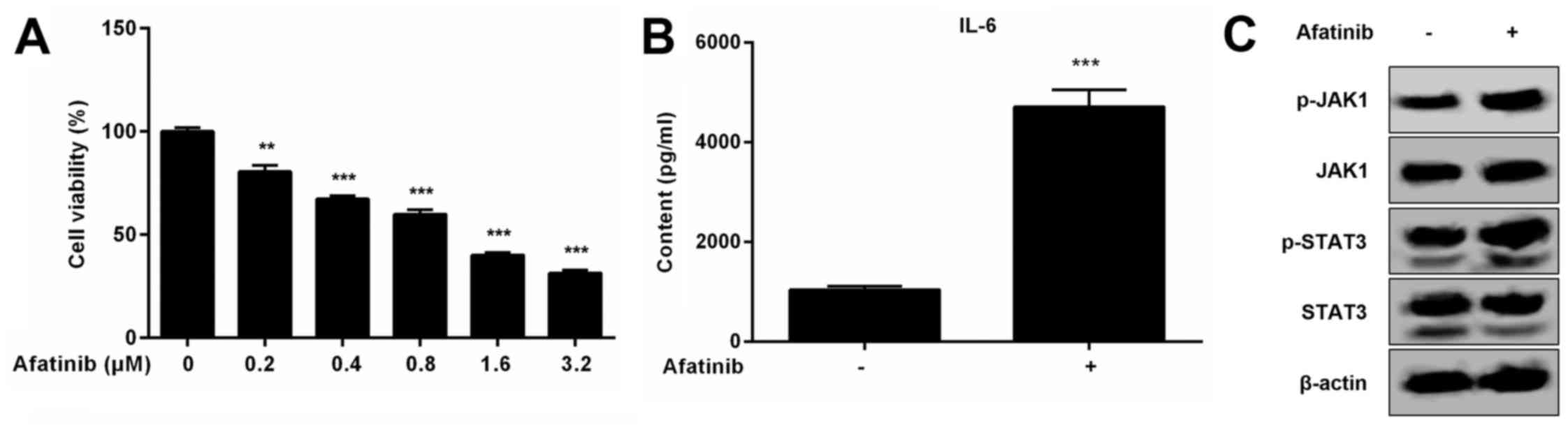
We have seen that afatinib treatment not only
decreases the viability of H1975 cells, but also induces the
expression of IL-6 and activation of the JAK1/STAT3 signaling
pathway (Fig. 6A-C). Activation of
the IL-6/JAK1/STAT3 signaling pathway likely increases the
resistance of H1975 cells to afatinib. To investigate whether
matrine can inhibit activation of the IL-6/JAK1/STAT3 signaling
pathway stimulated by afatinib (IC50 value of 1 µM) in
H1975 cells, the expression of IL-6 and phosphorylation of JAK1 and
STAT3 were detected by western blot and ELISA in H1975 cells
treated with matrine and afatinib. Our results demonstrated that
matrine can inhibit activation of the IL-6/JAK1/STAT3 signaling
pathway induced by afatinib and can also increase the inhibitory
effects of afatinib on H1975 cells resulting from the increased
rate of apoptosis (Fig. 7A-C). To
explore whether matrine can also inhibit the growth of H1975 cells
in vivo, we performed tumor xenograft studies. Treatment
with afatinib and matrine decreased the tumor volume more
dramatically than matrine or afatinib alone (Fig. 8). Therefore, matrine increased the
toxicity of afatinib by regulating the IL-6/JAK1/STAT3 signaling
pathway, and by decreasing the expression of Bcl-2 and the
resistance of H1975 cells to afatinib.
Discussion
Mutations occur in NSCLC cells and result in
resistance to EGFR inhibitors (TKIs), such as EGFR C797S mutation
happened in NSCLC, which was resistant to AZD9291 (21). These mutations, especially the T790
M mutation, are a threat to patients with NSCLC (7,8). It
is important to overcome EGFR (T790M) resistance in NSCLC (22). Consequently, understanding the
mechanism by which the mutated EGFR acquires resistance to TKI
treatment will be important for the development of new therapies to
cure NSCLC or prevent further progression of the disease. It has
been suggested that the T790M mutation changes the structure of the
EGFR, inhibiting its ability to bind TKIs, thus decreasing the
inhibitory effects of TKIs on EGFR activation (8). It has been shown recently that TKIs
can activate several signaling pathways, including the JAK1/STAT3
pathway, by inducing expression of IL-6, which results in a decline
to TKI toxicity and an increase in the survival of NSCLC cells with
the T790M mutation (9–11). The IL-6/JAK1/STAT3 signaling
pathway, stimulated by TKIs such as afatinib, can also promote the
EMT, thereby increasing cancer cell migration, invasion, and
ultimately metastasis (12,13).
Inhibiting the expression of IL-6 or inactivating the JAK1/STAT3
signaling pathway has been reported to increase the toxicity of
TKIs toward TKI-resistant NSCLC cells (10,14,23).
Targeting the IL-6/JAK1/STAT3 signaling pathway will be an
important strategy for the treatment of patients with TKI-resistant
NSCLC.
Matrine is an alkaloid prepared from the leguminous
plant radix Sophorae flavescentis. It plays an important
role in the regulation of IL-6 expression and activation of the
JAK1/STAT3 signaling pathway (17–19,24).
A number of previous studies have reported the effects of matrine
on NSCLC cells with the T790M mutation, including H1975 cells, in
the presence or absence of a TKI. However, there remain some
questions as to whether matrine can inhibit the expression of IL-6
or promote inactivation of the JAK1/STAT3 signaling pathway in
H1975 cells stimulated by afatinib, and whether a combination of
matrine and afatinib can further inhibit H1975 cells.
In the present study, we determined the effect of
matrine treatment on the growth of H1975 cells treated without
afatinib with the CCK-8 assay. Our results demonstrated that
matrine reduces the viability of H1975 cells, likely due to the
decreased expression of Bcl-2 and increased rate of apoptosis.
Activation of the JAK1/STAT3 signaling pathway was detected by
western blot and we found that matrine treatment inhibited
phosphorylation of JAK1 and STAT3 in a dose-dependent manner.
IL-6 participates in activation of the JAK1/STAT3
signaling pathway by binding the IL-6 receptor complexed with
glycoprotein 130 (gp130). Our results indicated that matrine
treatment decreased IL-6 protein levels as a result of its
dose-dependent inhibition of IL-6 transcription. To identify
inactivation of the JAK1/STAT3 signaling pathway as a result of
IL-6 inhibition and the role of the IL-6/JAK1/STAT3 pathway in
matrine-induced apoptosis in H1975 cells, we targeted IL-6 with
siRNA. Down regulation of IL-6 by siRNA inhibited phosphorylation
of JAK1 and STAT3. However, IL-6 siRNA could not enhance
inactivation of the JAK1/STAT3 signaling pathway, inhibition of
Bcl-2 expression, or apoptosis in H1975 cells treated with matrine.
In summary, matrine inhibits the IL-6/JAK1/STAT3 signaling pathway
and decreases Bcl-2 expression, causing an increased rate of
apoptosis and a decline in the viability of H1975 cells.
We then studied whether matrine treatment
significantly inhibited H1975 cells stimulated by afatinib. Our
results indicated that treatment with both matrine and afatinib can
trigger apoptosis of H1975 cells and that matrine treatment can
inhibit the IL-6/JAK1/STAT3 pathway induced by afatinib, thus
increasing the inhibitory effects of afatinib. In addition,
treatment with both matrine and afatinib had a more dramatic
inhibitory effect on mouse tumor volume in vivo.
We found that matrine could enhance the toxicity of
afatinib toward H1975 cells harboring the T790M mutation by
suppressing activation of the IL-6/JAK1/STAT3 signaling pathway
induced by afatinib. Treatment with both matrine and afatinib will
likely be successful for patients of NSCLC with the T790M
mutation.
Acknowledgements
The present study was supported by the Science and
Technology Development Fund Projects of The First Affiliated
Hospital, College of Medicine, Zhejiang University (grant no.
B1519). Experimental animals were provided by the Experimental
Animal Center of the Zhejiang Academy of Medical Sciences. We thank
Professor Jian-Ying Zhou for the experimental design.
References
|
1
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spira A and Ettinger DS: Multidisciplinary
management of lung cancer. N Engl J Med. 350:379–392. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lanaya H, Natarajan A, Komposch K, Li L,
Amberg N, Chen L, Wculek SK, Hammer M, Zenz R, Peck-Radosavljevic
M, et al: EGFR has a tumour-promoting role in liver macrophages
during hepatocellular carcinoma formation. Nat Cell Biol.
16:972–981. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pao W and Chmielecki J: Rational,
biologically based treatment of EGFR-mutant non-small-cell lung
cancer. Nat Rev Cancer. 10:760–774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pirker R, Pereira JR, von Pawel J,
Krzakowski M, Ramlau R, Park K, de Marinis F, Eberhardt WE,
Paz-Ares L, Störkel S, et al: EGFR expression as a predictor of
survival for first-line chemotherapy plus cetuximab in patients
with advanced non-small-cell lung cancer: Analysis of data from the
phase 3 FLEX study. Lancet Oncol. 13:33–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kobayashi S, Boggon TJ, Dayaram T, Jänne
PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG and Halmos
B: EGFR mutation and resistance of non-small-cell lung cancer to
gefitinib. N Engl J Med. 352:786–792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao SP, Mark KG, Leslie K, Pao W, Motoi N,
Gerald WL, Travis WD, Bornmann W, Veach D, Clarkson B and Bromberg
JF: Mutations in the EGFR kinase domain mediate STAT3 activation
via IL-6 production in human lung adenocarcinomas. J Clin Invest.
117:3846–3856. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim SM, Kwon OJ, Hong YK, Kim JH, Solca F,
Ha SJ, Soo RA, Christensen JG, Lee JH and Cho BC: Activation of
IL-6R/JAK1/STAT3 signaling induces de novo resistance to
irreversible EGFR inhibitors in non-small cell lung cancer with
T790M resistance mutation. Mol Cancer Ther. 11:2254–2264. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Lan T, Zhang C, Zeng C, Hou J, Yang
Z, Zhang M, Liu J and Liu B: Reciprocal activation between
IL-6/STAT3 and NOX4/Akt signaling promotes proliferation and
survival of non-small cell lung cancer cells. Oncotarget.
6:1031–1048. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abulaiti A, Shintani Y, Funaki S, Nakagiri
T, Inoue M, Sawabata N, Minami M and Okumura M: Interaction between
non-small-cell lung cancer cells and fibroblasts via enhancement of
TGF-β1 signaling by IL-6. Lung Cancer. 82:204–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen J, Lan T, Zhang W, Dong L, Kang N,
Zhang S, Fu M, Liu B, Liu K and Zhan Q: Feed-forward reciprocal
activation of PAFR and STAT3 regulates epithelial-mesenchymal
transition in non-small cell lung cancer. Cancer Res. 75:4198–4210.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang FQ, Yang WT, Duan SZ, Xia YC, Zhu RY
and Chen YB: JAK2 inhibitor TG101348 overcomes erlotinib-resistance
in non-small cell lung carcinoma cells with mutated EGF receptor.
Oncotarget. 6:14329–14343. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Xu Y, Ji W, Li X, Sun B, Gao Q and
Su C: Anti-tumor activities of matrine and oxymatrine: Literature
review. Tumour Biol. 35:5111–5119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang LP, Jiang JK, Tam JW, Zhang Y, Liu
XS, Xu XR, Liu BZ and He YJ: Effects of matrine on proliferation
and differentiation in K-562 cells. Leuk Res. 25:793–800. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu X, Zhu Z, Jiang L, Sun X, Jia Z, Qian
S, Li J and Ma L: Matrine increases NKG2D ligand ULBP2 in K562
cells via inhibiting JAK/STAT3 pathway: A potential mechanism
underlying the immunotherapy of matrine in leukemia. Am J Transl
Res. 7:1838–1849. 2015.PubMed/NCBI
|
|
18
|
Lin W, Zhang JP, Hu ZL and Qian DH:
Inhibitory effect of matrine on lipopolysacchride-induced tumor
necrosis factor and interleukin-6 production from rat Kupffer
cells. Yao Xue Xue Bao. 32:93–96. 1997.(In Chinese). PubMed/NCBI
|
|
19
|
Zhang Y, Wang S, Li Y, Xiao Z, Hu Z and
Zhang J: Sophocarpine and matrine inhibit the production of
TNF-alpha and IL-6 in murine macrophages and prevent
cachexia-related symptoms induced by colon26 adenocarcinoma in
mice. Int Immunopharmacol. 8:1767–1772. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu X, Ye F, Xiong H, Hu DN, Limb GA, Xie
T, Peng L, Zhang P, Wei Y, Zhang W, et al: IL-1β induces IL-6
production in retinal Müller cells predominantly through the
activation of P38 MAPK/NF-κB signaling pathway. Exp Cell Res.
331:223–231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thress KS, Paweletz CP, Felip E, Cho BC,
Stetson D, Dougherty B, Lai Z, Markovets A, Vivancos A, Kuang Y, et
al: Acquired EGFR C797S mutation mediates resistance to AZD9291 in
non-small cell lung cancer harboring EGFR T790M. Nat Med.
21:560–562. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jia Y, Yun CH, Park E, Ercan D, Manuia M,
Juarez J, Xu C, Rhee K, Chen T, Zhang H, et al: Overcoming
EGFR(T790M) and EGFR(C797S) resistance with mutant-selective
allosteric inhibitors. Nature. 534:129–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nan J, Du Y, Chen X, Bai Q, Wang Y, Zhang
X, Zhu N, Zhang J, Hou J, Wang Q and Yang J: TPCA-1 is a direct
dual inhibitor of STAT3 and NF-κB and regresses mutant
EGFR-associated human non-small cell lung cancers. Mol Cancer Ther.
13:617–629. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma L, Zhu Z, Jiang L, Sun X, Lu X, Zhou M,
Qian S and Jianyong L: Matrine suppresses cell growth of human
chronic myeloid leukemia cells via its inhibition of the
interleukin-6/Janus activated kinase/signal transducer and
activator of transcription 3 signaling cohort. Leuk Lymphoma.
56:2923–2930. 2015. View Article : Google Scholar : PubMed/NCBI
|