Introduction
Hepatocellular carcinoma (HCC) is one of the most
common forms of liver cancer and is one of the major causes of
mortality among patients with chronic liver disease worldwide
(1,2). It has been reported that the mean
survival rate of HCC is <5% and HCC is the third most common
cause of cancer-associated mortality worldwide (3,4). In
addition, HCC is one of the most common and malignant cancers, with
an increasing incidence rate, particularly in Europe and East Asia
(5). At present, chemotherapy with
synthetic drugs is commonly used to treat HCC; however, serious
side effects are associated with this treatment (6). HCC also exhibits a poor response to
treatment modalities and resistance to systemic chemotherapy
(7). Therefore, there is an urgent
requirement to develop novel anti-HCC agents with improved
therapeutic effects.
Traditional Chinese medicine (TCM) has been used for
centuries, and it has been reported that plant-derived medicines
are safer than synthetic drugs (8). In addition, TCM has been reported to
be effective in the treatment of various diseases, particularly
those that cannot be treated by modern synthetic drugs (9). The rhizome of Atractylodes
macrocephala, a recognized herbal medicine in China, has been
commonly used to treat conditions including edema,
spleen-deficiency, diarrhea and abdominal distention (10). Various other effects of the rhizome
of A. macrocephala have also been described, including
antibacterial, anti-aging and antitumor effects (10). It has previously been reported that
2-[(2E)-3,7-dimethyl-2,6-octadienyl]-6-methyl-2,5-cyclohexadiene-1,4-dione
(DMD), the structure of which is presented in Fig. 1, is a compound isolated from the
aerial part of A. macrocephala (APM), however, its antitumor
activities have not yet been investigated (11).
The present study obtained a quantity of DMD from
the APM and investigated its antitumor effects against H22 cells
in vitro and in vivo, and explored the potential
underlying pharmacological mechanism to provide a scientific basis
for the clinical use of DMD in the future.
Materials and methods
Chemicals
Dimethyl sulfoxide (DMSO) and MTT were purchased
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). RPMI-1640
medium and fetal bovine serum were purchased from Gibco (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Cleaved (c)-caspase-3
(cat. no. ab32499), c-caspase-9 (cat. no. ab2324) and c-caspase-7
(cat. no. ab69540) antibodies were purchased from Abcam (Cambridge,
MA, USA). Cytochrome c (cat. no. ab13575), B-cell lymphoma 2
(Bcl-2)-associated X protein (Bax; cat. no. ab32503), Bcl-2 (cat.
no. ab32124), c-Jun N-terminal kinase (JNK; cat. no. ab76125),
phosphorylated (p)-JNK (cat. no. ab4821), extracellular
signal-regulated kinase (ERK)1/2 (cat. no. ab17942), p-ERK1/2 (cat.
no. ab200807), p38 (cat. no. ab31828), p-p38 (cat. no. ab47363) and
GAPDH (cat. no. ab8245) antibodies were purchased from Abcam
(Cambridge, MA, USA). Bicinchoninic acid (BCA) protein assay
reagent was purchased from Beyotime Institute of Biotechnology
(Haimen, China). Silica-gel (100–200 mesh) was purchased from
Qingdao Haiyang Chemical Co., Ltd. (Qingdao, China). The Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) kit was
purchased from BD Biosciences (San Jose, CA, USA). All other
chemicals used in this study were of analytical reagent grade.
Animals
A total 12 BALB/C nude mice (5–6 weeks old; 8–9 g),
were purchased from the Shanghai Laboratory Animal Center
(Shanghai, China). The animals were maintained under controlled
conditions (21±1°C and 30–70%) with a 12-h light/dark cycle and
free access to food and water. All animal treatments were strictly
in accordance with international ethical guidelines and the
National Institutes of Health Guide concerning the Care and Use of
Laboratory Animals (12), and the
experiments were performed with the approval of the Animal
Experimentation Ethics Committee of The First Hospital of Lanzhou
University (Lanzhou, Gansu, China).
Preparation of DMD
The APM was collected from Jiande, China in August
2014. The dried APM was powdered and extracted five times with 75%
ethanol by percolation extraction for 3 days each time. The solvent
was evaporated under vacuum to obtain the crude extract.
Subsequently, the crude extract was suspended in water and
partitioned with petroleum ether, ethyl acetate (EtOAc) and
n-butanol sequentially. The EtOAc fraction was subjected to
repeated column chromatography over silica gel (100–200 mesh) and
eluted with petroleum ether-EtOAc (97%:3%, 90%:10%, 80%:20%,
70%:30% and 50%:50%). Combination of similar fractions on the basis
of thin layer chromatography (TLC) analysis afforded 5 fractions
(I–V). The TLC spots were visualized using a ZF-1 Ultraviolet
analyzer (Shanghai King Tech Industry Co., Ltd., Shanghai, China)
at 254 nm and 365 nm using the sulfuric acid-alcohol chromogenic
agent. As described previously (11), DMD was isolated from fraction IV
using further column chromatography over a silica gel (200–300
mesh) and eluted with petroleum ether:EtOAc (50%:50%).
Identification of the compound
The isolated DMD was identified by
1H-nuclear magnetic resonance (NMR) and
13C-NMR. The 1H-NMR and 13C-NMR
spectrum data of this compound are as follows: Brown oil;
1H-NMR (CDCl3, 600 MHz, J/Hz) d: 6.62 (1H, m,
H-6), 6.57 (1H, m, H-2), 5.11 (1H, t, J=7.3 Hz, H-2′), 5.03 (1H, t,
J=6.8 Hz, H-6′), 3.17 (2H, d, J=7.3 Hz, H2-1′), 2.18 (2H, m,
H2-5′), 2.13 (5H, m, H2-4′ and H3-7), 1.74 (3H, s, H-9′), 1.67 (3H,
s, H-10′), 1.64 (3H, s, H-8′); 13C-NMR
(CDCl3, 150M Hz) d: 189.52 (C-1), 182.54 (C-4), 148.81
(C-2), 143.59 (C-6), 134.96 (C-3′), 132.96 (C-3), 131.12 (C-5),
130.42 (C-7′), 122.43 (C-6′), 113.56 (C-2′), 40.11 (C-4′), 28.01
(C-1′), 25.99 (C-5′), 25.01 (C-9′), 18.03 (C-8′), 16.03 (C-10′),
14.93 (C-7). According to these spectral data, physicochemical
properties and a reported study (13), the compound was identified as
DMD.
Cell culture and determination of cell
viability
Human HL-60 myeloid leukemia, A549 lung cancer,
MCF-7 breast cancer, HCT-8 colon cancer and HeLa cervical carcinoma
cell lines, and the H22 mouse HCC cell line, were purchased from
the American Type Culture Collection (Manassas, VA, USA). The cells
were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.) and antibiotics (100 U/ml penicillin and
100 µg/ml streptomycin). The cell lines were maintained at 37°C in
an atmosphere containing 5% CO2/95% air.
Cell viability was determined using the MTT assay
according to the previously reported method (14). Cell suspension (100 µl;
5×105 cells/ml) were seeded in 96-well plates and
cultured for 24 h at 37°C. Cells were then treated with DMD at
various concentrations (10, 20, 40, 60, 80 and 100 µg/ml) and
cultured for 24 h at 37°C. The control cells were cultured without
DMD treatment for 24 h at 37°C. Subsequently, the MTT assay was
performed to determine the percentage of cell proliferation
inhibition (n=4) by detecting the optical density (OD) at 570 nm
using a microplate reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The half maximal inhibitory concentration
(IC50) values of DMD on HL-60, A549, MCF-7, HCT-8, HeLa
and H22 cells were calculated. In addition, to investigate the
dose-dependent and time-dependent effects of DMD, H22 cells were
treated with DMD (15, 30 and 60 µg/ml) for 12, 24, 36 and 48 h. The
inhibitory rate was calculated according to the following formula:
[(ODcontrol-ODtreatment)/ODcontrol]
x 100.
Xenograft model in mice
To evaluate the antitumor effects of DMD against H22
cells in vivo, mice were divided into two groups
(n=6/group), including control and DMD (40 mg/kg) groups. Nude mice
were subcutaneously injected in the back on the right-hand side
with H22 cells (0.2 ml; 1×107 cells per mouse). Once the
tumors had grown to 2–3 mm in diameter, mice were treated
intraperitoneally with DMD (40 mg/kg/day) for 20 days and an equal
volume of solvent control (0.5% DMSO). Mice were observed over 20
days, and tumor sizes were measured every 5 days after the tumor
inoculation. Tumor diameters were determined using a vernier
caliper, and the tumor volumes were calculated according to the
following formula (9): Volume =
(width2 × length)/2.
Apoptosis assay
Apoptotic cells were detected by flow cytometry on a
FACSCalibur cytometer (BD Biosciences). Firstly, 2 ml H22 cells
(5×105/ml) were seeded in 6-well plates for 24 h at
37°C. On the following day, the cells were treated with 15, 30 and
60 µg/ml DMD. After 48 h, cells were trypsinized, washed with PBS
and stained using the Annexin V-FITC/PI kit (200 µl Annexin V-FITC
and 10 µl PI for every 1×105 cells), according to the
manufacturer's protocol.
Western blotting
Cells were treated with DMD (15, 30 and 60 µg/ml)
for 24 h at 37°C. Cells (5×106) were then harvested and
homogenized with lysis buffer for 10 min and centrifuged at 4°C for
5 min (10,000 × g). Total protein was extracted from cells using
the cell lysis buffer for western blotting and IP (Beyotime
Institute of Biotechnology; cat. no. P0013), and the protein
concentration was determined using the BCA protein assay reagent
(Beyotime Institute of Biotechnology; cat. no. P0012S).
Subsequently, 35 µg protein was separated by 12% SDS-PAGE and
blotted onto polyvinylidene fluoride membranes. Membranes were
blocked with 5% fat-free dry milk in 1X TBST (containing 0.1%
Tween-20; Beyotime Institute of Biotechnology; cat. no. P0233) at
room temperature for 2 h. Then, membranes were incubated with the
following primary antibodies: Cytochrome c (dilution 1:1,000),
c-caspase-3 (dilution 1:1,000), c-caspase-9 (dilution 1:1,000),
c-caspase-7 (dilution 1:1,000), Bax (dilution 1:1,000), Bcl-2
(dilution 1:1,000), p38 (dilution 1:1,000), p-p38 (dilution
1:1,000), JNK (dilution 1:1,000), p-JNK (dilution 1:1,000), ERK1/2
(dilution 1:1,000), p-ERK1/2 (dilution 1:1,000) and GAPDH (dilution
1:2,000) at 4°C overnight. Followed by incubation with horseradish
peroxidase-conjugated secondary antibodies (1:2,000; Beyotime
Institute of Biotechnology; cat. no. A0286) at room temperature for
1 h. Proteins bands were visualized using Beyo
Electrochemiluminescence Star reagents (Beyotime Institute of
Biotechnology; cat. no. P0018A). Immunoblotting signals were
evaluated quantitatively using the ImageQuant™ LAS 4000
digital imaging system (GE Healthcare Bio-Sciences, Pittsburgh, PA,
USA). To normalize for protein loading, antibodies directed against
GAPDH were used, and protein expression was expressed relative to
GAPDH.
Statistical analysis
Data are presented as the mean ± standard deviation
and the significance of differences between groups was determined
by one-way analysis of variance followed by a Dunnett's multiple
comparisons post hoc test using SPSS software (SPSS for Windows
v19.0; IBM Corp., Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
IC50 values of DMD on
HL-60, A549, MCF-7, HCT-8, HeLa and H22 cell lines
The antiproliferative effects of DMD on HL-60, A549,
MCF-7, HCT-8, HeLa and H22 cell lines were investigated, and the
results are presented in Table I.
The results demonstrated that DMD exhibited an antiproliferative
effect against the H22 cell line, and the IC50 value was
44.29±3.97 µg/ml, which is <50 µg/ml. Furthermore, DMD displayed
moderate antiproliferative activities against HL-60 and A549 cell
lines (IC50 values were 87.43±3.12 µg/ml and 68.19±1.08
µg/ml, respectively). However, no obvious antitumor effects were
observed against MCF-7, HCT-8 and HeLa cell lines (IC50
>100 µg/ml).
 | Table I.IC50 values of DMD on
various cancer cell lines. |
Table I.
IC50 values of DMD on
various cancer cell lines.
| Cell line | IC50
(µg/ml) |
|---|
| HL-60 | 87.43±3.12 |
| A549 | 68.19±1.08 |
| MCF-7 | >100 |
| HCT-8 | >100 |
| HeLa | >100 |
| H22 | 44.29±3.97 |
Inhibitory effects of DMD against H22
cells in vitro and in vivo
The antitumor activity of DMD against H22 cells
in vivo and in vitro was evaluated in the present
study. The results demonstrated that DMD possesses significant
cytotoxicity against H22 cells, as cell viability was significantly
reduced, compared with control cells following treatment with 15
µg/ml (P<0.05), 30 µg/ml (P<0.01) and 60 µg/ml (P<0.01)
DMD, and viability was reduced in a concentration-dependent manner
(Fig. 2A). In addition, DMD (15,
30 and 60 µg/ml) also exhibited time-dependent cytotoxic effects
against H22 cells (Fig. 2B).
Furthermore, the antitumor activity of DMD against H22 cells in
vivo was further evaluated in a mouse xenograft model. As
demonstrated in Fig. 2C, DMD (40
mg/kg) significantly inhibited tumor growth, compared with the
control group (P<0.01).
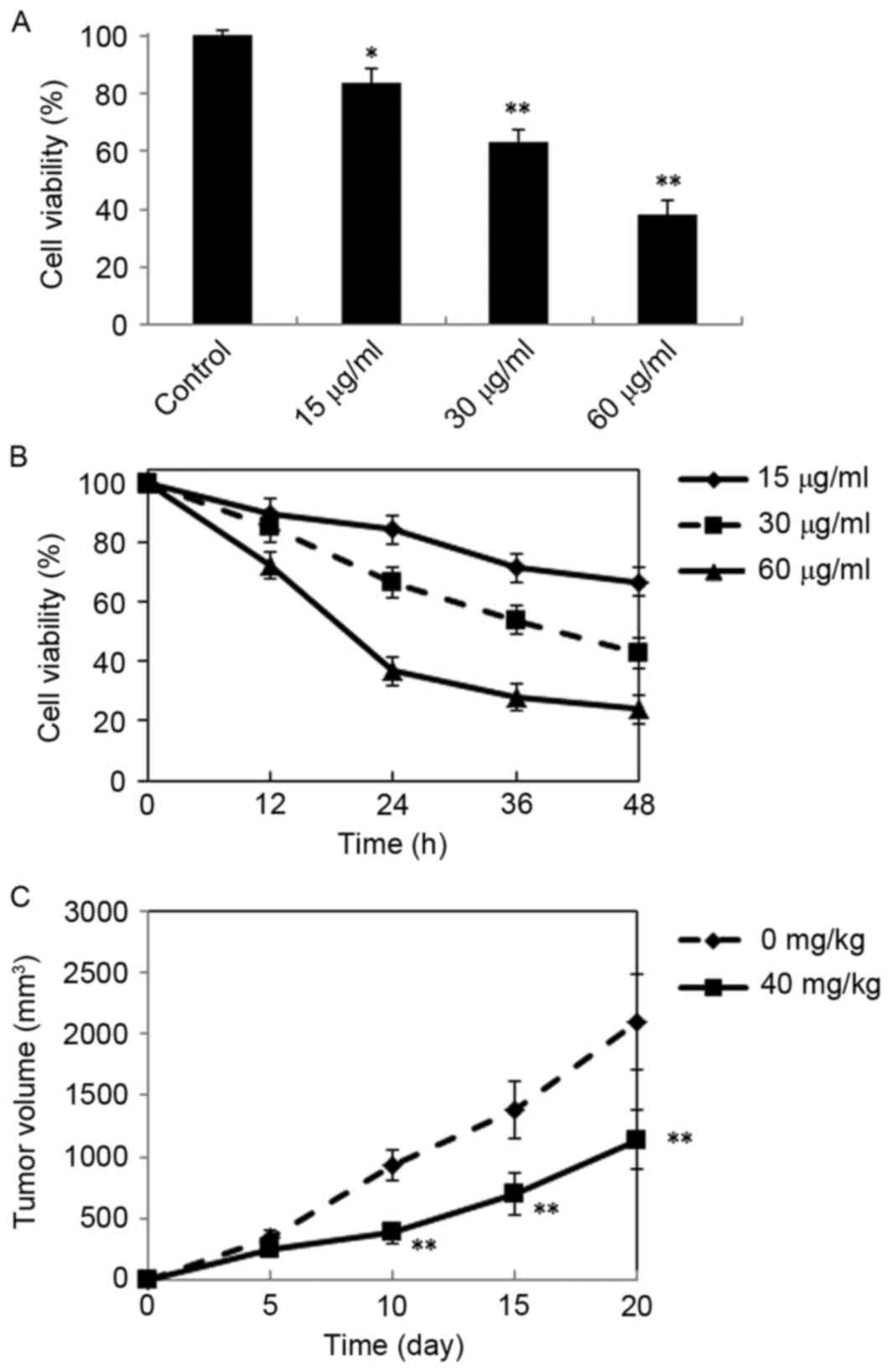 | Figure 2.Antitumor effects of DMD against H22
cells in vitro and in vivo. (A) Inhibitory effects of
DMD on the proliferation of H22 cells. Cells were treated with DMD
at three concentrations (15, 30 and 60 µg/ml) for 24 h and cell
viability was determined by MTT assay. (B) Cells were treated with
DMD (15, 30 and 60 µg/ml) for 12, 24, 36 and 48 h, and cell
viability was determined by MTT assay. (C) Tumor growth curves of
xenograft mice injected with H22 cells and treated with DMD (40
mg/kg/day, i.p.) for 20 days or solvent control. Data are presented
as the mean ± standard deviation (n=4). *P<0.05 and **P<0.01
vs. the control group. DMD,
2-[(2E)-3,7-dimethyl-2,6-octadienyl]-6-methyl-2,5-cyclohexadiene-1,4-dione. |
Proapoptotic effects of DMD on H22
cells
The aforementioned results demonstrated that DMD
exhibits notable antitumor activity against H22 HCC cells. To
determine whether the antitumor activity of DMD resulted from
induction of apoptosis, apoptotic H22 cells were detected by
staining with Annexin V-FITC/PI followed by flow cytometry. As
presented in Fig. 3, the
percentage of apoptotic cells increased gradually when treated with
increasing concentrations of DMD (15, 30 and 60 µg/ml), and the
percentage was significantly increased, compared with control cells
at all doses (P<0.01). These results indicated that DMD may
induce H22 cell death by inducing apoptosis.
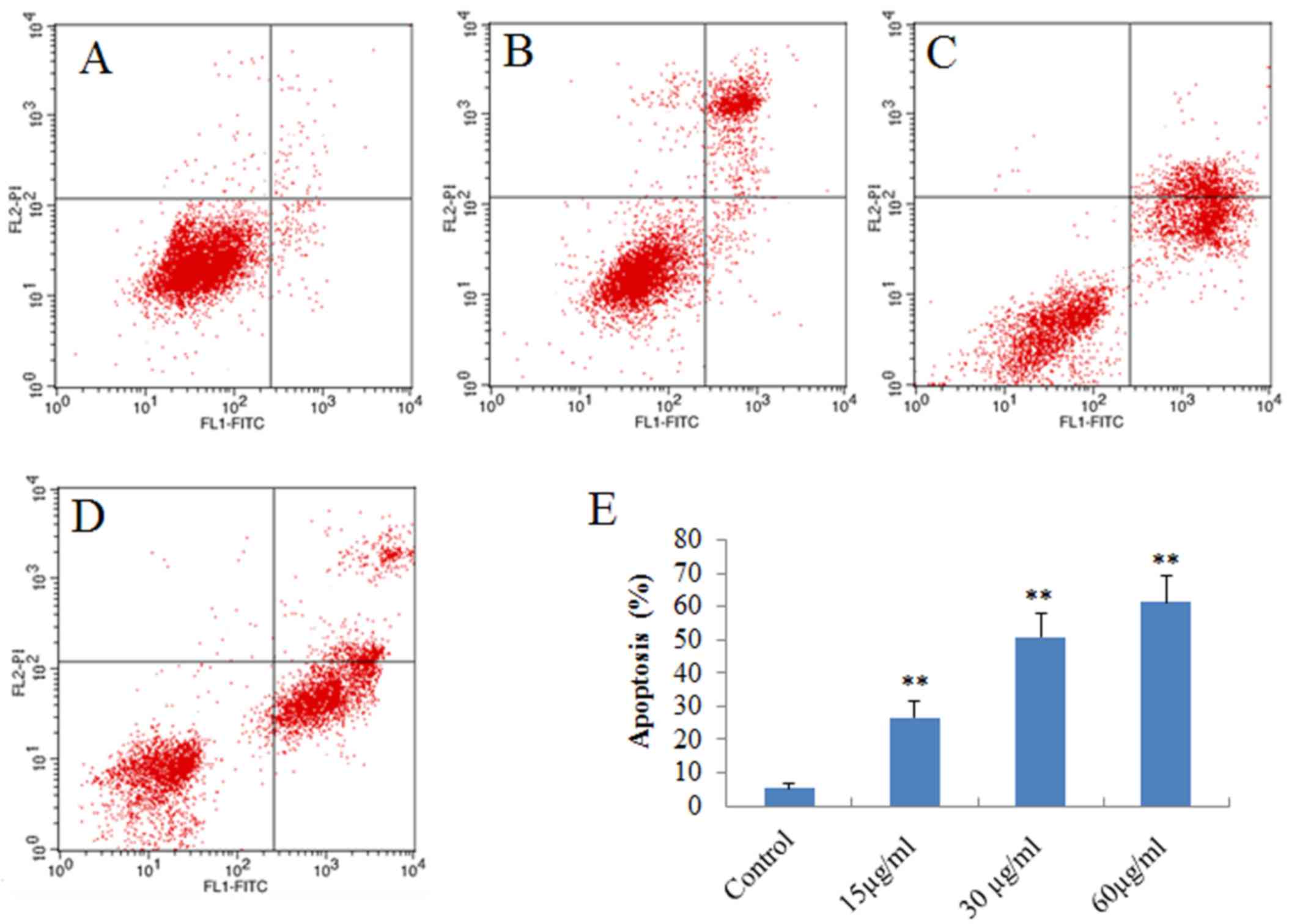 | Figure 3.Apoptotic effects of DMD on H22 cells,
as determined by flow cytometry. H22 cells were treated with DMD at
15, 30 and 60 µg/ml for 48 h, and apoptotic cells were detected by
flow cytometry. Flow cytometry scatter plots for H22 cells treated
with (A) control (B) 15 µg/ml DMD, (C) 30 µg/ml DMD and (D) 60
µg/ml DMD. (E) Percentage of apoptotic cells detected in each
group. Data are presented as the mean ± standard deviation (n=3).
**P<0.01 vs. the control group. DMD,
2-[(2E)-3,7-dimethyl-2,6-octadienyl]-6-methyl-2,5-cyclohexadiene-1,4-dione;
FITC, fluorescein isothiocyanate; PI, propidium iodide. |
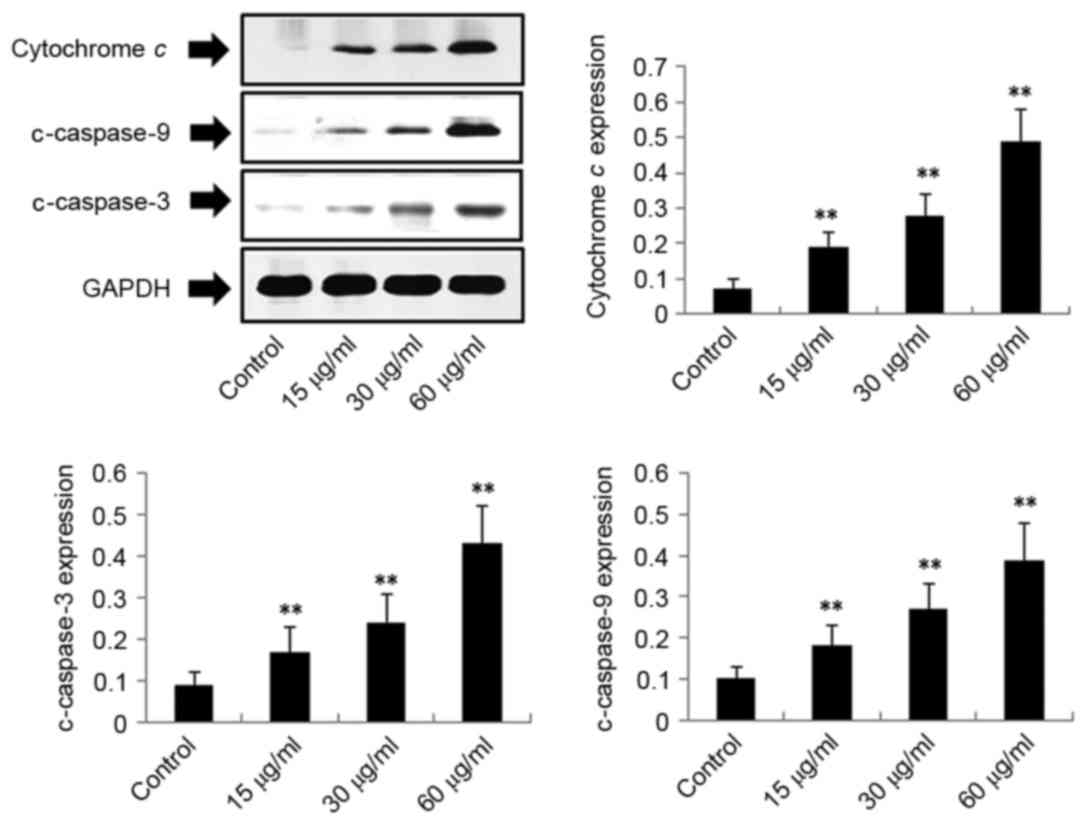
Exposure of H22 cells to DMD results
in upregulation of cytochrome c, c-caspase-3, c-caspase-9,
c-caspase-7 and Bax, and downregulation of Bcl-2
To investigate the potential mechanism by which DMD
induces apoptosis of H22 cells, the protein expression levels of
cytochrome c, c-caspase-3, c-caspase-9, c-caspase-7, Bax and Bcl-2
in H22 cells were detected. As demonstrated in Figs. 4–6, the expression levels of cytochrome c
(P<0.01), c-caspase-3 (P<0.01), c-caspase-9 (P<0.01) and
Bax (P<0.01) were significantly upregulated following treatment
with DMD (15, 30 and 60 µg/ml) in a concentration-dependent manner.
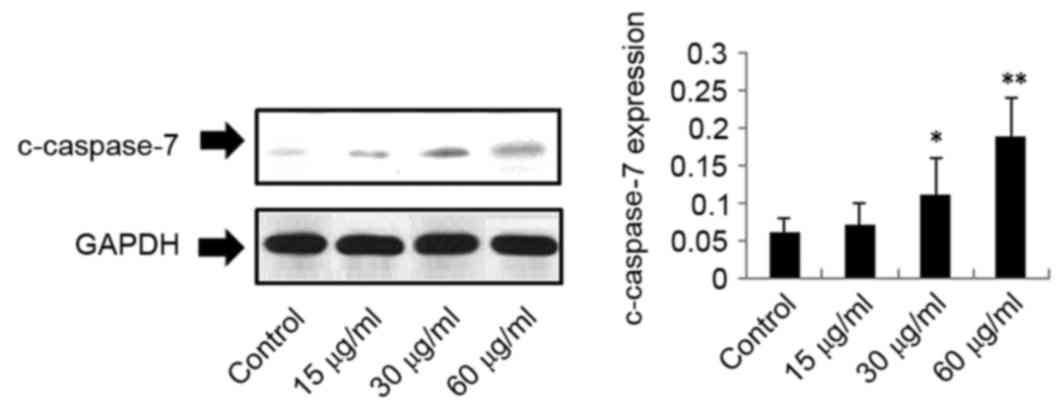
In addition, c-caspase-7 expression was upregulated in response to
DMD, in a concentration-dependent manner. C-caspase-7 was
significantly upregulated following treatment with 30 µg/ml
(P<0.05) and 60 µg/ml DMD (P<0.01), compared with the control
group; however, no significant difference was observed in
c-caspase-7 expression following treatment with 15 µg/ml DMD
(Fig. 5). However, as demonstrated
in Fig. 6, DMD significantly
reduced the expression of Bcl-2 in a concentration-dependent
manner, compared with the control group (P<0.01). These results
indicated that DMD-induced apoptosis of H22 cells may be associated
with the mitochondria-mediated apoptotic pathway.
Effects of DMD on p38, p-p38, JNK,
p-JNK, ERK1/2 and p-ERK1/2 protein expression
To investigate other potential mechanisms of DMD
action on H22 cells, the expression levels of proteins in the
mitogen-activated protein kinase (MAPK) pathway were examined,
including p38, JNK, ERK1/2, p-p38, p-JNK and p-ERK1/2. As presented
in Fig. 7, the effects of DMD on
p38, p-p38, JNK and ERK1/2 were not significant (P>0.05).
However, DMD (15, 30 and 60 µg/ml) significantly upregulated the
expression levels of p-JNK (P<0.01) and reduced the expression
of p-ERK1/2 (P<0.01) dose-dependently, compared with the control
group. Therefore, DMD-mediated apoptosis of H22 cells may also be
associated with the MAPK apoptotic pathway.
 | Figure 7.Effects of DMD on the protein
expression levels of (A) p-p38, (B) p38, (C) p-JNK, (D) JNK, (E)
p-ERK1/2, (F) ERK1/2 and (G) GAPDH. Total protein was extracted and
detected by western blot analysis using antibodies against ERK1/2,
p-ERK1/2, JNK, p-JNK, p38 and p-p38; GAPDH was used as an internal
control. Data are presented as the mean ± standard deviation (n=4).
**P<0.01 vs. the control group. DMD,
2-[(2E)-3,7-dimethyl-2,6-octadienyl]-6-methyl-2,5-cyclohexadiene-1,4-dione;
ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal
kinase; p-, phosphorylated-. |
Discussion
The present study systematically investigated the
antitumor activity and mechanism underlying the effects of DMD
isolated from the aerial part of APM on a HCC cell line in
vivo and in vitro. The results indicated that DMD
exhibited an antitumor effect against the H22 HCC cell line in
vivo and in vitro, which may be associated with
mitochondria-mediated intrinsic apoptosis and the MAPK pathway.
It has previously been reported that uncontrolled
cell proliferation and insufficient apoptosis may be regarded as
the leading causes behind the development of cancer (15). Apoptosis, which is a form of
programmed cell death, is a physiological cell suicide process that
is regulated by several proteins (16). In addition, it is considered to be
an ideal target for cancer therapy (17). Mitochondria-mediated apoptosis is
considered to be a major apoptotic pathway, and this pathway relies
on the Bcl-2 family proteins to control the release of cytochrome
c and activate the caspase family of proteins (18). Cytochrome c is a small
soluble heme-protein, which is present in the mitochondrial
intermembrane space under homeostatic conditions (19). It is involved in the electron
transport chain and carries electrons from the cytochrome
bc1 complex to cytochrome c oxidase
(20). In addition, it is
established that cytochrome c is the first protein released
from the mitochondria of apoptotic cells, and promotes the
activation of caspase-9 (21). The
caspase family consists of ≥14 members, including caspase-2, −3,
−6, −7, −8, −9 and −10 (22).
Caspases are well known for their roles in apoptosis and
inflammatory responses (23).
Caspase-9 is considered to be the initiator caspase in the caspase
cascade reaction and the presence of cytochrome c in the
cytoplasm causes the activation of caspase-9 (24). Upon activation of caspase-9,
caspase-3, which is an important death protease, becomes activated;
the activation of caspase-3 may be used as a biomarker to identify
cells that are undergoing apoptosis (14).
Bcl-2 family proteins have important roles in
mitochondria-mediated apoptosis, and are considered to be the
initial regulatory step in the induction of mitochondrial apoptosis
(17). Bcl-2 and Bax are
recognized as apoptosis-associated proteins. Bcl-2 directly binds
and suppresses the proapoptotic proteins of the Bcl-2 family;
however, Bax directly causes cytochrome c to be released
into the cytoplasm or inhibits antiapoptotic Bcl-2 proteins
(18). To explore the potential
mechanism underlying DMD-induced apoptosis of the H22 cell line,
the present study determined the expression of various proteins
associated with the mitochondria-mediated apoptotic pathway,
including cytochrome c, c-caspase-3, c-caspase-9,
c-caspase-7, Bax and Bcl-2. The results indicated that DMD
upregulated the expression of cytochrome c, c-caspase-3,
c-caspase-9, c-caspase-7 and Bax, and downregulated the expression
of Bcl-2, compared with control cells. Therefore, the results of
the present study indicated that DMD may induce
mitochondria-mediated apoptosis in the H22 mouse HCC cell line.
MAPKs are serine/threonine kinases that function in
the regulation of a wide range of cellular processes, including
proliferation, differentiation and apoptosis (9). MAPKs consist of three families: JNK,
ERK and p38 MAPK (25,26). ERK is involved in functions that
include cell proliferation and the prevention of apoptosis, and
exhibits a cytoprotective role in the MAPK apoptosis pathway
(27). Conversely, the JNK and p38
MAPK cascades are associated with proapoptotic effects (28). To investigate whether DMD-induced
apoptosis of H22 cells was associated with the MAPK pathway, the
present study detected the expression levels of JNK, ERK1/2 and p38
proteins, and the level of phosphorylation of these proteins. The
results indicated that DMD significantly upregulated the expression
levels of p-JNK and downregulated the expression levels of p-ERK1/2
dose-dependently, whereas DMD exhibited no obvious effect on JNK,
ERK1/2, p38 and p-p38 protein expression, compared with control
cells. These results indicated that DMD-mediated apoptosis may be
associated with the MAPK pathway.
In conclusion, the results of the present study
demonstrated that DMD isolated from APM exhibited a notable
antitumor effect against H22 cells, and the potential underlying
mechanism may be associated with mitochondria-mediated apoptosis
via increased expression of cytochrome c, c-caspase-3,
c-caspase-9, c-caspase-7 and Bax, and reduced expression of Bcl-2.
In addition, the antitumor effects of DMD against H22 cells may
also involve the MAPK pathway, as the results demonstrated
increased p-JNK expression and reduced p-ERK1/2 expression, which
serve proapoptotic and cytoprotective roles, respectively.
References
|
1
|
Xie QC and Yang YP: Anti-proliferative of
physcion 8-O-β-glucopyranoside isolated from Rumex japonicas Houtt.
on A549 cell lines via inducing apoptosis and cell cycle arrest.
BMC Complem Altern Med. 14:3772014. View Article : Google Scholar
|
|
2
|
Li JP, Zhao DL, Jiang HJ, Huang YH, Li DQ,
Wan Y, Liu XD and Wang JE: Assessment of tumor vascularization with
functional computed tomography perfusion imaging in patients with
cirrhotic liver disease. Hbpd Int. 10:43–49. 2011.PubMed/NCBI
|
|
3
|
Bruix J and Llovet JM: Prognostic
assessment and evaluation of the benefits of treatment. J Clin
Gastroenterol. 35 5 Suppl 2:S138–S142. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marra M, Sordelli IM, Lombardi A, Lamberti
M, Tarantino L, Giudice A, Stiuso P, Abbruzzese A, Sperlongano R,
Accardo M, et al: Molecular targets and oxidative stress biomarkers
in hepatocellular carcinoma: An overview. J Transl Med. 9:1712011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Katsuta E, Tanaka S, Mogushi K, Matsumura
S, Ban D, Ochiai T, Irie T, Kudo A, Nakamura N, Tanaka H, et al:
Age-related clinicopathologic and molecular features of patients
receiving curative hepatectomy for hepatocellular carcinoma. Am J
Surg. 208:450–456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kasai K, Kuroda H and Suzuki K: Adjuvant
therapy after treatment of hepatocellular carcinoma. Nippon
Shokakibyo Gakkai Zasshi. 105:787–794. 2008.(In Japanese).
PubMed/NCBI
|
|
7
|
Caraglia M, Giuberti G, Marra M, Addeo R,
Montella L, Murolo M, Sperlongano P, Vincenzi B, Naviglio S, Prete
SD, et al: Oxidative stress and ERK1/2 phosphorylation as
predictors of outcome in hepatocellular carcinoma patients treated
with sorafenib plus octreotide LAR. Cell Death Dis. 2:e1502011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fokunang CN, Ndikum V, Tabi OY, Jiofack
RB, Ngameni B, Guedje NM, Tembe-Fokunang EA, Tomkins P, Barkwan S,
Kechia F, et al: Traditional medicine: Past, present and future
research and development prospects and integration in the national
health system of Cameroon. Afr J Tradit Complement Altern Med.
8:284–295. 2011.PubMed/NCBI
|
|
9
|
Xie Q, Yang Y, Wang Z, Chen F, Zhang A and
Liu C: Resveratrol-4-O-D-(2′-galloyl)-glucopyranoside isolated from
Polygonum cuspidatum exhibits anti-hepatocellular carcinoma
viability by inducing apoptosis via the JNK and ERK pathway.
Molecules. 19:1592–1602. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhong YM and Feng YF: Advance on the
chemical constituents and pharmacological effects of Atractylodes
macrocephala Koidz. J Guangdong Coll Pharm. 1–221. 2012.
|
|
11
|
Peng W, Han T, Liu Q and Qin L: Chemical
constituents from aerial part of Atractylodes macrocephala.
Zhongguo Zhong Yao Za Zhi. 36:578–581. 2011.(In Chinese).
PubMed/NCBI
|
|
12
|
National Institute of Health (NIH), .
Public health service policy on humane care and use of laboratory
animals. NIH; Bethesda, MD, USA: 2002
|
|
13
|
Resch M, Steigel A, Chen ZL and Bauer R:
5-Lipoxygenase and cyclooxygenase-1 inhibitory active compounds
from Atractylodes lancea. J Nat Prod. 61:347–350. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang XK, Xu MY, Xu GS, Zhang YL and Xu ZX:
In vitro and in vivo antitumor activity of scutebarbatine A on
human lung carcinoma A549 cell lines. Molecules. 19:8740–8751.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mattern J and Volm M: Imbalance of cell
proliferation and apoptosis during progression of lung carcinomas.
Anticancer Res. 24:4243–4246. 2004.PubMed/NCBI
|
|
16
|
Liu YL, Tang LH, Liang ZQ, You BG and Yang
SL: Growth inhibitory and apoptosis inducing by effects of total
flavonoids from Lysimachia clethroides Du by in human chronic
myeloid leukemia K562 cells. J Ethnopharmacol. 131:1–9. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okada H and Mak TW: Pathways of apoptotic
and non-apoptotic death in tumor cells. Nat Rev Cancer. 4:592–603.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chipuk JE, McStay GP, Bharti A, Kuwana T,
Clarke CJ, Siskind LJ, Obeid LM and Green DR: Sphingolipid
metabolism cooperates with Bak and Bax to promote the mitochondrial
pathway of apoptosis. Cell. 148:988–1000. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Delivani P and Martin SJ: Mitochondrial
membrane remodeling in apoptosis: An inside story. Cell Death
Differ. 13:2007–2010. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guerra-Castellano A, Díaz-Moreno I,
Velázquez-Campoy A, De la Rosa MA and Díaz-Quintana A: Structural
and functional characterization of phosphomimetic mutants of
cytochrome c at threonine 28 and serine 47. Biochim Biophys Acta.
1857:387–395. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng TS and Li X: Research progress in
mitochondrial apoptosis pathway. Yixue Zongshu. 19:3282–3285.
2013.(In Chinese).
|
|
22
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 5:a0086562013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu J, Jiang S, Li Y, Li M, Cheng Q, Zhao
D, Yang B, Jia Z, Wang L and Song L: Caspase-3 serves as an
intracellular immune receptor specific for lipopolysaccharide in
oyster Crassostrea gigas. Dev Comp Immunol. 61:1–12. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao N, Budhraja A, Cheng S, Yao H, Zhang Z
and Shi X: Induction of apoptosis in human leukemia cells by grape
seed extract occurs via activation of c-Jun NH2-terminal kinase.
Clin Cancer Res. 15:140–149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pearson G, Robinson F, Gibson T Beers, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: Regulation and physiological functions.
Endocr Rev. 22:153–183. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boutros T, Chevet E and Metrakos P:
Mitogen-activated protein (MAP) Kinase/MAP kinase phosphatase
regulation: Roles in cell growth, death, and cancer. Pharmacol Rev.
60:261–310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao N, Budhraja A, Cheng S, Liu EH, Huang
C, Chen J, Yang Z, Chen D, Zhang Z and Shi X: Interruption of the
MEK/ERK signaling cascade promotes dihydroartemisinin-induced
apoptosis in vitro and in vivo. Apoptosis. 16:511–523. 2011.
View Article : Google Scholar : PubMed/NCBI
|