Introduction
Lung cancer is the main cause of cancer-associated
mortality worldwide, representing almost one in five cases of
cancer-associated mortality, with non-small cell lung cancer
(NSCLC) accounting for three in four cases of lung cancer. A large
proportion of patients with NSCLC succumb to mortality in the first
few years following diagnosis, and the morbidity and mortality
rates remain high at 5 years. Although advances in the treatment of
NSCLC have improved patient prognosis, >50% of patients with
NSCLC are diagnosed at an advanced stage of disease, and there is
currently no curative therapy for these patients (1,2). In
the treatment of lung cancer, >90% of patients with lung cancer
are managed with conventional therapy, including surgery, radiation
and chemotherapy, however, their efficacy remains limited and it is
difficult to achieve complete remission or cure (3–5).
Less invasive therapies to control local tumor
development and metastasis, including minimally invasive and less
radical removal, in addition to image-guided ablative approaches
are currently used in the treatment of early-stage NSCLC (6).
Compared with small interfering RNA (siRNA),
vector-based RNA interference (RNAi) provides a prolonged duration
of gene silencing and has attracted increased interest; the
application of RNAi to downregulate oncogene expression and alter
the biological behavior of tumor cells represents the newest type
of treatment for NSCLC (7).
Livin and Survivin, of the inhibitors of apoptosis
(IAP) family, are reported to be associated with the development
and prognosis of tumors, and are expressed at high levels in the
majority of tumor cells, including prostatic cancer, osteosarcoma,
bladder cancer and lung cancer (8–10).
Therefore, Survivin and Livin may be novel promising targets in
cancer therapy. Yang et al reported that the dual short
hairpin RNA (shRNA) silencing of Livin and Survivin genes
significantly inhibited their expression levels in PC-3M prostate
cancer cells, reduced proliferation and facilitated apoptosis of
the PC-3M cells (11). Guan et
al indicated that the mPEG-CSNPs mediated Livin and Survivin
interference, significantly decreasing their expression in MG-63
osteosarcoma cells, suppressing the proliferation and promoting the
apoptosis of osteosarcoma cells (10). It has been reported that Livin and
Survivin, in addition to X-linked inhibitor of apoptosis (XIAP),
have a synergistic effect reducing cellular proliferation and
promoting the apoptosis of human bladder cancer cells (12). Zhang et al also reported
that the overexpression of Sharpin activated the nuclear factor-κB
pathway and downstream targets Survivin and Livin, potentially
promoting the development of prostate cancer (13).
However, whether the double silencing of Livin and
Survivin has any synergistic effect on the growth, proliferation
and apoptosis of human NSCLC remains to be elucidated. In the
present study, eukaryotic expression vectors encoding Livin and
Survivin shRNAs were designed and constructed for transfection or
co-transfection into human NSCLC cells to examine the effects of
single shRNA silencing and double silencing on the biological
function of human NSCLC cells.
Materials and methods
Cell culture
The A549 human lung cancer cell line was purchased
from the Cell Bank of Shanghai Institutes for Biological Sciences,
Chinese Academy of Sciences (Shanghai, China). The A549 cells were
cultured in RPMI-1640 medium with 10% heat inactivated fetal bovine
serum (FBS) (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) in a humidified incubator with 5% CO2 at 37°C.
Construction of shRNA expression
vectors
The Livin (Gene ID: 79444) and Survivin (Gene ID:
332) mRNA sequences were obtained from the NCBI database
(http://www.ncbi.nlm.nih.gov/pubmed)
according to a previous study (14). The pSILENCER-3.0H1 expression
vector (Ambion; Thermo Fisher Scientific, Inc.) contained a human
H1 polymerase-III promoter for shRNA expression. According to the
methods described previously (15,16),
the following 19 nucleotide siRNA target sequences were designed:
Livin, 5′-GGAAGAGACTTTGTCCACA-3′; Survivin,
5′-GGACCACCGCATCTCTACA-3′. Each shRNA insert was designed as a
synthetic duplex with overhanging ends identical to those created
by BamHI restriction enzyme digestion at the 5′ end and
HindIII restriction enzyme digestion at the 3′ end. There
was a nine-nucleotide ring sequence (TTC AAG AGA) between the
strand and antisense strands. Following digestion by the
BamHI/HindIII restriction endonucleases, the two
sequences were inserted into the pSILENCER-3.0H1 plasmid by
ligating the same restriction enzyme-digested shRNA fragment to the
vector.
Transfection
Transfection was performed using
Lipofectamine® LTX reagent according to the
manufacturer's protocol. At 24 h prior to transfection, the A549
cells were harvested and 2.5×105 cells were plated in
each well of a 6-well plate in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) with 10% FBS at 5% CO2, 37°C, 95%
humidity. When the cells reached 50–80% confluence, the culture
medium was removed and replaced with 2 ml of complete culture
medium. For the transfection of cells in each well, 2.5 µg of
plasmid diluted in 100 µl of Opti-MEM® I reduced serum
medium (0% serum), following which Lipofectamine LTX®
reagent (3.75–8.75 µl) was added into the above diluted solution.
The DNA-Lipofectamine LTX® reagent complexes formed
following gentle mixing of the solution and incubation at room
temperature for 30 min. The DNA-Lipofectamine LTX®
reagent complexes (500 µl) were added directly to each well
containing 2.5×105 cells and mixed gently by rocking the
plate back and forth for 30 min. The complexes were not removed
following transfection. Prior to transgene expression assaying, the
transfected cells were incubated at 37°C, 5% CO2 for 24
h following transfection. The cells were divided into four groups:
pSilencer-Livin transfection group; pSilencer-Survivin transfection
group; pSilencer-Survivin and pSilencer-Livin double-transfection
group; and no transfection group (control group).
mRNA expression levels of livin and
survivin
Fluorescent reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis was performed to
determine the expression levels of Survivin and Livin according to
a previous study (17). Following
transfection for 72 h, the cells were harvested and total RNA
extraction was performed using TRIzol reagent (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany). PrimeScript RT Master Mix (Takara
Bio, Inc., Otsu, Japan) was used to perform reverse transcription
according to the manufacturer's protocol. Briefly, 2 µl 5X Primer
Script mix, 3 µl RNase-free water and 5 µl total RNA were added to
the reaction system. The mixture was incubated for 15 min at 37°C,
and then at 85°C for 5 sec, following which the mixture was
maintained at 4°C. The primer sequences were as follows: Survivin,
forward 5′-ATTCGCAGACTGGCCCTTTA-3′ and reverse
5′-AGAGGAAACCACAGTTGGCAG-3′; Livin, forward
5′-AGGGCGTGGTGGGTTCTTG-3′ and reverse 5′-CGGCACAAAGACGATGGACA-3′.
The ABI PRISM 7000 sequence detection system and SYBR-Green PCR
Master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.)
were used to perform RT-qPCR. The PCR sample volume of 25 µl
consisted of 12.5 µl SYBR-Green PCR Master mix, 300 nM forward
primers and reverse primers, and 2.0 µl template cDNA (diluted
1:20). The PCR cycle consisted of a total of 40 cycles, the
parameters were as follows: Pre-degeneration for 30 sec at 95°C;
followed by denaturation at 95°C for 5 sec and annealing at 60°C
for 20 sec, and then melting curve analysis for 15 sec at 65°C. The
2−ΔΔCq method (18),
with a reference gene (β-actin, forward 5′-GAACGGTGAAGGTGACAG-3′
and reverse 5′-TAGAGAGAAGTGGGGTGG-3′) as an internal standard, was
used to determine the relative gene expression.
Western blot analysis
The protein expression levels of Livin and Survivin
were determined using western blot analysis (19). At 48 h post-transfection, cells
were lysed with lysis buffer (Invitrogen; Thermo Fisher Scientific,
Inc.) with protease inhibitor cocktail tablets (Roche Diagnostics
GmbH, Mannheim, Germany) and phosphatase inhibitors (1 mM NaF and 1
mM Na3CV04). Protein concentration was measured using a protein
assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA) with
bovine serum albumin (Bio-Rad Laboratories, Inc.) as the standard.
Proteins (15 µg/lane) were separated on 10% SDS-PAGE gels, and
these were transferred onto nitrocellulose membranes. Subsequently,
5% w/v non-fat dry milk in Tris-buffered saline and Tween-20 (TBST)
buffer, containing 20 mmol/l Tris-HCl (pH 7.4), 150 mmol/l NaCl and
0.05% Tween-20, was used to block the membranes at room temperature
for 1 h. The membranes were then incubated with primary antibodies
at room temperature for 1 h. Primary antibodies were as follows:
Survivin rabbit mAb (cat. no. 2808; 1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA) and livin rabbit mAb (cat. no.
5471; 1:1,000; Cell Signaling Technology, Inc.). Following three
15-min washes in TBST, the membranes were incubated with individual
secondary antibodies [anti-rabbit IgG horseradish peroxidase
(HRP)-linked antibody, cat. no. 7074 and anti-biotin HRP-linked
antibody, cat. no. 7075; both 1:2,000; Cell Signaling Technology,
Inc.] at room temperature for 1 h, followed by three 10-min washes
with TBST. The subsequent signal generation was performed using a
Perfect Protein Western Blot kit (Novagen, Inc., Madison, WI, USA).
The membrane was visualized with ECL Western Blotting Detection
reagents (GERPN2209; Sigma-Aldrich; Merck KGaA). Image J software
(ImageJ2× 2.1.4.7; National Institutes of Health, Bethesda, MD,
USA) was used for densitometric analysis and the relative protein
levels were evaluated using GADPH (cat. no. 8884; 1:1,000; Cell
Signaling Technology, Inc.) as the control.
CCK assay
The cells were seeded into each well of a 96-well
plate (1×103 cells/well) 24 h prior to transfection and
were divided into the four groups described above. The transfection
procedure was performed according to the protocol described above.
Following transfection, the cells were cultured for 24, 48 or 72 h
at 37°C in 5% CO2, respectively. The optical density
(OD) values were measured at an absorbance of 490 nm. The
inhibition rate of cell proliferation was calculated using the
following formula: (1-experimental group OD value/control group OD)
× 100%.
Cancer cell xenograft model and
plasmid injection
The use of animals in the present study was approved
by the Institutional Approval Board of The Second Affiliated
Hospital of Fujian Medical University (Quanzhou, China). The human
cancer cell xenograft models were constructed according to a
previous study (20). In brief,
the A549 cells were obtained and washed with PBS three times,
re-suspended in RPMI-1640 medium with 10% FBS, and injected
(2×106 cells) into the flank regions of athymic nude
(nu/nu) mice (male; age, 8 weeks; weight, 20 g). A total of 36 mice
were supplied by the Laboratory Animal Center, Fujian Medical
University and housed under standard animal housing conditions,
with a temperature of 22±1°C, a 12-h light/dark cycle and free
access to food and water. Following tumor development, the tumor
fragments were transplanted subcutaneously into the flanks of both
sides of nude mice, following which the mice were randomly divided
into four groups (n=6/group): shRNA-Livin injection group;
shRNA-Survivin injection group; shRNA-Survivin and shRNA-Livin
double-injection group; and PBS group (control group). When the
volume of the tumors reached 50–100 mm3, the mice were
administered with shRNA plasmids in 500 µl PBS via injection into
the tail vein three times each week for 6 weeks. The tumor
diameters were calculated during the injection period and for 4–6
weeks following the final injection.
Analysis of apoptosis
The apoptosis of cells was detected using a TUNEL
assay. The cells were seeded onto a 25 cm2 plate 24 h
prior to transfection, and divided into the four groups. At 48 h
post-transfection, 4% paraformaldehyde was used to fixed
non-adherent cells and adherent cells, and the cells were
permeabilized with a solution of 0.1% Triton X-100 in 0.1% sodium
citrate, followed by incubation for 1 h in the TUNEL reaction
mixture at room temperature (Roche Diagnostics GmbH). The cells
were washed with PBS three times, and the TUNEL-positive (FL1
>101) cells were analyzed by FACS analysis using
CellQuest version 5.1 software following flow cytometry (BD
Biosciences, Franklin Lakes, NJ, USA) (1).
To examine apoptosis in the tumor tissues, the mice
were sacrificed following the final injection, and the tumor
tissues were harvested and fixed in 4% paraformaldehyde in PBS and
embedded in paraffin. The tumor tissues were cut into 5-µm thick
tissue sections, and the sections were de-paraffinized in xylene,
rehydrated in reduced grades of alcohol and then washed in PBS. The
rehydrated slides were permeabilized in 0.1% Triton X-100 and 0.1%
sodium citrate, and then incubated in the TUNEL reaction mixture at
37°C in humidified conditions for 1 h. The sections were then
washed in PBS and observed under a fluorescence microscope.
Fluorescence values were calculated from three randomly selected
fields per section and expressed as the mean ± standard
deviation.
Statistical analysis
The data are presented as the mean ± standard
deviation and were analyzed by one-way analysis of variance with a
Newman-Keuls post hoc test using SPSS 17.0 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Relative mRNA expression of livin and
survivin
In order to evaluate the effects of Livin shRNA
and/or Survivin shRNA transfection on mRNA levels, fluorescent
RT-qPCR was used to evaluate the relative mRNA expression levels of
Livin and Survivin. Following transfection for 48 h, the relative
mRNA expression of Livin was 0.18±0.02 in the Survivin shRNA and
Livin shRNA co-transfection group, which was lower than that in the
Livin shRNA transfection group (0.72±0.17; Fig. 1A). The relative mRNA expression of
Survivin was 0.21±0.02 in the Survivin shRNA and Livin shRNA
co-transfection group, which was lower than that in the Survivin
shRNA transfection group (0.75±0.15; Fig. 1B), indicating that shRNA
transfection decreased the mRNA expression levels of Livin and
Survivin. Co-transfection was more efficient than single
transfection with either Survivin shRNA or Livin shRNA alone
(Fig. 1).
Protein expression of livin and
survivin
Western blot analysis was performed to measure the
protein expression levels of Livin and Survivin. Following
transfection for 48 h, the relative protein expression of Livin was
0.32±0.03 in the co-transfection group, which was lower than that
in the Livin shRNA transfection group (0.69±0.18; Fig. 2A). The relative protein expression
of Survivin was 0.35±0.04 in the co-transfection group, which was
lower than that in the Survivin shRNA transfection group
(0.67±0.19; Fig. 2B), indicating
that shRNA transfection decreased the protein expression of Livin
and Survivin. Co-transfection was more efficient than single
transfection with either Survivin shRNA or Livin shRNA alone
(Fig. 2).
CCK assay
As shown in Fig. 3,
transfection with Livin shRNA and/or Survivin shRNA inhibited cell
proliferation in the transfected A549 cells. The proliferation of
cells in the co-transfection group exhibited a higher rate of
growth inhibition, compared with that in either the Livin shRNA or
Survivin shRNA transfection group (P<0.05).
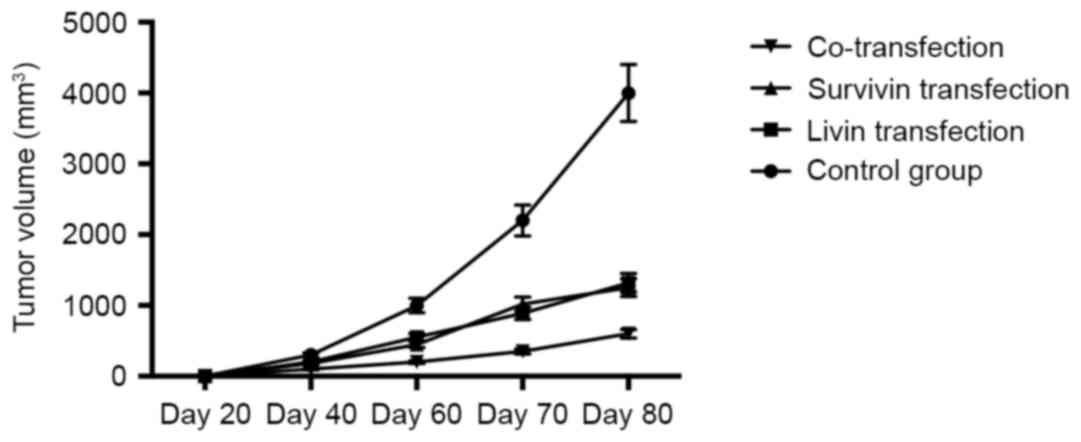
Antitumor effects of shRNAs in
vivo
In order to evaluate the antitumor effects of the
shRNAs in vivo, the nude mice, which were implanted with
tumor xenografts established using A549 cells, were injected with
shRNA plasmids. As shown in Fig.
4, shRNA plasmid injection inhibited tumor growth significantly
in the mice, compared with that in the control group, and double
injection with Livin ShRNA and Survivin shRNA exerted a more marked
effect, compared with that in the groups transfected with Livin
shRNA or Survivin shRNA alone.
Analysis of apoptosis
As shown in Fig.
5A, after 48 h, transfection with Livin shRNA and/or Survivin
shRNA enhanced cell apoptosis. The apoptotic rate was 55.40±5.48%
in the co-transfection group, which was higher than that in either
the Livin shRNA transfection group (37.80±4.11%) or the Survivin
shRNA transfection group (42.61±3.99%; P<0.05). This indicated
that Livin and Survivin shRNA co-transfection was more effective in
promoting apoptosis.
As shown in Fig.
5B, the injection of Livin shRNA and/or Survivin shRNA induced
apoptosis in the tumor tissues, and the number of apoptotic cells
in the Livin shRNA and Survivin shRNA double injection group was
higher, compared with the number of apoptotic cells in either the
Livin shRNA injection group or the Survivin shRNA injection group
(P<0.05). This suggested that Livin shRNA and Survivin shRNA
double injection was more effective than either Livin shRNA or
Survivin shRNA in inducing apoptosis in the tumor tissues.
Discussion
Lung cancer is one of the most common and
life-threatening malignances worldwide, and remains one of the
biggest challenges faced by physicians, with a mortality rate
similar to that of breast and colorectal cancer combined, despite
incidence being similar to that of breast or colorectal cancer
(21). NSCLC remains the main
subtype of lung cancer, accounting for ~75% of all lung cancer
cases. NSCLC is life threatening, with a five-year relative
survival rate of ~17% (22).
As cancer is one of the key targets of RNAi-based
therapy, RNAi is important in cancer treatment. RNAi has high gene
silencing efficiency, which can induce the silencing of advanced
stages of tumor growth, transmitting the silenced gene to
subsequent generations. It is also relatively low cost compared
with other gene therapies, and has high specificity compared with
cancer therapies, including chemotherapy (23). As RNAi-based therapy has high
efficiency and specificity for gene silencing, a precise functional
mechanism and fewer side effects, compared with chemotherapy,
oncogenes and certain other genes involved in tumor formation and
development are ideal gene silencing targets for RNAi-based therapy
(23).
The IAP family members are regulators of apoptosis,
cytokinesis and signal transduction, which are important in the
process of cancer resistance to apoptosis and are produced in the
processes of standard cytotoxic chemotherapy or radiotherapy.
Therefore, cancer cells acquire mutations that allow them to
survive apoptosis that are part of the transformation process or
that may affect the growth and dissemination of the tumor (9). It had been reported that Survivin and
Livin have are expressed at high levels in the majority of solid
tumors, and it has been suggested that their expression is
associated with prognostic significance (8). As Survivin and Livin appear to be
novel promising targets in cancer therapy, Livin and Survivin
shRNAs were established in the present study to silence their
respective expression to examine the synergistic effect of the two
IAPs in inhibiting apoptosis. The results of the RT-qPCR and
western blot analyses demonstrated that the relative mRNA and
protein expression levels were significantly decreased in the Livin
and Survivin shRNA groups, and the effects were more marked in the
Livin and Survivin shRNA co-transfection group, compared with
either the Livin shRNA or Survivin shRNA single transfection groups
alone. Yang et al previously reported that the knockdown of
Livin, X-linked inhibitor of apoptosis (XIAP), and Survivin reduced
the proliferation and transformation of high-grade bladder cancer
T24 cells, but enhanced the apoptotic sensitivity of the cells to
chemotherapy. In addition, the combined silencing of Livin, XIAP
and Survivin significantly increased the levels of active
caspase-3, active caspase-7, active caspase-9 and cytosolic second
mitochondria-derived activator of caspase (12).
In the present study, the results of xenograft tumor
experiments showed that transfection with Livin or Survivin shRNA
reduced tumor growth significantly, compared with that in the
untreated group, and Livin and Survivin shRNA co-transfection led
to a more marked reduction of tumor size, compared with that in the
cells transfected with either vector alone.
In conclusion, the knockdown of both the Livin and
Survivin genes by shRNAs may be a promising therapy in the
treatment of cancer.
Acknowledgements
The present study was supported partly by grants
from the National Natural Science Foundation of China (grant no.
81141093), the Science Foundation of the Fujian Province, China
(grant no. 2013J01290), Technology Foundation for Selected Overseas
Chinese Scholar, Ministry of Personnel of China (2012) and the
Nursery Research Fund of Second Affiliated Hospital of Fujian
Medical University (grant no. 2012MP73).
References
|
1
|
Gatti L, Cossa G, Tinelli S, Carenini N,
Arrighetti N, Pennati M, Cominetti D, De Cesare M, Zunino F,
Zaffaroni N and Perego P: Improved apoptotic cell death in
drug-resistant non-small-cell lung cancer cells by tumor necrosis
factor-related apoptosis-inducing ligand-based treatment. J
Pharmacol Exp Ther. 348:360–371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Janssen-Heijnen ML, van Steenbergen LN,
Steyerberg E, Visser O, De Ruysscher DK and Groen HJ: Long-term
excess mortality for survivors of non-small cell lung cancer in the
Netherlands. J Thorac Oncol. 7:496–502. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chung JH, Choi YS, Cho JH, Kim HK, Kim J,
Zo JI and Shim YM: Uniportal video-assisted thoracoscopic
lobectomy: An alternative to conventional thoracoscopic lobectomy
in lung cancer surgery? Interact Cardiovasc Thorac Surg.
20:813–819. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tacelli N, Santangelo T, Scherpereel A,
Duhamel A, Deken V, Klotz E, Cortot A, Lafitte JJ, Wallyn F, Remy J
and Remy-Jardin M: Perfusion CT allows prediction of therapy
response in non-small cell lung cancer treated with conventional
and anti-angiogenic chemotherapy. Eur Radiol. 23:2127–2136. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takenaka T, Takenoyama M, Inamasu E,
Yoshida T, Toyokawa G, Nosaki K, Hirai F, Yamaguchi M, Shimokawa M,
Seto T and Ichinose Y: Role of surgical resection for patients with
limited disease-small cell lung cancer. Lung Cancer. 88:52–56.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Donington JS, Koo CW and Ballas MS: Novel
therapies for non-small cell lung cancer. J Thorac Imaging.
26:175–185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takahashi Y, Yamaoka K, Nishikawa M and
Takakura Y: Quantitative and temporal analysis of gene silencing in
tumor cells induced by small interfering RNA or short hairpin RNA
expressed from plasmid vectors. J Pharm Sci. 98:74–80. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gazzaniga P, Gradilone A, Giuliani L,
Gandini O, Silvestri I, Nofroni I, Saccani G, Frati L and Aglianò
AM: Expression and prognostic significance of LIVIN, SURVIVIN and
other apoptosis-related genes in the progression of superficial
bladder cancer. Ann Oncol. 14:85–90. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
LaCasse EC, Mahoney DJ, Cheung HH,
Plenchette S, Baird S and Korneluk RG: IAP-targeted therapies for
cancer. Oncogene. 27:6252–6275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guan HP, Sun JZ, Feng XL, Chen JS, Chen
FJ, Cheng XF, Liu XW and Ni B: Effects of RNA interference-mediated
knockdown of livin and survivin using monomethoxypolyethylene
glycol-chitosan nanoparticles in MG-63 osteosarcoma cells. Mol Med
Rep. 13:1821–1826. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang AQ, Wang PJ, Huang T, Zhou WL and
Landman J: Effects of monomethoxypolyethylene glycol-chitosan
nanoparticle-mediated dual silencing of livin and survivin genes in
prostate cancer PC-3M cells. Genet Mol Res. 5:2016.doi:
10.4238/gmr.15027430.
|
|
12
|
Yang D, Song X, Zhang J, Ye L, Wang S, Che
X, Wang J, Zhang Z, Wang L and Shi W: Therapeutic potential of
siRNA-mediated combined knockdown of the IAP genes (Livin, XIAP,
and Survivin) on human bladder cancer T24 cells. Acta Biochim
Biophys Sin (Shanghai). 42:137–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Huang H, Zhou H, Du T, Zeng L,
Cao Y, Chen J, Lai Y, Li J, Wang G and Guo Z: Activation of nuclear
factor kB pathway and downstream targets survivin and livin by
SHARPIN contributes to the progression and metastasis of prostate
cancer. Cancer. 120:3208–3218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McIntyre GJ and Fanning GC: Design and
cloning strategies for constructing shRNA expression vectors. BMC
Biotechnol. 6:12006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao X, Yuan Y, Zhang Z, Feng X, Zhang J,
Yuan X and Li J: Effects of shRNA-silenced livin and survivin on
lung cancer cell proliferation and apoptosis. J BUON. 19:757–762.
2014.PubMed/NCBI
|
|
16
|
Xu W, Chang H, Qin CK and Zhai YP: Impact
of Co-transfection with livin and survivin shRNA expression vectors
on biological behavior of HepG2 cells. Asian Pac J Cancer Prev.
14:5467–5472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kong X, Li L, Sun L, Fu K, Long J, Weng X,
Ye X, Liu X, Wang B, Yan S, et al: Rapid diagnosis of aneuploidy
using segmental duplication quantitative fluorescent PCR. PLoS One.
9:e889322014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mavridis K, Stravodimos K and Scorilas A:
Downregulation and prognostic performance of microRNA 224
expression in prostate cancer. Clin Chem. 59:261–269. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tirrò E, Consoli ML, Massimino M, Manzella
L, Frasca F, Sciacca L, Vicari L, Stassi G, Messina L, Messina A
and Vigneri P: Altered expression of c-IAP1, survivin, and Smac
contributes to chemotherapy resistance in thyroid cancer cells.
Cancer Res. 66:4263–4272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Spänkuch B, Matthess Y, Knecht R, Zimmer
B, Kaufmann M and Strebhardt K: Cancer inhibition in nude mice
after systemic application of U6 promoter-driven short hairpin RNAs
against PLK1. J Natl Cancer Inst. 96:862–872. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Custodio A, Méndez M and Provencio M:
Targeted therapies for advanced non-small-cell lung cancer: Current
status and future implications. Cancer Treat Rev. 38:36–53. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van der Drift MA, Karim-Kos HE, Siesling
S, Groen HJ, Wouters MW, Coebergh JW, de Vries E and
Janssen-Heijnen ML: Progress in standard of care therapy and modest
survival benefits in the treatment of non-small cell lung cancer
patients in the Netherlands in the last 20 years. J Thorac Oncol.
7:291–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mansoori B, Shotorbani S Sandoghchian and
Baradaran B: RNA interference and its role in cancer therapy. Adv
Pharm Bull. 4:313–321. 2014.PubMed/NCBI
|